Abstract
Background: Emergency department (ED) human immunodeficiency virus (HIV) screening programs are challenged by the unsustainable cost of exogenous staff and the relatively low penetration rates. Kiosk systems have increased registration efficiency in various clinical settings and have shown promising results for advancing various public health initiatives. This study evaluated the usability of kiosks within the existing HIV testing program and assessed patients' perceived acceptability of kiosk-based screening in the ED. Subjects and Methods: ED patients (n=88) were asked to complete both a Registration Module (intended to integrate into the ED's pending kiosk registration system) and a Risk Assessment Module using a pen-based touchscreen tablet platform. Participants provided feedback upon program completion. All comments, questions, and errors were documented. Kiosk programs tracked time spent on each screen. Quantitative (chi-squared test or t test) and qualitative data analyses were performed. Results: Consented subjects (n=62) were 60% female, 69% were black, the mean±standard deviation age was 37.8±11.4 years, 52% had a high school degree or less, and 50% reported no prior kiosk experience. Mean time spent on the Registration and Risk Assessment Modules was 2:35±1:24 min and 5:09±1:58 min, respectively. The leading technical challenge identified was login: 84% of patients required assistance. Removal of the login screen reduced times to 1:05±0:36 min and 4:10±1:38 min. Ninety-five percent of subjects reported length of use as “just right,” and over 75% of patients found the software easy to use, answered questions without help, and preferred screening on the kiosk to in-person interviews. Favorite aspects of the program included ease of use (52%), privacy (48%), and speed (30%). Sixty-six percent of patients reported there was nothing they disliked or would change. Conclusions: ED patient response to the kiosk system was favorable. Subjects easily and quickly navigated the program, with the exception of a login screen, which could be eliminated via automated login using ID bracelet scanners.
Key words: : human immunodeficiency virus testing, emergency department, kiosk, usability
Introduction
The latest estimates from the Centers for Disease Control and Prevention (CDC) indicate there are approximately 48,000 new human immunodeficiency virus (HIV) infections every year in the United States,1 and approximately 18% of the more than 1.1 million Americans infected with HIV are unaware of their infectious status.2 These estimated 200,000 plus unrecognized HIV-infected individuals serve as one of the most important “transmission pools” for the country's ongoing HIV epidemic. Accordingly, the CDC has introduced and implemented a wide range of aggressive national screening strategies.3–5 A cornerstone of the current CDC approach for identifying patients with unrecognized HIV is widespread screening in emergency department (ED) settings.5 ED screening programs have identified hundreds of previous undiagnosed HIV-infected individuals since the 2006 CDC recommendations for HIV testing.6 However, existing ED programs are challenged by the inability to carry out true universal screening due to resource demands, as well as unsustainable costs for program funding, particularly those programs that require use of exogenous testing staff.7
Patient-centered touchscreen kiosk systems have been identified as an innovative approach to optimize ED “front-end” operation efficiency, including ED patient registration processing.8 Furthermore, computerized kiosks have been shown to be an excellent adjunctive tool in ED settings for provision of focused public health screening as well as patient-driven support for specific clinical conditions. Targeted kiosk-based initiatives in EDs have included screening, brief interventions and/or referral to treatment for intimate partner violence,9 child safety,10 suicide,11 and alcohol abuse,12 and asthma treatment,13,14 as well as appropriate antibiotic use for respiratory tract infections.15 To date, only one study of which we are aware has evaluated use of kiosks as part of an ED-based HIV testing program.16 Findings of this study suggest that although triage-based kiosks could be leveraged to obtain informed consent, patients' understanding of kiosk-based opt-out consent was suboptimal at best. Other uses of the kiosk to streamline HIV screening have yet to be studied.
In this pilot study, our group designed and customized kiosks to integrate with our rapid HIV screening services; the role of these kiosks included offering patients the opportunity for screening at ED registration and collecting behavioral risk data associated with our testing program. To avoid potential stigma associated with HIV screening, the kiosks were designed to include other public health screening information. Thus, our aim was to evaluate the usability of our computerized kiosks to offer HIV screening and collect risk assessment information to help streamline existing ED-based HIV screening services. In this usability study, the Registration Module and Risk Assessment Module were operated via a pen-based touchscreen tablet platform, as a prototype for the near-future patient-centered touchscreen computerized kiosks.
Subjects and Methods
Study Population and Setting
The usability study took place in an inner-city academic adult ED in Baltimore, MD, with an annual census of approximately 65,000 visits. The ED population is socioeconomically disadvantaged, with >75% African Americans, 15% prior or current injection drug users, and up to a 2.2% rate of newly diagnosed HIV from the existing ED rapid HIV screening program.17 In November 2005, the department established a nontargeted, rapid, oral fluid HIV screening program. ED patients were considered eligible for this study if also eligible for the existing HIV screening program: 18–64 years old; no previous diagnosis of HIV; no test in the past 3 months; not critically ill; and able to provide informed consent. Study participation was further restricted to English-speaking patients.
Study Design and Procedures
The usability study design included the evaluation of participants' ability to complete two prototype kiosk software modules via documented staff observations as well as embedded program data collection, a short constructive survey, and a short open-ended survey. ED patients were recruited by convenience sampling after registration between 8 a.m. and 11 p.m. during 10 weekdays from July 6, 2011 to July 22, 2011. Patients approached bedside under the existing HIV screening program were also asked to participate in this usability study. Those who agreed were verbally consented and then asked to complete a two-part survey on a CREOSO C5 computer tablet with pen-based touchscreen functionality (CREOSO, Phoenix, AZ) (Fig. 1). Trained research assistants observed participants and documented all patient comments and questions as well as any errors encountered while using the tablets. Upon completion of both program modules, participants provided further feedback via staff-administered interview. The study was approved by The Johns Hopkins University School of Medicine Institutional Review Board.
Fig. 1.

Prototype kiosk: a computer tablet with pen-based touchscreen functionality used in the usability study with the login screen displayed.
Study Tools
Study software includes two distinct survey module programs, both built using Visual Basics® within the Microsoft® (Redmond, WA) Access® platform and designed for optimal use with touchscreen technology. Questions appear in white text against a dark blue background, and users indicate answers by touching large yellow buttons that change color when selected. Patients navigate within modules by touching buttons in the lower corners of each screen labeled with forward and back arrows or labeled “continue.” The program forces patients to complete each portion of the module prior to advancing. If a patient attempts to advance to a new screen prior to answering all required questions, a pop-up indicating omitted answers appears. Patients can elect to exit the module at any time by touching a button labeled “X” in the upper right corner of each screen and are prompted to indicate why they desired to stop. Answer selection, as well as time spent on each screen, was directly imported into secure linked Access databases for analysis.
The first program, the “Registration Module,” was intended to integrate HIV test offers into the ED's planned kiosk registration by offering HIV tests to all eligible patients upon entry into the ED after initial registration. The module consisted of five screens: (1) “login,” where patients entered first and last names, date of birth, last four digits of social security number, and zip code; (2) a question assessing interest in services and information regarding other public health screening initiatives (including hypertension, diabetes, sexually transmitted diseases, substance abuse, depression, and domestic violence); (3) a question assessing patient comfort level with entering and updating health information (i.e., medical records) via kiosk; (4) a screen highlighting recommendations for regular HIV screening as well as the ease of testing and then offered a free test in the ED; and (5) instructions and information dependent on prior answer selections.
The second program, the “Risk Assessment Module,” was designed to replace the staff-administered risk assessment survey and demographic data collection, as well as subsequent data entry for patients who agree to test. The module includes up to 21 screens, with the exact number of screens varying based on user responses. Screens included the following: login; confirmation of the patient's desire to test; sociodemographic data collection (including computer and kiosk experience); HIV risk assessment questions; and a survey on the patient's preferences and ease of use regarding the kiosk program.
Data Analysis
Sociodemographic characteristics and time spent per module were summarized by descriptive statistical analysis. Qualitative data analysis was performed to analyze participants' overall experience with, as well as their three favorite and least favorite aspects of, the kiosk system. Chi-squared tests or t tests followed by multivariate regression analysis were performed to determine association between sociodemographic factors and perceptions of kiosk programs as well as time spent completing each module.
Results
Characteristics of Participants
Overall, 62 ED patients were recruited, and 61 completed both program modules among the 88 patients who were invited to participate in the study. The majority of these patients were female, African American, and under 40 years old (Table 1). Approximately half of the participants had an education level of a high school degree or less. Fifty percent of the subjects had some prior kiosk experience, most commonly at bank automated teller machines and grocery store self-checkout kiosks.
Table 1.
Sociodemographic Characteristics of 62 Emergency Department Patient Participants
| CHARACTERISTIC, CATEGORY | MEAN (SD) OR NUMBER (%) |
|---|---|
| Age (years) | |
| Mean | 37.8±11.4 |
| 20–29 | 16 (26) |
| 30–39 | 20 (33) |
| 40–49 | 12 (20) |
| 50–59 | 12 (20) |
| ≥60 | 1 (2) |
| Unknown | 1 (NC) |
| Gender | |
| Male | 25 (40) |
| Female | 37 (60) |
| Race/ethnicity | |
| African American | 42 (72) |
| White | 12 (21) |
| Hispanic | 2 (3) |
| Other | 2 (3) |
| Unknown | 4 (NC) |
| Highest education level | |
| Some high school or less | 16 (28) |
| High school degree | 14 (24) |
| Some college/trade school | 17 (29) |
| College degree or more | 11 (19) |
| Unknown | 4 (NC) |
| Prior kiosk experience | |
| None | 29 (50) |
| Some | 29 (50) |
| Bank ATM | 20 |
| Grocery | 10 |
| Healthcare setting | 5 |
| Airport | 5 |
| Other | 5 |
| Unknown | 4 (NC) |
ATM, automated teller machine; NC, not calculated; SD, standard deviation.
Module Program Performance
Mean time spent on the Registration and Risk Assessment Modules was 2:35±1:24 min and 5:09±1:58 min, respectively. Removal of the login screen significantly reduced times to 1:05±0:36 min and 4:10±1:38 min. Ninety-five percent of subjects reported length of use as “just right.” In addition, approximately 80% of participants agreed the following statements: “I found this software easy to use” (80%); “I answered these questions without help” (78%); and “I prefer doing this on the kiosk than in person” (80%).
User Comments and Feedbacks
Most of the participants (92%) reported positive experiences with our kiosk-based program modules (Table 2). Typical patient statements include “I liked it,” “It was fairly easy,” “100% cool,” “Good,” or “It was OK.” Only three participants stated that HIV screening was uncomfortable when completed via kiosk versus by in-person interview. Only two participants described the software program as hard or difficult.
Table 2.
Some Quotes from Participants on Their Experience in the Use of Kiosks for Offering Human Immunodeficiency Virus Testing in the Emergency Department
| FEATURE | QUOTE |
|---|---|
| Ease of use | “Extremely easy” |
| “Fine, quite easy” | |
| “Pretty easy. I love computers, so.” | |
| “Quick and easy” | |
| Favorable views | “100% liked” |
| “100% cool” | |
| “Fast quick, moved quickly between screens” | |
| “Fun, interesting, challenging because never used one before” | |
| All right, cool machine for person that doesn't know how to work it” | |
| “I like it because don't really have computer—novel experience like” | |
| “I liked it. Private, don't have to worry about others listening” | |
| “It's fine, less uncomfortable to use kiosk instead of person interview” | |
| “Extremely easy, more comfortable doing that than talking person” | |
| Less favorable views | “Unremarkable” |
| “Uncomfortable because of nature of (sex) questions” | |
| Unease with use | “No opinion. Hard because not computer inclined.” |
| “Very different, 5/5 difficulty” |
Overall, 66% of participants reported there was nothing that they disliked or would change about the program. Favorite aspects of the program reported by participants included ease of use (52%), privacy (48%), and speed (30%). Least favorite aspects of the program included the tablet's use of a “pen” rather than true touchscreen technology (10%), login screen (10%), and tablet weight (5%). Suggested changes from participants included addition of headphones for audio functionality (7%), replacement of the “pen” with true touchscreen functionality (7%), and elimination of the login screen (7%). Although roughly a quarter (26%) of participants stated they would prefer to wear headphones to hear the questions on the kiosk, the majority (54%) indicated that they would not.
Observational Notes
Research assistants observed that more than 95% of participants were able to maneuver through most screens on the Registration and Risk Assessment Modules (15 out of 27 screens) without help. The leading technical issue was login—85% and 25% of subjects required assistance for login for the Registration or Risk Assessment Module, respectively. The three other screens where a significant portion (>20%) of subjects required assistance include patient interest assessment for other public health screening initiatives in ED (for the Registration Module), prior kiosk experience, and receptive anal sex questions in the Risk Assessment Module. Additionally, two screens on the Risk Assessment Module regarding sexual behaviors and sexually transmitted diseases presented some difficulty, with over 10% of subjects requesting assistance.
Factors Associated With Time Spent and Participants' Feedback
Higher education levels and prior kiosk experience were associated with less time spent on kiosks. Participants with at least a high school degree spent approximately 1 min less each on both the Registration and Risk Assessment Modules compared with those with less than a high school degree (Registration, 2:09 min versus 3:19 min [p<0.05]; Risk Assessment, 4:52 min versus 5:42 min [p<0.05]). Participants who reported prior kiosk experience also spent approximately 1 min less per module than those without prior kiosk experience (4:41 min versus 5:51 min). No other factor was associated with time spent on the kiosks.
Both education level and prior kiosk experience also correlated with reported ease of use. Participants with at least a high school degree were significantly (p=0.014) more likely to rate the programs “Easy to Use” (93%) than participants with less than a high school degree (67%). Similarly, 90% of participants reporting prior kiosk experience rated the program “Easy to Use” compared with only 69% of those with no prior kiosk experience (p=0.052). Education level also inversely correlated with assistance needed to complete kiosk surveys. Participants with a high school degree or greater were more likely to report being able to answer questions without help compared with those with less than a high school degree (89% versus 67% [p=0.039]). Additionally, participants with at least a high school degree were more likely to report to prefer the kiosk program to an in-person interview than those with less than a high school degree (93% versus 67% [p=0.014]). Age, gender, and race did not correlate with a participant's feedback.
Improvement of Registration and Risk Assessment Module Software and Hardware
Based on the findings from the usability study, we have implemented the following improvements to the kiosk program to minimize technical assistance and streamline the process to allow further integration with ED care flow. First, we replaced the tablet with a free-standing touchscreen kiosk. This has allowed for a larger screen and font presentation, eliminated suboptimal tablet “pen” performance, and permitted for greater usability because the user no longer has to support the tablet's weight while navigating through screens. Second, we have worked with manufacturers to equip the kiosk with a wristband scanner. Because every ED patient receives a wristband after initial brief ED registration (Fig. 2), this allows for the elimination of manual login. Finally, we made slight revisions in the wording and layout of screens that participants found difficult to understand or had trouble navigating and also fine-tuned question to minimize patient discomfort with completing to more sensitive risk assessment questions.
Fig. 2.
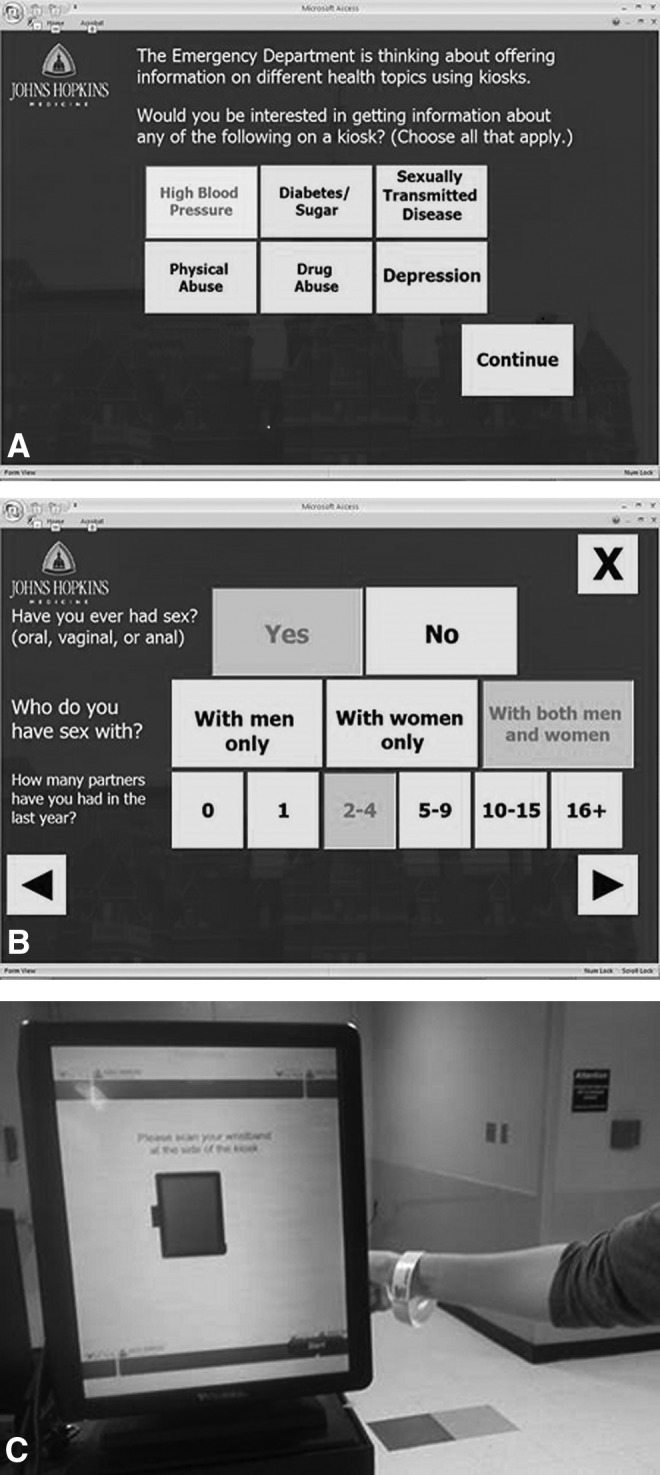
(A) Screenshot from the Registration Module of the prototype kiosk: the computer tablet. (B) Screenshot from the Risk Assessment Module of the prototype kiosk: the computer tablet. (C) Free-standing touchscreen kiosk equipped with the wristband scanner.
Discussion
The results of this usability study demonstrate favorable patient response to incorporation of kiosk-based methods for HIV testing offer, as well as screening and risk assessment in the ED. Participants reported the prototype system was easy to use, and we observed most participants easily maneuvered through the majority of screens without any assistance. We also found that average time spent on each module by participants, after excluding manual login screen (easily achievable via wristband scanner), was approximately 1 and 4 min, respectively. These findings highlight the potential valuable role kiosk systems could play in developing an integrated method of HIV testing into ED operations.
The positive participant response regarding this novel approach for offering and streamlining HIV screening was particularly interesting given the fact that the majority of our inner-city population is socioeconomically disadvantaged. This overwhelming acceptance and ease of use even among less educated and less kiosk-experienced individuals suggest great potential for future implementation in diverse settings. Implementation could be optimized for each population based on fine-tuning and modification of the prototype software modules. It is notable, however, that we did find that lower education level and lack of prior kiosk experience were correlated with neutral or unfavorable perception regarding the maneuverability of the prototype kiosks as well as need some assistance on kiosk use. One reason for this could be from use of some “jargon” wording and terms used in the prototype modules. We have accordingly revised some language to minimize need of assistance and adjusted question skipping functions to reduce level of discomfort. As noted above, we also replaced the manual login screen (where many of the difficulties were encountered among those with lower educational levels) with wristband scanner login, which offsets those challenges. An audio feature and a pop-up function to explain some terms are additional features that will be considered in future refinements of the system.
One of the most important features of the kiosk system for offering HIV screening in EDs was its flexibility. The kiosk software modules can be easily customized for different screening approaches according to each individual ED's patient flow and program requirements. If a targeted screening approach can be adopted, the registration module could be easily programmed to offer tests to only “high-risk” ED patients selected based on inputted sociodemographic information (e.g., a risk score).18 If an ED does not need to collect extensive risk information for a funding agency as we do, a more simplified Risk Assessment Module could be tailored without difficulty (or even it can be completely removed from the screening program). Other public health screening initiatives as well as health education could also be added to the software modules. For example, we have successfully modified our Risk Assessment Module with add-on screens for patient rapid HIV self-testing instructions19,20 after the recent U.S. Food and Drug Administration's approval of the over-the-counter use of the rapid oral fluid HIV test.21 Finally, for more severely ill patients, this kiosk system can be equipped onto a movable cart or installed on an iPad® (Apple, Cupertino, CA)-like lightweight touchscreen tablet for bedside use, with or without assistance of a healthcare provider.
Limitations
The current study has several limitations. First, our study may be biased as the population acuity level distribution does not fully reflect that of the greater ED patient population. Because of time required to complete not only program modules, but also consent and pilot surveys, only patients already stabilized and in semiprivate long-term beds were enrolled in the study. These patients might have more favorable views of our novel kiosk-based HIV screening modules compared with those with more severe acute illnesses or those who are less ill (ambulatory fast track patients) who have shorter ED lengths of stay. Second, our study also suffered from selection bias because it is likely that we enrolled more computer-savvy ED patients as volunteers who might be more interested in our study than those who do not use or infrequently use computers or other high-tech products (e.g., smartphones or tablets). Such computer-savvy patients would be more likely to have more easily maneuvered through our prototype kiosk software modules and report our tool as “easy to use” than those who were not. Third, this was a single institution study. Therefore, our prototype kiosk system might not be suitable for use in other EDs without further tailoring. However, as previously mentioned, the kiosk system's flexibility, given the ease of software modification, makes it easily adaptable to diverse settings. Our findings should provide other EDs with guidance in tailoring this kiosk-based screening program to best integrate and help streamline some aspects of the testing process in the context of their own ED operations.
Conclusions
Our study findings suggest that use of kiosks could help streamline patient-directed HIV screening in acute care settings. Kiosks could also be used for other public health initiatives in both acute and routine clinical care settings, such as screening for sexually transmitted infections. The information provided here will allow others to assess the feasibility of incorporating kiosks for similar public health services in various locations and to identify key factors related to the successful integration of these programs. Additional work to streamline kiosk interface with the information technology systems unique to each healthcare setting is needed to optimize integration into clinical practice.
Acknowledgments
Support was provided by Gilead Sciences, Inc.'s HIV FOCUS program.
Disclosure Statement
D.B. and W.L. are employees of Vecna Technologies, Inc., which manufactures the kiosk. R.E.R., M.G.-K., A.W., S.P., B.T., J.K., C.A.G., K.D., and Y.-H.H. declare no competing financial interests exist.
References
- 1.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One 2011;6:e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 U.S. dependent areas, 2010. HIV Surveillance Supplemental Report 2012;17(No. 3, part A). Available at http://www.cdc.gov/hiv/topics/surveillance/resources/reports (last accessed October31, 2013)
- 3.Centers for Disease Control and Prevention. Recommendations for HIV testing services for inpatients and outpatients in acute-care hospital settings. MMWR Recomm Rep 1993;42:1–10 [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Revised recommendations for HIV counseling, testing, and referral. MMWR Recomm Rep 2001;50:1–62 [PubMed] [Google Scholar]
- 5.Branson B, Handsfield H, Lampe M, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006;55:1–17 [PubMed] [Google Scholar]
- 6.Kelen G, Rothman R. Emergency department-based HIV testing: Too little, but not too late. Ann Emerg Med 2009;54:65–71 [DOI] [PubMed] [Google Scholar]
- 7.Moran G, Talan D. Processes and models for HIV screening in the emergency department: Can and should we do this? Ann Emerg Med 2011;58(1 Suppl 1):S172–S173 [DOI] [PubMed] [Google Scholar]
- 8.Wiler J, Gentle C, Halfpenny J, et al. Optimizing emergency department front-end operations. Ann Emerg Med 2010;55:142–160.e1. [DOI] [PubMed] [Google Scholar]
- 9.Houry D, Kemball R, Rhodes K, Kaslow N. Intimate partner violence and mental health symptoms in African American female ED patients. Am J Emerg Med 2006;24:444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gielen A, McKenzie L, McDonald E, et al. Using a computer kiosk to promote child safety: Results of a randomized, controlled trial in an urban pediatric emergency department. Pediatrics 2007;120:330–339 [DOI] [PubMed] [Google Scholar]
- 11.Kemball R, Gasgarth R, Johnson B, Patil M, Houry D. Unrecognized suicidal ideation in ED patients: Are we missing an opportunity? Am J Emerg Med 2008;26:701–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaca F, Winn D, Anderson C, Kim D, Arcila M. Feasibility of emergency department bilingual computerized alcohol screening, brief intervention, and referral to treatment. Subst Abus 2010;31:264–269 [DOI] [PubMed] [Google Scholar]
- 13.Porter S, Cai Z, Gribbons W, Goldmann D, Kohane I. The asthma kiosk: A patient-centered technology for collaborative decision support in the emergency department. J Am Med Inform Assoc 2004;11:458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi A, Weng W, Lichenstein R, Arora M, Sears A. Prospective tracking of a pediatric emergency department e-kiosk to deliver asthma education. Health Informatics J 2009;15:282–295 [DOI] [PubMed] [Google Scholar]
- 15.Price E, Mackenzie T, Metlay J, Camargo CJ, Gonzales R. A computerized education module improves patient knowledge and attitudes about appropriate antibiotic use for acute respiratory tract infections. Patient Educ Couns 2011;85:493–498 [DOI] [PubMed] [Google Scholar]
- 16.Haukoos J, Hopkins E, Bender B, et al. Use of kiosks and patient understanding of opt-out and opt-in consent for routine rapid human immunodeficiency virus screening in the emergency department. Acad Emerg Med 2012;19:287–293 [DOI] [PubMed] [Google Scholar]
- 17.Hsieh Y-H, Jung J, Shahan J, et al. Outcomes and cost analysis of three operational models for rapid HIV testing services in an academic inner-city emergency department. Ann Emerg Med 2011;58(1 Suppl 1):S133–S139 [DOI] [PubMed] [Google Scholar]
- 18.Haukoos J, Lyons M, Lindsell C, et al. Derivation and validation of the Denver human immunodeficiency virus (HIV) risk score for targeted HIV screening. Am J Epidemiol 2012;175:838–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaydos C, Solis M, Hsieh Y-H, et al. Use of tablet-based kiosks in the emergency department to guide patient HIV self-testing with a point-of-care oral fluid test. Int J STD AIDS 2013;21:716–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh Y-H, Gauvey-Kern M, Gaydos C, et al. Kiosk-Facilitated Patient Self Testing for HIV in an Emergency Department Rapid HIV Screening Program. 2012 National Summit on HIV and Viral Hepatitis Diagnosis, Prevention and Access to Care, Washington, DC [Google Scholar]
- 21.Roehr B. FDA approves “instant” HIV home test. BMJ 2012;345:e4636. [DOI] [PubMed] [Google Scholar]


