Abstract
Background
Although lymph node transplantation has been shown to improve lymphatic function the mechanisms regulating lymphatic vessel reconnection and functional status of lymph nodes remains poorly understood.
Methods
We developed and used LacZ lymphatic reporter mice to examine the lineage of lymphatic vessels infiltrating transferred lymph nodes. In addition, we analyzed lymphatic function, expression of vascular endothelial growth factor (VEGF-C), maintenance of T and B cell zone, and anatomic localization of lymphatics and high endothelial venules (HEVs).
Results
Reporter mice were specific and highly sensitive in identifying lymphatic vessels. Lymph node transfer was associated with rapid return of lymphatic function and clearance of Tc99 secondary to a massive infiltration of recipient mouse lymphatics and putative connections to donor lymphatics. T and B cell populations in the lymph node were maintained. These changes correlated with marked increases in the expression of VEGF-C in the perinodal fat and infiltrating lymphatics. Newly formed lymphatic channels in transferred lymph nodes were in close anatomic proximity to HEVs.
Conclusions
Transferred lymph nodes have rapid infiltration of functional host lymphatic vessels and maintain T and B cell populations. This process correlates with increased endogenous expression of VEGF-C in the perinodal fat and infiltrating lymphatics. Anatomic proximity of newly formed lymphatics and HEVs supports the hypothesis that lymph node transfer can improve lymphedema by exchanges with the systemic circulation.
Keywords: Lymph node, transfer, lymphatic regeneration, lymphatic flow, lymphedema
Introduction
Lymphedema is a condition characterized by progressive swelling and adipose deposition that occurs commonly after lymphadenectomy. It is estimated that 1 in 3 patients who undergo lymph node dissection for breast cancer go on to develop lymphedema.(1) These patients require life-long palliative interventions with the goal being preventing disease progression rather than a cure.
Recent clinical studies have suggested that the transfer of lymph nodes from an unaffected area to the lymphedematous limb can improve lymphatic function. For example, Cheng, et al., recently reported on ten patients with post-mastectomy lymphedema who underwent vascularized lymph node transfer and found that patients treated surgically had a significant reduction in their lymphedema as compared with controls.(2) Similarly, Becker and colleagues reported long-term improvements in 20/24 patients with lymphedema after lymph node transplantation.(3)
Animal studies have provided insight into the mechanisms by which lymph node transfer can restore lymphatic function. For example, using a sheep model, Tobbia, et al., showed that microsurgical transfer of lymph nodes after lymphadenectomy results in reconnection of lymphatic channels and systemic uptake of peripherally injected I125.(4) Similarly, using a porcine model, Saarasito, et al., found that lymphatic regeneration after microsurgical lymph node transfer can be significantly augmented by exogenously delivered vascular endothelial growth factor C (VEGF-C).(5) However, although these results are exciting, several questions remain unanswered. For example, the source and mechanism of lymphatic vessel re-anastomosis after lymph node transplantation has not been described. Furthermore, although previous studies have analyzed lymphatic function, these studies have relied primarily on systemic absorption of radioactive particles which may not accurately reflect lymph node uptake because interstitial fluid will eventually enter circulation and drain without functional lymphatic drainage. This gap in our knowledge is important since a better understanding of the cellular mechanisms regulating lymphatic regeneration after lymph node transfer may lead to the discovery of interventions that improve this process.
The purpose of this study was to determine the cellular sources of lymphatic vessel re-anastomosis after lymph node transplantation. To accomplish this goal, we developed a novel lymphatic reporter mouse that enables us to trace the origin of lymphatic vessel and ingrowth of lymphatic endothelial cells. In addition, we used this model to analyze lymphatic uptake and function after lymph node transplantation.
Methods
Animals
All procedures were approved by the IACUC at Memorial Sloan-Kettering Cancer Center. Adult male C57/BL6 mice (10-12 weeks) were purchased from Jackson Labs (Bar Harbor, Maine).
Transgenic lymphatic reporter mice using a tamoxifen-inducible Cre-Lox system were developed by crossing C57B6 transgenic mice that expressed the bacterial Cre recombinase gene driven by the promoter of the mouse vascular endothelial growth factor receptor 3 gene (VEGF-R3 or FLT4) under the control of tamoxifen (a gift of Dr. Ortega), with C57B6 LacZLoxP transgenic mice (Jackson Labs, Bar Harbor, ME) (Figure 1A).(6) This approach enabled us to target lymphatic endothelial cells (LECs) and obtain lymphatic specific expression of the LacZ gene since FLT4 or VEGF-R3 is expressed by all LECs.(7) In addition, we were able to regulate expression of Cre recombinase in these mice using tamoxifen, thereby allowing the temporal modulation of the recombination and expression of LacZ.
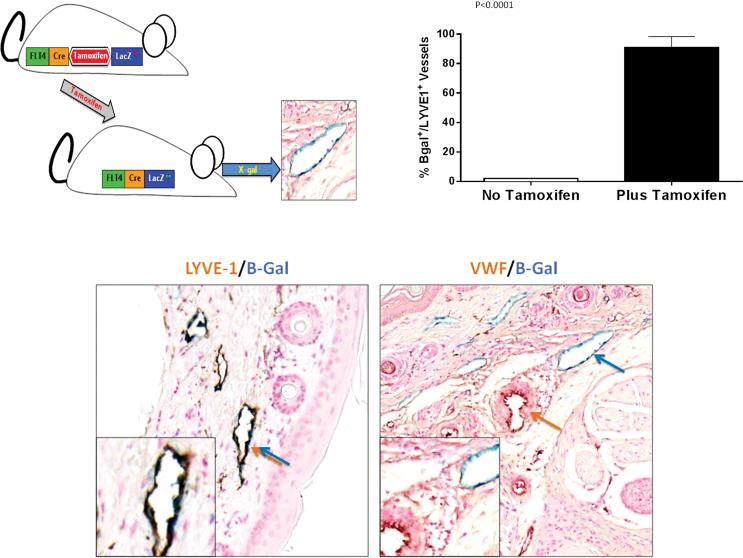
Figure 1. FLT-4-LacZ mice can be used as an inducible lymphatic reporter mouse.
(A) Schematic figure of FLT-4Cre/LacZ LoxP mice. Treatment with tamoxifen activates LacZ expression and enables visualization of lymphatic vessels with B-gal.
(B) High level activation of LacZ expression in LYVE-1 positive lymphatics is inducible with tamoxifen.
(C) Representative high power (40×) images of skin sections double stained for B-galactosidase (blue) and LYVE-1 (brown stain, left panel) or Von-Willebrand Factor (VWF; brown stain, right panel) demonstrate specificity of Lac-Z expression only in lymphatics vessels (blue/brown double arrow on the left). Notice no overlap with VWF (separate brown and blue arrows) on the right panel.
Analysis of Cre-Lox Reporter Mice
To activate Cre-lox recombination, homozygous FLT4-Cre/LacZ-LoxP mice (FLT4-LacZ mice) were injected intraperitoneally with 200mg/kg tamoxifen (Sigma Aldrich, St. Louis MO) for 5 days followed by sacrifice.(6) LacZ expression was visualized by incubating tissues in Lac-Z buffer solution, followed by embedding and sectioning. To analyze the penetrance/specificity of Cre-expression, we stained tissues using antibodies directed against the lymphatic specific marker lymphatic vessel endothelial receptor 1 (LYVE-1; R&D Systems, Minneapolis, MN), or Von-Willibrand factor (VWF), a marker of blood vessels (Abcam, Cambridge, MA).(8)
Lymph Node Transfer
To determine the source of lymphatic vessels in transplanted lymph nodes, we harvested axillary lymph nodes from adult male wild-type C57B6 mice and implanted them in recipient FLt4cre-Lac-Z loxP transgenic mice immediately after axillary lymphadenectomy.(9) Control mice underwent an axillary incision without lymph node removal/ implantation. Five days prior to sacrifice, animals were treated with tamoxifen to activate Cre-expression and label recipient LECs with B-gal.
Analysis of Lymphatic Function and Histology
We used a modification of our previous methods for lymphoscintraphy to quantify lymphatic transport.(10). Briefly, 14 or 28 days after surgery, 50μl of technetium 99m (99mTc) labeled sulfur colloid was injected into the distal forearm and uptake of 99mTc in the axillary lymph node was assessed using a X-SPECT camera (Gamma Medica, Northridge, CA) for 90 minutes (n=4/group). Region-of-interest analysis was performed using ASIPro software (CTI Molecular Imaging, Knoxville, TN).
To visualize functional lymphatic vessels in the transferred lymph nodes, we injected Prussian blue (Sigma-Aldrich, St. Louis, MO) in the distal extremity 60 minutes prior to sacrifice. This compound enables identification of functional lymphatic vessels because there is an iron III component that is transported selectively in lymphatics.(11) Staining was visualized using 5% potassium ferrocyanide according to published protocols.(12)
In order to determine the sources of LECs responsible for revascularization of transferred lymph nodes, we co-stained harvested lymph nodes for LYVE-1 and B-gal as outlined above. Importantly, using this analysis we were able to identify both donor (B-gal−/LYVE-1+) and recipient (B-gal+/LYVE-1+) lymphatic vessels using our FLT4-LacZ mice. We confirmed specificity of B-gal expression using an antibody directed against VEGF-R3 (R&D Systems, Minneapolis, MN).
In order to determine if immune cells survive after lymph node transfer, we stained harvested lymph nodes with an antibody directed against T cells (CD3) or B cells (B220); Abcam, Cambridge MA).(10) To determine if endogenously expressed VEGF-C expression correlates with lymphatic regeneration after lymph node transplant, we stained freshly frozen lymph nodes with an antibody against the mouse VEGF-C protein (Abcam, Cambridge, MA).(13)
Although the mechanisms by which lymph node transfers ameliorate lymphedema remain unknown, one hypothesis is that lymphatic fluid is filtered through lymphatic vessels into adjacent high endothelial vessels (HEVs) of the lymph node, thereby shifting the fluid to vascular compartment.(2) Therefore, to examine potential interactions between lymphatic and vascular systems, we co-stained harvested lymph node specimens for LYVE-1 and MECA32 to identify lymphatic vessels and HEVs, respectively.(14)
Statistical Analysis
All data are presented as mean +/− standard deviation with p<0.05 considered significant. A minimum of 4 animals per group was used for all experiments. Comparison of multiple groups was performed using ANOVA with Tukey-Cramer post hoc tests to compare all values.
Results
FLT4-LacZ mice can be used as an inducible lymphatic reporter mouse
Histological analysis of skin sections was performed to analyze the degree and specificity of Cre expression. We chose the skin for this analysis because discrete capillary lymphatics are easily visualized, enabling us to quantify cre expression levels. As expected, we found that the expression of Flt4-Cre was tamoxifen dependent, and as a result, control mice that were not primed with tamoxifen prior to sacrifice showed no B-gal staining in LYVE-1+ lymphatic vessels (Figure 1B). In contrast, treatment of transgenic mice with tamoxifen resulted in high-level expression of Cre-recombinase observed in almost all lymphatic endothelial cells thereby identifying more than 90% of capillary lymphatics as evidenced by double staining with LYVE-1, another lymphatic specific marker. The expression of B-gal was limited and specific to lymphatic vessels because we found no B-gal staining of blood vessels stained with Von Willebrand factor (Figure 1C).
Lymph node transplantation restores lymphatic transport capacity and function
Using lymphoscintigraphy, we found progressive and statistically significant increases in Tc99 uptake in transplanted nodes with peak values approximating non-operated controls by day 28 (Figure 2A). Because of the increase in lymphatic function that was observed between days 14 and 28, we chose to limit our remaining analysis to lymph nodes harvested on day 28.
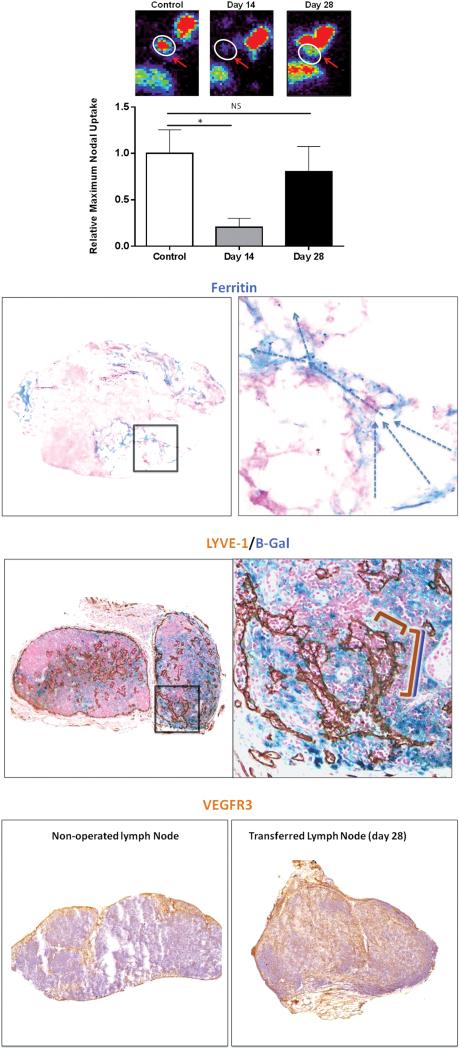
Figure 2. Lymph node transplantation restores lymphatic transport capacity and function by promoting infiltration of lymphatics from the recipient mouse.
(A) Representative heat maps (top) and quantification of relative Tc99 uptake in axillary lymph nodes after injection in the distal upper extremity of control mice (axillary incision without lymphadenectomy), and experimental mice 14 and 28 days after lymph node transplantation. Note recovery of Tc99 uptake by day 28.
(B) Representative low (left panel; 2.5×) and high (right panel; 40×) powered images of ferritin staining in lymph nodes harvested 28 days after transplantation (arrows denote direction of lymphatic flow from lymphatic sinuses toward the cortex).
(C) Representative low (left panel; 2.5×) and high powered (right panel; 40× zoom of boxed region in left panel) images of transplanted lymph nodes double stained with the lymphatic specific marker LYVE-1 (brown stain) and B-gal (blue stain). Note connection of double stained vessels (overlapping blue/brown brackets) with single stained brown vessel (brown bracket) suggesting connection of recipient and donor lymphatics within the lymph node.
(D) Representative low power (2.5×) images of control (sham-operated; left panel) and transplanted lymph node (right panel) stained for VEGF-R3 (brown stain) demonstrating massive infiltration of VEGF-R3 positive cells in transplanted node.
Ferritin staining demonstrated lymphatic vessels in and around transplanted lymph nodes and confirmed our Tc99 studies. Significantly, because the transport of ferritin from the injection site in the distal arm is dependent on uptake in the distal lymphatics, this finding suggests that the lymphatic channels in the transplanted lymph node are functional. Using this analysis and the gradients of ferritin expression, we could further infer that the direction of lymphatic flow in these nodes was from external to internal (Figure 2B).
Using B-gal staining we found that day 28 transplanted lymph nodes were filled with recipient mouse LECs (i.e., B-gal+) (Figure 2C, D). We found anastomoses between B-gal+/LYVE-1+ (i.e., recipient) and B-gal−/LYVE-1+ (i.e., donor) vessels, and double staining with LYVE-1 confirmed that B-gal expression was limited to LECs. These findings suggest that recipient and donor mouse lymphatic vessels connected spontaneously within the transferred lymph node. We also confirmed that B-gal staining in these lymph nodes correlated with VEGFR3 expression, and found that transferred lymph nodes had a massive increase in VEGF-R3+ cell infiltration as compared to control (i.e., non-operated) nodes (Figure 2D).
Transplanted lymph nodes retain T and B cells
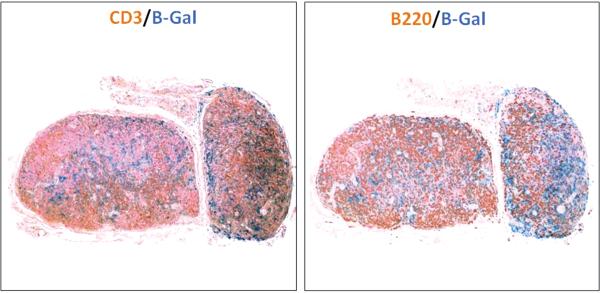
We stained transferred lymph nodes with antibodies directed against T cells (CD3) and B cells (B220) to determine if immune components of the lymph node survive after transfer (Figure 3). Our analysis demonstrated that although the histological architecture of the lymph node was altered, most predominantly by a decrease in the follicular pattern of B cell distribution, both T and B cell populations survived after transfer and were found in all regions of the nodes.
Figure 3. Transplanted lymph nodes retain T and B cells.
Representative low power (2.5×) images of transplanted lymph nodes double stained with B-galactosidase (blue) and CD3 (brown staining of T cells; left panel) or B220 (brown staining of B cells; right panel). Note that transplanted lymph nodes retain both cell populations in addition to maintenance of lymph node cellularity.
Lymphatic regeneration correlates with expression of VEGF-C
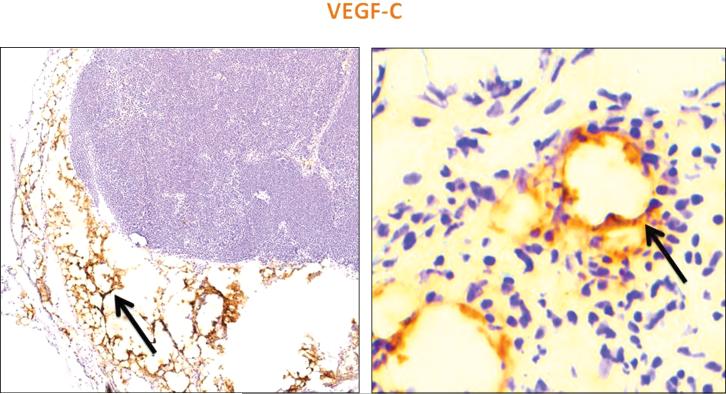
Although previous studies have shown that exogenously applied VEGF-C increases lymphatic regeneration in transferred lymph nodes (5, 15), it remains unknown if the endogenous expression of VEGF-C is increased after lymph node transfer. Interestingly, we found that the expression of VEGF-C in transplanted lymph nodes was markedly increased in the perinodal fat as well as in newly formed lymphatic vessels when compared to control non-operated lymph nodes (Figure 4A, B). Analysis of the interstitial space of the transferred lymph nodes also demonstrated VEGF-C staining along the areas infiltrated by lymphatic vessels suggesting that this growth factor may help in LEC migration and tubule formation after lymph node transfer.
Figure 4. Lymphatic regeneration correlates with expression of VEGF-C.
Representative low (left panel; 5×) and high power (right panel; 40×) images of transplanted lymph nodes stained for VEGF-C (brown staining). Note high-level expression in perinodal fat and in infiltrating lymphatic vessels (black arrows).
Lymphatic vessels in lymph nodes are in close proximity to high endothelial venules
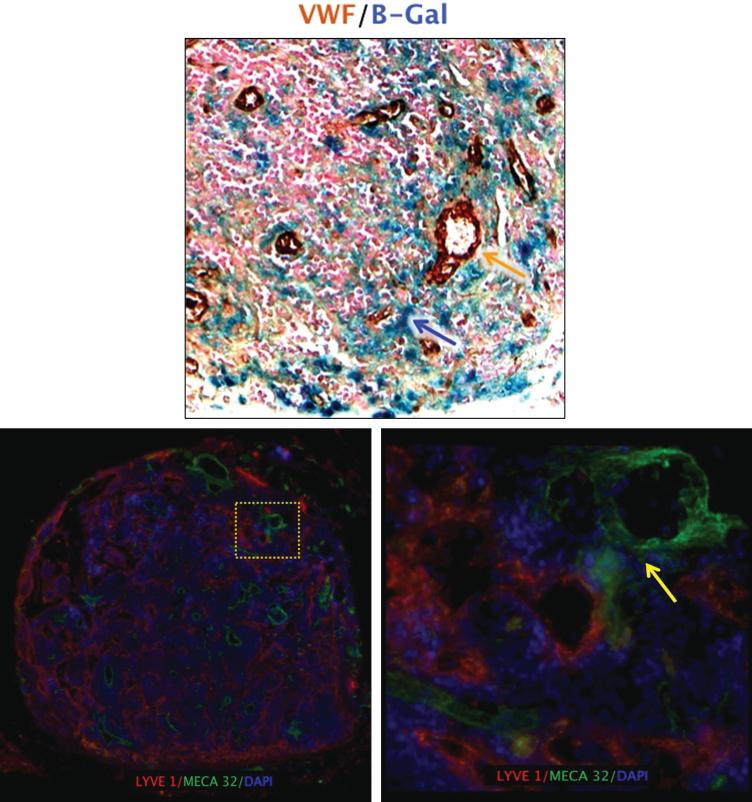
We found close proximity of B-gal positive lymphatics and blood vessels stained with Von Willebrand Factor (VWF) in transplanted lymph nodes (Figure 5A). In order to better analyze the spatial arrangement of lymphatic vessels and HEVs, we co-localized LYVE-1 and Meca-32, an HEV marker. Similar to our observations with VWF, we found close approximation of lymphatic vessels and HEVs in the medulla of non-operated lymph nodes (not shown). In fact, in some sections there appeared to be some overlap in these vessels. Interestingly, when we co-localized lymphatic vessels and HEVs in transplanted lymph nodes harvested 28 days after surgery, we noted a marked increase in the number of both blood and lymphatic vessels (Figure 5B). Moreover, similar to our observations with VWF staining, we found very close approximation of lymphatic vessels and HEVs in the transplanted nodes (Figure 5C). These findings demonstrate that lymphatic vessels and HEVs exist in close proximity in transferred lymph nodes. Future studies will determine if there is an exchange of lymphatic fluid between these vessels.
Figure 5. Lymphatic vessels in lymph nodes are in close proximity to high endothelial venules (HEVs).
(A) Representative high power (40×) images of transplanted lymph nodes double stained with B-galactosidase (blue) and Von Willebrand factor (VWF; brown). Note close proximity of lymphatic vessels shown in blue and blood vessels stained brown.
(B) Representative low-power (2.5×) florescent images of transplanted lymph nodes double stained with LYVE-1 (red) and HEV marker MECA-32 (green). Note large numbers of lymphatic channels
(C) Representative high-power (40×) image of yellow-boxed area shown in B. Note close proximity of lymphatics and HEVs (yellow arrow).
Discussion
We have developed an inducible lymphatic reporter mouse in an effort to track the lineage of lymphatic endothelial cells (LECs) after lymph node transfer. We chose the FLT4 promoter (also known as VEGF-R3) for this approach since VEGF-R3 expression is limited primarily to LECs in adult humans and mice (7), and more importantly, unlike other lymphatic markers, it is expressed by nearly all LECs regardless of whether they originated from capillary or collecting vessels. Although previous studies have used other lymphatic reporter constructs (i.e., Prox-1), we felt that these mice were less useful for our study since they have very limited inducible expression in adult mice. (16)
Previous studies have suggested that transfer of lymph nodes in a variety of models can restore lymphatic flow to the lymph node as demonstrated by histological analysis or lymphangiography. (5, 15) However, these studies have not analyzed the functional potential of transferred lymph nodes or the time course of lymphatic vessel regeneration in this process. In the current study we show that even as early as 14 days after lymph node transfer, there is low-level uptake of Tc99 after peripheral injection. Lymphatic function improved rapidly over the next two weeks as was evidenced by lymph node uptake of Tc99 being nearly equal to non-operated control lymph nodes 4 weeks after transplantation. More importantly, since the peripheral injection of ferritin was rapidly and efficiently picked up by lymphatic vessels in the transferred node, it was evident that the uptake of Tc99 is associated with functional lymphatic vessel regeneration.
Functional regeneration of lymphatic vessels to the sub-capsular lymph node sinus is important since these connections are known to regulate immune cell homeostasis. (17) The fact that we found that T and B cell populations are maintained in transplanted lymph nodes is, therefore, important since it suggests that these nodes are immunologically competent. Our specific staining for T and B cells add to those of Tobbia, et al., who have shown that microvascularly transplanted lymph nodes remain viable based on histologic studies.(4) This finding is relevant in the clinical treatment of lymphedema since bacterial infections and localized immune incompetence in the lymphedematous limb are a major pathologic component of lymphedema. Studies after long term follow up will determine if the minor architectural changes we observed are restored, if transplanted T/B cells are of donor or recipient origin, and if these cells maintain the potential to respond to immunological challenges.
Although previous studies have shown that lymphatic regeneration occurs spontaneously in transplanted lymph nodes (4, 15), our findings add to and expand upon these studies by demonstrating the source of newly formed lymphatics. Using our lymphatic reporter mice, we found that transplanted lymph nodes are rapidly and widely infiltrated by recipient LECs, that these cells coalesce into lymphatic vessels, and that these new vessels spontaneously reconnect with existing lymphatics within the lymph node.
We found very high levels of VEGF-C expression in the perinodal fat and in the newly formed lymphatic vessels suggesting that endogenous expression of this growth factor plays a critical role in the regulation of lymphatic regeneration and migration of LECs from the recipient into the donor lymph node. This finding is consistent with the known roles of VEGF-C in the regulation of LEC migration and lymphatic regeneration (18, 19). Interestingly, our finding that endogenous VEGF-C expression is adequate for restoration of lymphatic circulation in transplanted lymph nodes contrasts with those of Tammela, et al., who, using a similar model, found that lymphatic regeneration only occurred after exogenous delivery of VEGF-C. (15) These differences are likely due to technical differences between models , but require further analysis.
Although clinical and experimental studies have provided evidence that lymph node transplantation can improve lymphatic flow in lymphedematous limbs, the mechanisms regulating this effect remain unknown. Our study provides anatomic evidence that HEVs and lymphatics in transferred lymph nodes are in close proximity to each other thereby suggesting that interstitial fluid may be exchanged. This finding is consistent with previous studies demonstrating that lymph and blood vessels can exchange cellular and fluid components in the lymph node. (20)
In conclusion, we have developed an inducible lymphatic reporter mouse and used it to analyze the cellular origin of lymphatic vessels after lymph node transplantation. We have shown that newly formed lymphatic vessels are functional and that T/B cells remain viable in transplanted lymph nodes. In addition, we have shown that endogenous VEGF-C expression gradients correlate with LEC migration lymphatic vessel invasion. Finally, we have shown that newly formed lymphatic vessels are in close proximity to HEVs and provide anatomic support for the hypothesis that interstitial fluid can be exchanged between lymphatic and HEVs within the lymph node.
Acknowledgments
Sources of Funding: NIH R01 HL111130-02 awarded to BJM
Footnotes
Disclosures: None of the authors have any conflicts of interest or disclosures.
References
- 1.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83:2776–2781. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg. 2013;131:1286–1298. doi: 10.1097/PRS.0b013e31828bd3b3. [DOI] [PubMed] [Google Scholar]
- 3.Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313–315. doi: 10.1097/01.sla.0000201258.10304.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobbia D, Semple J, Baker A, et al. Experimental assessment of autologous lymph node transplantation as treatment of postsurgical lymphedema. Plast Reconstr Surg. 2009;124:777–786. doi: 10.1097/PRS.0b013e3181b03787. [DOI] [PubMed] [Google Scholar]
- 5.Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg. 2012;255:468–473. doi: 10.1097/SLA.0b013e3182426757. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Corral I, Olmeda D, Dieguez-Hurtado R, et al. In vivo imaging of lymphatic vessels in development, wound healing, inflammation, and tumor metastasis. Proc Natl Acad Sci U S A. 2012;109:6223–6228. doi: 10.1073/pnas.1115542109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohela M, Saaristo A, Veikkola T, et al. Lymphangiogenic growth factors, receptors and therapies. Thromb Haemost. 2003;90:167–184. doi: 10.1160/TH03-04-0200. [DOI] [PubMed] [Google Scholar]
- 8.Avraham T, Zampell JC, Yan A, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27:1114–1126. doi: 10.1096/fj.12-222695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zampell JC, Yan A, Avraham T, et al. Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am J Physiol Cell Physiol. 2011;300:C1107–1121. doi: 10.1152/ajpcell.00378.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avraham T, Daluvoy S, Zampell J, et al. Blockade of Transforming Growth Factor-{beta}1 Accelerates Lymphatic Regeneration during Wound Repair. Am J Pathol. 2010;177:3202–3214. doi: 10.2353/ajpath.2010.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaka N, Padera TP, Hagendoorn J, et al. Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res. 2004;64:4400–4404. doi: 10.1158/0008-5472.CAN-04-0752. [DOI] [PubMed] [Google Scholar]
- 12.Leu AJ, Berk DA, Lymboussaki A, et al. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60:4324–4327. [PubMed] [Google Scholar]
- 13.Zampell JC, Yan A, Avraham T, et al. HIF-1alpha coordinates lymphangiogenesis during wound healing and in response to inflammation. FASEB J. 2011 doi: 10.1096/fj.11-195321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallmann R, Mayer DN, Berg EL, et al. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Developmental dynamics : an official publication of the American Association of Anatomists. 1995;202:325–332. doi: 10.1002/aja.1002020402. [DOI] [PubMed] [Google Scholar]
- 15.Tammela T, Saaristo A, Holopainen T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 16.Harvey NL, Srinivasan RS, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nature genetics. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 17.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 18.Rutkowski JM, Boardman KC, Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ Physiol. 2006;291:H1402–1410. doi: 10.1152/ajpheart.00038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan A, Avraham T, Zampell J, et al. Mechanisms of Lymphatic Regeneration after Tissue Transfer. PlosOne. 2011;6:e17201. doi: 10.1371/journal.pone.0017201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohtani O, Ohtani Y. Structure and function of rat lymph nodes. Archives of histology and cytology. 2008;71:69–76. doi: 10.1679/aohc.71.69. [DOI] [PubMed] [Google Scholar]