Abstract
A growing body of clinical and epidemiological research suggests that two of the most common diseases of aging, type 2 diabetes (T2DM) and Alzheimer disease (AD), are linked. The nature of the association is not known, but this observation has led to the notion that drugs developed for the treatment of T2DM may be beneficial in modifying the pathophysiology of AD and maintaining cognitive function. Recent advances in the understanding of the biology of T2DM have resulted in a growing number of therapies that are approved or in clinical development for this disease. This review summarizes the evidence that T2DM and AD are linked, with a focus on the cellular and molecular mechanisms in common, and then assesses the various clinical-stage diabetes drugs for their potential activity in AD. At a time when existing therapies for AD offer only limited symptomatic benefit for some patients, additional clinical trials of diabetes drugs are needed to at least advance the care of T2DM patients at risk for or with comorbid AD and also to determine their value for AD in general.
Introduction
Epidemiologic Data Linking Alzheimer Disease and Type 2 Diabetes
Alzheimer disease (AD) and type 2 diabetes (T2DM) are two of the most common diseases of aging around the world. In the U.S., an estimated 5.4 million people of all ages have AD, and the risk of this disease increases with age. An estimated one in eight people aged older than 65 and one in two people 85 and older have AD. In the U.S., diabetes affects 25.8 million people of all ages (8.3% of the population), and the cumulative incidence of diabetes is 26.9% among people 65 and older. T2DM accounts for more than 90% of cases of diabetes in the U.S. and in many other developed countries. Given the frequency with which T2DM and AD occur, the notion that people with T2DM may be at increased risk for AD has large societal consequences, and understanding mechanistic links between these diseases is imperative for the development of effective AD prevention and treatment strategies.
One of the first reports to provide strong evidence that patients with T2DM are at a significantly increased risk of developing AD was the Rotterdam study (1). This prospective cohort study of 6,370 elderly subjects found that the presence of diabetes almost doubled the risk of developing AD. The risk of AD was even higher among patients treated with insulin, a group that likely had a longer history of diabetes and were refractory to oral agents. Several other large studies have also investigated the relationship between AD and elevated glucose levels, impaired glucose tolerance, and diabetes, and a systematic review and meta-analysis of these studies reported similar overall findings (2). More recently, Crane et al. (3) showed that blood glucose level is positively associated with accelerated cognitive decline, even among individuals without clinical diabetes, after adjustment for multiple possible covariates including age, sex, blood pressure, smoking, and other determinants of macrovascular risk. Therefore, there is an increased risk of AD dementia with each serial increase in glucose level through the entire spectrum of possible glucose levels.
Evidence of Insulin-Signaling Abnormalities in AD Brain
Diabetes is a group of metabolic diseases characterized by insufficiency of the hormone insulin. T2DM is thought to arise in its earliest stage from decreased sensitivity of peripheral tissues to circulating insulin, leading to impaired glucose tolerance, compensatory hyperinsulinemia in an attempt to maintain glucose homeostasis, and relative insulin insufficiency (4). That an analogous process of cellular insulin resistance and insulin insufficiency is occurring in the brain in AD is becoming evident, including in those without systemic diabetes, and for this reason some have referred to AD as “type 3 diabetes” (5–7). Here we will review the growing evidence that insulin resistance and downstream abnormalities in the insulin pathway are present in the AD brain and contribute to the development of cognitive dysfunction in this disease.
When insulin binds to the insulin receptor, it activates a complex intracellular-signaling pathway that leads to the downstream activation of insulin receptor substrate 1 (IRS-1), extracellular signal–related kinase/mitogen-activated protein kinase (ERK/MAPK), and PI3 kinase/Akt pathways (PI3K/AKT), and inhibits glycogen synthase kinase-3 (GSK-3). These same insulin-signaling pathways are conserved in virtually all tissues and cell types that express the insulin receptor, including human neurons. Using a novel ex vivo stimulation paradigm that exposes human postmortem hippocampal tissues to physiologic concentrations of insulin, we were able to study the activation of insulin pathways in brain tissues from people with AD, from those with normal cognitive aging, and from control brains in response to insulin (8). In normal brain tissue, the phosphorylation of insulin receptor-β subunit, IRS-1, AKT, and other insulin-signaling proteins increases robustly in the presence of insulin. However, this response is blunted in the AD brain, similar to insulin resistance in peripheral tissues in T2DM.
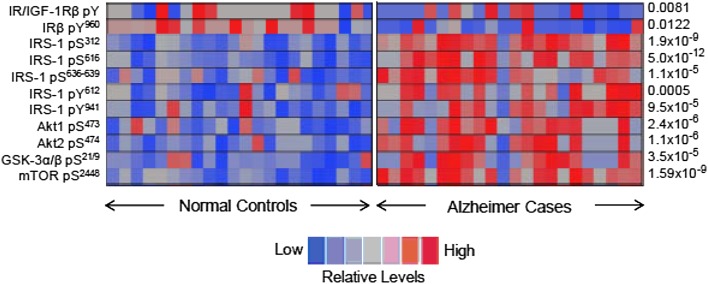
Postmortem studies of AD brains have demonstrated reduced insulin receptor expression (5) and reduced cytosolic levels of PI3K subunits p85α and p110α (9), consistent with insulin resistance. We and others have also shown significant abnormalities in the phosphorylation of IRS-1 in the AD brain (Fig. 1) (8). The phosphorylation of IRS-1 on tyrosine residues is required for insulin-stimulated responses, whereas the phosphorylation of IRS-1 on multiple serine residues causes a reduced response to insulin consistent with insulin resistance (10). Using antibodies specifically targeting phospho-serine residues, we have observed dramatic increases in serine phosphorylation of IRS-1 in the AD hippocampus and cerebral cortex compared with normal matched control subjects. Serine phosphorylation of IRS-1 in this analysis was also robustly and inversely associated with memory and global cognition scores measured in subjects before their deaths. This association with cognitive impairment was stronger than even the density of brain paired helical filament (PHF)-tau neurofibrillary tangles or amyloid-β plaques, the defining pathologic lesions of AD, and the association remained after controlling for plaques and tangles, indicating an independent contribution.
Figure 1.
Heat map showing abnormal phosphorylation of multiple insulin-signaling proteins in postmortem hippocampal brain tissue from 24 normal and 24 matched human AD case subjects. The significant increase in basal serine phosphorylation of protein kinases within the IRS-1 → AKT insulin-signaling cascade in the AD case subjects signifies impaired brain insulin signaling and brain insulin resistance as a feature of this disease. The P values for differences between normal and AD case subjects are given to the right of each row. mTOR, mammalian target of rapamycin. Reproduced and adapted from Talbot et al. (8).
The link between abnormal insulin signaling and AD is further supported in preclinical models. For example, the crossing of T2DM mouse models, such as the ob/ob (leptin deficient) or Nagoya-Shibata-Yasuda (NSY) mouse into an APP23 transgenic mouse background, an AD mouse model, exacerbates the cognitive dysfunction of the AD background (11). In these cognitively impaired mice, phosphorylation of key insulin-signaling domains is impaired, and cerebrovascular inflammation and Aβ plaque density are increased. Another preclinical model that demonstrates a role for insulin-signaling abnormalities in AD is the streptozotocin (STZ)-induced rat model of AD. STZ is a naturally occurring alkylating agent that damages insulin-producing cells and insulin receptors. Rats exposed to intracerebral STZ exhibit impaired cognitive function and histopathological features of AD. Some notable features include marked neurodegeneration and gliosis, abnormal GSK-3 activation, and increased PHF-τ neurofibrillary tangles and Aβ plaques (7,12). Intracerebral STZ seems to act only on the central nervous system (CNS) in these studies, because these mice do not show elevated blood glucose or diabetes. Therefore, induction of abnormal insulin signaling within the CNS appears to directly produce cognitive dysfunction.
Pathogenesis Models of Abnormal Brain Insulin Signaling and AD
The most commonly used pathogenesis models of AD to date focus on amyloid-β production and aggregation, and to a lesser degree, hyperphosphorylated tau fibrillization in neurites and neurofibrillary tangles, leading to synaptic loss, cell death, and cognitive dysfunction (13). Although the data linking abnormal insulin signaling to AD in epidemiological studies, animal studies, and human postmortem studies are compelling, how insulin-signaling dysfunction relates to other aspects of AD pathology and cognitive decline remains somewhat unclear.
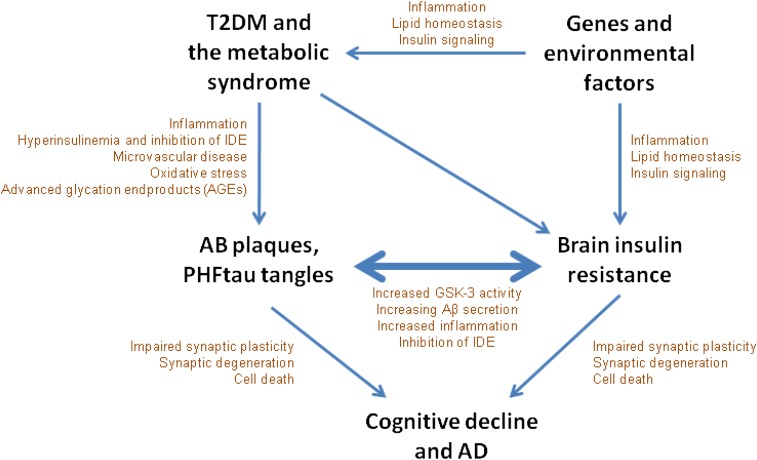
One model linking T2DM, brain insulin resistance, and AD pathology is shown in Fig. 2. T2DM and the metabolic syndrome may promote AD pathology through chronic inflammation, hyperinsulinemia, microvascular disease, oxidative stress, and advanced glycation end products (AGEs) (6). Because the insulin-degrading enzyme (IDE) is a principle regulator of amyloid-β levels in neuronal cells, hyperinsulinemia in T2DM may reduce clearance of amyloid-β through competitive inhibition of IDE. Brain insulin resistance may develop in parallel with T2DM through a number of common or concurrent mechanisms involving predisposing genes and environmental factors. Hyperinsulinemia related to T2DM may induce brain insulin resistance by causing a reduction in insulin receptor expression and receptor kinase activity (14) and ultimately promoting the development of amyloid-β and tau pathology. Conversely, or perhaps reciprocally, brain insulin resistance may arise as a result of pathological oligomeric or fibrillar amyloid-β. Amyloid-β shares a consensus sequence with insulin and can directly bind to the insulin receptor, leading to insulin resistance (15). Amyloid-β has also been shown to activate GSK-3, thereby further promoting brain insulin resistance indirectly (16), and to activate the JNK/tumor necrosis factor-α pathway, which in turn promotes serine phosphorylation of IRS-1 (17).
Figure 2.
T2DM, brain insulin resistance, and AD have been linked in a number of different epidemiological, clinical, and animal studies. One model for this link is displayed. Initially, genes and lifestyle promote insulin resistance in peripheral and brain tissue. Insulin resistance in peripheral tissues leads to T2DM and the metabolic syndrome, which further promotes brain insulin resistance as well as AD pathology though hyperinsulinemia, reactive oxygen species, and AGEs. AD pathology and brain insulin resistance may drive one another in a reciprocal cycle that eventually leads to synaptic loss and clinical dementia due to AD.
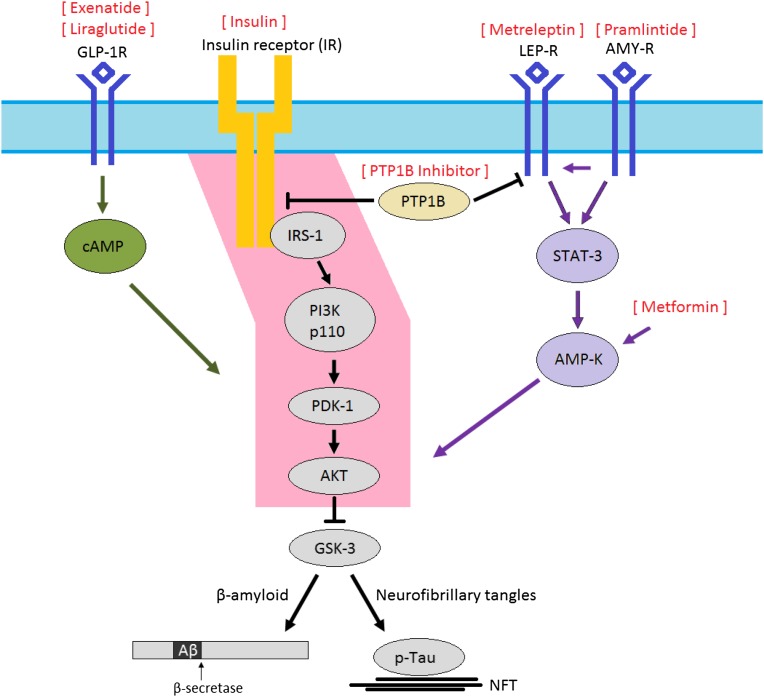
Regardless of whether brain insulin resistance initially develops as a primary or secondary process, brain insulin resistance may promote AD pathology and clinical AD through several mechanisms. At the molecular level, abnormal insulin signaling may promote amyloid-β and hyperphosphorylated tau through the multifunctional serine/threonine kinase GSK-3. This kinase is normally constitutively active, and its activity is regulated primarily through inhibition by AKT. Therefore, dysfunctional insulin signaling through the IRS-1 → AKT pathway in AD may lead to increased GSK-3 activity (Fig. 3). GSK-3 has been most extensively studied as a kinase for tau, but it is also involved in amyloid-β production (18,19). Insulin resistance in the brain may also promote amyloid-β by increasing its secretion and decreasing degradation by amyloid-β–degrading enzymes, including IDE (20,21). Recent work from our group has focused on the relationship between insulin resistance and hyperphosphorylated tau. We have found that cellular markers of insulin resistance frequently colocalized with pathologic tau in tangle-bearing neurons and that abnormal insulin signaling may promote hyperphosphorylated tau (22). Others have demonstrated that insulin resistance may lead to decreased synapse formation and increased cell death (23). Additional work is needed to understand the precise mechanisms through which insulin resistance promotes AD and its related neurodegeneration and dementia.
Figure 3.
All diabetes drugs are likely to have indirect effects in the CNS by affecting circulating concentrations of glucose and insulin. However, the especially intimate relationship between brain insulin resistance and AD, and the relative “paralysis” of brain insulin signaling through the IRS-1 → AKT pathway (pink) in MCI and AD, suggests that restoring signaling through this pathway with therapeutic agents originally developed for the treatment of diabetes may be of particular benefit. One approach is to overcome brain insulin resistance with exogenous insulin, but a theoretical concern of this approach is that in the long-run the chronic hyperinsulinemic environment will actually perpetuate brain insulin resistance. Other therapies, including GLP-1 agonists (e.g., exenatide, liraglutide), metformin, leptin analogs (metreleptin), amylin analogs (pramlintide), and PTP1B inhibitors may circumvent insulin-signaling impairment and reestablish signaling through the IRS-1 → AKT pathway. Peroxisome proliferator–activated receptor-γ agonists, such as rosiglitazone and pioglitazone, which reduce blood glucose by increasing GLUT-4 translocation but have been unsuccessful in improving outcomes in well-powered studies of AD, are somewhat removed from this impaired insulin-signaling pathway. NFT, neurofibrillary tangles.
New Role for Diabetes Drugs As a Treatment for AD
Insulin has important roles in normal brain functioning, and insulin-signaling dysfunction is increasingly recognized for its association with AD in preclinical and comparative postmortem neuropathology studies. These discoveries have given way to a growing interest in restoring insulin signaling in AD with therapeutic agents originally developed for the treatment of T2DM. All diabetes drugs may affect AD indirectly through effects on circulating concentrations of glucose, insulin, inflammatory markers, and by generation of reactive oxygen species and AGEs. However, because insulin resistance of the brain is most closely linked to AD, targeting insulin signaling in the brain may be most important. Therefore, diabetes drugs that pass the blood-brain barrier and are active in brain tissue, and especially those that affect signaling through the IRS-1 → AKT pathway (Fig. 3), are of particular interest for study in AD. Here we assess the various clinical-stage diabetes drugs for their potential activity in AD.
Intranasal Insulin
Although many classes of drugs are now approved for management of diabetes, a primary focus of efforts to treat insulin-signaling dysfunction in AD has been the administration of exogenous insulin. There is abundant anecdotal evidence that insulin administration in people with diabetes may acutely affect mood, behavior, and cognitive performance. In 2001, the cognitive effects of insulin in nonimpaired adults were demonstrated by Kern et al. (24) using a 6-h insulin infusion. Subjects exposed to higher insulin infusion rates demonstrated changes in auditory-evoked brain potentials, enhanced memory as evidenced by improved word recall, and improved cognitive flexibility and attention as measured by the Stroop test. Similar benefits of acute insulin administration were demonstrated by Craft et al. (25) in patients with AD. In their study, the AD patients showed improved story recall and attention during insulin infusion relative to saline infusion. Although theoretically interesting, the feasibility of treating AD with peripherally administered insulin is doubtful. Most peripherally administered insulin does not enter the CNS, and the dose of peripherally infused insulin is limited by induction of hypoglycemia. In both studies, hypoglycemia was mitigated by a simultaneous glucose infusion, which is obviously impractical outside of the research setting.
Interesting recent efforts to study the effects of insulin on cognitive function have concentrated on intranasal insulin delivery. Insulin that is administered intranasally bypasses the blood-brain barrier and is rapidly delivered into the cerebrospinal fluid (CSF) compartment (26). Intranasal insulin is believed to enter the CNS along axons and their sheaths in the olfactory nerve that extend through the cribriform plate to the olfactory bulb and in the trigeminal nerve. Because intranasal insulin is preferentially delivered to the CNS, it may theoretically be possible to achieve clinically relevant concentrations of insulin in the CNS without causing systemic hypoglycemia. In the acute setting, one dose of intranasal insulin induces changes in auditory-evoked brain potentials in healthy cognitively intact adults (27). More recent pilot clinical studies have also shown that chronic administration of intranasal insulin may improve memory function. In a study of 38 young, cognitively intact adults exposed to 8 weeks of regular intranasal insulin (4 × 40 IU/day), word recall was significantly improved compared with vehicle nasal spray (28). Peripheral glucose levels were not significantly affected by intranasal insulin. These studies in cognitively normal adults supported the importance of insulin in normal brain functioning and raised interest in the use of intranasal insulin in cognitively impaired adults.
Results of recent pilot studies of intranasal insulin in mild cognitive impairment (MCI) and AD have been encouraging. The most notable of these studies was a double-blind, randomized trial of 104 older adults with MCI or AD who received placebo, low-dose (20 IU), or high-dose (40 IU) intranasal insulin for 4 months (29). Compared with placebo, participants who received either dose of insulin demonstrated significant improvements in memory as assessed by the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) and the Alzheimer’s Disease Cooperative Study (ADCS) activities of daily living scales. These benefits of intranasal insulin were apparent not only at the end of the treatment period but also 2 months after treatment cessation, suggesting that intranasal insulin has lasting effects on CNS functioning. A subset of subjects also underwent positron emission tomography (PET) and lumbar puncture to assess for changes in AD CSF biomarkers. Compared with participants who received intranasal insulin, less radioactive glucose uptake during the PET scan was noted in multiple brain regions in the placebo group, indicative of cerebral metabolic dysfunction and consistent with AD progression. However, no significant differences were noted in amyloid-β levels in CSF fluid among the three participant groups.
One theoretical concern about intranasal insulin is that chronic hyperinsulinemic conditions in the brain may actually promote brain insulin resistance. For example, excessive exposure to insulin in mice leads to phosphorylation of key components of the insulin cascade, such as AKT, GSK-3β, and p70S6K, consistent with insulin resistance (14). Therefore, it is possible that longer-term studies of intranasal insulin will produce different results from the published shorter-term pilot studies or that escalating doses of intranasal insulin will be required to show continued benefit. However, given the promising results of intranasal insulin in small pilot studies, longer-term studies of intranasal insulin are warranted. In 2012, the U.S. National Institutes of Health allocated $7.9 million for a pivotal trial of intranasal insulin called the Study of Nasal Insulin in the Fight Against Forgetfulness (SNIFF; ClinicalTrials identifier: NCT01767909). This multicenter phase 2/3 study will be conducted by the ADCS. It is expected to recruit 250 participants with AD or MCI and to randomize them for 12 months to intranasal insulin or placebo, followed by an open-label extension of 6 months in which all participants will receive intranasal insulin. The study should be completed in late 2014.
Peroxisome Proliferator–Activated Receptor-γ Agonists
Thiazolidinediones (TZDs) are a class of oral diabetes drugs that increase insulin sensitivity by activating the nuclear receptor peroxisome proliferator–activated receptor-γ, leading to increased expression of the glucose transporter GLUT-4. Two TZDs, rosiglitazone (Avandia) and pioglitazone (Actos), are approved for treatment of T2DM, although the use of rosiglitazone was restricted in many countries, including the U.S., due to concerns about increased cardiovascular events. Pioglitazone is now generic and remains widely used, but there has been an increased recognition of adverse effects with it as well, including fluid retention, bone fractures, and bladder cancer (30).
In preclinical studies, TZDs improved biomarkers of AD as well as memory and cognition (31). The first pilot studies in humans were also generally encouraging, including a study by Watson et al. (32) that showed improved memory and modulation of amyloid-β levels in CSF compared with placebo after 6 months of treatment with rosiglitazone. On the basis of these preliminary studies, the maker of rosiglitazone sponsored two adequately powered phase 3 studies of rosiglitazone in AD as monotherapy or as adjunctive therapy to acetylcholinesterase inhibitors in mild-to-moderate AD. These larger trials failed to replicate the positive findings of the smaller pilot studies (33). One of the phase 3 studies (AVA102672) found a nominally statistically significant benefit of low-dose rosiglitazone (2 mg) in one of the prespecified outcome measures, the ADAS-Cog score, although this difference was reported as not significant after applying prespecified adjustments for possible confounding variables. The second phase 3 trial (AVA102670) found no evidence of efficacy.
Many explanations have been proposed for why rosiglitazone does not appear to be effective as a treatment for AD in cognitively impaired adults. Perhaps the most convincing explanation is that rosiglitazone has only modest blood-brain barrier penetration, and in fact, rosiglitazone is actively pumped out of the brain by an endogenous efflux system (34). Therefore, rosiglitazone should be expected to have only a mild insulin-sensitizing effect in the human brain. Furthermore, because rosiglitazone is highly effective at sensitizing peripheral tissues to insulin, it causes a significant decrease in circulating blood insulin levels. By reducing circulating insulin, rosiglitazone likely decreases brain exposure to insulin. In the long-run, decreased brain exposure to insulin might ameliorate neuronal insulin resistance (14); however, in the short-run, it would be expected to decrease brain insulin signaling and worsen cognitive impairment.
Metformin
Metformin is an oral, generically available diabetes drug that is the first-line treatment for T2DM in the American Diabetes Association treatment algorithm (30). It is generally well tolerated and safe, although gastrointestinal upset and diarrhea are common adverse effects. Its glucose-lowering actions include increasing glucose uptake in muscle and other peripheral tissues and decreasing liver gluconeogenesis. Although metformin’s cellular mechanism of action has not been completely clarified, it is thought to affect glucose metabolism by activating AMP-activated protein kinase (AMPK) in liver and other tissues. AMPK is a central regulator of lipid and glucose metabolism. Activation of AMPK may increase insulin sensitivity through interactions with a number of intracellular targets such as mTOR, p38 MAPK, and protein kinase C.
Relatively little is known about metformin’s effects in the CNS. Although human studies about metformin’s CNS penetration are lacking, there is evidence from rodent studies that metformin does cross the blood-brain barrier and activates AMPK in CNS tissue (35,36). In neuronal cell lines, exposure to metformin sensitizes neurons to insulin and also prevents AD pathology in neurons chronically exposed to a hyperinsulinemic environment (37). However, metformin has also been reported to increase β-secretase 1 (BACE1) transcription and associated generation of amyloid-β in neuronal cell lines (38). A pilot study of metformin in 80 subjects with amnestic MCI was recently completed (ClinicalTrials identifier: NCT00620191) but has not yet been published, and our group is currently conducting a pilot randomized controlled crossover study of metformin at the University of Pennsylvania on cognition, neuroimaging, and CSF biochemical biomarkers in MCI and AD (ClinicalTrials identifier: NCT01965756).
GLP-1, Amylin, and Leptin Analogs
A different approach for restoring insulin signaling in the AD brain is to modulate the insulin-signaling pathway with insulin-like metabolic hormones. Three metabolic hormones have shown promise in preclinical models of AD: GLP-1, amylin, and leptin. These hormones are structurally unrelated to insulin and each binds to independent receptors in the brain, thereby circumventing insulin-signaling impairment. After binding to their respective receptors, they activate signaling pathways that converge with the insulin-signaling pathway and facilitate insulin signaling. Analogs of all three hormones are approved or are in the process of regulatory approval in the U.S. All readily cross the blood-brain barrier. One advantage of these agents (and metformin) over insulin is that they do not cause significant hypoglycemia and can therefore be safely administered at relatively high doses peripherally.
The hormone GLP-1 is secreted by intestinal L cells in response to a meal. Its glucose-lowering effects include enhanced insulin secretion and decreased glucagon secretion, delayed gastric emptying, increased satiety, and increased insulin sensitivity in multiple tissues (30). At a cellular level, GLP-1 binds to the G-protein–coupled GLP-1 receptor, stimulating adenylyl cyclase and leading to the downstream modulation of PKA, PI3K, MAPK, PKC, and AKT. The GLP-1 receptor is nearly ubiquitous and is widely expressed in the brain. Although endogenous GLP-1 is rapidly degraded by dipeptidyl peptidase-4 (DPP-4, also called CD26), injectable analogs of GLP-1 with prolonged half-lives have been developed and approved for the treatment of T2DM including exenatide and liraglutide. Multiple oral inhibitors of DPP-4 that indirectly augment endogenous GLP-1 levels have also been approved for T2DM.
Preclinical studies of GLP-1 agonists in cell culture and mouse models of AD have been encouraging (39,40). These agents have been shown to act as a growth factor in the brain, causing synaptogenesis and neurogenesis and protecting against oxidative injury. In AD mouse models, GLP-1 agonists reduce levels of AD pathologic markers, including oligomeric amyloid-β and amyloid-β plaque load, decrease microglial activation, and improve memory behaviors. We are aware of two ongoing pilot studies of GLP-1 analogs for AD. One is a 3-year 230-patient randomized trial using exenatide sponsored by the National Institute on Aging (ClinicalTrials identifier: NCT01255163). The other is a 12-month, 200-patient trial of liraglutide conducted by the Imperial College London (ClinicalTrials identifier: NCT01843075).
Leptin is an adipokine that is produced in adipose tissue and activates CNS networks to regulate food consumption and energy expenditure. Although leptin has been most extensively studied in the context of obesity, recent evidence suggests that leptin has neuronal functions that are unrelated to its effects on energy homeostasis. Leptin receptors are highly expressed in areas of the brain that are involved in learning and memory, such as the CA1 region of the hippocampus, and leptin receptor–knockout mice demonstrate poor performance on memory tasks (41). Leptin has also been shown to reduce BACE1 activity and extracellular amyloid-β and tau phosphorylation in AD mouse models (42). Although congenital leptin or leptin receptor deficiency in humans is an extremely rare condition, the phenotypic findings of leptin deficiency are well described. They include hyperphagia, obesity, severe insulin resistance, and/or hypertriglyceridemia, as well as cognitive delay, and that leptin replacement may restore the rate of intellectual development in many neurocognitive domains has been reported (43). Interestingly, increased circulating leptin was also recently associated with a reduced incidence of AD after adjustment for basic and vascular risk factors in a prospective study of 785 asymptomatic older individuals without dementia who were followed up for an average of 8.3 years (44). Together these epidemiologic observations, preclinical findings, and case reports support a role for leptin as a possible therapy for AD in humans. At the time of this writing, an analog of leptin (metreleptin) is undergoing review as a treatment for metabolic disorders associated with lipodystrophy. The safety of metreleptin remains unclear because it has not been extensively studied in large placebo-controlled trials. Some potential concerns include possible increased risks of lymphoma and immune-related reactions.
Abnormal amylin has recently been recognized in brain tissue in AD. Jackson et al. (45) described oligomeric and plaque-like accumulations of amylin in brain parenchyma and cerebral vasculature that was present in patients with diabetes as well as in people with AD who were nondiabetic compared with normal, nondiabetic control subjects. Interestingly, these plaques were sometimes colocalized with amyloid-β plaques but were often independent. We recently investigated an amylin agonist in a rodent model of accelerated aging as a potential therapeutic approach for the treatment of AD and observed salutary effects (46). Amylin, also called islet amyloid polypeptide (IAPP), is a peptide hormone that is cosecreted with insulin from β-cells in the pancreas. Similar to GLP-1, amylin lowers blood glucose levels through delayed gastric emptying, decreased glucagon secretion, and increased satiety. Although amylin contributes to glucose control, the utility of endogenous amylin is limited due to its propensity to aggregate and form amylin oligomers and plaques. However, pramlintide, a soluble, nonaggregating synthetic analog of this hormone, is approved as an adjunctive therapy to insulin for the treatment of type 1 and T2DM.
Amylin readily crosses the blood-brain barrier, and its receptors are distributed widely throughout the brain in the area postrema, nucleus of the solitary tract, parabrachial nucleus, amygdala, hypothalamus, nucleus accumbens, and dorsal raphe. Amylin may play a larger role in the CNS than previously appreciated, and a growing body of evidence has implicated amylin in a variety of CNS functions, including memory, mood, anxiety, and satiety. We have found that chronic infusion of pramlintide in mice improves memory performance in the novel object recognition task and also modulates synapses, decreases oxidative stress and inflammatory markers in the hippocampus (46), and increases hippocampal neurogenesis (unpublished data). Pramlintide causes minimal hypoglycemia, and its excellent safety profile and tolerability are appealing for the study and treatment of AD.
Amylin and leptin activate overlapping signaling cascades that include STAT3 and AMPK, and ultimately converge on the insulin-signaling pathway by activating AKT (Fig. 3) and increasing insulin sensitivity. Interestingly, amylin and leptin signaling appear to be synergistic in multiple preclinical and clinical models. For example, the weight loss caused by the combination of metreleptin and pramlintide in mice and in humans is greater than the additive weight loss of each drug used alone (47). Amylin is thought to sensitize neurons to the effects of leptin, because amylin pretreatment of neurons augments leptin signaling. This unique synergy suggests that achieving even greater improvements in memory may be possible by using pramlintide and leptin as a combined therapy.
Earlier-Stage Diabetes Compounds
More than 200 drugs, belonging to dozens of novel drug classes, are in preclinical through phase 3 development for diabetes. Although little if anything is known about the CNS effects of most of these drugs, many are intriguing as potential treatments of MCI and AD given their possible effects on brain insulin signaling.
As discussed previously, we and others have shown that insulin signaling through multiple pathways, including the IRS-1 → AKT pathway, is severely affected in AD. Unfortunately, there are still no approved drugs that directly modulate the intracellular IRS-1 → AKT insulin-signaling pathway. However, one early-stage diabetes drug class that may modulate this pathway is the inhibitors of tyrosine-protein phosphatase nonreceptor type 1, also known as protein-tyrosine phosphatase 1B (PTP1B). PTP1B is a negative regulator of insulin and leptin signaling, and PTP1B inhibitors have been shown in preclinical models to reduce inflammation and restore insulin and leptin signaling in the brain (48). Several PTP1B inhibitors in early-stage development for T2DM include ISIS-PTP1BRx (phase 2), TTP814 (phase 2), and trodusquemine (phase 1). We believe that PTP1B inhibitors are especially appealing for use in neurodegenerative diseases.
Other drugs that are worthy of study for the treatment of neurodegenerative disease are the oxidoreductase 11-β-hydroxysteroid dehydrogenase type 1 (11-β-HSD1) inhibitors such as LY2523199 (phase 2), INCB13739 (phase 2), or MK-0736 and MK-0916 (both phase 1). This drug class inhibits the conversion of inactive 11-keto forms of cortisone to the active glucocorticoids (cortisol, corticosterone). Cortisol is well known to have a pathogenic role in T2DM, and chronic elevation of glucocorticoids have also been linked to neuronal damage, cognitive deficits, and hippocampal atrophy (49). Although brief elevations in cortisol are necessary for coping with acute stress, chronic stress resulting in sustained overactivity of the hypothalamic-pituitary-adrenal axis and hypercortisolemia has been associated with memory impairment (50). We believe that pilot studies of an 11-β-HSD1 inhibitor for treatment of MCI or AD are warranted.
Conclusions
AD is the most common form of dementia, and the current treatment options for this disease offer only marginal clinical benefit. Novel treatment approaches for AD are needed. A growing body of epidemiological, preclinical, and pathologic studies indicates that AD and T2DM share common cellular and molecular mechanisms. Targeting brain insulin signaling with pharmacologic therapies used in the management of T2DM is a compelling approach to the treatment of AD. Many diabetes drugs are in clinical use, and which therapies—if any—will be effective at improving cognitive function is unclear. The results of clinical studies of the diabetes drug rosiglitazone suggest that controlling peripheral glucose levels is insufficient to maintain cognitive function. Multiple ongoing clinical trials of other diabetes drugs that more directly target brain insulin signaling are planned or recommended and may provide the ultimate test for the AD-T2DM hypothesis.
Article Information
Acknowledgments. The authors acknowledge Kelly L. Close (Close Concerns, Inc.) for her advice with this manuscript.
Funding. This work was supported in part by grants from the BrightFocus Foundation and the National Institute on Aging (P30 AG10124) and by a gift from the Allen H. and Selma W. Berkman Charitable Foundation. S.E.A. receives institutional research funding from BrightFocus Foundation to conduct a clinical trial on metformin for mild cogntive impairment and early Alzheimer disease.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.Y. and S.E.A. drafted and revised the manuscript. M.Y. and S.E.A. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
References
- 1.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet 1997;349:151–154 [DOI] [PubMed] [Google Scholar]
- 2.Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol 2008;585:97–108 [DOI] [PubMed] [Google Scholar]
- 3.Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. N Engl J Med 2013;369:540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 5.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease – is this type 3 diabetes? J Alzheimers Dis 2005;7:63–80 [DOI] [PubMed] [Google Scholar]
- 6.de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes—evidence reviewed. J Diabetes Sci Technol 2008;2:1101–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis 2006;10:89–109 [DOI] [PubMed] [Google Scholar]
- 8.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012;122:1316–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 2010;31:224–243 [DOI] [PubMed] [Google Scholar]
- 10.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–336 [DOI] [PubMed] [Google Scholar]
- 11.Takeda S, Sato N, Uchio-Yamada K, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A 2010;107:7036–7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labak M, Foniok T, Kirk D, et al. Metabolic changes in rat brain following intracerebroventricular injections of streptozotocin: a model of sporadic Alzheimer’s disease. Acta Neurochir Suppl (Wien) 2010;106:177–181 [DOI] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 2010;9:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim B, Sullivan KA, Backus C, Feldman EL. Cortical neurons develop insulin resistance and blunted Akt signaling: a potential mechanism contributing to enhanced ischemic injury in diabetes. Antioxid Redox Signal 2011;14:1829–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci 2002;22:RC221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci U S A 1997;94:9660–9664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bomfim TR, Forny-Germano L, Sathler LB, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. J Clin Inves 2012;122:1339–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 2006;9(Suppl.):309–317 [DOI] [PubMed] [Google Scholar]
- 19.Phiel CJ, Wilson CA, Lee VM-Y, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature 2003;423:435–439 [DOI] [PubMed] [Google Scholar]
- 20.Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A 2003;100:4162–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho L, Qin W, Pompl PN, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J 2004;18:902–904 [DOI] [PubMed] [Google Scholar]
- 22.Arnold SE, Yarchoan M, Talbot K, et al. Markers of brain insulin resistance in Alzheimer’s disease and other tauopathies (Program 136/Poster F40). In Neuroscience Meeting Planner, New Orleans, LA, 2013. Washington, DC, Society for Neuroscience, p. 136.01 [Google Scholar]
- 23.Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 2008;58:708–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology 2001;74:270–280 [DOI] [PubMed] [Google Scholar]
- 25.Craft S, Asthana S, Newcomer JW, et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry 1999;56:1135–1140 [DOI] [PubMed] [Google Scholar]
- 26.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002;5:514–516 [DOI] [PubMed] [Google Scholar]
- 27.Kern W, Born J, Schreiber H, Fehm HL. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes 1999;48:557–563 [DOI] [PubMed] [Google Scholar]
- 28.Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 2004;29:1326–1334 [DOI] [PubMed] [Google Scholar]
- 29.Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 2012;69:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Standards of medical care in diabetes–2013. Diabetes Care 2013;36(Suppl. 1):S11–S66. [DOI] [PMC free article] [PubMed]
- 31.Nicolakakis N, Aboulkassim T, Ongali B, et al. Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci 2008;28:9287–9296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 2005;13:950–958 [DOI] [PubMed] [Google Scholar]
- 33.Harrington C, Sawchak S, Chiang C, et al. Effects of rosiglitazone-extended release as adjunctive therapy to acetylcholinesterase inhibitors over 48 weeks on cognition in Apoe4-stratified subjects with mild-to-moderate Alzheimer’s disease. Alzheimer Dement 2009;5:e17–e18 [Google Scholar]
- 34.Festuccia WT, Oztezcan S, Laplante M, et al. Peroxisome proliferator-activated receptor-gamma-mediated positive energy balance in the rat is associated with reduced sympathetic drive to adipose tissues and thyroid status. Endocrinology 2008;149:2121–2130 [DOI] [PubMed] [Google Scholar]
- 35.Łabuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopień B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep 2010;62:956–965 [DOI] [PubMed] [Google Scholar]
- 36.Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol 2009;182:8005–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta A, Bisht B, Dey CS. Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology 2011;60:910–920 [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Zhou K, Wang R, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A 2009;106:3907–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry TA, Greig NH. A new Alzheimer’s disease interventive strategy: GLP-1. Curr Drug Targets 2004;5:565–571 [DOI] [PubMed] [Google Scholar]
- 40.McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci 2011;31:6587–6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X-L, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 2002;113:607–615 [DOI] [PubMed] [Google Scholar]
- 42.Greco SJ, Bryan KJ, Sarkar S, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis 2010;19:1155–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paz-Filho GJ, Babikian T, Asarnow R, et al. Leptin replacement improves cognitive development. PLoS ONE 2008;3:e3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 2009;302:2565–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson K, Barisone GA, Diaz E, Jin LW, DeCarli C, Despa F. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol 2013;74:517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adler BL, Yarchoan M, Hwang HM, et al. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol Aging 2014;35:793–801 [DOI] [PubMed] [Google Scholar]
- 47.Ravussin E, Smith SR, Mitchell JA, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picardi PK, Calegari VC, Prada PO, et al. Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology 2008;149:3870–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuchs E, Flügge G, Ohl F, Lucassen P, Vollmann-Honsdorf GK, Michaelis T. Psychosocial stress, glucocorticoids, and structural alterations in the tree shrew hippocampus. Physiol Behav 2001;73:285–291 [DOI] [PubMed] [Google Scholar]
- 50.Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998;1:69–73 [DOI] [PubMed] [Google Scholar]