Abstract
Diagnosis of the autoimmune disease type 1 diabetes (T1D) is preceded by the appearance of circulating autoantibodies to pancreatic islets. However, almost nothing is known about events leading to this islet autoimmunity. Previous epidemiological and genetic data have associated viral infections and antiviral type I interferon (IFN) immune response genes with T1D. Here, we first used DNA microarray analysis to identify IFN-β–inducible genes in vitro and then used this set of genes to define an IFN-inducible transcriptional signature in peripheral blood mononuclear cells from a group of active systemic lupus erythematosus patients (n = 25). Using this predefined set of 225 IFN signature genes, we investigated the expression of the signature in cohorts of healthy controls (n = 87), patients with T1D (n = 64), and a large longitudinal birth cohort of children genetically predisposed to T1D (n = 109; 454 microarrayed samples). Expression of the IFN signature was increased in genetically predisposed children before the development of autoantibodies (P = 0.0012) but not in patients with established T1D. Upregulation of IFN-inducible genes was transient, temporally associated with a recent history of upper respiratory tract infections (P = 0.0064), and marked by increased expression of SIGLEC-1 (CD169), a lectin-like receptor expressed on CD14+ monocytes. DNA variation in IFN-inducible genes altered T1D risk (P = 0.007), as exemplified by IFIH1, one of the genes in our IFN signature for which increased expression is a known risk factor for disease. These findings identify transient increased expression of type I IFN genes in preclinical diabetes as a risk factor for autoimmunity in children with a genetic predisposition to T1D.
Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by the T-cell–mediated destruction of insulin-producing β-cells in pancreatic islets. Seroconversion to islet autoantibody positivity occurs in the first years of life, often many years before insulin dependence and diagnosis of disease (1,2). Although first characterized in the 1970s, islet autoantibodies remain the only established disease-specific markers. However, the precise mechanisms that lead to the break in tolerance and infiltration of the pancreas by autoreactive T cells remain poorly understood in humans. Genetic studies have implicated type I interferon (IFN) signaling and antiviral immune responses in the etiology of T1D through the association of several candidate genes in this biological pathway (IFIH1, TLR7/TLR8, FUT2, and GPR183) (1,3). These data support the hypothesis that viral infections could be an environmental factor for T1D (4,5). Furthermore, IFN-α and increased HLA class I can be readily detected in the islet β-cells of patients with T1D, which is consistent with a model involving direct cytotoxic T-cell killing of β-cells in the pathogenesis of the disease (6,7). These findings are remarkably consistent with data from the spontaneous mouse model of diabetes (nonobese diabetic mice) in which increased IFN production is directly involved in the pathogenesis of the disease (8,9).
An IFN-inducible transcriptional signature, originally characterized in patients with systemic lupus erythematosus (SLE) and found to correlate with increased disease severity (10,11), now has been identified in the peripheral blood of patients with other autoimmune and infectious diseases (12,13). Here we investigated the expression of an IFN signature in the onset of autoimmunity and progression to a clinical diagnosis of T1D in a large prospective birth cohort of children at high risk of developing T1D (14). Our findings provide novel evidence for the role of an exacerbated IFN response in the earlier stages of the autoimmune response in T1D and show for the first time that a disease-associated transcriptional signature can be detected in genetically predisposed children before the development of autoantibodies.
Research Design and Methods
Subjects
All samples and information were collected with written and signed informed consent. Study participants included 49 patients with recently diagnosed T1D (disease duration ≤3 years); 15 adult patients with long-standing T1D; 93 adult healthy volunteers; 25 adult patients with active SLE; and 109 children genetically predisposed to T1D who were enrolled in the BABYDIET study (14). All BABYDIET children were prospectively measured from the age of 3 months for four T1D-specific autoantibodies: IAA, GADA, IA2A, and ZnT8 (further details on the BABYDIET study design and patient selection are provided in the Supplementary Material).
Patients with recently diagnosed T1D were enrolled in the Diabetes-Genes, Autoimmunity and Prevention (D-GAP) study. The D-GAP study was approved by the Royal Free Hospital & Medical School research ethics committee (REC; 08/H0720/25). Adult patients with long-standing T1D and healthy volunteers were enrolled in the Cambridge BioResource (CBR; www.cambridgebioresource.org.uk). The study was approved by the local Peterborough and Fenland REC (05/Q0106/20).
Patients with SLE meeting at least four American College of Rheumatology SLE criteria attended or were referred to the Addenbrooke’s Hospital (UK) specialist vasculitis unit between July 2004 and May 2008 (15). All presented with active disease, and immunosuppressive therapy was commenced or increased. The patients with SLE were recruited under the following two ethics applications: Cambridge/Hinxton Centre for Translational Research in Autoimmune Disease, approved 23 January 2004 by the Cambridge local REC (ref. 04/023); Cambridge/Hinxton Centre for Translational Research in Autoimmune Disease 2, approved 27 June 2008 by the Cambridgeshire 3 REC (ref. 08/H0306/21).
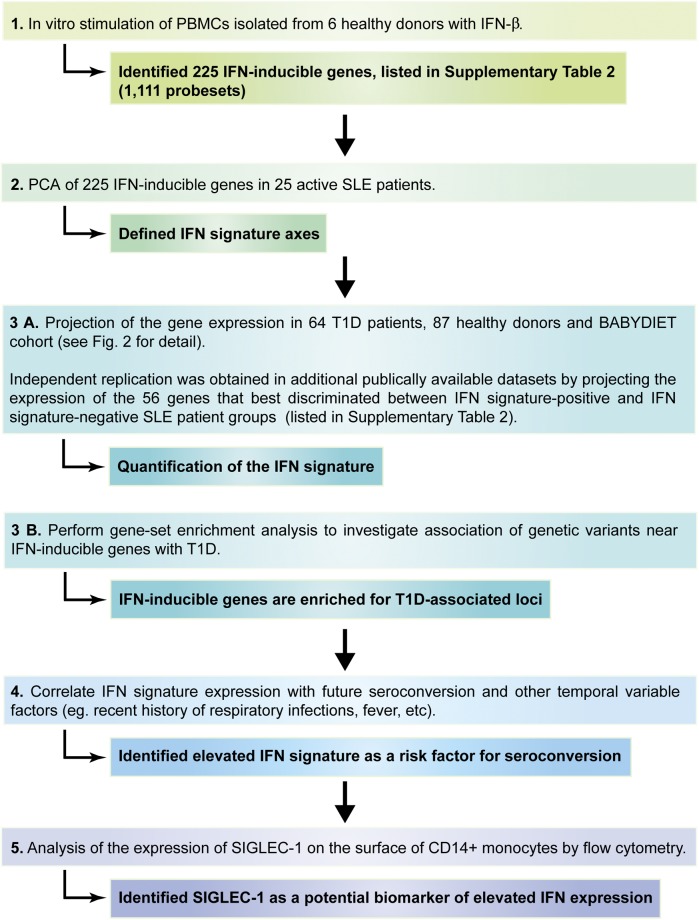
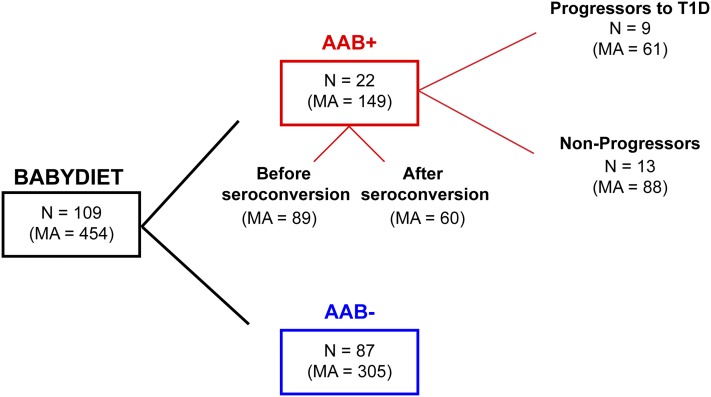
A diagram of the study design and workflow is depicted in Fig. 1, and baseline characteristics and demographics of all study participants and cohorts are summarized in Fig. 2 and Table 1.
Figure 1.
Workflow of the study’s experimental design. The diagram depicts the different stages of the study and the main outcomes of each stage.
Figure 2.
Summary diagram of the BABYDIET cohort. The diagram represents the division of the 109 BABYDIET children and microarray samples according to seroconversion and progression to T1D. AAB, T1D-specific autoantibodies (see Research Design and Methods for details); MA, number of microarray measurements; N, number of individuals.
Table 1.
Baseline characteristics of study participants
| Cohort | Individuals (n) | Microarray measurements (n) | Age (years) | Male, n (%) | Disease duration (years) | ||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | ||||
| Patients with TID | |||||||
| D-GAP* | 49 | 49 | 12 | 6–34 | 29 (59) | 1.4 | 0–3 |
| CBR† | 15 | 15 | 31 | 22–35 | 5 (33) | 13 | 0–23 |
| Combined | 64 | 64 | 13 | 6–35 | 34 (53) | 1.7 | 0–23 |
| Healthy controls from CBR | |||||||
| IFN-β stimulation‡ | 6 | 6 | 35 | 22–37 | 3 (50) | N/A | N/A |
| IFN signature§ | 87 | 87 | 42 | 22–52 | 27 (31) | N/A | N/A |
| Patients with SLE | 25 | 25 | 43 | 19–61 | 5 (20) | N/A | N/A |
| BABYDIET‖ | 109 | 454 | 1.5 | 0.2–9.1 | 45 (41) | N/A | N/A |
Baseline characteristics for the study participants were stratified by the study cohorts.
Patients with newly diagnosed T1D (duration of disease ≤3 years) enrolled in the Diabetes-Genes, Autoimmunity and Prevention (D-GAP) study.
Long-standing adult patients with T1D enrolled in the Cambridge BioResource (CBR).
Healthy donors selected from the CBR for the IFN-β stimulation assay
Healthy donors selected from the CBR for the microarray assays.
BABYDIET is a prospective birth cohort of children genetically predisposed to T1D with at least one first-degree relative diagnosed with T1D and a carrier of the high-risk HLA-DRB1*03 and/or HLA-DRB1*04 alleles. In the BABYDIET cohort, there were 22 seroconverters (children who persistently developed at least one of four T1D-specific autoantibodies during the course of the study [i.e., IAA, GAD, IA2A, and ZnT8]) and 9 children who progressed to TID. Median age of seroconversion was 2.1 years, and median age at TID diagnosis was 6.2 years. N/A, not applicable.
Peripheral Blood Mononuclear Cell Preparation and RNA Isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by density gradient centrifugation using Lympholyte (Cedarlane). Total RNA was isolated from 106 PBMCs using the phenol-chloroform method according to the manufacturer’s instructions. RNA concentrations were measured by NanoDrop (Thermo Scientific), and RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies). All RNA samples analyzed in this study were isolated from PBMCs collected directly into TRIZOL reagent (Life Technologies) and stored at −80°C within less than 24 h of venipuncture to avoid alterations of the gene expression profile caused by freezing and resuscitation of viable PBMCs. The duration of storage of stable denatured PBMC lysates in TRIZOL before RNA isolation varied according to the sample cohort: 1) BABYDIET: median 90.4 months, minimum 57.1 months, maximum 136.9 months; 2) patients with T1D from CBR: median 6.8 months, minimum 1.8 months, maximum 15.7 months; 3) patients with SLE: median 12.9 months, minimum 2.1 months, maximum 58.8 months. The investigators were blinded to group allocation during sample preparation.
In Vitro Stimulation of PBMCs
Two million PBMCs from six adult healthy donors (see Table 1) were cultured in X-VIVO medium (Lonza) containing 1% heat-inactivated human AB serum (Sigma-Aldrich) in the presence or absence of IFN-β (100 U/mL; Peprotech) on flat-bottom 24-well plates (BD Biosciences). Cells were harvested at 2, 6, and 18 h after stimulation and were immediately stored in TRIZOL reagent (Life Technologies) at −80°C. In addition, resting PBMCs from each donor (unstimulated; t = 0 h) also were stored in TRIZOL reagent at −80°C.
cDNA Synthesis Microarray Hybridization
Single-stranded cDNA was synthesized from 200 ng total RNA using a whole-transcript expression kit (Ambion) according to the manufacturer’s instructions. cDNA (3.44 μg) was fragmented and labeled using the GeneChip terminal labeling and hybridization kit and hybridized to 96-sample Titan Affymetrix Human Gene 1.1 ST arrays, which provide comprehensive whole-transcriptome coverage with more than 750,000 unique 25-mer oligonucleotide probes interrogating more than 28,000 annotated genes (median 22 probes/gene). All gene expression data generated in this study are on record with ArrayExpress (http://www.ebi.ac.uk/arrayexpress, accession no. E-MTAB-1724).
PBMC Immunostaining and Flow Cytometry
Surface expression of SIGLEC-1, IL-15R, PD-L1, TRAIL, CD69, and CD38 was measured in cryopreserved PBMCs from three patients with T1D showing upregulated expression of IFN-inducible genes and three age- and sex-matched patients with low expression of the IFN-inducible genes. Aliquots of 5 × 106 cryopreserved PBMCs from the same preparation used for microarray hybridization were thawed as described previously (16) and were stained for 1 h at 4°C. The investigators were blinded to the sample group allocation during the experimental procedure and data analysis.
Expression of these six IFN-inducible surface proteins also was measured in fresh PBMCs from one healthy donor after in vitro stimulation with IFN-α (10 ng/mL), IFN-β (10 ng/mL; Peprotech), or culture medium (unstimulated) for 3, 6, 24, 72, 120, and 168 h. Cells were harvested at 3, 6, 24, 72, 120, and 168 h after stimulation and stained for 1 h at 4°C, as described previously (16).
Antibodies used in this study are summarized in Supplementary Table 1. Positivity for SIGLEC-1 was determined on the basis of the upper one percentile of the isotype control (phycoerythrin-conjugated mouse IgG1k; BD Biosciences) immunostaining in the respective donor. Immunostained samples were analyzed using a Fortessa flow cytometer (BD Biosciences) with FACSDiva software (BD Biosciences). Flow cytometry data were exported in the format 3.0 and analyzed using FlowJo software (Tree Star, Inc.). Doublet exclusion was performed for all assessed populations.
Gene Expression Data Analysis
Data were summarized by exon-level probe sets and normalized using variance stabilizing normalization (17) separately for BABYDIET samples and other samples. In addition, we downloaded public microarray data from ArrayExpress, as detailed in the Supplementary Material.
We omitted from the analysis 70 of 524 BABYDIET hybridizations based on large median normalized unscaled standard errors (suggestive of poor RNA quality) and principal component (PC) analysis indicating outliers. Of these, 62 samples belonged to a single batch in which RNA isolation was compromised because of contaminated chloroform leading to inaccurate gene expression measurement in these samples. Hierarchical clustering was performed in R (http://www.R-project.org) (18) using the function hclust, and PCs were used to summarize gene expression signatures either directly (SLE and independent data sets) or by projection onto PCs defined in patients with SLE (T1D, control, and BABYDIET samples), as detailed in the Supplementary Material. A sample’s projection on the first PC, which correlated with upregulation of IFN-inducible genes, was used to quantify its IFN signature. When analyzing the IFN signature in independent external data sets, we could not project them onto the IFN signature defined in our SLE patient data because different array technologies have different gene coverage. Instead, we applied independent PC analysis using the subset of probes from these data sets that overlapped the 56 IFN-inducible genes (fold-change >2 and false discovery rate <0.05; listed in Supplementary Table 1) that most strongly discriminated between groups of patients with SLE according to the microarray gene symbol annotation provided in each data set.
The R package “wGSEA” (http://cran.r-project.org/web/packages/wgsea/index.html) was used to perform gene-set enrichment analysis to assess whether IFN-inducible genes were enriched for variants associated with T1D compared with a control set of probe sets generated by 10-to-1 matching according to the coefficient of variation in the unstimulated (time = 0 h) PBMC samples from the same donors. Probe sets were assigned to genes using Ensembl (version 67); to capture potential regulatory variation, genic regions were extended by ±200 kb centered on the most 5′ transcriptional start site (19).
Paired and unpaired moderated t tests (20) were used to test for expression differences at individual probe sets between cells cultured in the presence or absence of IFN-β at each time point and between subgroups of patients with SLE, respectively. For longitudinal data sets we tested the association between the IFN signature and factors of interest by fitting a linear mixed model, using a random intercept to allow for within-individual correlation and including age and sex as covariates.
Correlation Between Infectious Events and the Expression of the IFN Signature in the BABYDIET Cohort
At each visit, parents completed a detailed questionnaire about their child’s history of infections, fever, and medications. In particular they were asked about fever, infectious symptoms (such as diarrhea, vomiting, constipation, and allergies), and the name of administered pharmaceutical agents or their active ingredient with starting date and duration of infections and medications. Infectious disease was defined as an acute event according to the ICD-10 code or by a symptom indicating an infectious genesis. Infectious events were assigned to a specific time interval by their date of onset. We defined three categories of infectious diseases: 1) infections of the respiratory tract, ear, nose, throat, and eye (if inflammatory symptoms of the respiratory tract were reported); 2) gastrointestinal infections (if the main symptoms were diarrhea and/or vomiting); and 3) other infections (e.g., with symptoms of skin or mucosa lesions). For the current analysis, gastrointestinal and other infections were considered nonrespiratory tract infections. Other disease events such as allergies or accidents were not considered infectious diseases. Separate infectious diseases of one category were defined as one infectious event if there were <6 days of potential remission between the respective infections because these seemed likely to be caused by the same infectious agent. Infectious events that could be matched to microarray samples were included for analysis. Because we wanted to maximize the power to investigate whether the expression of IFN-inducible genes was associated with the occurrence of an infectious event, we included in the analysis only infectious events that were measured within 1.5 months of the microarray measurements. We excluded from analysis 48 microarray measurements because they were taken more than 1.5 months after the infection report. Incidences of infections were subdivided according to whether they were respiratory. Samples with two or more recent infections were grouped as “2+.” Counts of both nonrespiratory and respiratory infections were fitted in a single model, including age, sex, and a random intercept to allow for within-individual correlation, and either type of infection was examined individually, stratifying by the count of the other infection.
Results
Characterization of a Type I IFN Transcriptional Signature in PBMCs
We identified 1,111 probe sets mapping to 225 unique genes that were differentially expressed (false discovery rate <0.001) at one or more time points in PBMCs isolated from six healthy donors in response to IFN-β stimulation for 2, 6, and 18 h (Supplementary Table 1). IFN-α and IFN-β, the two main type I IFNs, are known to regulate a similar set of genes. Expression of these 225 genes in PBMCs stimulated with IFN-α or IFN-β was strongly correlated in a public data set (21) (ρ = 0.86; Supplementary Fig. 1), and we therefore describe them as type I IFN-inducible. We then used P values from a genome-wide association study of 7,514 cases and 9,045 controls (3) to examine the evidence for association between these genes and T1D. Three IFN-inducible genes—IFIH1, CD69, and IL2RA—have been previously characterized as candidate causal genes in known T1D susceptibility loci (www.T1DBase.org). A gene set enrichment analysis showed that variants near these 225 type I IFN-inducible genes showed stronger association with T1D than variants near a control set of genes (P = 4 × 10−5 overall; P = 0.007 excluding the major histocompatibility complex region).
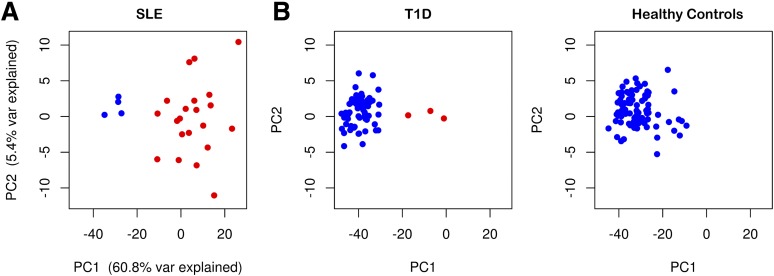
An IFN-inducible transcriptional signature has been previously identified in whole blood from patients with SLE (10,11). We clustered 25 patients with SLE based on expression of the IFN-inducible probe sets and found that patients clustered into two distinct groups, with 21 (84%) showing an upregulation of the IFN-inducible genes (Fig. 3). Increased expression was seen across the vast majority of IFN-inducible genes and was specific to these genes (Supplementary Fig. 2 and Supplementary Table 1). Our summary IFN signature measure, represented by an individual’s projection of the 225 identified IFN-inducible genes onto to the first PC, explained more than 60% of the variation in expression and correlated strongly with increased expression of the IFN-inducible genes. An IFN signature can also be induced by treatment with recombinant IFN-β and infection, which we demonstrated using public data from patients with multiple sclerosis (MS) and controls (22) (Supplementary Fig. 3) and two large cohorts of healthy donors followed longitudinally after vaccination against influenza (23) (Supplementary Fig. 4). After vaccination, the IFN induction is rapid (seen within 24 h) and transient, returning close to baseline at day 3 (Supplementary Fig. 4). Given the use of different gene expression platforms, the IFN signature was calculated in external data sets using the expression of the probes mapping to a subset of 56 discriminatory IFN-inducible genes (Supplementary Table 1), which we found to recapitulate the IFN signature obtained from the expression of all 225 IFN-inducible genes.
Figure 3.
An IFN signature can be detected in peripheral blood of patients with SLE and T1D. A: Plot depicting the two first PCs obtained from the expression of the 1,111 IFN-inducible probe sets defined in patients with SLE (n = 25) in this study. The percentage of variance explained by each PC is shown on the respective axis. B: Plots depicting the first two PCs obtained from the projection of the expression of the IFN-inducible probe sets in subjects from cross-sectional cohorts of patients with T1D (n = 64; left panel) and adult healthy controls (n = 87; right panel) onto the PC axes defined in the analysis of the patients with SLE (see Research Design and Methods for details). The cross-sectional cohorts of patients with T1D include 15 adult patients with long-standing T1D (median 13 years since diagnosis; median age 31 years) enrolled from the Cambridge BioResource and 49 recently diagnosed patients (median 1.4 years since diagnosis; median age 12 years) recruited from the Diabetes-Genes, Autoimmunity and Prevention study. Samples that show clear evidence of upregulation of IFN-inducible genes by hierarchical clustering are depicted in red.
Expression of the IFN Signature in Prediabetes is Transient and Precedes Seroconversion
We found evidence for the expression of the IFN signature in a subset of the 64 samples from patients with T1D and the 87 samples from healthy controls (Fig. 3) but at a lower frequency than in those from patients with SLE. While the samples from patients with T1D could clearly be separated into two groups (three samples showed strong upregulation of the IFN-inducible genes), there was no clear grouping among the controls, and it is possible that IFN signature genes were not strongly upregulated in any control samples. Transient expression of the IFN signature also was observed in 454 longitudinal measurements from 109 unique children in BABYDIET (Supplementary Fig. 5). In contrast to the patients with SLE, there was no clear grouping of the samples (Supplementary Fig. 6). Instead, the pattern resembled that found in patients with MS (22), with a large group of samples with relatively homogeneous expression and a smaller group showing variable expression (Supplementary Fig. 3).
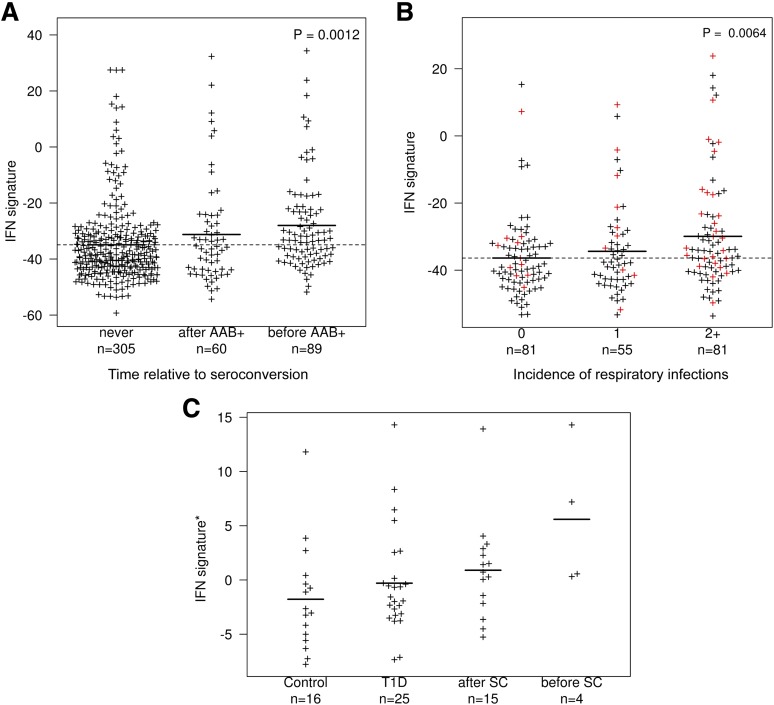
Compared with patients with T1D, the expression of the IFN signature was increased in BABYDIET children, but we cannot separate the effects of disease stage and age in this comparison. Instead, we compared the 22 individuals who seroconverted during the course of the study to the 87 subjects who did not (Fig. 2) and observed that the mean expression of the IFN signature was highest in samples collected before seroconversion and lowest in those from children who did not seroconvert during the course of the study (P = 0.0012; Fig. 4A). The IFN signature in children was correlated with recent self-reported incidence of respiratory infections (P = 0.0064; Fig. 4B) but not with age, recent self-reported incidence of nonrespiratory infections, or fever (P = 0.444, P = 0.524, and P = 0.478, respectively; Supplementary Fig. 7). Similarly, a correlation was observed only with respiratory infection when stratifying by nonrespiratory infections and not vice versa (Supplementary Fig. 8). We found no evidence for an effect of time of first gluten exposure in the children’s diet and the development of an IFN signature in peripheral blood (P = 0.211; data not shown).
Figure 4.
Expression of the IFN signature is increased before seroconversion and correlates with a recent history of respiratory infections. A: A quantitative summary IFN signature score (IFN signature; depicted in the y-axis) was obtained by the individual’s projection of the 225 identified IFN-inducible genes onto the first PC defined in the SLE group. The IFN signature is shown for all 454 samples from 109 children in BABYDIET who were analyzed for gene expression using microarrays, stratified according to time to seroconversion, including 305 microarray measurements from 87 donors who did not seroconvert (“never”), 89 microarray measurements from 22 T1D-specific autoantibody–positive (AAB+) donors taken before seroconversion (“before AAB+”), and 60 microarray measurements from the same 22 AAB+ donors taken after seroconversion (“after AAB+”). The number of microarray measurements taken before and after seroconversion from each of the 22 AAB+ donors is depicted in Supplementary Fig. 5. The P value represents a two-sided two degrees of freedom test comparing the average IFN signature of the three groups. B: IFN signature score of all 219 samples from the 109 children in BABYDIET are shown, stratified according to number (0, 1, or 2+) of self-recorded episodes of respiratory infections in the 3-month period nearest to, and within 1.5 months of, the date of collection of the blood sample used for microarray analysis. The P value represents a two-sided one degree of freedom test treating incidence as a quantitative variable and comparing the average IFN signature of the three groups using a linear mixed model. Samples from AAB+ children collected before seroconversion (red) were enriched for 2+ respiratory infections (P = 0.0128, χ2 test). C: Expression of the IFN signature was measured in an independent longitudinal cohort comprising two AAB+ Finnish children (19 samples) compared with three patients with T1D (25 samples) and three matched control children (16 samples). Samples from the AAB+ children were further stratified according to whether they were before (n = 4) or after (n = 15) seroconversion (SC). Data were downloaded from E-TABM-666. *Expression of the IFN signature was obtained from independent principle component analysis from 54 genes present in E-TABM-666 that overlapped with the 56 most discriminatory IFN-inducible genes in the SLE cohort (listed in Supplementary Table 1). n, number of samples with microarray measurements in each group.
To obtain independent support for our results, we analyzed published data from two autoantibody-positive children before clinical diagnosis of T1D, three children who developed T1D during the course of the study, and three age-matched controls in a longitudinal birth cohort from Finland (24). Although the limited sample size precludes formal statistical analysis, visual inspection provides obvious support for our results in BABYDIET: expression of the IFN signature was again highest in samples before seroconversion, lowest in control samples, and intermediate in samples taken after seroconversion and from children with clinical T1D (Fig. 4C and Supplementary Fig. 9).
SIGLEC-1 Expression by Monocytes Marks the IFN Signature
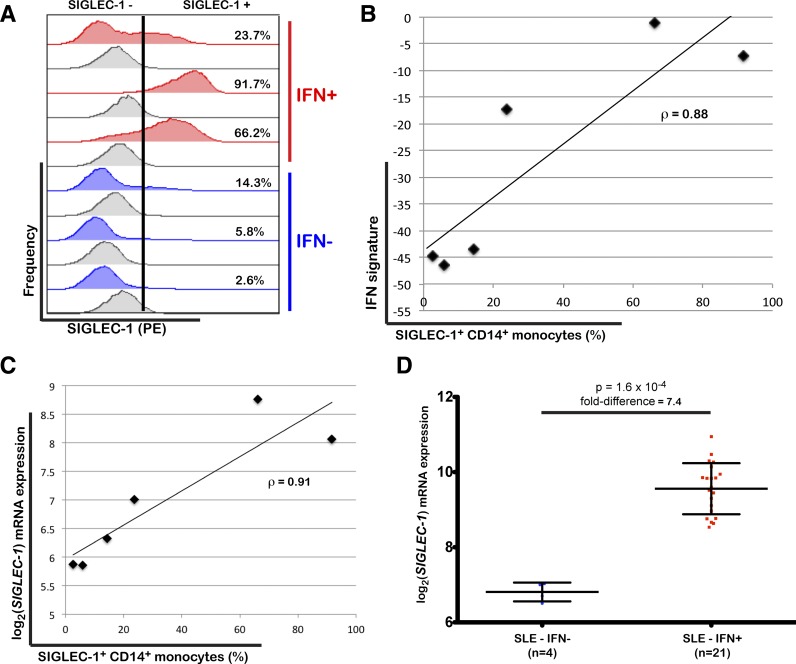
Among the 225 IFN-inducible genes, we identified six encoding for surface proteins: SIGLEC-1, IL-15R, PD-L1, TRAIL, CD69, and CD38. We assessed the expression levels of these proteins on the major subsets of circulating leukocytes by flow cytometry in all three of the patients with T1D in whom we found evidence for the upregulation of IFN-inducible genes (Fig. 2) and in three age- and sex-matched patients with low expression of these genes. Expression of SIGLEC-1 (CD169, sialoadhesin) was increased on the surface of CD14+ monocytes of the three patients with an IFN signature (Fig. 5A) and correlated strongly with the expression levels of IFN-inducible genes, defined by the first PC (ρ = 0.88; Fig. 5B). Furthermore, we found a strong correlation between the frequency of SIGLEC-1+ CD14+ monocytes and SIGLEC-1 mRNA expression levels in PBMCs (ρ = 0.91; Fig. 5C) as measured by microarray in these six donors, suggesting that mRNA expression in whole blood is a good surrogate for the surface expression of the protein on monocytes. In SLE, SIGLEC-1 mRNA expression was, on average, 7.4-fold higher in the 21 IFN signature–positive patients than in the four IFN signature–negative patients (P = 1.6 × 10−4; Fig. 5D), which further supports the specificity of the expression of this protein in individuals with increased IFN signaling in the peripheral blood.
Figure 5.
SIGLEC-1 expression by CD14+ monocytes is a marker of increased IFN responses. A: Frequency of SIGLEC-1+ CD14+ monocytes was measured by flow cytometry in cryopreserved PBMCs from three patients with T1D with increased expression of IFN-inducible genes (IFN+) and three age- and sex-matched patients with T1D with low expression of IFN-inducible genes (IFN-). Histograms for the isotype control immunostainings are depicted in gray immediately below the respective volunteer. Positivity for SIGLEC-1 is defined on the basis of the upper one percentile of the respective isotype control (illustrated by the vertical black bar in one representative example). The percentage of SIGLEC-1+ CD14+ monocytes is indicated for each volunteer. B: Correlation between the frequency of SIGLEC-1+ CD14+ monocytes and the expression of the IFN signature in peripheral blood of the same six patients with T1D as measured by the projection of the expression of the IFN-inducible genes from each sample onto the first PC defined in the SLE group. C: Correlation between the frequency of SIGLEC-1+ CD14+ monocytes measured by flow cytometry and the normalized SIGLEC-1 mRNA expression in PBMCs from the six assessed patients with T1D as measured by DNA microarray. D: Scatter plot (mean ± SD) depicting the normalized SIGLEC-1 mRNA expression in PBMCs isolated from 25 patients with SLE stratified by the presence of an IFN signature (IFN+; red squares) or the absence of an IFN signature (IFN-; blue circles). The P value was calculated using a two-sided Wilcoxon test. ρ, correlation coefficient.
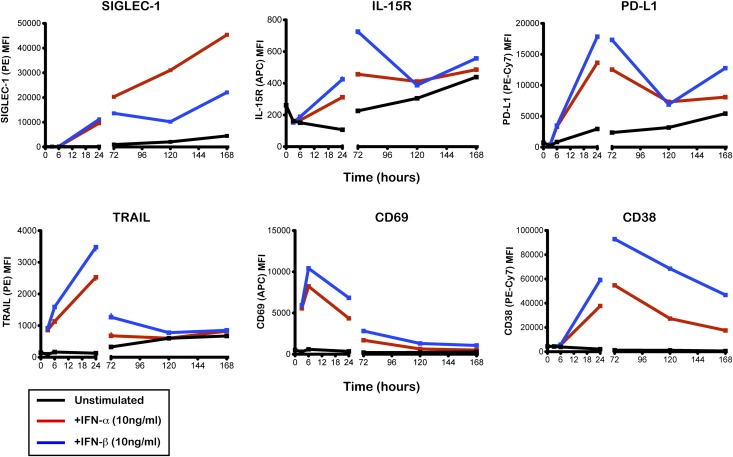
We found no evidence for differential expression of the other five surface markers in any of the assessed immune subsets 24–72 h after stimulation. In contrast, SIGLEC-1 expression on monocytes increased linearly with IFN-α or IFN-β stimulation during up to 7 days of culture (Fig. 6), suggesting that expression of this protein is sustained for a long period of time following an IFN response.
Figure 6.
Time-course of the expression of six IFN-inducible proteins on the surface of CD14+ monocytes. Surface expression of the IFN-inducible proteins SIGLEC-1, IL-15R, PD-L1, TRAIL, CD69, and CD38 was measured by flow cytometry on CD14+ monocytes. PBMCs isolated from a healthy donor were cultured with IFN-α (10 ng/mL; red line), IFN-β (10 ng/mL; blue line), or culture medium (unstimulated; black line). Protein expression was quantified on CD14+ monocytes at 3, 6, 24, 72, 120, and 168 h after stimulation. MFI, median fluorescence intensity.
Discussion
We have shown for the first time that expression of a type I IFN-inducible transcriptional signature is increased in the peripheral blood before the development of islet autoimmunity. The results from our study are supported by data from the study by Kallionpää et al. (25), which also reports increased expression of IFN-inducible genes in Finnish children at increased risk of T1D before the onset of autoimmunity and seroconversion. Together, these two independent data sets provide convincing evidence linking an activated innate immune response with the development of islet autoimmunity, which is currently the strongest known predictive risk factor for T1D. Other gene expression studies have implicated the interleukin-1B signaling pathway in the onset of clinical diabetes (26) and increased type I IFN signaling, oxidative phosphorylation (27), and a systematic suppression of the immune response (24) before the onset of the disease. To date, however, such studies have had limited sample sizes, have not been reproduced independently, and have not been able to investigate transcriptional changes associated with the course of the disease, in particular in the earliest stages preceding the first autoimmune manifestations, which remain poorly characterized. One of the greatest strengths of this study is the access to samples taken longitudinally from children before and after the first clinical signs of T1D. This has enabled us not only to depict the transient nature of IFN signature expression but also to demonstrate the temporal association of the expression of the IFN signature with recent respiratory infections and its correlation with future seroconversion.
Increased expression of type I IFN is a normal response to both viral and bacterial infections and can occur in healthy individuals, in particular children, who are more likely to be exposed to viral infections. Comparison with a recent study of adult patients with MS and controls (22) shows that the pattern of upregulation in patients with MS, patients with T1D, healthy controls, and children at risk for T1D is distinct and often less extreme to that found in patients with SLE, an autoimmune disease characterized by systemic and chronic inflammation. Instead, there is a range of expression of the signature, which in MS correlates with IFN treatment. Increased expression of the IFN signature could reflect a history of IFN responses, but the pattern of expression suggests that individuals in the population may have variable sensitivities to IFN stimulation, with hypersensitive subjects being at higher risk of developing a more pronounced type I IFN response. Our results also suggest that the frequency of circulating SIGLEC-1–expressing monocytes, which correlates with the presence of an IFN signature in peripheral blood, could be a marker of a recent antiviral immune response. In support of this hypothesis, expression of SIGLEC-1 was previously shown to be increased in other diseases associated with chronic IFN signaling, such as SLE, systemic sclerosis, and MS, and suggested to be a potential biomarker of disease activity (28–30).
The increased expression of SIGLEC-1 at the protein level observed on monocytes from individuals with an IFN signature provides evidence that the upregulation of probably many of the IFN-inducible RNAs corresponds to upregulated protein levels. Figure 6 also shows upregulation of five other surface proteins—IL-15R, PD-L1, TRAIL, CD69, and CD38—in response to IFN stimulation. Furthermore, increased serum concentrations of IFN-α in patients with SLE is well established (31,32), and this serum IFN-α has recently been shown to be bioactive and increased in the serum of patients with SLE and an active IFN signature (33). Expression of the IFN receptor and phosphorylation of the respective downstream signaling molecule STAT1 also have been shown in patients with MS treated with IFN-β (34). Although in this study we were not able to test for the secretion of proteins encoded by IFN signature genes because of limited sample availability, our flow cytometric data and the published literature strongly support that the activation of downstream IFN-inducible pathways is occurring in the IFN signature–positive individuals identified in our studies.
In mice, activation of the innate IFN immune response has been associated with class switching of IgM antibodies to the IgG isotype (35) and to the development of pathogenic IgG autoantibodies in murine SLE (36). Similarly, it is plausible that an increased innate IFN immune response in prediabetic individuals can lead to the class switching of naturally occurring IgM autoantibodies into IgG islet-specific autoantibodies that characterize patients with T1D. The average increase in IFN signature in children who subsequently seroconvert could reflect an increased frequency, duration, or strength of IFN upregulation or some combination thereof. The transient expression of the IFN signature shown here suggests the presence of flares of IFN signaling, which would be in agreement with a relapsing-remitting model of T1D etiology (37,38).
There is a long-held belief that viral infection is an environmental factor in the etiology of T1D (4,5). An increased incidence of respiratory infections during childhood has recently been shown to be associated with the development of islet autoimmunity in the BABYDIET study (5). Consistent with this observation, we observed an enrichment of samples collected from AAB+ children with two or more reported respiratory infections before seroconversion (P = 0.0128; Fig. 4B). Upper tract respiratory infections are mainly caused by common viral infections targeting the upper respiratory tract and therefore can be used as a surrogate marker for a viral infection. In this study we found evidence for an increased expression of type I IFN-inducible genes in the same children with a recent history of respiratory infections, which supports the hypothesis that common viral infections could be a key environmental factor in T1D. One potential weakness of the study was the recording of respiratory infection incidence, which was self-reported in daily diaries. While the prospective nature of recording protects against recall bias, there may be variability between families in the interpretation of symptoms. Furthermore, data were aggregated into quarterly incidence before analysis, whereas our analysis of the influenza vaccination cohort suggests that the upregulation of IFN-inducible genes is likely to persist for only days or weeks. Although neither factor can plausibly induce a spurious relationship with the IFN signature, together they should dilute any relationship between infection and gene upregulation. In light of these limitations, we cannot infer a direct causal link between respiratory infections and seroconversion. This hypothesis will need to be validated in larger prospective studies using more sensitive assays for the screening of specific viral infections and designed to investigate the occurrence of respiratory infections and the development of T1D-specific autoantibodies. Nevertheless, together with the data from Kallionpää et al. (25), our findings implicate heightened IFN responses, manifested by an IFN signature, in the development of islet autoimmunity; we have obtained some evidence for an association (P = 0.0064) between respiratory infections and increased IFN response. We reported previously an association between respiratory infections in early life and seroconversion in the BABYDIET cohort (5). We thus hypothesize that IFN upregulation could represent a mechanistic link for the increased risk of islet autoimmunity with increased incidence of viral infections, which is consistent with a recent report establishing a pathogenic role for the enterovirus coxsackievirus B1 in the induction of β-cell autoimmunity (39).
In support of this hypothesis, a recent study characterized the whole blood transcriptional signature associated with three common viral respiratory infections: respiratory syncytial virus (RSV), influenza, and human rhinovirus (40). Notably, there was a strong overlap between the genes that define the IFN signature described in our study and the three gene expression modules associated with IFN response that were found to be modulated upon viral infection (M1.2 = 83.33%, M3.4 = 84.09%, and M5.12 = 57.45%; Supplementary Table 1), even though these modules were defined from whole blood RNA and our IFN signature was obtained from PBMCs. Interestingly, the transcriptional changes caused by RSV infection remained altered 1 month after acute infection (40), suggesting that virally induced IFN signatures may persist in the periphery for some time after the viral infection has been cleared. This is in sharp contrast with the transient increase in IFN-induced transcriptional changes induced by influenza vaccination, which were nearly undetectable in peripheral blood as early as 3 days after vaccination (Supplementary Fig. 4). RSV is the most common cause of respiratory infections in children and may thus represent an important cause of chronic IFN signaling in humans, in particular, in very young children.
Expression of the IFN signature was strongest in children in BABYDIET who went on to develop T1D-specific autoantibodies before seroconversion. Previous studies (24,26) have failed to find statistical support for increased IFN signaling in patients after T1D diagnosis compared with healthy controls. This likely reflects both the relationship between the upregulation of IFN-inducible genes and infection, which is more frequent in children, and the inability to capture a transient transcriptional signature in a cross-sectional patient population. Nevertheless, the genetic association of T1D risk with polymorphisms near IFN-inducible genes and the previously reported genetic association with an IFN regulatory factor 7 (IRF7)–driven inflammatory transcriptional network in monocytes (41) suggest an intriguing possibility: sensitivity to an antiviral immune response could be genetically determined such that an increased sensitivity to initiate an overt IFN response predisposes to T1D. Such a model is supported by the established T1D candidate gene IFIH1, which is among the IFN-inducible genes we identified here and encodes the intracellular pathogen recognition receptor MDA5, a major receptor for viral double-stranded RNA. Its binding to RNA induces the production of type I IFN, which is a key requirement for CD8+ T-cell responses (42,43). Furthermore, expression of IFIH1 mRNA correlates with increased T1D risk (44). Nevertheless, larger longitudinal studies of adults and children—such as TEDDY (http://teddy.epi.usf.edu/), which is already underway—will be required to properly address the exact link between upregulation of IFN-inducible genes and infection and the putative genetic regulation of this response.
It is important to note that the transient nature of the IFN signature precludes at this time an obvious clinical application as a diagnostic biomarker of anti-islet autoantibody seroconversion or progression to T1D. Nevertheless, our findings clearly implicate increased type I IFN signaling in the pathogenesis of T1D and advance our understanding of the interaction between genes and environmental risk factors in disease etiology. Consistent with this hypothesis, in the nonobese diabetic mouse model, IFN signaling has been shown to be critical for the initiation of the disease (8,9). In this disease model, IFN-α production in the pancreas was detected as early as 3 weeks, leading to the regulation of an IFN signaling network (9,45), and preceded the recruitment of diabetogenic T cells to the pancreas. Blockade of IFN signaling was sufficient to delay or even prevent disease onset in this model (8,9). In humans, the detection of IFN-α in the pancreas of patients with T1D has been well documented (7), and patients undergoing therapy with recombinant IFN-α/β for MS or hepatitis have been reported to be at increased risk of developing autoantibodies and secondary autoimmune diseases such as SLE and T1D (46–48). These paradoxical effects of IFN-β in MS underlie the complex nature of type I IFN signaling and highlight the immunoregulatory potential of these molecules, which can have both beneficial and detrimental effects in vivo (49–51). Although the mechanism for the therapeutic effect of IFN-β in MS is unclear, its role in promoting autoimmunity is thought to involve the activation of dendritic cells, leading to increased uptake and cross-presentation of self-antigen from apoptotic cells, which is critical for the activation of autoreactive cytotoxic CD8+ T cells (52). In T1D, HLA class I hyperexpression in the pancreatic islet β-cells in response to IFN signaling is a hallmark of disease (7). These observations provide support for direct killing of β-cells by cytotoxic CD8+ T cells via the recognition of HLA class I molecules with bound peptides from β-cell antigens on the β-cell surface by autoreactive T-cell receptors. In addition, a recent study indicates that IFN-α can inactivate T regulatory cell function (53), which, on the basis of genetic (1) and immunological (54) results, is a central pathway in the pathogenesis of T1D. Two other T1D genes, PTPN22 and TYK2, encode molecules central to the amplification of the type I IFN response (55).
Our findings, and those reported in the study by Kallionpää et al. (25), implicate the activation of innate IFN immune pathways in the initiation of islet autoimmunity. They support the long-established hypothesis that viral infections are major environmental risk factors in T1D and suggest IFN upregulation as a mechanistic link. Together, these two independent longitudinal cohorts provide unique insight into the preclinical stage of T1D and show for the first time that temporal changes in the expression of type I IFN-inducible genes are associated with the earliest stages of disease pathogenesis.
Article Information
Acknowledgments. The authors appreciate the participation of all the patients, BABYDIET families, and control subjects. The authors thank Dr. Sandra Hummel and Dr. Maren Pflüger for coordination of the BABYDIET study; Dr. Cand. Med. F. Wehweck for extracting infectious history data from BABYDIET weekly records; J. Stock, A. Knopff, M. Bunk, C. Ramminger, and the staff of the Clinical Study Center of the Institute of Diabetes Research for recruitment and follow-up of BABYDIET children; S. Krause, M. Schulz, C. Matzke, A. Wosch, and A. Gavrisan for sample processing, preparation of PBMC samples, and antibody measurement; Dr. Christiane Winkler for data management; Dr. Ramona Puff for laboratory management; C. Peplow for consent and regulatory issues; Dr. Michael Hummel, Dr. Ruth Chmiel, Dr. Anna Huppert, and Dr. Miriam Krasmann for clinical care of children with islet autoantibodies; and all pediatricians and family doctors in Germany for participating in the BABYDIET study. The authors thank staff of the National Institute for Health Research (NIHR) Cambridge BioResource recruitment team for assistance with volunteer recruitment and K. Beer, T. Cook, S. Hall, and J. Rice for blood sample collection. The authors thank M. Woodburn and T. Attwood for their contribution to sample management and N. Walker and H. Schuilenburg for data management. The authors thank members of the NIHR Cambridge BioResource SAB and management committee for their support and the NIHR Cambridge Biomedical Research Centre for funding. Access to NIHR Cambridge BioResource volunteers and their data and samples is governed by the NIHR Cambridge BioResource SAB. Documents describing access arrangements and contact details are available at http://www.cambridgebioresource.org.uk/. The authors also thank H. Stevens, P. Clarke, G. Coleman, S. Dawson, S. Duley, M. Maisuria-Armer, and T. Mistry for preparation of PBMC samples. They thank Dr. James Lee (Cambridge Institute for Medical Research and Department of Medicine, University of Cambridge School of Clinical Medicine) for providing critical feedback on the manuscript.
Funding. This work was supported by the JDRF UK Centre for Diabetes Genes, Autoimmunity and Prevention (D-GAP, 4-2007-1003); the JDRF; the Wellcome Trust (WT061858/091157 and 083650/Z/07/Z); the National Institute for Health Research Cambridge Biomedical Research Centre; the Medical Research Council Cusrow Wadia Fund; and the Medical Research Council and Kidney Research UK. The Cambridge Institute for Medical Research (CIMR) received a Wellcome Trust Strategic Award (100140). The BABYDIET study was supported by grants from the Deutsche Forschungsgemeinschaft (DFG ZI-310/14-1 to-4), the Juvenile Diabetes Research Foundation (JDRF 17-2012-16 and 1-2006-665), the Competence Network for Diabetes Mellitus funded by the Federal Ministry of Education and Research (FKZ 01GI0805-07), and the German Center for Diabetes Research. R.C.F. is funded by a JDRF postdoctoral fellowship (3-2011-374). E.B. is supported by the DFG Research Center and Cluster of Excellence–Center for Regenerative Therapies Dresden (FZ 111). C.W. is funded by the Wellcome Trust (WT089989).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.C.F., H.G., L.S.W., J.A.T., and C.W. designed the experiments and interpreted the data. R.C.F., D.J.S., M.L.P., and A.J.C. performed experiments. H.G., R.M.R.C, O.S.B., J.D.D., D.G.C., and C.W. analyzed the data. S.F., E.F.M., P.A.L., K.G.C.S., P.A., A.B., D.B.D., E.B., and A.-G.Z. provided samples and clinical outcome data. R.C.F., H.G., J.A.T., E.B., C.W., and A.-G.Z. conceived the study and wrote the article. C.W. and A.-G.Z. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-1777/-/DC1.
R.C.F. and H.G. contributed equally to this work.
J.A.T., E.B., C.W., and A.-G.Z. co-directed the project.
See accompanying articles, pp. 2203 and 2402.
References
- 1.Virgin HW, Todd JA. Metagenomics and personalized medicine. Cell 2011;147:44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett JC, Clayton DG, Concannon P, et al. Type 1 Diabetes Genetics Consortium Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009;41:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dotta F, Galleri L, Sebastiani G, Vendrame F. Virus infections: lessons from pancreas histology. Curr Diab Rep 2010;10:357–361 [DOI] [PubMed] [Google Scholar]
- 5.Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatr 2013;167:800–807 [DOI] [PubMed] [Google Scholar]
- 6.Foulis AK, Farquharson MA, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet 1987;2:1423–1427 [DOI] [PubMed] [Google Scholar]
- 7.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-α initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A 2008;105:12439–12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diana J, Simoni Y, Furio L, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med 2013;19:65–73 [DOI] [PubMed] [Google Scholar]
- 10.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 2003;100:2610–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 2003;197:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaussabel D, Quinn C, Shen J, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity 2008;29:150–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry MPR, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 2010;466:973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hummel S, Pflüger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care 2011;34:1301–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–1277 [DOI] [PubMed] [Google Scholar]
- 16.Ferreira RC, Freitag DF, Cutler AJ, et al. Functional IL6R 358Ala allele impairs classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet 2013;9:e1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber W, von Heydebreck A, Sültmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 2002;18(Suppl. 1):S96–S104 [DOI] [PubMed] [Google Scholar]
- 18.R Core Team R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing, 2013 [Google Scholar]
- 19.Stranger BE, Montgomery SB, Dimas AS, et al. Patterns of cis regulatory variation in diverse human populations. PLoS Genet 2012;8:e1002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004;3:Article3. [DOI] [PubMed] [Google Scholar]
- 21.Waddell SJ, Popper SJ, Rubins KH, et al. Dissecting interferon-induced transcriptional programs in human peripheral blood cells. PLoS One 2010;5:e9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nickles D, Chen HP, Li MM, et al. Blood RNA profiling in a large cohort of multiple sclerosis patients and healthy controls. Hum Mol Genet 2013;22:4194–4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franco LM, Bucasas KL, Wells JM, et al. Integrative genomic analysis of the human immune response to influenza vaccination. eLife 2013;2:e00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elo LL, Mykkänen J, Nikula T, et al. Early suppression of immune response pathways characterizes children with prediabetes in genome-wide gene expression profiling. J Autoimmun 2010;35:70–76 [DOI] [PubMed] [Google Scholar]
- 25.Kallionpää H, Elo LL, Laajala E, et al. Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. Diabetes 2014;63:2402–2414 [DOI] [PubMed] [Google Scholar]
- 26.Kaizer EC, Glaser CL, Chaussabel D, Banchereau J, Pascual V, White PC. Gene expression in peripheral blood mononuclear cells from children with diabetes. J Clin Endocrinol Metab 2007;92:3705–3711 [DOI] [PubMed] [Google Scholar]
- 27.Reynier F, Pachot A, Paye M, et al. Specific gene expression signature associated with development of autoimmune type-I diabetes using whole-blood microarray analysis. Genes Immun 2010;11:269–278 [DOI] [PubMed] [Google Scholar]
- 28.York MR, Nagai T, Mangini AJ, Lemaire R, van Seventer JM, Lafyatis R. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum 2007;56:1010–1020 [DOI] [PubMed] [Google Scholar]
- 29.Biesen R, Demir C, Barkhudarova F, et al. Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis Rheum 2008;58:1136–1145 [DOI] [PubMed] [Google Scholar]
- 30.Malhotra S, Castilló J, Bustamante M, et al. SIGLEC1 and SIGLEC7 expression in circulating monocytes of patients with multiple sclerosis. Mult Scler 2013;19:524–531 [DOI] [PubMed] [Google Scholar]
- 31.Preble OT, Black RJ, Friedman RM, Klippel JH, Vilcek J. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science 1982;216:429–431 [DOI] [PubMed] [Google Scholar]
- 32.Hooks JJ, Jordan GW, Cupps T, Moutsopoulos HM, Fauci AS, Notkins AL. Multiple interferons in the circulation of patients with systemic lupus erythematosus and vasculitis. Arthritis Rheum 1982;25:396–400 [DOI] [PubMed] [Google Scholar]
- 33.Morimoto AM, Flesher DT, Yang J, et al. Association of endogenous anti-interferon-α autoantibodies with decreased interferon-pathway and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:2407–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comabella M, Lünemann JD, Río J, et al. A type I interferon signature in monocytes is associated with poor response to interferon-β in multiple sclerosis. Brain 2009;132:3353–3365 [DOI] [PubMed] [Google Scholar]
- 35.Le Bon A, Thompson C, Kamphuis E, et al. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol 2006;176:2074–2078 [DOI] [PubMed] [Google Scholar]
- 36.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med 2006;203:553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonifacio E, Scirpoli M, Kredel K, Füchtenbusch M, Ziegler AG. Early autoantibody responses in prediabetes are IgG1 dominated and suggest antigen-specific regulation. J Immunol 1999;163:525–532 [PubMed] [Google Scholar]
- 38.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol 2007;7:988–994 [DOI] [PubMed] [Google Scholar]
- 39.Laitinen OH, Honkanen H, Pakkanen O, et al. Coxsackievirus B1 is associated with induction of β-cell autoimmunity that portends type 1 diabetes. Diabetes 2014;63:446–455 [DOI] [PubMed] [Google Scholar]
- 40.Mejias A, Dimo B, Suarez NM, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med 2013;10:e1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinig M, Petretto E, Wallace C, et al. Cardiogenics Consortium A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature 2010;467:460–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Swiecki M, Cella M, et al. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe 2012;11:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hervas-Stubbs S, Mancheño U, Riezu-Boj J-I, et al. CD8 T cell priming in the presence of IFN-α renders CTLs with improved responsiveness to homeostatic cytokines and recall antigens: important traits for adoptive T cell therapy. J Immunol 2012;189:3299–3310 [DOI] [PubMed] [Google Scholar]
- 44.Downes K, Pekalski M, Angus KL, et al. Reduced expression of IFIH1 is protective for type 1 diabetes. PLoS One 2010;5:e12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS One 2013;8:e59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fabris P, Betterle C, Greggio NA, et al. Insulin-dependent diabetes mellitus during alpha-interferon therapy for chronic viral hepatitis. J Hepatol 1998;28:514–517 [DOI] [PubMed] [Google Scholar]
- 47.Crow MK. Type I interferon in organ-targeted autoimmune and inflammatory diseases. Arthritis Res Ther 2010;12(Suppl. 1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura K, Kawasaki E, Imagawa A, et al. Research Committee on Type 1 Diabetes of the Japan Diabetes Society Type 1 diabetes and interferon therapy: a nationwide survey in Japan. Diabetes Care 2011;34:2084–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Decker T, Müller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol 2005;5:675–687 [DOI] [PubMed] [Google Scholar]
- 50.Trinchieri G. Type I interferon: friend or foe? J Exp Med 2010;207:2053–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.González-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol 2012;12:125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Bon A, Etchart N, Rossmann C, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol 2003;4:1009–1015 [DOI] [PubMed] [Google Scholar]
- 53.Bacher N, Raker V, Hofmann C, et al. Interferon-α suppresses cAMP to disarm human regulatory T cells. Cancer Res 2013;73:5647–5656 [DOI] [PubMed] [Google Scholar]
- 54.Tree TIM, Roep BO, Peakman M. A mini meta-analysis of studies on CD4+CD25+ T cells in human type 1 diabetes: report of the Immunology of Diabetes Society T Cell Workshop. Ann N Y Acad Sci 2006;1079:9–18 [DOI] [PubMed] [Google Scholar]
- 55.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol 2013;14:36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]