Abstract
The neurovascular unit is now well accepted as a conceptual framework for investigating the mechanisms of ischemic stroke. From a molecular and cellular perspective, three broad mechanisms may underlie stroke pathophysiology – excitotoxicity, oxidative stress and inflammation. To date, however, most investigations of these basic mechanisms have focused on neuronal responses. In this mini-review, we ask whether these mechanisms of excitotoxicity, oxidative stress and inflammation can also be examined in terms of non-neuronal interactions in the neurovascular unit, including the release of extracellular vesicles for cell-cell signaling.
Keywords: Neurovascular unit, stroke, neuronal cell death, neuroprotection, extracellular vesicles, cell-cell interaction
1. INTRODUCTION
Stroke is one of the most serious life-threatening diseases in the developed countries [1]. Over the past two decades, advances in molecular and cellular biology have defined many potential mechanisms and targets for stroke. But it has been very difficult to translate these gains in basic knowledge into true clinical applications [2, 3]. Therapeutic options for stroke patients remain limited to reperfusion with tissue plasminogen activator or mechanical catheter-devices.
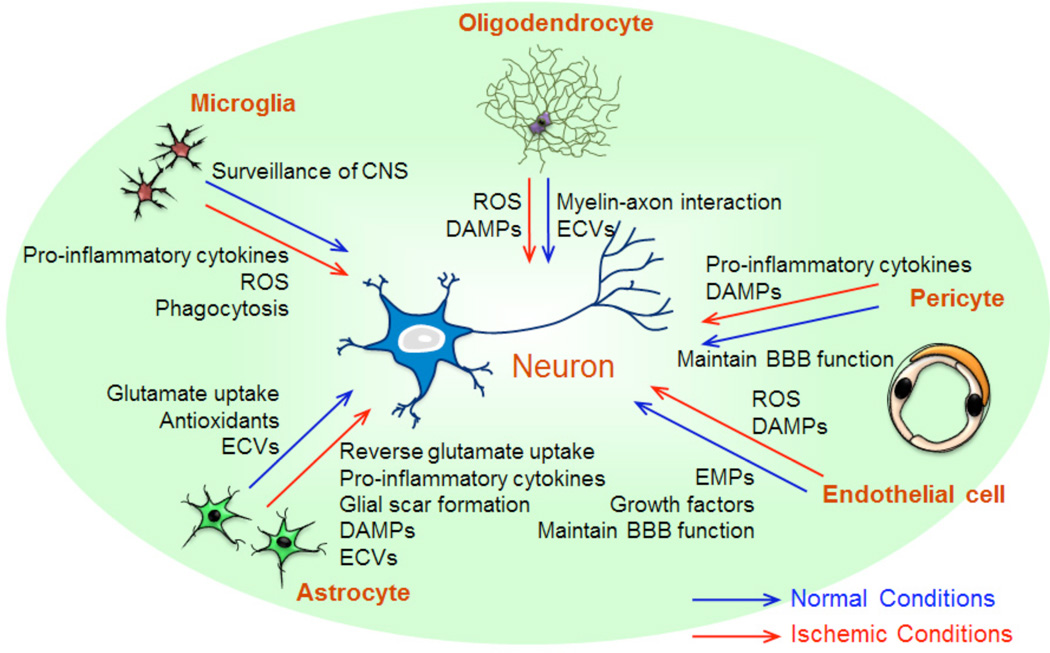
There are many reasons why neuroprotection trials have mostly failed [4, 5], but one major reason may be related to the idea that stroke damages not only neurons but also all cell types in the neurovascular unit (NVU), i.e. neurons, glia and vascular cells. Cell-cell interactions between neurons, glia (astrocytes, microglia, and oligodendrocytes), vascular compartments (endothelial cells, pericytes, and smooth muscle cells), and extracellular matrix underlies central nervous system (CNS) homeostasis. For signaling purposes, a large array of endogenous molecules such as cytokines, growth factors, chemokines, and microparticles (MPs) are proposed to be essential mediators within this modular concept (Fig. 1). Hence, any disruption of these complex interactions may lead to NVU dysfunction during neurodegenerative conditions including brain ischemia. In this regard, therapeutic approaches for stroke should aim to not only protect neurons but also rescue all cell types and restore function in the entire NVU [4, 6].
Fig. (1).
Neurovascular Unit (NVU) is composed with neurons, glia (astrocytes, microglia, and oligodendrocytes), vascular components (endothelial cells, pericytes, and smooth muscle cells), and extracellular matrix. Non-neuronal components have cell-cell interactions with neurons through endogenous molecules, such as cytokines, growth factors, chemokines, and microparticles. Neuronal function is maintained on the basis of mutual interactions, but after ischemic stroke, non-neuronal compartments may lead to neuronal damage, resulting in overall NVU/brain dysfunction.
Research at the molecular and cellular level, as well as in animal models, have revealed many interesting mechanisms and targets for neuroprotection. After ischemic stroke, the initial loss of blood flow in the central core regions is very severe, so neuronal cell death rapidly occurs due to lack of oxygen and glucose. However, in the surrounding penumbral areas, energy loss is relatively moderate or mild and it has been proposed that brain cells here are potentially salvageable. Within the evolving penumbra, neurons slowly die because a complex cascade of cell death mechanisms becomes triggered. Broadly speaking, these mechanisms can be grouped into general categories involving excitotoxicity, oxidative stress and inflammation [7]. To date, however, dissection of these overall mechanisms has mostly been centered on neuronal responses. In this mini-review, we attempt to explore how cell-cell interactions within the NVU, especially in non-neuronal cells, can modify and amplify these pathophysiologic mechanisms by affecting the ability of NVU components to regulate signaling mediators such as glutamate, free radicals, growth factors, cytokines, chemokines and extracellular vesicles.
2. EXCITOTOXICITY
Glutamate is a major excitatory neurotransmitter in the mammalian brain. It contributes to cellular function, synaptic plasticity, cell death and survival, neuronal development, and learning and memory [8]. Under normal conditions, neurons are exposed to small amount of glutamate to maintain their function. Although exposure to low-dose glutamate does not lead to neuronal damage [9, 10], excessive levels of glutamate cause excitotoxicity by over-stimulating various glutamate receptors of neurons, such as N-methyl-D-aspartate (NMDA) receptors, alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors and kainate receptors of ionotropic receptors, and metabotropic receptors. The calcium overload by excessive glutamate exposure gives neurons oxidative stress, mitochondrial dysfunction, calpain activation, and DNA fragmentation [11]. Following an ischemic insult, the subsequent ATP loss causes ionic imbalance of certain ion, such as sodium, potassium, calcium and chloride ions [12]. This imbalance inhibits the uptake of glutamate by glutamate transporters, and even causes the reversal of the transporters, which induce over-accumulation of extracellular glutamate [13].
Astrocytes are key players in the regulation of glutamate in CNS brains. They express several glutamate transporters such as glutamate-aspartate transporter (GLAST, EAAT 1 in human) and glial glutamate transporter 1 (GLT-1, EAAT 2 in human) [14]. After astrocytes take up glutamate, glutamate is converted to glutamine by astrocytic glutamine synthase followed by being released from the astrocytes [15]. The released glutamine is then removed from the extracellular space into neurons [16]. After that, neurons convert glutamine back to glutamate and proceed to release it into synapse [17, 18]. Ischemic conditions disrupt this glutamate-glutamine cycle [19]. Rossi DJ et al. suggested that transporter-mediated extracellular glutamate homeostasis failed dramatically under ischemic conditions and that the astrocytic transporters were the major source of extracellular glutamate to trigger the death of neurons [13]. The reverse uptake of glutamate in astrocytes was demonstrated by the evidence of increased vesicular and non-vesicular release of glutamate in the extracellular space [20]. NMDA receptors are proposed as the predominant glutamate receptor for neuronal death because of the high permeability of calcium. Indeed, multiple NMDA receptor subtypes, such as NR2Aand NR2B-containing NMDA receptors, have different function in epileptogenesis and ischemic stroke [21, 22]. Reverse glial glutamate uptake may trigger neuronal cell death through preferential activation of extra-synaptic NR2Bcontaining NMDA receptor-related pathways [23]. Furthermore, astrocytes may also assist neurons in terms of energy regulation [24]. Because astrocytes are less susceptible than neurons to ischemic conditions, astrocytes may try to protect neurons through glutamate uptake, glycogen hydrolysis to lactate for energy, and conduction of protective molecules through gap junctions, even under the ischemic conditions. In addition, glutamate activates metabotropic glutamate receptors (mGluRs) in astrocytes, which releases arachidonic acid derivatives to modulate cerebral blood flow (CBF). Under stroke conditions, both competitive and non-competitive NMDA receptor antagonists increase in CBF while attenuating injury. Therefore, NMDA receptor antagonists may also contribute to neuroprotection through augmenting CBF in affected brain areas [25]. Taken together, these observations suggest that restoring astrocyte function in terms of glutamate handling and energy regulation may provide potential therapeutic targets for ischemic stroke.
Oligodendroglial lineage cells, which are one of the main cell types in cerebral white matter, also express AMPA and kinate glutamate receptors. AMPA/kainate receptor blockade protects cultured oligodendrocytes from hypoxic injury [26]. Recently, it was reported that oligodendrocytes also expressed functional NMDA receptors containing NR1, NR2C and NR3 subunits in ischemia [27]. NMDA receptors have higher glutamate affinity than AMPA or kinate receptors. Hence, oligodendrocytic NMDA receptors could contribute to brain injury, especially white matter damage, which occurs when the glutamate level is elevated in stroke. Because mature oligodendrocytes myelinate axons to support axonal signal transduction, oligodendrocyte-neuron interaction should be very important in maintaining normal neuronal function. Indeed, oligodendrocytes can signal to neurons via myelin-axon interactions [28, 29]. Mouse models of oligodendrocyte injury have demonstrated axonal loss without considerable demyelination [30, 31], suggesting that oligodendrocytes support axonal survival through a myelinindependent mechanism [32]. Moreover, oligodendrocytes may serve as a principal metabolic supplier of lactate, which is integral for axonal energy support through monocarboxy-late transporter 1 [33]. In addition, oligodendrocyte-derived trophic factors promote neuron survival and axon outgrowth in vitro [34]. Hence, oligodendrocyte death by glutamate toxicity may eventually lead to neuronal damage and dysfunction.
Glutamate function was originally described in terms of neuronal neurotransmission. But as discussed in the preceding paragraphs, it is now known that this signaling system applies to many other cell types in the CNS. So in the context of stroke and brain injury, excitotoxicity mechanisms should operate in multiple cell types. Besides astrocytes and oligodendrocytes, glutamate transporters also exist in pericytes [35], and over-activation of ionotropic glutamate channels can trigger apoptosis in cerebral endothelium as well [36, 37]. Excitotoxic cascades are not only a neuronal phenomenon but comprise all compartments in the neurovascular unit. Taken together, suppression of glutamate toxicity under ischemic conditions would promote neuronal survival. However, clinical application of glutamate receptor antagonists have been tested without successful results [13, 38]. One possible reason why glutamate antagonists were not successful would be partly because the treatable target focused on only neuronal NMDA glutamate receptors [38]. To achieve effective neuronal protection, we may need to consider how to control the glutamate-mediated excitotoxicity through non-NMDA receptors in neurons as well as glutamate receptors/transporters in non-neuronal brain cells.
3. OXIDATIVE STRESS
Reactive oxygen species (ROS) are associated with many diseases, including ischemic stroke and other neurodegenerative diseases. Ischemic insults produce an excess amount of free radicals, especially in the reperfused ischemic regions [39, 40]. Oxidative stress causes neuronal cell death by attacking key cellular components. For example, ROS open mitochondrial permeability transition pore, and subsequently cause mitochondrial swelling, resulting in necrosis [41]. ROS also initiate apoptosis through activating p53 and p38 MAPK [42]. Hence, protecting neurons from oxidative stress have been considered as a promising therapeutic approach for stroke. Indeed, a great number of antioxidants were evaluated in clinical trials. For example, a radical-spin trap NXY-059 improved disability significantly 3 months following stroke [43]. However, in the next SAINT-II trial, the compound showed no significant effects [3]. Nevertheless, research of oxidative stress in ischemic stroke field continues to reveal novel possibilities for antioxidant therapies [44]. In this section, we will discuss how non-neuronal cells regulate oxidative stress within the NVU compartment.
Astrocytes possess a large capacity of endogenous antioxidants. The release of antioxidants, such as glutathione and superoxide dismutase (SOD), from astrocytes may play an important role in maintaining and enhancing neuronal survival [45–49]. In this regard, nuclear factor-erythroid 2 related factor 2 (Nrf2) is a promising target to attenuate brain damage and neurological deficits following stroke [50]. Nrf2 is the redox-sensitive transcription factor for phase II defense enzymes and antioxidant stress proteins, and oxidative stress induces nuclear translocation and binding of the antioxidant response element in the promoter of protective genes [50]. Indeed, astrocytic Nrf2 mediates several gene expressions to represent a major contributors of endogenous defense system for neurons against oxidative stress [51–54]. In a rat stroke model, Nrf2 expression was higher in peri-infarct region than ischemic core [55]. In addition, transient ischemia activated Nrf2 in astrocytes both in vivo and in vitro and increased the expression of Nrf2 target genes, which contribute to neuroprotection [54]. Metallothioneins (MTs) in astrocytes also play essential roles in protecting neurons against ROS stress. MTs are a family of metal binding proteins and have multiple function including the regulation of metal concentration and detoxification of heavy metal ions [56]. MT overexpressed mice showed significant reduction of infarct size and better motor function after transient middle cerebral artery occlusion [57]. In turn, both MT-I- and MT-II-deficient mice developed larger infarcts and worse neurological outcomes compared to wild-type mice [58]. Interestingly, MT was shown to be transferred from astrocytes to neurons in response to injury [59]. These MTs from astrocytes may protect neurons from oxidative stress [60]. These findings suggest that intercellular communications between astrocytes and neurons exist, and because of that, neurons are guarded by astrocytes against oxidative stress.
In contrast to astrocytes, endothelial cells could be a major source of ROS under pathological conditions due to ample amount of Nox [61]. Nox is a catalytic subunit of NADPH oxidases, whose identified isoforms are Nox1, 2, 4, and 5 [62]. Endothelial cells also produce nitric oxide (NO), which is essential to maintain vascular tone. NO improves cerebral blood flow during and after ischemic onset. However, NO together with superoxide would form the much more powerful oxidant peroxynitrite [63] to attack neighboring cells including neurons under acute phase of stroke. Additionally, an inflammatory cytokine tumor necrosis factor-α (TNF-α), which is released after brain ischemia, significantly increases intracellular level of ROS in endothelial cells [64]. Under normal conditions, cerebral endothelial cells nourish neighboring neurons through growth factor release. But under pathological conditions, those endothelial-derived ROS may in turn induce neuronal cell death.
The cerebral white matter consists of abundance of oligodendrocytes and lipid-rich contents of myelin sheath, and intrinsic antioxidant properties in the white matter are relatively low. Therefore, cerebral white matters can be also an enormous source of ROS. Hence, under ischemic conditions, axons in the white matter may be damaged due to oligoden-drocyte-derived ROS release. In addition, oligodendrocytes and their precursors themselves appeared to be relatively susceptible to oxidative stress [65, 66]. As noted, oligodendrocytes support neuronal function in the white matter via myelin-axon interactions. Moreover, precursor cells of oligodendrocytes exist even in adult white matters to repair axonal function after injury through differentiating mature oligodendrocytes. Therefore, ROS accumulation in the white matter after stroke may cause neuronal (axonal) damage in both direct and indirect manners.
In general, excessive production of ROS may be damaging in stroke and a wide range of CNS disorders. But it is becoming evident that like other mediators in the neuroscience of disease, ROS can be detrimental or beneficial, depending on concentration and cellular context [67]. For example, low levels of ROS promote OPC migration [68] and oligodendrocyte differentiation and myelin formation [69], and homeostatic levels of nitric oxide may support EPC function [70]. Recently, there is an important movement to more carefully define not only sources but also different types of radicals [71]. Not all free radicals are the same. Investigating these different cellular and chemical categories will be critical as we continue to dissect these mechanisms in the neurovascular unit for the future development of diagnostics and therapeutics.
4. INFLAMMATORY RESPONSES
Focal ischemia induces a potent inflammatory response within a few hours after onset of ischemia. Inflammation after ischemic insults composes reperfusion injury, and is an important part of stroke [72]. The insufficient blood supply after initial ischemic insult causes the development of coagulation cascades, which induce impact-induced shear stress and cellular damages in endothelial cells primarily. Expressions of adhesion molecules, such as P-selectin are increased in platelets and endothelial cells as early as 15 minutes after ischemic onset, and then, pro-inflammatory cascade is promptly initiated [73]. Endothelial cells are the major component of blood-brain barrier (BBB), and inflammatory cascades including platelet aggregation and leukocyte adhesion lead to BBB breakdown [73]. Once BBB is disrupted, various blood immune cells infiltrate into ischemic area [74–76]. These migrated immune cells (along with injured brain cells) produce inflammatory mediators, resulting in promoting neuronal death [77].
Microglial cells, which derive from bone marrow stem cells, are the resident immune cells of the CNS. Basically, resting microglial cells scan the environment, and play an active part in ischemic stroke because of their very low threshold of activation [78]. Neurons have been thought as victims of activated microglia (i.e. activated microglia are known as phagocytes of neurons after ischemia)[79]. Wake H. et al. examined the mechanisms of direct and activity-dependent connections between microglia and neuronal synapses. In vivo two-photon imaging analysis demonstrated that the duration of these interactions was prolonged in ischemic brain [80]. Under normal conditions, healthy neurons actively protect themselves from phagocytosis by displaying “don’t-eat-me” signals (CD200, CD47, and CD22 etc.) on their surface [81, 82]. Microglia have receptors for these signal molecules, and with these receptors’ activation, microglial phagocytosis is maintained at low level. However, under ischemic conditions, reduction of these “don’t-eat-me” signals exacerbates microglial activation to accelerate neuronal damage.
Other brain cells than microglia may also contribute to inflammatory responses to cause neuronal damage after ischemia. Following the onset of ischemia, a variety of molecules are released from the intracellular part of dead/damaged cells. These endogenous molecules are called danger-associated molecular patterns (DAMPs), and are regarded as activators of microglia and infiltrating peripheral immune cells [83]. Among DAMPs, high mobility group box 1 (HMGB1) has been relatively well investigated to understand the pathophysiology under ischemic conditions [84, 85]. Most brain cell types along with microglia and peripheral immune cells express HMGB1 receptors, such as advanced glycation end products (RAGE), toll-like receptor-2 (TLR-2), and TLR-4 [86, 87]. Once these receptors are activated, several pro-inflammatory cytokines are released to accelerate inflammatory responses. For example, activated microglia by HMGB1 contribute to post-ischemic inflammation by secreting TNF-α, ineteleukin-1β (IL-1β), ROS, and many pro-inflammatory cytokines [88]. Reactive astrocytes are also harmful after stroke in producing pro-inflammatory cytokines, such as IL-6, TNF-α, IL-1β and interferon-γ [89]. In addition, reactive astrocytes form glial scars, which inhibit axon function [89]. Pericytes, which exist intermittently along the wall of capillaries to support endothelial function, such as BBB tightness, are also known to release proinflammatory cytokines after injury [90, 91]. Released cytokines can promote inflammatory responses via inducing further release of DAMPs. These vicious cycles may accelerate inflammatory reactions and exacerbate neuronal loss.
Taken together, suppression of inflammatory responses remains a promising therapeutic approach in acute phase. Unlike other stress cascades, such as oxidative stress and glutamate excitotoxicity, inflammation occurs over hours and continue for days or weeks [92]. This wider therapeutic time window may provide potential opportunities for anti-inflammatory therapy for ischemic stroke [93]. However, it may be important to recognize that some mediators/responses in neuroinflammation may have biphasic characteristics, i.e. deleterious at acute phase but beneficial at chronic remodeling phase after brain injury [94, 95]. For example, matrix metalloproteinases represent a highly conserved component of inflammation post-stroke [96–98]. Early elevations in MMPs may be deleterious by degrading blood-brain barrier integrity and overall neurovascular function [99]. But during stroke recovery, MMPs may be essential for neurovascular plasticity and remodeling [100]. Therapeutic approaches that target inflammation should be carefully titrated so as not to interfere with the beneficial endogenous mechanisms of remodeling within the NVU.
5. EXTRACELLULAR VESICLES AND NEUROVASCULAR UNIT IN ISCHEMIC STROKE
As mentioned, various kinds of endogenous molecules are secreted from cells within the NVU. In this regard, extracellular vesicles (ECVs) have attracted our attentions on understanding the detail mechanisms of trophic coupling within this conceptual framework. A large amounts of ECVs are found within or out of eukaryotic cells [101]. They are called either MPs or exosomes according to their origins, sizes, and release mechanisms. MPs were first described in 1967 as “platelet dust” [102], whereas exosome was first named in 1980s as 5’-nucleotidase activity-containing vesicles [103]. Recently, microvesicles (MVs) is generally used for vesicles with limited diameters regardless of their origins and innate characteristics. However, as new contents and function in ECVs emerge, the simple distinction based on their size does not represent a complete explanation of ECVs any longer. It has been confirmed that MVs contain necessary signaling to exert physiological and pathogenic effects. Indeed, lipids, proteins, and nucleic acids in the vesicles are considered to have pivotal roles in cellular communication and contribute to pathogenesis in a broad scope of diseases including tumor, immunity disorders, cardiovascular diseases, neurodegeneration, infectious diseases, renal diseases and blood diseases [104–108]. For example, in the case of diabetic patients, the losses of endothelial micro RNA (miRNA) in MPs, which are involved in the endothelial function, are related to the impairment of peripheral angiogenic signaling [109].
MVs/MPs may contribute to NVU function/dysfuntion after ischemic stroke. Almost all the cell types composing NVU can produce ECVs [110], and therefore, they may mediate the inter-cellular communication within the NVU. Thus far, endothelial-derived MPs (EMPs) have been prominently researched. EMPs are highly organ-dependent [111], and are suggested as markers of endothelial dysfunction [112]. Production of EMPs is known to be stimulated by mediators such as TNF-α [113], thereafter increased EMPs convey proteases of matrix metalloproteinase family or mRNA that may promote recovery via angiogenesis under certain circumstances [114, 115]. Circulating EMPs account for about 17.1% of total MPs. Although EMPs have been categorized into groups according to their surface markers detected by flow cytometry (Table 1) [113], more studies are needed for clarification. Due to sampling limitations, EMPs profiling within ischemic region is still difficult to be completed, however, the correlation of circulating EMPs phenotype with intracranial and extracranial arterial stenosis was already identified [116]. Hence, circulating EMPs may be used as prognostic marker for acute ischemic patients when analyzed within 18.5–51.8 hours after ischemic onset [117].
Table 1.
Categorization of EMPs According to their Surface Markers Recognized by Flow Cytometry
| EMP Name |
Surface Markers Recognized by Flow Cytometry |
|---|---|
| E+ EMP | CD105+, CD41a−, CD45− |
| C+ EMP | CD105+, CD144+ |
| PS+ EMP | CD105+, PS+, CD41a− |
| I+ EMP | CD105+, CD54+, CD45− |
E+ EMP: endoglin-positive EMP; C+ EMP: specific endothelial EMP expressing VE-cadherin and endoglin; PS+ EMP: EMP expressing phosphatidylserine; I+ EMP: EMP expressing ICAM-1.
Other NVU component cells may be involved in the ECV phenomena after injury. As noted, astrocytes communicate with surrounding cells by direct contact and by releasing soluble factors. But they also shed ECVs that may induce angiogenesis and apoptosis [118, 119]. Within larger ECVs shed from astrocytes, mitochondria and other membranous organelles were identified [120]. Oligodendrocytes also secrete exosomes to balance myelin proteins and lipids and to relieve cell stress. These secreted exosome-like vesicles can inhibit morphological differentiation and myelin sheath formation [121]. Moreover, some exosomes released from oligodendrocytes would be selectively transferred from oligodendrocytes to microglia [122]. Research in NVU and ECVs is relatively new, and therefore, mechanisms by which ECVs from non-neuronal cells affect neuronal survival/ function are still mostly unknown. But recently, conditioned media from neuronal cells was shown to dramatically reduce the release of exosomes from oligodendrocytes [123]. Hence, deeper comprehension of ECV roles on cell-cell signaling in the NVU may hopefully lead to novel therapeutic strategies to protect neurons and all other NVU components against ischemic stress.
6. CONCLUSIONS
The concept of NVU provides an integrated framework for understanding cerebral function and dysfunction in both normal and pathological conditions. In this regard, salvage of only neurons is insufficient for treating stroke or other neurodegenerative diseases. Because cell-cell interactions are essential for proper functioning of the NVU, function and crosstalk between all cell types must be rescued. In this minireview, we have tried to summarize and re-interpret the major mechanisms of stroke pathophysiology in the context of cell-cell signaling in the NVU. A deeper understanding of these signals and substrates will be required for further development of stroke therapies.
ACKNOWLEDGEMENTS
Supported in part by grants from the National Institutes of Health, the SUMITOMO Life Social Welfare Service Foundation, the National Natural Science Foundation of China (Grant No. 81200915), the Beijing Natural Science Foundation, and the China National Basic Research 973 Program.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. New Engl. J. Med. 2000;343(10):710–722. doi: 10.1056/NEJM200009073431007. [DOI] [PubMed] [Google Scholar]
- 2.Lyden P, Shuaib A, Ng K, Levin K, Atkinson RP, Rajput A, Wechsler L, Ashwood T, Claesson L, Odergren T, Salazar-Grueso E Investigators, C.-I.H.T. Clomethiazole Acute Stroke Study in ischemic stroke (CLASS-I): final results. Stroke. 2002;33(1):122–128. doi: 10.1161/hs0102.101478. [DOI] [PubMed] [Google Scholar]
- 3.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T, Wasiewski WW, Emeribe U Investigators, S.I.T. NXY-059 for the treatment of acute ischemic stroke. New Engl. J. Med. 2007;357(6):562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 4.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nature reviews. Neuroscience. 2003;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 5.Gladstone DJ, Black SE, Hakim AM Heart; Stroke Foundation of Ontario Centre of Excellence in Stroke, R. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33(8):2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 6.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitagawa K. CREB and cAMP response element-mediated gene expression in the ischemic brain. The FEBSJ. 2007;274(13):3210–3217. doi: 10.1111/j.1742-4658.2007.05890.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15(11):1382–1402. doi: 10.1007/s10495-010-0481-0. [DOI] [PubMed] [Google Scholar]
- 9.Mabuchi T, Kitagawa K, Kuwabara K, Takasawa K, Ohtsuki T, Xia Z, Storm D, Yanagihara T, Hori M, Matsumoto M. Phosphorylation of cAMP response element-binding protein in hippocampal neurons as a protective response after exposure to glutamate in vitro and ischemia in vivo. J. Neuroscience. 2001;21(23):9204–9213. doi: 10.1523/JNEUROSCI.21-23-09204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J. Neuroscience. 1987;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kostandy BB. The role of glutamate in neuronal ischemic injury: the role of spark in fire. Neurol. Sci. 2012;33(2):223–237. doi: 10.1007/s10072-011-0828-5. [DOI] [PubMed] [Google Scholar]
- 12.Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69(4):369–381. doi: 10.1016/s0024-3205(01)01142-0. [DOI] [PubMed] [Google Scholar]
- 13.Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403(6767):316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32(1):1–14. [PubMed] [Google Scholar]
- 15.Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195(4284):1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhry FA, Schmitz D, Reimer RJ, Larsson P, Gray AT, Nicoll R, Kavanaugh M, Edwards RH. Glutamine uptake by neurons: interaction of protons with system a transporters. J. Neuroscience. 2002;22(1):62–72. doi: 10.1523/JNEUROSCI.22-01-00062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kvamme E, Roberg B, Torgner IA. Phosphate-activated glutaminase and mitochondrial glutamine transport in the brain. Neurochem. Res. 2000;25(9–10):1407–1419. doi: 10.1023/a:1007668801570. [DOI] [PubMed] [Google Scholar]
- 18.McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J. Neuroscience Res. 2007;85(15):3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- 19.Gorovits R, Avidan N, Avisar N, Shaked I, Vardimon L. Glutamine synthetase protects against neuronal degeneration in injured retinal tissue. Proc. Natl. Acad. Sci. USA. 1997;94(13):7024–7029. doi: 10.1073/pnas.94.13.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabaudon D, Scanziani M, Gahwiler BH, Gerber U. Acute decrease in net glutamate uptake during energy deprivation. Proc. Natl. Acad. Sci. USA. 2000;97(10):5610–5615. doi: 10.1073/pnas.97.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH, Xiong ZQ. Differential roles of NR2Aand NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J. Neuroscience. 2007;27(3):542–552. doi: 10.1523/JNEUROSCI.3607-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Feng XY, Xu L, Duan WH, Xiong ZQ. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke. 2008;39(11):3042–3048. doi: 10.1161/STROKEAHA.108.521898. [DOI] [PubMed] [Google Scholar]
- 23.Gouix E, Leveille F, Nicole O, Melon C, Had-Aissouni L, Buisson A. Reverse glial glutamate uptake triggers neuronal cell death through extrasynaptic NMDA receptor activation. Mol. Cell. Neuroscience. 2009;40(4):463–473. doi: 10.1016/j.mcn.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Stobart JL, Anderson CM. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front. Cell. Neuroscience. 2013;7:38. doi: 10.3389/fncel.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutherland BA, Papadakis M, Chen RL, Buchan AM. Cerebral blood flow alteration in neuroprotection following cerebral ischaemia. J. Physiol. 2011;589(Pt 17):4105–4114. doi: 10.1113/jphysiol.2011.209601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J. Cereb. Blood Flow Metab. 2003;23(3):263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- 27.Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438(7071):1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nature reviews. Neuroscience. 2008;9(11):839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 29.Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330(6005):779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- 30.Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 2003;33(3):366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 31.Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, Nave KA. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280(5369):1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 32.Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J. Neurosci. 2003;23(12):4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathur BN, Deutch AY. Rat meningeal and brain microvasculature pericytes co-express the vesicular glutamate transporters 2 and 3. Neurosci. Lett. 2008;435(2):90–94. doi: 10.1016/j.neulet.2008.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharp CD, Hines I, Houghton J, Warren A, Jackson THt, Jawahar A, Nanda A, Elrod JW, Long A, Chi A, Minagar A, Alexander JS. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am. J. Physiol. Heart Circ. Physiol. 2003;285(6):H2592–H2598. doi: 10.1152/ajpheart.00520.2003. [DOI] [PubMed] [Google Scholar]
- 37.Basuroy S, Leffler CW, Parfenova H. CORM-A1 prevents blood-brain barrier dysfunction caused by ionotropic glutamate receptor-mediated endothelial oxidative stress and apoptosis. Am. J. Physiol. Cell. Physiol. 2013;304(11):C1105–C1115. doi: 10.1152/ajpcell.00023.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1(6):383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 39.Cao W, Carney JM, Duchon A, Floyd RA, Chevion M. Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci. Lett. 1988;88(2):233–238. doi: 10.1016/0304-3940(88)90132-2. [DOI] [PubMed] [Google Scholar]
- 40.Floyd RA, Towner RA, He T, Hensley K, Maples KR. Translational research involving oxidative stress and diseases of aging. Free Radic. Biol. Med. 2011;51(5):931–941. doi: 10.1016/j.freeradbiomed.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gouriou Y, Demaurex N, Bijlenga P, De Marchi U. Mitochondrial calcium handling during ischemia-induced cell death in neurons. Biochimie. 2011;93(12):2060–2067. doi: 10.1016/j.biochi.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Wang H, Li J, Jimenez DA, Levitan ES, Aizenman E, Rosenberg PA. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J. Neuroscience. 2004;24(47):10616–10627. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, Shuaib A, Hardemark HG, Wasiewski WW Stroke-Acute Ischemic, N.X.Y.T.T.I. NXY-059 for acute ischemic stroke. New Engl. J. Med. 2006;354(6):588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- 44.Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem. Int. 2013;62(5):712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Lindenau J, Noack H, Possel H, Asayama K, Wolf G. Cellular distribution of superoxide dismutases in the rat CNS. Glia. 2000;29(1):25–34. doi: 10.1002/(sici)1098-1136(20000101)29:1<25::aid-glia3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 46.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000;267(16):4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Vartiainen NE, Ying W, Chan PH, Koistinaho J, Swanson RA. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J. Neurochem. 2001;77(6):1601–1610. doi: 10.1046/j.1471-4159.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- 48.Bambrick L, Kristian T, Fiskum G. Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem. Res. 2004;29(3):601–608. doi: 10.1023/b:nere.0000014830.06376.e6. [DOI] [PubMed] [Google Scholar]
- 49.Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr. Mol. Med. 2004;4(2):193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- 50.Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J. Physiol. 2011;589(Pt17):4125–4136. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neuroscience. 2003;23(8):3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neuroscience. 2004;24(5):1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calkins MJ, Vargas MR, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol. Sci. 2010;115(2):557–568. doi: 10.1093/toxsci/kfq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bell KF, Fowler JH, Al-Mubarak B, Horsburgh K, Hardingham GE. Activation of Nrf2-regulated glutathione pathway genes by ischemic preconditioning. Oxid. Med. Cell. Longev. 2011;2011:689524. doi: 10.1155/2011/689524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srivastava S, Alfieri A, Siow RC, Mann GE, Fraser PA. Temporal and spatial distribution of Nrf2 in rat brain following stroke: quantitation of nuclear to cytoplasmic Nrf2 content using a novel immunohistochemical technique. J. Physiol. 2013;591(Pt14):3525–3538. doi: 10.1113/jphysiol.2013.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West AK, Hidalgo J, Eddins D, Levin ED, Aschner M. Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. Neurotoxicology. 2008;29(3):489–503. doi: 10.1016/j.neuro.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Lookeren Campagne M, Thibodeaux H, van Bruggen N, Cairns B, Gerlai R, Palmer JT, Williams SP, Lowe DG. Evidence for a protective role of metallothionein-1 in focal cerebral ischemia. Proc. Natl. Acad. Sci. USA. 1999;96(22):12870–12875. doi: 10.1073/pnas.96.22.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trendelenburg G, Prass K, Priller J, Kapinya K, Polley A, Muselmann C, Ruscher K, Kannbley U, Schmitt AO, Castell S, Wiegand F, Meisel A, Rosenthal A, Dirnagl U. Serial analysis of gene expression identifies metallothionein-II as major neuroprotective gene in mouse focal cerebral ischemia. J. Neuroscience. 2002;22(14):5879–5888. doi: 10.1523/JNEUROSCI.22-14-05879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung RS, Penkowa M, Dittmann J, King CE, Bartlett C, Asmussen JW, Hidalgo J, Carrasco J, Leung YK, Walker AK, Fung SJ, Dunlop SA, Fitzgerald M, Beazley LD, Chuah MI, Vickers JC, West AK. Redefining the role of metallothionein within the injured brain: extracellular metallothioneins play an important role in the astrocyte-neuron response to injury. J. Biol. Chem. 2008;283(22):15349–15358. doi: 10.1074/jbc.M708446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sohn EJ, Kim DW, Kim MJ, Jeong HJ, Shin MJ, Ahn EH, Kwon SW, Kim YN, Kim DS, Han KH, Park J, Hwang HS, Eum WS, Choi SY. PEP-1-metallothionein-III protein ameliorates the oxidative stress-induced neuronal cell death and brain ischemic insults. Biochimica et Biophysica Acta. 2012;1820(10):1647–1655. doi: 10.1016/j.bbagen.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Chrissobolis S, Banfi B, Sobey CG, Faraci FM. Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J. Appl. Physiol. 2012;113(2):184–191. doi: 10.1152/japplphysiol.00455.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller AA, Drummond GR, Sobey CG. Novel isoforms of NADPH-oxidase in cerebral vascular control. Pharmacol. Ther. 2006;111(3):928–948. doi: 10.1016/j.pharmthera.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am. J. Pathol. 2008;173(1):2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christov A, Ottman JT, Grammas P. Vascular inflammatory, oxidative and protease-based processes: implications for neuronal cell death in Alzheimer's disease. Neurol. Res. 2004;26(5):540–546. doi: 10.1179/016164104225016218. [DOI] [PubMed] [Google Scholar]
- 65.Mronga T, Stahnke T, Goldbaum O, Richter-Landsberg C. Mitochondrial pathway is involved in hydrogen-peroxide-induced apoptotic cell death of oligodendrocytes. Glia. 2004;46(4):446–455. doi: 10.1002/glia.20022. [DOI] [PubMed] [Google Scholar]
- 66.Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22(4):371–378. doi: 10.1002/(sici)1098-1136(199804)22:4<371::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 67.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat. Med. 2008;14(5):497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 68.Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, Arai K. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J. Neurosci. 2011;31(29):10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cavaliere F, Urra O, Alberdi E, Matute C. Oligodendrocyte differentiation from adult multipotent stem cells is modulated by glutamate. Cell. Death Dis. 2012;3:e268. doi: 10.1038/cddis.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fleissner F, Thum T. Critical role of the nitric oxide/reactive oxygen species balance in endothelial progenitor dysfunction. Antioxid. Redox. Signal. 2011;15(4):933–948. doi: 10.1089/ars.2010.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 2012;52(1):1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromol. Med. 2010;12(2):193–204. doi: 10.1007/s12017-009-8074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, Obrenovitch TP, Contreras TJ. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986;17(2):246–253. doi: 10.1161/01.str.17.2.246. [DOI] [PubMed] [Google Scholar]
- 75.Clark RK, Lee EV, Fish CJ, White RF, Price WJ, Jonak ZL, Feuerstein GZ, Barone FC. Development of tissue damage, inflammation and resolution following stroke: an immunohistochemical and quantitative planimetric study. Brain Res. Bull. 1993;31(5):565–572. doi: 10.1016/0361-9230(93)90124-t. [DOI] [PubMed] [Google Scholar]
- 76.Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33(2):586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- 77.Shichita T, Ago T, Kamouchi M, Kitazono T, Yoshimura A, Ooboshi H. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J. Neurochem. 2012;123(Suppl 2):29–38. doi: 10.1111/j.1471-4159.2012.07941.x. [DOI] [PubMed] [Google Scholar]
- 78.Graeber MB. Changing face of microglia. Science. 2010;330(6005):783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 79.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trend. Neurosci. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neuroscience. 2009;29(13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neher JJ, Neniskyte U, Brown GC. Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Front. Pharmacol. 2012;3:27. doi: 10.3389/fphar.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, Ehrhart J, Mullan M, Tan J. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46(4):369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- 83.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neuroscience. 2006;26(24):6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann. N. Y. Acad. Sci. 2010;1207:50–57. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158(3):1007–1020. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res. Rev. 2009;59(2):278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012;87(1):10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barreto G, White RE, Ouyang Y, Xu L, Giffard RG. Astrocytes: targets for neuroprotection in stroke. Cent. Nerv. Syst. Agents Med. Chem. 2011;11(2):164–173. doi: 10.2174/187152411796011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol. Nneurobiol. 2012;45(2):327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- 91.Kovac A, Erickson MA, Banks WA. Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J. Neuroinflammation. 2011;8:139. doi: 10.1186/1742-2094-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann. N. Y. Acad. Sci. 2012;1268:21–25. doi: 10.1111/j.1749-6632.2012.06691.x. [DOI] [PubMed] [Google Scholar]
- 93.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol. Res. 2008;30(8):783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xing C, Hayakawa K, Lok J, Arai K, Lo EH. Injury and repair in the neurovascular unit. Neurol. Res. 2012;34(4):325–330. doi: 10.1179/1743132812Y.0000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maki T, Hayakawa K, Pham LD, Xing C, Lo EH, Arai K. Biphasic Mechanisms of Neurovascular Unit Injury and Protection in CNS Diseases. CNS. Neurol. Disord. Drug Targets. 2013;12(3):302–315. doi: 10.2174/1871527311312030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Ann. N. Y. Acad. Sci. 2010;1207:46–49. doi: 10.1111/j.1749-6632.2010.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Romi F, Helgeland G, Gilhus NE. Serum levels of matrix metalloproteinases: implications in clinical neurology. Eur. Neurol. 2012;67(2):121–128. doi: 10.1159/000334862. [DOI] [PubMed] [Google Scholar]
- 98.Seo JH, Guo S, Lok J, Navaratna D, Whalen MJ, Kim KW, Lo EH. Neurovascular matrix metalloproteinases and the blood-brain barrier. Curr. Pharm. Des. 2012;18(25):3645–3648. doi: 10.2174/138161212802002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001;21(19):7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metallo proteinases in delayed cortical responses after stroke. Nat. Med. 2006;12(4):441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 101.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnel J. Molecular Cell Biology. 4th edition. New York: W.H. Freeman and Company; 2000. [Google Scholar]
- 102.Wolf P. The nature and significance of platelet products in human plasma. Brit. J. Haematol. 1967;13(3):269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 103.Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et Biophysica Acta. 1981;645(1):63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 104.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 105.Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front. Physiol. 2012;3:228. doi: 10.3389/fphys.2012.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ling ZL, Combes V, Grau GE, King NJ. Microparticles as immune regulators in infectious disease - an opinion. Front. Immunol. 2011;2:67. doi: 10.3389/fimmu.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shai E, Varon D. Development, cell differentiation, angio genesis--microparticles and their roles in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2011;31(1):10–14. doi: 10.1161/ATVBAHA.109.200980. [DOI] [PubMed] [Google Scholar]
- 108.Tushuizen ME, Diamant M, Sturk A, Nieuwland R. Cell-derived microparticles in the pathogenesis of cardiovascular disease: friend or foe? Arterioscler. Thromb. Vasc. Biol. 2011;31(1):4–9. doi: 10.1161/ATVBAHA.109.200998. [DOI] [PubMed] [Google Scholar]
- 109.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circulation Res. 2010;107(6):810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 110.Fruhbeis C, Frohlich D, Kramer-Albers EM. Emerging roles of exosomes in neuron-glia communication. Front. Physiol. 2012;3:119. doi: 10.3389/fphys.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo S, Zhou Y, Xing C, Lok J, Som AT, Ning M, Ji X, Lo EH. The vasculome of the mouse brain. PloS One. 2012;7(12):e52665. doi: 10.1371/journal.pone.0052665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bulut D, Maier K, Bulut-Streich N, Borgel J, Hanefeld C, Mugge A. Circulating endothelial microparticles correlate inversely with endothelial function in patients with ischemic left ventricular dysfunction. J. Card. Fail. 2008;14(4):336–340. doi: 10.1016/j.cardfail.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 113.Horstman LL, Jy W, Jimenez JJ, Ahn YS. Endothelial microparticles as markers of endothelial dysfunction. Front. Biosci. 2004;9:1118–1135. doi: 10.2741/1270. [DOI] [PubMed] [Google Scholar]
- 114.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 115.Taraboletti G, D'Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 2002;160(2):673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jung KH, Chu K, Lee ST, Park HK, Bahn JJ, Kim DH, Kim JH, Kim M, Kun Lee S, Roh JK. Circulating endothelial microparticles as a marker of cerebrovascular disease. Ann. Neurol. 2009;66(2):191–199. doi: 10.1002/ana.21681. [DOI] [PubMed] [Google Scholar]
- 117.Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J. Thromb. Haemost. 2006;4(6):1296–1302. doi: 10.1111/j.1538-7836.2006.01911.x. [DOI] [PubMed] [Google Scholar]
- 118.Proia P, Schiera G, Mineo M, Ingrassia AM, Santoro G, Savettieri G, Di Liegro I. Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int. J. Mol. Med. 2008;21(1):63–67. [PubMed] [Google Scholar]
- 119.Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, Mayer-Proschel M, Bieberich E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD) J. Biol. Chem. 2012;287(25):21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Falchi AM, Sogos V, Saba F, Piras M, Congiu T, Piludu M. Astrocytes shed large membrane vesicles that contain mitochondria, lipid droplets and ATP. Histochem. Cell Biol. 2013;139(2):221–231. doi: 10.1007/s00418-012-1045-x. [DOI] [PubMed] [Google Scholar]
- 121.Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, Nave KA, Schild H, Trotter J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteom. Clin. Appl. 2007;1(11):1446–1461. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- 122.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK, Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011;124(Pt 3):447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 123.Bakhti M, Winter C, Simons M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 2011;286(1):787–796. doi: 10.1074/jbc.M110.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]