Abstract
Monoacylglycerol acyltransferase (MGAT) enzymes convert monoacylglycerol to diacylglycerol (DAG), a lipid that has been linked to the development of hepatic insulin resistance through activation of protein kinase C (PKC). The expression of genes that encode MGAT enzymes is induced in the livers of insulin-resistant human subjects with nonalcoholic fatty liver disease, but whether MGAT activation is causal of hepatic steatosis or insulin resistance is unknown. We show that the expression of Mogat1, which encodes MGAT1, and MGAT activity are also increased in diet-induced obese (DIO) and ob/obmice. To probe the metabolic effects of MGAT1 in the livers of obese mice, we administered antisense oligonucleotides (ASOs) against Mogat1 to DIO and ob/ob mice for 3 weeks. Knockdown of Mogat1 in liver, which reduced hepatic MGAT activity, did not affect hepatic triacylglycerol content and unexpectedly increased total DAG content. Mogat1 inhibition also increased both membrane and cytosolic compartment DAG levels. However, Mogat1 ASO treatment significantly improved glucose tolerance and hepatic insulin signaling in obese mice. In summary, inactivation of hepatic MGAT activity, which is markedly increased in obese mice, improved glucose tolerance and hepatic insulin signaling independent of changes in body weight, intrahepatic DAG and TAG content, and PKC signaling.
Introduction
Obesity is associated with intrahepatic lipid accumulation, which has been linked to the development of insulin resistance and metabolic dysfunction. For example, accumulation of diacylglycerol (DAG) is associated with insulin resistance, and experimentally modulating DAG levels affects hepatic insulin sensitivity (1–5). Similarly, other lipid mediators activate signaling cascades, leading to impairment in insulin sensitivity in liver (6). However, given the impossibility of modulating the concentration of one lipid species in isolation and the potential number of candidate lipids, the identity of lipids that link hepatic lipid accumulation and insulin resistance is still not completely understood. Furthermore, even the cause-and-effect relationship between hepatic steatosis and insulin resistance can be debated (7).
Triacylglycerol (TAG) is the primary storage form of intracellular lipids, and TAG is solely generated from acylation of DAG. In most cells of the body, DAG destined for TAG synthesis is produced primarily from the sequential acylation and dephosphorylation of glycerol-3-phosphate (Fig. 1A) (8,9). However, there are also alternative pathways for synthesizing DAG, including acylation of monoacylglycerol, which is catalyzed by monoacylglycerol acyltransferase (MGAT) enzymes (Fig. 1A). The human and mouse genomes each contain three MGAT family genes (Mogat1, 2, and 3), but mouse Mogat3 is a pseudogene and not analogous to the human MOGAT3 (10). The MGAT enzymes are important for dietary fat absorption by intestinal enterocytes, and Mogat1 and Mogat2 are most highly expressed in the gastrointestinal system (11–13). MGAT enzymes may also be an important mechanism for recycling remnants of lipolytic processes in nonintestinal cells (14,15), but relatively little is known about their effects in extraintestinal tissues.
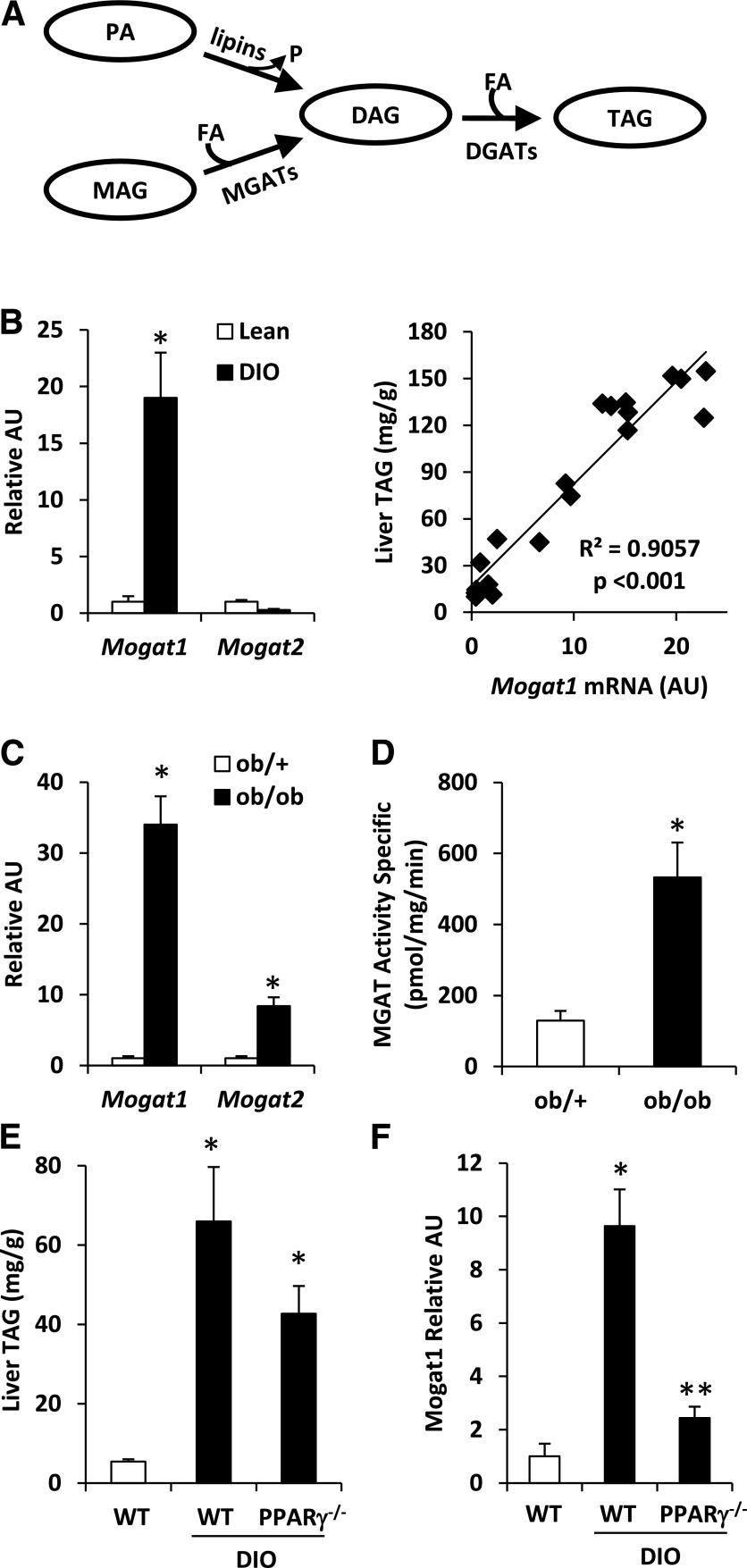
Figure 1.
Mogat1 expression is increased in obese mice in a PPARγ-dependent manner. A: The schematic depicts the two metabolic pathways for triglyceride synthesis. B: Hepatic Mogat1 expression in mice fed chow containing 60% fat or 10% fat for 14 weeks. The scatter plot (right) depicts the relationship between liver TAG content and Mogat expression in individual mice. C: Hepatic Mogat expression in ob/ob and lean ob/+ control mice. D: Hepatic MGAT activity in ob/ob and lean ob/+ control mice. E: Hepatic TAG content in WT or liver-specific PPARγ−/− mice fed a low- or high-fat diet for 14 weeks. F: Hepatic Mogat1 expression in WT or liver-specific PPARγ−/− mice fed a low- or high-fat diet for 14 weeks. *P < 0.05 vs. lean controls; **P < 0.05 vs. lean and DIO WT mice. AU, arbitrary unit; FA, fatty acid; MAG, monoacylglycerol; P, phosphate; PA, phosphatidic acid.
MGAT enzymes are of potential relevance to mechanisms of obesity-related hepatic steatosis for a number of reasons. First, as noted, the product of MGAT activity (DAG) has been linked to the development of insulin resistance in a variety of tissues (3). Second, the expression of genes encoding MGAT enzymes has been shown to be induced in steatotic liver in mice (16) and human subjects (11). We have shown that marked weight loss in obese subjects after gastric bypass surgery leads to reduced expression of the MOGAT genes and that this coincides with insulin sensitization and resolution of hepatic steatosis (11). Finally, mice null for Mogat2 are protected from diet-induced obesity due to delayed absorption of dietary fat and increased systemic energy expenditure (17).
To examine the metabolic consequences of reversing the activation of Mogat1 in liver, antisense oligonucleotides (ASOs) targeting Mogat1 for knockdown were administered to diet-induced obese (DIO) or ob/ob mice in which hepatic expression of Mogat1 is markedly induced. To our surprise, attenuation of hepatic Mogat1 expression led to increased DAG content in obese liver but significantly improved glucose tolerance and hepatic insulin signaling. These data suggest that targeting MGAT activity could be a novel strategy for improving obesity-related insulin resistance.
Research Design and Methods
Animal Studies
All mice were maintained in accordance with the Animal Use and Care Committees of Washington University School of Medicine. To cause DIO, C57BL/6J male mice were fed chow providing 60% of calories from fatty acids (D12492; Research Diets, Inc.) starting at 6 weeks of age. Age-matched mice were maintained on a matched 10% fat chow (D12450B; Research Diets, Inc.). Ob/ob and ob/+ control mice were obtained at 6 weeks of age from The Jackson Laboratory (Bar Harbor, ME). Mice with liver-specific peroxisome proliferator–activated receptor (PPAR) γ deficiency have been described (18) and were compared with littermate PPARγflox/flox mice not expressing Cre.
Mice received intraperitoneal injections of ASO directed against Mogat1 or a scrambled control ASO 25 mg/kg body weight (ISIS Pharmaceuticals, Inc., Carlsbad, CA) twice a week for 3 weeks. Treatments were initiated after 14 weeks of high-fat-diet feeding or at 6 weeks of age in ob/ob mice. After treatment with ASOs for 18 days, mice were subjected to a glucose tolerance test. Mice were killed after 3 weeks of injections with ASOs, and tissues were harvested, frozen in liquid nitrogen, and stored at −80°C for further analyses.
Glucose tolerance tests were performed as described (19). Before the tests, mice were fasted for 4 h and then injected with a 10% solution of d-glucose (1 g/kg). Tail blood glucose was determined at 0, 30, 60, and 120 min after challenge using a OneTouch Ultra glucometer (LifeScan, Inc.). Total area under the curve was calculated using the trapezoidal rule. In one study, plasma was collected by mandibular bleed at the 15-min time point for assessment of insulin concentration.
Insulin Signaling
To assess insulin signaling in peripheral tissues, mice were fasted for 4 h before being killed. At time 0, mice received an intraperitoneal injection of 10 mU/g body weight insulin or an equal volume of saline. Five minutes after the injection, tissues (liver, gastrocnemius, and white adipose tissue [WAT]) were removed, immediately frozen in liquid nitrogen, and stored at −80°C for further analyses.
Plasma Metabolite Quantification
Nonesterified fatty acids were measured using commercially available kits (NEFA-HR; Wako Diagnostics, Richmond, VA). TAG and cholesterol content were determined using colorimetric kits from Thermo Scientific as previously described (20). Plasma insulin concentrations were determined by a commercially available ELISA (Crystal Chemical Company, Wakefield, MA).
MGAT and DAG Acyltransferase Enzymatic Assays
Membrane fractions were isolated from mouse liver by homogenization in 50 mmol/L Tris-HCl (pH 7.4), 1 mmol/L EDTA, and 0.25 mol/L sucrose and centrifuging at 100,000g for 1 h. Fifty micrograms of protein were incubated in 5 mmol/L MgCl2, 1.25 mg/mL BSA, 200 mmol/L sucrose, 100 mmol/L Tris-HCl (pH 7.4), 25 μmol/L [14C]oleoyl-CoA, and 200 μmol/L sn-2-oleoylglycerol (for MGAT assay) or 200 μmol/L sn-1,2-dioleoylglycerol (for DAG acyltransferase [DGAT] assay) for 5 min. The reaction was terminated by the addition of 50 μL 1% phosphoric acid. Lipids were extracted with 300 μL CHCl3/MeOH (2:1 volume for volume) and separated by thin-layer chromatography (TLC) with hexane/ethyl ether/acetic acid (80:20:1 volume for volume for volume). Lipids were extracted and separated on Linear-K preadsorbent TLC plates. The spots corresponding to DAG and TAG were scraped from the TLC plates and 14C-radioactivity was quantified by a scintillation counter. Reaction mixtures in which liver membrane proteins were omitted were used as blanks for background correction.
mRNA Isolation and Gene Expression Analyses
Total liver RNA was extracted with RNA-BEE (Iso-Tex Diagnostics, Friendswood, TX) according to the manufacturer’s instructions. First-strand cDNA synthesis was performed using Taqman reagents, and quantitative real-time RT-PCR was performed using the ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, CA) with Power SYBR green. Arbitrary units of target mRNA were corrected to the corresponding level of the 36B4 mRNA. The sequences of the primers used in these studies are available upon request.
Western Blot Analyses
For Western blot analyses using whole-cell lysates, protein extracts were obtained from liver by using the following lysis buffer containing a protease inhibitor cocktail: 10 mmol/L HEPES (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EDTA, 0.1% sodium deoxycholate, and 1% Triton X-100. For subcellular protein kinase C (PKC) localization studies, livers from lean, DIO, and DIO ASO–treated mice were fractionated as previously described (21). Briefly, 100 mg of tissue was homogenized by a Potter-Elvehjem homogenizer in lysis buffer (10 mmol/L HEPES, 1 mmol/L EDTA [pH 7.4]) with 60% sucrose. The homogenates were transferred to 2-mL centrifuge tubes. The tubes were then filled to capacity with lysis buffer. The gradients were centrifuged at 20,000g for 3 h at 4°C. Fractions were collected as described previously and stored at −80°C until Western blot analyses (21). Antibodies to Akt, pSer473 Akt, GSKα/β, pSer21/9 GSKα/β (Cell Signaling Technology, Danvers, MA), actin (Sigma-Aldrich, St. Louis, MO), PKCε (BD Biosciences, San Jose, CA), PKCδ (Santa Cruz Biotechnology, Dallas, TX), and calnexin (Enzo Life Sciences, Farmingdale, NY) were used according to the manufacturers’ instructions.
PKC Enzymatic Assay
Total membrane-associated PKC activity was quantified using the Protein Kinase C Biotrak Enzymatic Assay System (GE Healthcare, Chalfont St. Giles, Buckinghamshire, U.K.) following the manufacturer’s protocol. The assay was initiated by the addition of 1 μg of membrane proteins to the reaction component mixture and incubated at 37°C for 15 min. Reactions were terminated with the addition of 300 mmol/L orthophosphoric acid and processed as described by the manufacturer. Protein lysates from mouse brain, which contain high PKC activity, were used as positive control.
Tissue Glycerolipid Analyses
Total hepatic TAG content was determined using a colorimetric assay as previously described (20). Hepatic DAG content was separated into membrane and cytoplasmic fractions as previously described (22). Briefly, samples were homogenized on ice using a dounce homogenizer in 500 μL of lysis buffer containing 10 mmol/L Tris base, 0.5 mmol/L EDTA, 250 mmol/L sucrose, and protease inhibitors. Samples were centrifuged at 100,000g for 1 h. The supernatant and lipid layers were removed and designated as the cytoplasmic fraction. The pellet was resuspended in lysis buffer and designated as the membrane fraction.
Concentrations of DAG molecular species in each fraction were determined by using mass spectrometry as previously described (23). Neutral lipids were extracted by hexane, and the free hydroxyl group in DAG species was protected with 2,4-difluorophenyl isocyanate. The analysis was performed on an Agilent 1100 LC system connected to an Agilent 6460 TSQ mass spectrometer operated in electrospray ionization and positive ion mode (24). The liquid chromatography system allows separation of DAG species from other neutral lipids and resolution of sn-1,2 DAG and sn-1,3 DAG species.
Statistical Analyses
All data are presented as the mean ± SE. Statistical significance was calculated using an unpaired Student t test, with a statistically significant difference defined as P ≤ 0.05.
Results
Expression of Mogat1 Is Induced in Liver of Obese Mice
We examined Mogat1 expression in the livers of mice made obese by 14 weeks of high-fat-diet feeding. As expected, mice fed a high-fat diet were obese and had elevated liver TAG content compared with low-fat-diet–fed control mice (data not shown). The hepatic expression of Mogat1 was strongly induced in DIO mice compared with lean controls, whereas the expression of Mogat2 was not markedly affected (Fig. 1B). The expression of Mogat1 was tightly correlated (R2 = 0.9057) with hepatic TAG content in the livers of lean and DIO mice (Fig. 1B). Hepatic Mogat1 expression and MGAT activity were also increased in ob/ob mice compared with lean controls (Fig. 1C and D). Mogat2 was induced in ob/ob liver compared with that in lean controls (Fig. 1C). Collectively, these data indicate that hepatic expression of Mogat1 and MGAT activity are increased in livers of mice that are obese due to dietary intervention or genetic defects.
Induction of Mogat1 Expression by DIO Requires PPARγ
Mogat1 has been shown to be a direct target gene for PPARγ (16). To evaluate the requirement for PPARγ in regulating Mogat1 expression in liver of high-fat-diet–fed mice, we generated mice with liver-specific PPARγ deletion (LS-PPARγ−/− mice) (18) and induced them to become obese by feeding with a high-fat diet for 16–20 weeks. Although LS-PPARγ−/− mice tended to accumulate less hepatic TAG than wild-type (WT) DIO mice, hepatic TAG levels remained elevated in DIO LS-PPARγ−/− mice compared with lean controls (Fig. 1E). The induction of Mogat1 in response to DIO was markedly attenuated in LS-PPARγ−/− mice compared with WT DIO mice (Fig. 1F). These data suggest that the induction of Mogat1 in the livers of DIO mice requires PPARγ and are consistent with previous work demonstrating that Mogat1 is a direct target of PPARγ.
Mogat1 Knockdown Abolishes the Increase in Hepatic MGAT Activity in Liver of Obese Mice
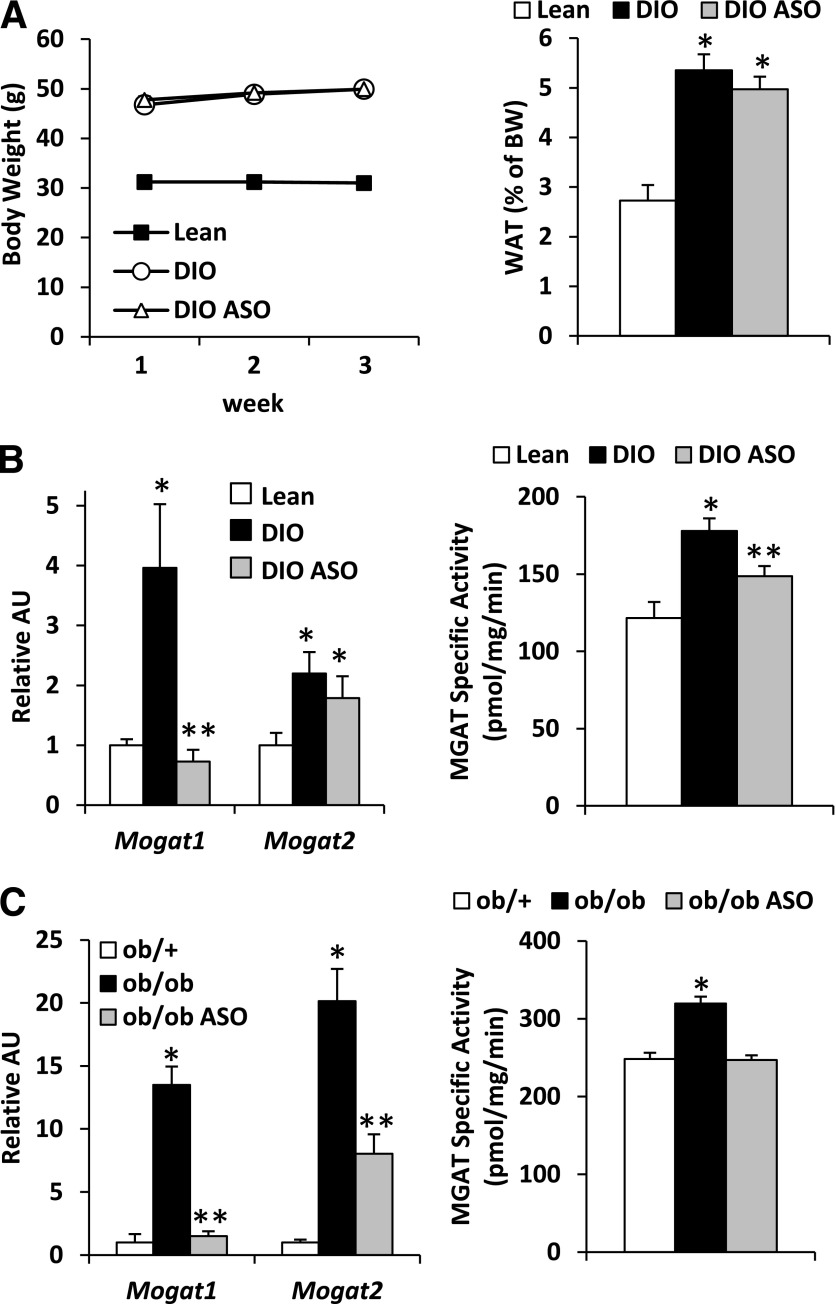
We evaluated the consequences of knocking down Mogat1 expression using ASO technology. Administration of ASO targeting Mogat1 to DIO mice that had been fed a high-fat diet for 14 weeks did not affect body weight gain or epididymal fat pad weight (Fig. 2A) but did result in a marked reduction in hepatic Mogat1 expression (DIO ASO) compared with DIO mice injected with scrambled control ASO (DIO) (Fig. 2B). Mogat1 ASO did not affect the expression of Mogat2 (Fig. 2B), and knockdown of Mogat1 was restricted to liver because the expression of Mogat1 was not affected in stomach, skeletal muscle (SKM), or WAT (data not shown). Mogat1 ASO administration resulted in a reduction in hepatic MGAT activity (Fig. 2B). ASO treatment also reduced expression of Mogat1 and Mogat2 as well as MGAT activity in the livers of ob/ob mice (Fig. 2C).
Figure 2.
Mogat1 ASO reduces hepatic MGAT activity in DIO and ob/ob mice. A: Body weight and epididymal fat pad weight of mice fed chow containing 60% fat or 10% fat for 14 weeks and treated with ASO against Mogat1 or scrambled control ASO for 3 weeks. B: Mogat expression and MGAT activity in liver of mice treated with ASO against Mogat1 or scrambled control ASO. *P < 0.05 vs. lean controls; **P < 0.05 vs. lean and DIO control ASO mice. C: Hepatic Mogat expression and MGAT activity of ob/ob mice treated with ASO against Mogat1 or scrambled control ASO. *P < 0.05 vs. lean controls; **P < 0.05 vs. lean and ob/ob control ASO mice. AU, arbitrary unit; BW, body weight.
Diminished Mogat1 Expression in Obese Liver Does Not Affect Hepatic TAG Content and Increases DAG Levels
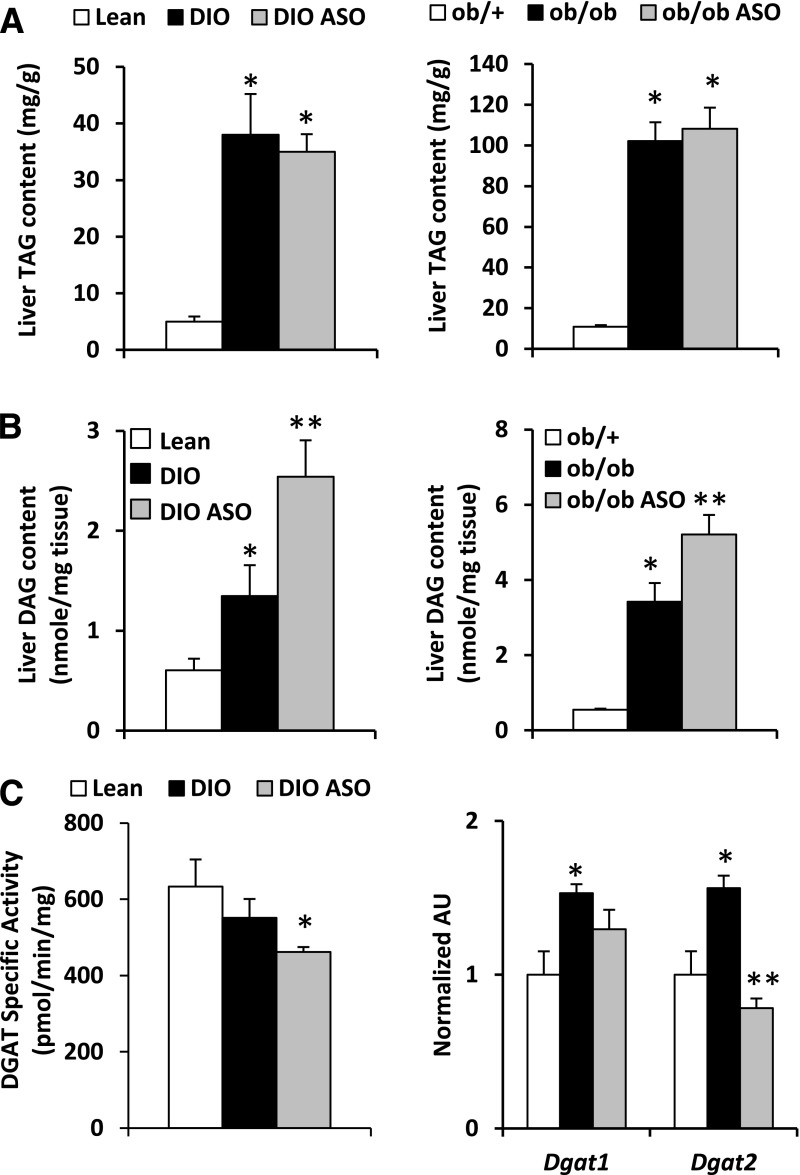
Hepatic TAG content in DIO and ob/ob mice was markedly increased compared with lean controls, and treatment with Mogat1 ASO did not affect TAG levels (Fig. 3A). Hepatic ceramide content was also not affected by Mogat1 ASO (data not shown). Mass spectrometry analyses demonstrated that hepatic DAG content, which was increased in the livers of DIO and ob/ob mice compared with lean controls, was unexpectedly increased further by Mogat1 knockdown in both types of obese mice (Fig. 3B). These data suggest that knockdown of Mogat1 does not reduce but actually increases hepatic DAG content. One possible explanation for this observation is that hepatic DGAT activity was also decreased by Mogat1 ASO coincident with reduced expression of Dgat2 in the livers of mice treated with Mogat1 ASO (Fig. 3C).
Figure 3.
Mogat1 knockdown does not affect liver TAG content but increases DAG levels. A: Hepatic TAG content in mice fed chow containing 60% fat or 10% fat for 14 weeks (left) or ob/ob mice (right) and treated with ASO against Mogat1 or scrambled control ASO. B: Hepatic DAG content in mice fed chow containing 60% fat or 10% fat for 14 weeks (left) or ob/ob mice (right) and treated with ASO against Mogat1 or scrambled control ASO. C: Hepatic DGAT activity and Dgat expression of mice fed chow containing 60% fat or 10% fat for 14 weeks and treated with ASO against Mogat1 or scrambled control ASO. *P < 0.05 vs. lean controls; **P < 0.05 vs. lean and obese control ASO mice. AU, arbitrary unit.
Inhibition of Mogat1 Expression Reduces the Expression of Several Known Genes Activated in Steatotic Liver
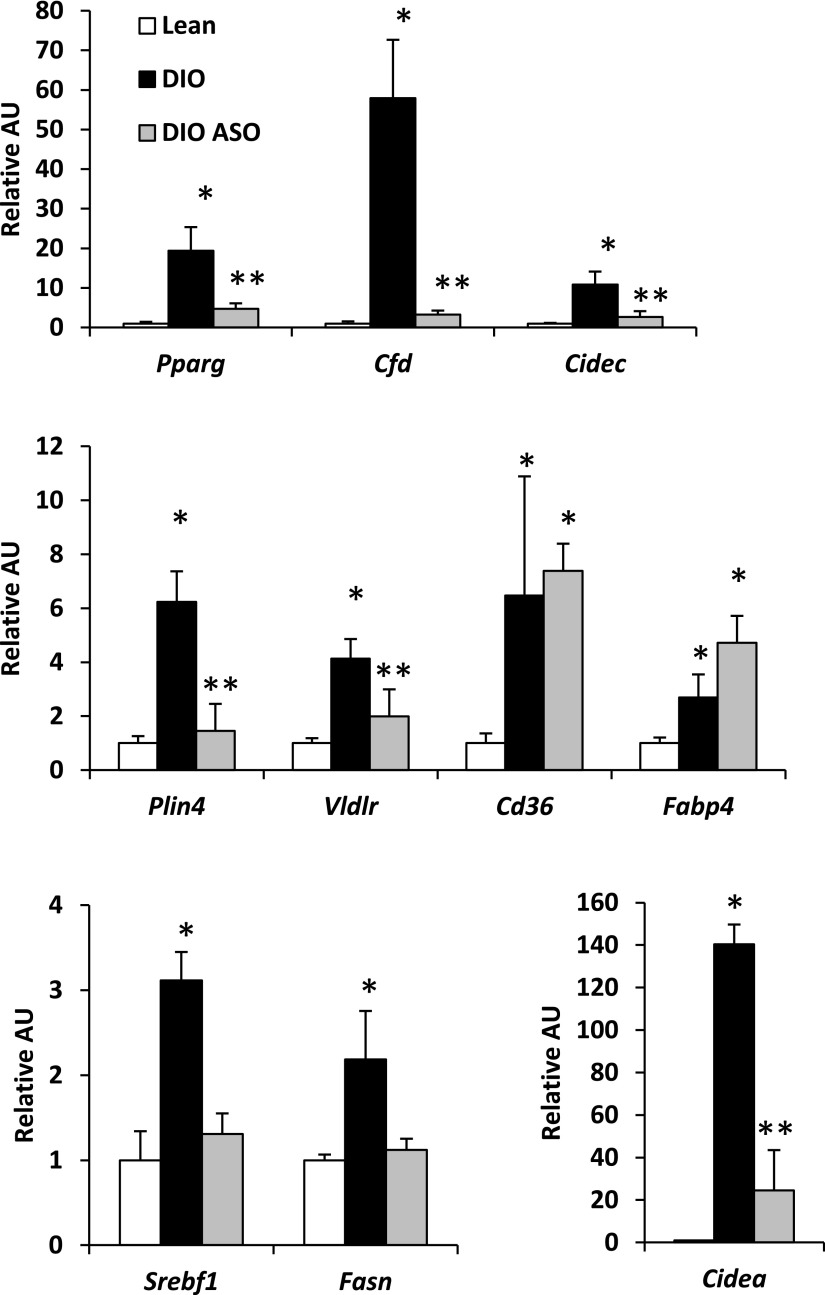
We next evaluated the effects of Mogat1 knockdown on the hepatic expression of a number of lipogenic genes known to be activated in insulin-resistant liver. Of note, Mogat1 knockdown reduced the expression of PPARγ (Pparg) and its known target genes Cidec, Cfd, Vldlr, and Plin4 but not Cd36 and Fabp4 (Fig. 4). The expression of sterol regulatory element–binding protein 1 (SREBP1 [Srebf1]) and its target genes Fasn and Cidea, which were increased in DIO mice compared with lean control mice, was also corrected by Mogat1 ASO treatment. These data are consistent with reduced expression and activity of the lipogenic transcription factors PPARγ and SREBP1 in DIO liver after treatment with Mogat1 ASO.
Figure 4.
Mogat1 ASO reduces hepatic expression of lipogenic genes in DIO mice. Hepatic expression of the indicated genes in mice fed chow containing 60% fat or 10% fat for 14 weeks and treated with ASO against Mogat1 or scrambled control ASO. *P < 0.05 vs. lean controls; **P < 0.05 vs. lean and DIO control mice. AU, arbitrary unit.
Inhibition of Mogat1 Expression Improves Insulin Sensitivity
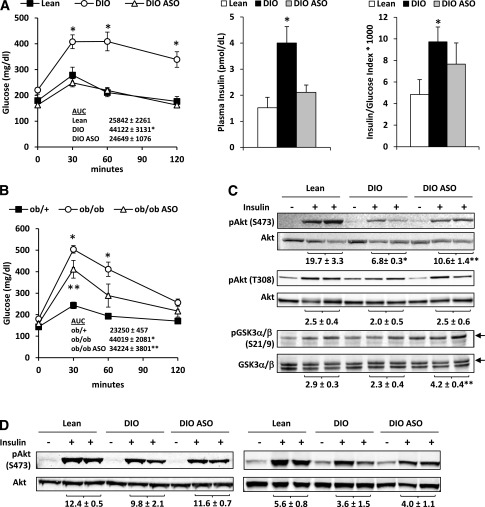
We next examined the effects of Mogat1 knockdown on measures of circulating metabolite levels, glucose metabolism, and insulin sensitivity. Mogat1 ASO treatment did not significantly affect plasma TAG, free fatty acid, or cholesterol concentration in DIO mice (Table 1). Ob/ob mice, which were hypertriglyceridemic, exhibited a marked decrease in plasma TAG concentration in response to Mogat1 knockdown, whereas plasma cholesterol and free fatty acid concentrations were not affected. Plasma insulin concentration was reduced in DIO and ob/ob mice by treatment with Mogat1 ASO compared with control ASO-treated mice (Table 1), suggesting that sensitivity to this hormone might be increased. Glucose tolerance testing demonstrated that Mogat1 ASO treatment markedly improved glucose tolerance compared with control ASO treatment in DIO mice (Fig. 5A). The improvement in glucose tolerance was not associated with increased insulin secretion because the plasma insulin concentration 15 min after glucose injection was actually reduced in Mogat1 ASO-treated mice compared with DIO controls and the insulin-to-glucose ratio was unchanged (Fig. 5A). Glucose tolerance was also improved in ob/ob mice treated with Mogat1 ASO compared with control ASO-treated mice (Fig. 5B).
Table 1.
Plasma parameters
| Lean | DIO control ASO | DIO Mogat1 ASO | |
|---|---|---|---|
| TAG (mg/dL) | 60.4 ± 4.0 | 53.8 ± 2.5 | 57.1 ± 4.1 |
| NEFA (mmol/L) | 0.57 ± 0.07 | 0.51 ± 0.04 | 0.51 ± 0.03 |
| Cholesterol (mg/dL) | 48.8 ± 2.5 | 101.1 ± 13.5* | 107.2 ± 12.4* |
| Insulin (ng/mL) | 1.6 ± 0.2 | 4.5 ± 1.1* | 1.9 ± 0.4** |
| ob/+ | ob/ob control ASO | ob/ob Mogat1 ASO | |
| TAG (mg/dL) | 55.5 ± 17.6 | 130.1 ± 19.2* | 71.9 ± 7.3** |
| NEFA (mmol/L) | 0.9 ± 0.1 | 1.8 ± 0.2* | 1.6 ± 0.1* |
| Cholesterol (mg/dL) | 99.5 ± 12.8 | 168.9 ± 18.0* | 190.2 ± 24.4* |
| Insulin (ng/mL) | 0.9 ± 0.1 | 9.9 ± 0.8* | 7.6 ± 1.0** |
Data are mean ± SE. Plasma was collected at sacrifice after a 4-h fast. NEFA, nonesterified fatty acid.
*P < 0.05 vs. lean controls.
**P < 0.05 vs. ASO control and lean controls.
Figure 5.
Inhibition of Mogat1 improves systemic glucose tolerance and hepatic insulin signaling. A: Glucose tolerance tests (GTTs) in DIO mice treated with ASO against Mogat1 or scrambled control ASO. AUC values are inset. Plasma insulin concentration (middle panel) and insulin/glucose index (right panel) were determined at the 15-min time point during the GTT. *P < 0.05 vs. lean controls. B: GTTs in ob/ob mice treated with ASO against Mogat1 or scrambled control ASO. AUC values are inset. *P < 0.05 vs. lean controls; **P < 0.05 vs. lean and ob/ob control ASO mice. C: Representative Western blots for liver lysates and the indicated antibodies. Five minutes before being killed, mice were injected with saline or insulin. Quantification of insulin-stimulated phosphorylation (relative to the total protein) is inset below each blot. Arrows indicate the bands for GSK3α. *P < 0.05 vs. lean controls; **P < 0.05 vs. DIO control ASO mice. D: Representative blots of gastrocnemius SKM and epididymal WAT from mice fed chow containing 60% fat or 10% fat for 14 weeks and treated with ASO against Mogat1 or scrambled control ASO. AUC, area under the curve.
To examine hepatic insulin signaling, we evaluated the phosphorylation of proteins downstream of the insulin receptor in liver after maximal insulin stimulation. The insulin-stimulated phosphorylation of Akt at Ser473, Akt at Thr308, and GSK3α at Ser21 was increased in the livers of DIO mice treated with Mogat1 ASO compared with mice injected with control ASO (Fig. 5C). In contrast, insulin-stimulated phosphorylation of Akt at Ser473 in SKM and WAT was not affected by Mogat1 knockdown (Fig. 5D). These data indicate that Mogat1 inhibition in liver improves systemic glucose tolerance and hepatic insulin signaling.
Mogat1 ASO Increases the Ratio of Membrane-to-Cytosolic–Associated PKCs
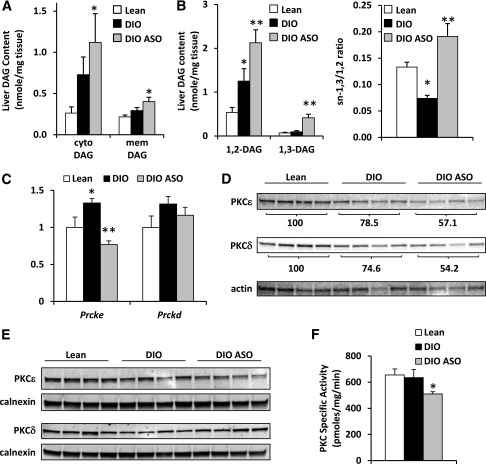
The data presented previously suggest that Mogat1 knockdown improves glucose tolerance and hepatic insulin signaling despite increases in total cellular DAG content. Previous studies also detecting increased DAG in insulin-sensitized mice reconciled these findings by identifying correlations between DAG in membrane or cytoplasmic compartments in the cell and insulin resistance (22). However, we found that both membrane and cytosolic DAG content was increased by Mogat1 knockdown (Fig. 6A). In addition, DAG with saturated fatty acids esterified at the sn-1,2 position are believed to be more robust activators of PKC signaling than sn-1,3 DAG species (25–27). We found that Mogat1 inhibition led to an accumulation of total sn-1,2 DAG (Fig. 6B), and all sn-1,2 DAG species tended to be or were significantly increased in DIO versus lean liver and further increased by Mogat1 ASO treatment, including a number of saturated fatty acid species (Supplementary Fig. 1). The only remarkable change in DAG species was that sn-1,3 DAG was specifically increased in DIO mice treated with Mogat1 ASO, and the ratio of sn-1,3 to sn-1,2 DAG, which is reduced in DIO compared with lean mice, was increased by Mogat1 knockdown (Fig. 6B).
Figure 6.
Mogat1 knockdown increases membrane DAG content and association with PKC isoforms. A: Cytoplasmic and membrane DAG content in livers of mice fed chow containing 60% fat or 10% fat for 14 weeks (left) and treated with ASO against Mogat1 or scrambled control ASO. *P < 0.05 vs. lean mice. B: Hepatic sn-1,2 and sn-1,3 DAG content in mice fed chow containing 60% fat or 10% fat for 14 weeks (left) and treated with ASO against Mogat1 or scrambled control ASO. The ratio of sn-1,3 to sn-1,2 DAG was also calculated (right). *P < 0.05 vs. lean controls; **P < 0.05 vs. lean and obese control ASO mice. C: Hepatic expression of Prcke and Prckd from liver of mice fed chow containing 60% fat or 10% fat for 14 weeks and treated with ASO against Mogat1 or scrambled control ASO for 3 weeks. *P < 0.05 vs. lean controls; **P < 0.05 vs. lean and obese control ASO mice. D: Representative Western blots for total cellular PKCε and PKCδ from livers of mice fed chow containing 60% fat or 10% fat for 14 weeks and treated with ASO against Mogat1 or scrambled control ASO for 3 weeks. Quantification of PKC isoform expression (relative to actin expression) is inset below each blot. E: Representative Western blots for PKCε and PKCδ in membrane protein fractions from livers of mice fed chow containing 60% fat or 10% fat for 14 weeks and treated with ASO against Mogat1 or scrambled control ASO for 3 weeks. F: Membrane-associated PKC-specific activity from liver of mice fed chow containing 60% fat or 10% fat for 14 weeks and treated with ASO against Mogat1 or scrambled control ASO for 3 weeks. *P < 0.05 vs. lean and obese control ASO mice. Cyto, cytoplasmic; mem, membrane.
Other work has suggested that activation of PKCε and PKCδ by DAG is involved in the development of hepatic insulin resistance (1,28–32). We found that the expression of mRNA encoding PKCε (Prcke) but not PKCδ (Prckd) was increased by DIO and significantly reduced by treatment with Mogat1 ASO (Fig. 6C). However, the total cellular protein abundance of PKCε and PKCδ was not affected by DIO and was diminished after Mogat1 ASO treatment (Fig. 6D). The activation of PKCs often is assessed by quantifying the membrane association of PKC protein (1,28–30). Although we found no effect of DIO on PKCε or PKCδ membrane association or membrane-associated PKC activity, Mogat1 knockdown significantly decreased membrane-associated PKC activity in DIO mice (Fig. 6F), which tracks with the abundance of membrane-associated PKCε (Fig. 6C). Collectively, the data suggest that knocking down Mogat1 in liver of obese mice improves glucose metabolism and insulin signaling independent of reductions in intrahepatic DAG or TAG content.
Discussion
The mechanisms whereby caloric excess and obesity lead to hepatic steatosis continue to emerge. Work from our group and others has shown that obese mice and human subjects exhibit a marked activation in the expression of the genes encoding MGAT enzymes, which catalyze the penultimate step in TAG synthesis (11,16). In this work, we found that Mogat1, which is expressed at low levels in normal mouse liver, is robustly induced by obesity through activation of PPARγ. Knockdown of Mogat1 in liver of obese mice improves circulating TAG concentration, glucose tolerance, and hepatic insulin signaling without reducing body weight or intrahepatic DAG or TAG content. These data suggest that targeting hepatic MGAT activity, which is also increased in the livers of obese humans, might have promise in treating dyslipidemia, insulin resistance, and diabetes.
Intrahepatic lipid content is well correlated with systemic insulin sensitivity and the circulating concentrations of cardiometabolic risk factors (33). Although the cause-and-effect relationship between hepatic steatosis and insulin sensitivity is unclear, there is evidence that several lipid intermediates in the TAG synthesis pathway may activate signaling pathways that lead to impaired insulin signaling (34). For example, DAG has been linked to insulin resistance through activation of novel-type PKCs (25). Membrane-associated DAG is known to activate PKCs (25–27), and the PKCε isoform has been shown to translocate from the cytosol to the membrane fraction, which suggests activation, in response to high-fat-diet feeding in rats (35). PKCε is believed to act at the membrane to impair insulin receptor kinase activity to blunt downstream effects on insulin signaling (35). A relationship between PKCε membrane localization and insulin resistance has been detected in a number of models (1,29,36,37). In support of this, administration of ASOs to knock down PKCε in rats fed a high-fat diet for 3 days improved hepatic insulin sensitivity (30), and PKCε knockout mice showed improved glucose tolerance while on a high-fat diet (32). However, the increased glucose tolerance in PKCε knockout mice seems to be due to exaggerated insulin secretion, suggesting a β-cell effect (32). Similarly, PKCδ is activated in liver of some obesity models, and PKCδ null mice are insulin sensitive while on a high-fat diet (31). In the current study, we found no increase in PKCε or PKCδ protein abundance or membrane localization or membrane-associated PKC activity after 17 weeks of feeding a 60% fat diet to mice. However, treatment with Mogat1 ASO reduced the total cellular PKCε and PKCδ abundance and membrane-associated PKC activity, which is surprising given the link between DAG and PKC signaling and the observed increase in membrane DAG content. These findings suggest that the effects of Mogat1 knockdown on the hepatic insulin signaling cascade are actually associated with increased hepatic DAG content.
In the current study, Mogat1 knockdown indeed led to increased total hepatic DAG content. The molecular explanation for this is unclear because we did not see a compensatory increase in phosphatidic acid phosphohydrolase or TAG hydrolase activity (data not shown). We did observe a decrease in the activity of DGAT, which could account for DAG accumulation. A counterintuitive accumulation of DAG was also observed with DGAT overexpression in muscle and liver (38,39) and with knockdown of adipose tissue triglyceride lipase (40); thus, the regulation of the intracellular concentration of this lipid remains enigmatic. Other studies have also detected normal or increased insulin sensitivity in the context of increased DAG (4,39–43). Some work suggested that sequestering DAG in cytosolic lipid droplets, possibly in an innocuous storage depot, prevents accumulation of DAG in the membrane where it could attract PKCε translocation and lead to impaired insulin signaling (22). This contradicts previous work that found a positive correlation between cytosolic DAG content and insulin resistance and PKCε activation (44). Nonetheless, in the current study, Mogat1 knockdown led to insulin sensitization despite causing increases in both membrane and cytosolic DAG content, suggesting that DAG sequestration does not explain the observed phenotype. We also found increases in all species of DAG after ASO treatment, including 16:0/18:1, 16:1/18:1, and other saturated fatty acid–containing DAG, which are best correlated with insulin resistance in SKM (45). Because most lipids are intermediates in metabolic pathways and are rapidly interconverted, it is nearly impossible to affect the concentration of one lipid in isolation, suggesting that changes in other lipid species mediate the effects of Mogat1 knockdown. Future work will be needed to identify the species involved.
While the present article was in preparation, Lee et al. (16) reported that Mogat1 expression is activated by PPARγ and that adenovirus-mediated knockdown of Mogat1 improved insulin sensitivity in obese mice. In their work, hepatic Mogat1 knockdown with adenoviral-driven short hairpin RNA led to dramatic body weight loss and attenuated hepatic steatosis, whereas we note no effect on these parameters after 3 weeks of Mogat1 ASO treatment. It is likely that the observed reduction in intrahepatic TAG content in the work of Lee et al. is secondary to weight loss because this can rapidly reduce hepatic steatosis (46), and overexpression of Mogat1 in liver is not sufficient to drive increased TAG content (A.M.H., unpublished observations). Indeed, the primary pathway for TAG synthesis in liver is through the sequential acylation of glycerol-3-phosphate, and this pathway does not require MGAT activity to synthesize DAG or TAG. It is still unclear why knockdown of Mogat1 in liver in the Lee et al. study led to body weight loss because food intake and energy expenditure were not examined. It remains to be determined whether this was a nonspecific effect of the adenovirus infection.
In summary, inactivation of hepatic MGAT activity, which is markedly increased in obese mice, improved glucose tolerance and hepatic insulin signaling independent of changes in body weight, intrahepatic DAG and TAG content, or PKC signaling. Although it should be noted that the human ortholog of MGAT1 is poorly expressed and that MGAT3 may be the MGAT family member most highly expressed in human liver (11), targeting this enzymatic activity by pharmacologic means might be a viable therapeutic target for the treatment of diabetes. Additional work is needed to examine the mechanisms of insulin sensitization and to determine whether this approach is efficacious.
Article Information
Funding. This work was supported by National Institutes of Health grants K01-DK-087821 to A.M.H. and R01-DK-078187 to B.N.F., and the core services of the Digestive Diseases Research Core Center (P30-DK-52574) and the Nutrition Obesity Research Center (P30-DK-56341) at Washington University School of Medicine. K.T.C. is an American Liver Foundation Liver Scholar.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.M.H. researched data, contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript. N.S., G.G.S., and K.S.M. researched data. K.T.C. and X.S. researched data and reviewed/edited the manuscript. Z.C. contributed to the discussion and reviewed/edited the manuscript. D.M.E. researched the lipidomics data and reviewed/edited the manuscript. M.J.G. reviewed/edited the manuscript. B.N.F. researched data, contributed to the discussion, and wrote the manuscript. A.M.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of data and the accuracy of data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-1502/-/DC1.
References
- 1.Jornayvaz FR, Birkenfeld AL, Jurczak MJ, et al. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci U S A 2011;108:5748–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jornayvaz FR, Lee HY, Jurczak MJ, et al. Thyroid hormone receptor-α gene knockout mice are protected from diet-induced hepatic insulin resistance. Endocrinology 2012;153:583–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell Metab 2012;15:574–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wendel AA, Li LO, Li Y, Cline GW, Shulman GI, Coleman RA. Glycerol-3-phosphate acyltransferase 1 deficiency in ob/ob mice diminishes hepatic steatosis but does not protect against insulin resistance or obesity. Diabetes 2010;59:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neschen S, Morino K, Hammond LE, et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab 2005;2:55–65 [DOI] [PubMed] [Google Scholar]
- 6.Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol 2010;21:128–135 [DOI] [PubMed] [Google Scholar]
- 7.Farese RV, Jr, Zechner R, Newgard CB, Walther TC. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab 2012;15:570–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y, Cheng D. Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am J Physiol Endocrinol Metab 2009;297:E10–E18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao J, Hawkins E, Brozinick J, et al. A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J Biol Chem 2004;279:18878–18886 [DOI] [PubMed] [Google Scholar]
- 10.Yue YG, Chen YQ, Zhang Y, et al. The acyl coenzymeA:monoacylglycerol acyltransferase 3 (MGAT3) gene is a pseudogene in mice but encodes a functional enzyme in rats. Lipids 2011;46:513–520 [DOI] [PubMed] [Google Scholar]
- 11.Hall AM, Kou K, Chen Z, et al. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J Lipid Res 2012;53:990–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen CL, Stone SJ, Cases S, Zhou P, Farese RV., Jr Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc Natl Acad Sci U S A 2002;99:8512–8517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen CL, Farese RV., Jr MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J Biol Chem 2003;278:18532–18537 [DOI] [PubMed] [Google Scholar]
- 14.Mostafa N, Bhat BG, Coleman RA. Increased hepatic monoacylglycerol acyltransferase activity in streptozotocin-induced diabetes: characterization and comparison with activities from adult and neonatal rat liver. Biochim Biophys Acta 1993;1169:189–195 [DOI] [PubMed] [Google Scholar]
- 15.Xia T, Mostafa N, Bhat BG, Florant GL, Coleman RA. Selective retention of essential fatty acids: the role of hepatic monoacylglycerol acyltransferase. Am J Physiol 1993;265:R414–R419 [DOI] [PubMed] [Google Scholar]
- 16.Lee YJ, Ko EH, Kim JE, et al. Nuclear receptor PPARγ-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc Natl Acad Sci U S A 2012;109:13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yen CL, Cheong ML, Grueter C, et al. Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat Med 2009;15:442–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Vigueira PA, Chambers KT, et al. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor γ-sparing thiazolidinedione. J Biol Chem 2012;287:23537–23548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finck BN, Bernal-Mizrachi C, Han DH, et al. A potential link between muscle peroxisome proliferator- activated receptor-alpha signaling and obesity-related diabetes. Cell Metab 2005;1:133–144 [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Gropler MC, Norris J, Lawrence JC, Jr, Harris TE, Finck BN. Alterations in hepatic metabolism in fld mice reveal a role for lipin 1 in regulating VLDL-triacylglyceride secretion. Arterioscler Thromb Vasc Biol 2008;28:1738–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris LA, Shew TM, Skinner JR, Wolins NE. A single centrifugation method for isolating fat droplets from cells and tissues. J Lipid Res 2012;53:1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantley JL, Yoshimura T, Camporez JP, et al. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc Natl Acad Sci U S A 2013;110:1869–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magkos F, Su X, Bradley D, et al. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 2012;142:1444–1446 [DOI] [PMC free article] [PubMed]
- 24.Leiker TJ, Barkley RM, Murphy RC. Analysis of diacylglycerol molecular species in cellular lipid extracts by normal-phase LC-electrospray mass spectrometry. Int J Mass Spectrom 2011;305:103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boni LT, Rando RR. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J Biol Chem 1985;260:10819–10825 [PubMed] [Google Scholar]
- 26.Rando RR, Young N. The stereospecific activation of protein kinase C. Biochem Biophys Res Commun 1984;122:818–823 [DOI] [PubMed] [Google Scholar]
- 27.Nomura H, Ase K, Sekiguchi K, et al. Stereospecificity of diacylglycerol for stimulus-response coupling in platelets. Biochem Biophys Res Commun 1986;140:1143–1151 [DOI] [PubMed] [Google Scholar]
- 28.Choi CS, Savage DB, Abu-Elheiga L, et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A 2007;104:16480–16485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jornayvaz FR, Jurczak MJ, Lee HY, et al. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab 2010;299:E808–E815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuel VT, Liu ZX, Wang A, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest 2007;117:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bezy O, Tran TT, Pihlajamäki J, et al. PKCδ regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J Clin Invest 2011;121:2504–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frangioudakis G, Burchfield JG, Narasimhan S, et al. Diverse roles for protein kinase C delta and protein kinase C epsilon in the generation of high-fat-diet-induced glucose intolerance in mice: regulation of lipogenesis by protein kinase C delta. Diabetologia 2009;52:2616–2620 [DOI] [PubMed] [Google Scholar]
- 33.Abdeen MB, Chowdhury NA, Hayden MR, Ibdah JA. Nonalcoholic steatohepatitis and the cardiometabolic syndrome. J Cardiometab Syndr 2006;1:36–40 [DOI] [PubMed] [Google Scholar]
- 34.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med 2006;119(Suppl. 1):S10–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 2004;279:32345–32353 [DOI] [PubMed] [Google Scholar]
- 36.Choi CS, Savage DB, Kulkarni A, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem 2007;282:22678–22688 [DOI] [PubMed] [Google Scholar]
- 37.Nagai Y, Yonemitsu S, Erion DM, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab 2009;9:252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monetti M, Levin MC, Watt MJ, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 2007;6:69–78 [DOI] [PubMed] [Google Scholar]
- 39.Timmers S, de Vogel-van den Bosch J, Hesselink MK, et al. Paradoxical increase in TAG and DAG content parallel the insulin sensitizing effect of unilateral DGAT1 overexpression in rat skeletal muscle. PLoS One 2011;6:e14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong KT, Mashek MT, Bu SY, Mashek DG. Hepatic ATGL knockdown uncouples glucose intolerance from liver TAG accumulation. FASEB J 2013;27:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown JM, Betters JL, Lord C, et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res 2010;51:3306–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voshol PJ, Haemmerle G, Ouwens DM, et al. Increased hepatic insulin sensitivity together with decreased hepatic triglyceride stores in hormone-sensitive lipase-deficient mice. Endocrinology 2003;144:3456–3462 [DOI] [PubMed] [Google Scholar]
- 43.Monetti M, Canavesi M, Camera M, et al. Rosuvastatin displays anti-atherothrombotic and anti-inflammatory properties in apoE-deficient mice. Pharmacol Res 2007;55:441–449 [DOI] [PubMed] [Google Scholar]
- 44.Kumashiro N, Erion DM, Zhang D, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A 2011;108:16381–16385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergman BC, Hunerdosse DM, Kerege A, Playdon MC, Perreault L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia 2012;55:1140–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]