Abstract
The dipeptidyl peptidase-4 inhibitor sitagliptin, an antidiabetic agent, which lowers blood glucose levels, also reduces postprandial lipid excursion after a mixed meal. The underlying mechanism of this effect, however, is not clear. This study examined the production and clearance of triglyceride-rich lipoprotein particles from the liver and intestine in healthy volunteers in response to a single oral dose of sitagliptin. Using stable isotope tracer techniques and with control of pancreatic hormone levels, the kinetics of lipoprotein particles of intestinal and hepatic origin were measured. Compared with placebo, sitagliptin decreased intestinal lipoprotein concentration by inhibiting particle production, independent of changes in pancreatic hormones, and circulating levels of glucose and free fatty acids. Fractional clearance of particles of both intestinal and hepatic origin, and production of particles of hepatic origin, were not affected. This pleiotropic effect of sitagliptin may explain the reduction in postprandial lipemia seen in clinical trials of this agent and may provide metabolic benefits beyond lowering of glucose levels.
Introduction
Insulin resistance and type 2 diabetes are associated with increased risk of cardiovascular disease (CVD) (1). The underlying causes include dyslipidemia, which is composed primarily of elevated fasting and postprandial triglyceride (TG)-rich lipoproteins (TRLs), decreased level of HDL cholesterol, and increased number of small, dense LDL particles (2,3). Patients with type 2 diabetes commonly have postprandial hyperlipemia, and nonfasting TG level poses as a strong predictor of CVD risk (4,5). The overproduction of apolipoprotein (apo) B-100–containing VLDL particles by the liver and apoB-48–containing chylomicrons by the intestine accentuates the accumulation of atherogenic lipoprotein particles in the circulation (6–9). We and others have demonstrated in humans that TRL production is regulated by several factors, including circulating free fatty acids (FFAs), monosaccharides, and gut and pancreatic hormones (glucagon-like peptide [GLP]-1, insulin, and glucagon) (10–18). Understanding the regulation of TRL production and strategies to ameliorate TRL overproduction could lead to new therapies to treat dyslipidemia and prevent CVD.
Incretin hormones, including GLP-1 and gastric inhibitory polypeptide (GIP), are secreted by the intestinal L and K cells, respectively. After secretion, both GLP-1 and GIP are rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4). Incretins have many physiological actions that affect glucose metabolism, including enhancing glucose-dependent insulin secretion, suppressing glucagon secretion, delaying gastric emptying, and promoting satiety (19). Incretin-based therapies have been in widespread clinical use for the treatment of type 2 diabetes (20). The mechanism of action of such therapies centers on enhancing GLP-1 receptor (GLP-1R) activation, either by providing exogenous GLP-1 activity with GLP-1R agonists or by preserving endogenous GLP-1 activity with DPP-4 inhibitors. Besides lowering glucose levels, both approaches have been shown to beneficially affect lipid profiles in clinical trials in patients with type 2 diabetes (21,22). The GLP-1 agonist exenatide decreased both fasting and postprandial TG (23,24), and DPP-4 inhibitors lowered postprandial TG (25,26).
The exact mechanism of GLP-1R modulation in lipid homeostasis remains poorly defined. Chronic studies are confounded by the multiple actions of incretins, including improved glycemic control, reduced plasma FFA levels, enhanced satiety, and weight loss (the latter two with GLP-1R agonists but not with DPP-4 inhibitors), which can independently improve lipid profiles. In short-term studies in humans, GLP-1 infusion or a single dose of its mimetic exenatide ameliorates postprandial lipid excursions and lipoprotein responses after a test meal (27,28). We further demonstrated that single-dose exenatide inhibits intestinal lipoprotein production in healthy individuals (29). Although long-term administration of DPP-4 inhibitors improves postprandial lipemia (25,26), whether a similar mechanism to exenatide is involved has not been explored. DPP-4 inhibition directly suppressed TRL secretion in ex vivo animal experiments (30), while such an effect has not been examined in humans. The current study was therefore designed to examine the mechanism of the DPP-4 inhibitor sitagliptin in TRL particle production in healthy humans, in conjunction with a pancreatic clamp to stabilize glucoregulatory hormone levels and thus reduce many of the known confounding effects of the drug that could indirectly affect lipid metabolism.
Research Design and Methods
Subjects
A total of 21 male volunteers were recruited and 15 completed both arms of the study. Of the premature withdrawals, four volunteers did not show up for the study after signing the consent form and being randomized to sitagliptin or placebo for the first of their two studies, and two volunteers did not meet the inclusion criteria. The demographic characteristics and fasting biochemical profiles of those who completed the study are shown in Table 1. Subjects had a BMI between 21 and 27 kg/m2 and normal glucose tolerance in response to a 75-g, 2-h oral glucose tolerance test performed immediately prior to enrollment in the study. None of the participants had any history of CVD, systemic illness, surgical intervention within the 6 months prior to the studies, or was taking any medications. The Research Ethics Board of the University Health Network, University of Toronto, approved the study, and all subjects gave written informed consent prior to their participation.
Table 1.
Demographic characteristics and fasting biochemical parameters of subjects (all men)
| Characteristics | Values |
|---|---|
| n | 15 |
| Age (years) | 42.3 ± 2.6 |
| Body weight (kg) | 78.5 ± 3.5 |
| BMI (kg/m2) | 24.6 ± 0.6 |
| Glucose (mmol/L) | 4.90 ± 0.1 |
| Insulin (pmol/L) | 48.7 ± 5.7 |
| Plasma TG (mmol/L) | 1.2 ± 0.2 |
| Plasma FFA (mmol/L) | 0.32 ± 0.04 |
| Plasma TC (mmol/L) | 3.79 ± 0.20 |
| TRL TG (mmol/L) | 0.96 ± 0.10 |
| TRL apoB-100 (mg/L) | 119.4 ± 11.1 |
| TRL apoB-48 (mg/L) | 2.44 ± 0.92 |
Data are mean ± SEM, unless otherwise indicated.
TC, total cholesterol.
Experimental Protocol
The experimental protocol is illustrated in Fig. 1A. Each subject underwent two separate lipoprotein kinetic studies in random order, 4 to 6 weeks apart. In each study, after an overnight fast, the subject was admitted to the Metabolic Test Center at Toronto General Hospital at approximately 9:00 a.m. on day 1 of the study. A 30-mL fasting blood sample was drawn. During the stay in the hospital, the subjects were provided with an American Heart Association phase 1 diet and refrained from exercise. At 4:00 p.m., two intravenous catheters were inserted into a superficial vein in each forearm, one for infusion and one for sampling. The subjects were fasted after 7:00 p.m. Starting at 4:00 a.m. the next day, a liquid formula (total fat 10% by weight, saturated fat 1.5%, trans fat 0%, monounsaturated fat 2.6%, polyunsaturated fat 5.6%, cholesterol 0%; 49% calories from fat, 38% from carbohydrates, 13% from proteins; Hormel Health Labs, Savannah, GA) was ingested every hour during the study, for the following 15 h. Each aliquot was calculated to evenly spread the total daily caloric requirement, estimated with the Harris-Benedict equation, across the course of the study. TRL kinetics were studied in a constant fed state because fasting TRL apoB-48 concentrations are too low to allow accurate quantification of stable isotope enrichment.
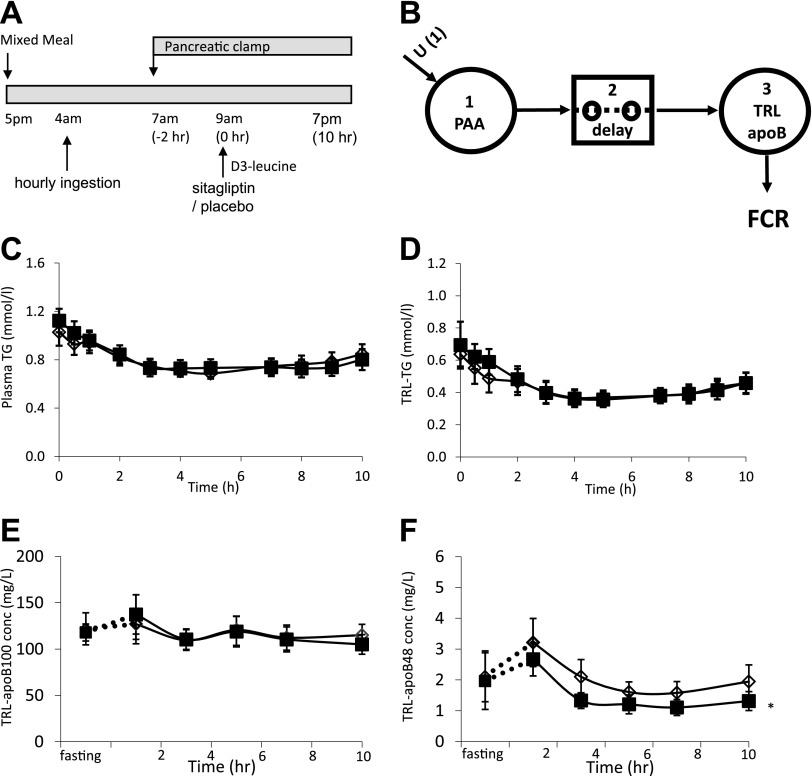
Figure 1.
A: Schematic representation of the experimental design. Hourly ingestion of a liquid formula was started at 4:00 a.m. to achieve a constant fed state. A pancreatic clamp (with infusion of somatostatin, insulin, glucagon, and growth hormone) was started 3 h later. Two hours into the pancreatic clamp, sitagliptin (100 mg) or placebo was administered orally, and a primed, constant infusion of d3-leucine was started. Frequent blood samples were drawn for isolation of TRL fractions, gel separation of apoB-100 and apoB-48, and gas chromatography–mass spectrometry quantification of stable isotopic enrichment. B: Compartmental model for estimating TRL apoB-100 and apoB-48 FCRs. The model consists of 1) a plasma amino acid (PAA; leucine) precursor pool, 2) a delay compartment, and 3) a plasma TRL compartment. Plasma TG (C), TRL TG (D), TRL apoB-100 (E), and TRL apoB-48 (F) concentrations during the kinetic study. The “fasting” sample was taken after a 14-h overnight fast the day prior to the study. Open diamond, placebo; solid squares, sitagliptin; PAA, plasma amino acids; conc, concentration. *P < 0.05, sitagliptin vs. placebo.
At 7:00 a.m. (i.e., 3 h after starting the liquid formula ingestion), a pancreatic clamp was started with the infusion of somatostatin. Insulin, glucagon, and growth hormone were infused at basal rates (29). The pancreatic clamp was performed to prevent the known effects of incretins on the secretion of islet hormones, which could then have independent effects on TRL metabolism, as we have demonstrated for insulin and glucagon in previous studies (10,29). Because plasma glucose levels did not decrease during the pancreatic clamp, dextrose infusion was not needed.
At 9:00 a.m. (5 h after starting to ingest the liquid formula and 2 h after starting the pancreatic clamp), 100 mg sitagliptin (Merck, Rahway, NJ) or matching placebo was administered by mouth. At the same time, a primed constant infusion (10 µmol/kg bolus followed by 10 μmol/kg/h for 10 h) of L-[5,5,5-2H3]-leucine (d3-leucine; Cambridge Isotope Laboratories, Andover, MA) was started. The total volume of infusion for hormones and tracer was 715 mL. Blood samples were collected at 0, 0.5, 1, 2, 3, 4, 5, 7, 8, 9, and 10 h after the administration of d3-leucine for isolation of lipoproteins. Blood samples for TG, FFA, and hormone analysis were collected at regular intervals.
Laboratory Methods
TRLs were isolated from plasma by centrifugation at a density of 1.006 g/mL for 16 h, and 39,000 rotations per minute at 12°C. Aliquots (∼1 mg protein) were delipidated and separated by preparative 3.3% SDS-PAGE. Gel bands corresponding to apoB-48 and apoB-100 were excised, hydrolyzed at 110°C with 6N HCl for 24 h, and dried under vacuum. Amino acids were then derivatized with a 100-µL mixture (1:1) of acetonitrile and N-tert-butyldimethyl-N-methyltrifluoracetamide (Sigma-Aldrich). Derivatized samples were analyzed with electron impact ionization gas chromatography–mass spectrometry (Agilent 5975/6890N; Agilent Technologies Canada, Mississauga, ON, Canada) using helium as the carrier gas. Selective ion monitoring at charge/mass ratios of 200 and 203 was performed. Tracer-to-tracee ratios (TTRs) were calculated from isotopic ratios for each sample according to a standard curve of isotopic enrichment. Plasma free amino acids were recovered from 0.25 mL plasma after precipitation of proteins with acetone and extraction of the aqueous phase with hexane, and derivatized as above.
Commercial kits were used to measure TG (Roche Diagnostics, Mannheim, Germany), FFAs (Wako Industrials, Osaka, Japan), cholesterol (Roche Diagnostics), insulin (Millipore, Billerica, MA), C-peptide (Millipore), glucagon (Millipore), and biologically active forms of GLP-1 (Millipore). TRL apoB-100 and apoB-48 mass were measured with ELISA kits specific for human apoB-100 (Mabtech, Mariemont, OH; intra-assay coefficient of variation [CV] 2%, interassay CV 10%) and apoB-48 (Shibayagi, Shibukawa, Japan; intra-assay CV 3.5%, interassay CV 5.6%).
Kinetic Analysis
A multicompartmental model (Fig. 1B) was fitted to TRL apoB-100 or apoB-48 leucine TTR time course curves during the entire kinetic study to derive the fractional catabolic rate (FCR), using SAAM II software (version 1.2; University of Washington, Seattle, WA), as previously described (29). The model consisted of the synthesis of TRL apoB from the precursor pool via a 30-min delay compartment. The plasma free leucine TTR (Supplementary Fig. 1) measured for each subject during each visit was used as a forcing function. The FCR was derived with the above model in SAAM II, and production rates (PRs) were calculated as PR = FCR × pool size, where pool size is the average plasma concentration (in milligrams per liter) over the kinetic study × plasma volume (estimated as 0.045 L/kg body weight).
Statistics
Results are presented as the mean ± SEM. Repeated-measures ANOVA was performed to compare the time course of parameters during the kinetic experiments. The primary outcome was the TRL apoB PR, which was compared between the two treatments using a paired t test, with P < 0.05 considered to be significant. Paired t tests with Bonferroni corrections were applied for comparisons of fasting TG, FFA, cholesterol, hormones, TRL apoB pool sizes, and FCR between treatments. All statistical analyses were performed with SAS (version 9; SAS Institute, Cary, NC).
Results
Effects of Sitagliptin on Plasma Glucose, Insulin, C-Peptide, and Active GLP-1 Levels and on Plasma and TRL TG and apoB Concentrations
During the kinetic study, plasma TG (Fig. 1C), TRL TG (Fig. 1D), glucose (Supplementary Fig. 2A), FFA (Supplementary Fig. 2B), and insulin (Supplementary Fig. 2C) levels were not significantly different between sitagliptin and placebo treatments. Sitagliptin, administered under pancreatic clamp conditions in the presence of somatostatin, did not stimulate insulin secretion, as evidenced by similar levels of plasma C-peptide in the sitagliptin and placebo studies (Supplementary Fig. 2D). Plasma glucagon and growth hormone concentrations were also not different between treatments (data not shown). Active GLP-1 levels were similar between treatments before the start of the pancreatic clamp. At 2 h into the pancreatic clamp and prior to the administration of sitagliptin or placebo, GLP-1 levels decreased by 20–30%, possibly because of somatostatin inhibition of GLP-1 secretion, resulting in comparable baseline GLP-1 levels before treatment (placebo 4.0 vs. sitagliptin 4.1 pmol/L). GLP-1 levels were increased after sitagliptin treatment, while levels remained stable after placebo treatment, resulting in significantly higher active GLP-1 incremental area under the curve (placebo −0.3 vs. sitagliptin 6 pmol/h/L, P < 0.05). The time courses of TRL apoB-100 and TRL apoB-48 concentrations are shown in Fig. 1E and F. No significant effects of sitagliptin on TRL apoB-100 levels were observed. In contrast, sitagliptin treatment significantly decreased the TRL apoB-48 concentration during the kinetic study (P < 0.05 sitagliptin vs. placebo).
Effects of Sitagliptin on TRL apoB-100 Pool Size, Fractional Clearance, and PRs
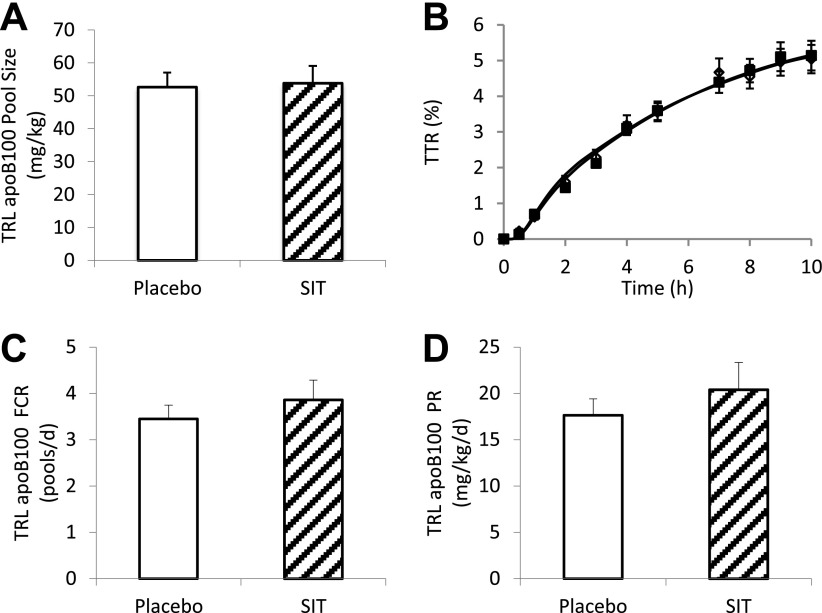
The pool sizes of TRL apoB-100 (placebo 56.2 ± 4.4 vs. sitagliptin 53.8 ± 5.2 mg/kg, P = 0.79) were comparable between sitagliptin and placebo (Fig. 2A). The TTR was not significantly different throughout the kinetic study between sitagliptin and placebo (Fig. 2B). Consistent with the superimposing TTR time-course curves, the FCR (placebo 3.45 ± 0.30 vs. sitagliptin 3.86 ± 0.43 pools/day, P = 0.37) was not significantly different between treatments (Fig. 2C). As a result, the PRs (placebo 17.6 ± 1.8 vs. sitagliptin 20.4 ± 2.9 mg/kg/day, P = 0.41) were not significantly affected by sitagliptin treatment (Fig. 2D).
Figure 2.
TRL apoB-100 pool size (A), TTR time course (B), FCR (C), and PR (D) after sitagliptin (SIT) (100 mg) or placebo treatment during a pancreatic clamp. White bars, placebo; hatched bars, sitagliptin; open diamond, placebo; solid squares, sitagliptin.
Effects of Sitagliptin on TRL apoB-48 Pool Size, Fractional Clearance, and PRs
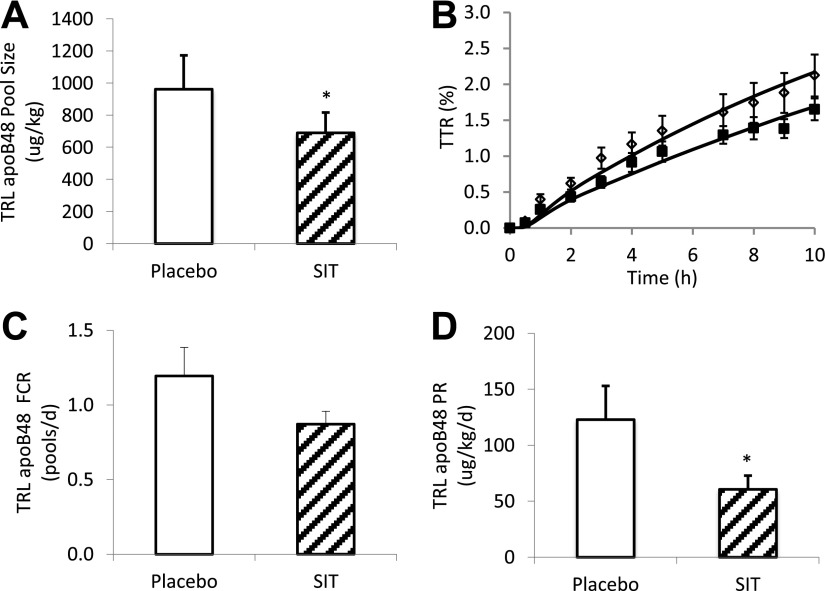
The pool size of TRL apoB-48 (placebo 961.4 ± 211.2 vs. sitagliptin 689.4 ± 127.6 μg/kg, P = 0.02) was lower in the sitagliptin study compared with placebo (Fig. 3A). The TTR time course diverged between treatments, with relatively lower TTR in sitagliptin than in placebo (Fig. 3B). The FCR in the sitagliptin study tended to be lower but was not statistically significant from placebo (placebo 1.19 ± 0.19 vs. sitagliptin 0.87 ± 0.09 pools/day, P = 0.15) (Fig. 3C). Calculated PRs (placebo 122.9 ± 30.4 vs. sitagliptin 60.6 ± 12.2 µg/kg/day, P = 0.02) were decreased by ∼50% with sitagliptin treatment (Fig. 3D), which accounted for the decreased TRL apoB-48 concentrations. When the mean integral apoB-48 concentrations (the area under the curve divided by 10 h) were used for the calculation, sitagliptin treatment resulted in similar reductions in apoB-48 pool size (−29%) and PR (−51%) as calculated using the arithmetic mean.
Figure 3.
TRL apoB-48 pool size (A), TTR time course (B), FCR (C), and PR (D) after sitagliptin (SIT) (100 mg) or placebo treatment during a pancreatic clamp. White bars, placebo; hatched bars, sitagliptin; open diamond, placebo; solid squares, sitagliptin. *P < 0.05, vs. placebo.
Discussion
DPP-4 inhibitors, although used for glycemic control in patients with type 2 diabetes, have demonstrated the potential to improve lipid profiles and postprandial lipemia. The current study is the first in humans to examine the mechanism of DPP-4 inhibition on TRL particle production in vivo. We demonstrate here that a single oral dose of 100-mg sitagliptin reduces the production of intestinally derived, apoB-48–containing lipoprotein particles in healthy males. These results, obtained under conditions of constant feeding and a pancreatic clamp, reveal that treatment with sitagliptin can reduce intestinal lipoprotein production through mechanisms independent of changes in pancreatic hormones, glycemia, and circulating fatty acids. These findings expand our understanding of the mechanism of action of sitagliptin and suggest a potentially clinically beneficial effect of sitagliptin that goes beyond its glucose-lowering properties.
DPP-4 inhibitors have been shown to have the potential to affect lipid profiles in clinical trials in patients with type 2 diabetes. For instance, treatment with sitagliptin resulted in lower fasting levels of plasma cholesterol, LDL cholesterol, apoB, and apoB-48, as well as postprandial plasma levels of TG, total apoB, and apoB-48 during oral fat tests (25). Vildagliptin did not significantly affect fasting lipid profiles; however, it decreased postprandial levels of plasma TG, chylomicron TG, and cholesterol after oral fat load (26); reduced remnant-like particles; and increased LDL particle size (31). While these studies demonstrated the efficacy of chronic DPP-4 inhibition in ameliorating postprandial lipid responses and modest improvement in fasting lipid profiles, the underlying mechanisms were not fully explored. In the current study, we examined the kinetics of hepatic and intestinal lipoprotein metabolism after a single oral dose of sitagliptin in healthy, nondiabetic men. We specifically chose to study the acute effects of sitagliptin in nondiabetic subjects in order to exclude the confounding effects of improved glycemic control, which is known to ameliorate hepatic TRL overproduction (32) in prolonged treatment of patients with diabetes. This allowed us to determine whether our previously observed effect of exenatide, a GLP-1R agonist, in acutely inhibiting chylomicron production in humans (29), is also a feature of the related class of therapeutic agents, the DPP-4 inhibitors. In our previous study with exenatide (29), a constant fed state was achieved by infusing the nutrient formula into the duodenum through a nasoduodenal tube to circumvent the well-documented effect of exenatide on slowing gastric emptying (33). Since sitagliptin does not delay gastric emptying (34), the intraduodenal infusion approach was not necessary in the current study. Instead, a constant fed state was achieved by hourly ingestion of the formula. Under these experimental conditions, sitagliptin acutely inhibited the production of chylomicron particles, thereby contributing to a lowering of postprandial lipemia. A reduction in TRL apoB-48 levels without concomitant reduction in plasma and TRL TG levels was also observed with the GLP-1 agonist exenatide in the constant fed state (29). This may be due to a differential inhibitory effect of sitagliptin and exenatide on the protein (apoB-48) versus the lipid (TG) moiety of the chylomicron particle during its assembly and secretion, resulting in the secretion of fewer chylomicron particles of larger size. Alternatively, since the source of the TRL TG includes both intestinal chylomicron and hepatic VLDL, a small effect of sitagliptin on chylomicron TG secretion may not have been detected. In addition, in these healthy subjects receiving somatostatin, which might have reduced fat absorption, plasma TG levels were <1.0 mmol/L for the duration of the study. This may have contributed to an absence of further TG lowering. Interestingly, hepatic (apoB-100–containing) lipoprotein particle production was not significantly affected by sitagliptin. In longer-term clinical trials in patients with type 2 diabetes, sitagliptin treatment decreased VLDL apoB-100 levels, along with improved glycemic control and decreased circulating levels of postprandial FFAs (25). In contrast, vildagliptin treatment of patients with type 2 diabetes for 4 weeks did not affect postprandial apoB-100 or FFA levels (26). In the current study, similar levels of plasma FFA in the sitagliptin and placebo arms may have contributed to the lack of acute effect of sitagliptin on TRL apoB-100 production.
The kinetics study was performed under pancreatic clamp conditions with the infusion of somatostatin. The effects of somatostatin on carbohydrate and lipid metabolism are mainly through its modulation of the secretion of insulin, glucagon, and growth hormone (35), which were simultaneously replaced in the current study. There are no known “direct” effects of somatostatin on intestinal or hepatic lipoprotein secretion, although, to our knowledge, this issue has not previously been systematically studied. Somatostatin or activation of its receptors may inhibit gastric emptying, gut motility, intestinal blood flow, and secretion of gut hormones including GLP-1 (36–38). In the current study, participants did not report any nausea, a side effect of reduced gastric emptying and gut motility associated with somatostatin infusion. Plasma intact GLP-1 concentration declined equally in both treatment arms during the first 2 h of the pancreatic clamp, prior to administration of sitagliptin or placebo, possibly due to somatostatin-mediated inhibition of GLP-1 secretion. After treatment, sitagliptin administration resulted in significantly higher GLP-1 levels than did placebo administration. It is not possible to compare the magnitude of sitagliptin-induced elevation of intact GLP-1 in the current study to that in previously published studies since there were major differences in the rate, quantity, and duration of nutrient administration between studies, in addition to differences in the GLP-1 assays used in the various studies. It is reassuring, however, that the same protocol was used in both the control and active treatment arms. Therefore, any effects of somatostatin on intestinal lipoprotein production would be unlikely to affect the conclusion of the study.
The cellular mechanisms whereby sitagliptin inhibits chylomicron production could not be examined in the present in vivo study in humans. Sitagliptin is a potent and selective DPP-4 inhibitor, a single oral dose of which results in sustained DPP-4 inhibition and elevated levels of active GLP-1 (39). In short-term or acute studies, GLP-1R agonists decreased intestinal lipid absorption and apoB-48 secretion in rodents (30,40). In addition, GLP-1 agonists also inhibited apoB-48 secretion from primary hamster enterocytes (30). In humans, GLP-1 and exenatide acutely attenuated postprandial lipemia in healthy subjects or patients with impaired glucose tolerance or type 2 diabetes (27,28). In our previous study, we demonstrated that a single subcutaneous dose of exenatide suppressed intestinal lipoprotein production (29). The effects of sitagliptin on TRL metabolism in the current study were very similar to those observed in our previous study with exenatide. Specifically, single doses of exenatide and sitagliptin both decreased intestinal but not hepatic lipoprotein particle production in healthy individuals in the fed state and under the pancreatic clamp condition, despite several differences in experimental protocols. It is known that insulin and FFAs could affect TRL particle production (13,15,17,18). In the study with exenatide, insulin levels were transiently increased after exenatide injection, presumably because of the potent stimulation of insulin secretion (29). In the current study, however, insulin and C-peptide levels were all matched between treatments. Plasma FFA levels in both treatments decreased after the start of the pancreatic clamp and remained low throughout the study, possibly because of the inhibition of fat absorption by somatostatin. In addition, blood glucose levels were not different between active and placebo treatments. Therefore, the reduction in TRL apoB-48 production with sitagliptin was not likely confounded by any effect of insulin or FFAs, or secondary to improvements in glycemic control. The current study thus further substantiates the notion that incretin-based therapies can reduce intestinal lipoprotein production beyond their effects on pancreatic hormones, FFAs, and improvements in metabolic status. Although studies in humans cannot conclusively demonstrate the cellular mechanisms, these studies, along with those in animals, suggest that a GLP-1R signaling pathway may be operating in human intestinal enterocytes to regulate lipoprotein synthesis, assembly, and secretion.
DPP-4 cleaves a variety of other substances besides GLP-1, including GIP and GLP-2 (41). In addition to enhanced GLP-1 activities, long-term administration of sitagliptin increased circulating intact GIP levels in patients with type 2 diabetes (25). GIP infusion decreases chylomicron TGs via accelerated clearance in dogs (42). The potential contribution from elevated GIP levels to decreased TRL apoB-48 levels is not expected to be substantial, since the FCR was not significantly affected in the current study. GLP-2 is cosecreted with GLP-1 in a 1:1 molar ratio, but is degraded less rapidly than GLP-1 (41). Based on animal studies, GLP-2 promotes intestinal lipid absorption and TRL apoB-48 secretion (30). Preliminary results in humans also suggest that GLP-2 stimulates the rapid release of stored intestinal lipid and preformed apoB-48 (43). Despite the potential counteracting actions of GLP-1 and GLP-2 on intestinal lipoprotein metabolism, sitagliptin decreased both the concentration and PR of TRL apoB-48 in this study, indicating a predominant effect of GLP-1 under these experimental conditions. This is in agreement with the findings in animal models where prolonged equimolar infusion of GLP-1 and GLP-2, or administration of sitagliptin, led to an overall hypolipidemic effect (44).
The efficacy of incretin-based therapies on cardiovascular outcomes is currently being actively investigated. In the first two adequately powered clinical outcome trials of the effects of DPP-4 inhibitors on CVD events, neutral effects on CVD events were reported with alogliptin in patients with type 2 diabetes after acute coronary syndrome (45) and with saxagliptin in patients with type 2 diabetes who had a history of or were at increased risk of CVD events (46). The lipid effects of DPP-4 inhibitors, therefore, do not appear to be sufficient to reduce CVD events in patients with type 2 diabetes and established CVD. A cardiovascular outcomes study with sitagliptin is currently underway. It remains to be studied in patients with type 2 diabetes whether DPP-4 inhibitors, including sitagliptin, lower TRL apoB-48 PRs and elicit CVD benefits when used earlier in the course of diabetes and for a longer duration.
In the current study, we have demonstrated in healthy males that a single dose of the DPP-4 inhibitor sitagliptin acutely decreases postprandial TRL apoB-48 levels by reducing particle PRs, possibly through enhanced GLP-1 action, and independent of changes in pancreatic hormones and circulating levels of FFA or improvement in glycemic control. These results further support the existence of a GLP-1R signaling pathway that may regulate lipoprotein particle production in human intestinal enterocytes, either directly or indirectly. This effect of incretin therapy, which goes beyond their well-established glucose-lowering effect, may provide additional therapeutic benefits in the patient with type 2 diabetes.
Article Information
Acknowledgments. The authors thank Brenda Hughes and Patricia Harley for conducting the clinical protocol and Linda Szeto for technical assistance.
Funding. S.D. and C.M. are recipients of postdoctoral fellowship awards from the Banting and Best Diabetes Centre, University of Toronto, and S.D. is the recipient of a Focus on Stroke 12 Fellowship Award from the Heart and Stroke Foundation of Canada. This work was supported by National Institutes of Health grant DK056341 (Washington University Nutrition Obesity Research Center, to B.W.P.) and by an operating grant from the Canadian Institutes of Health Research (to G.F.L.) and Merck Canada Inc. (to G.F.L.). G.F.L. holds the Sun Life Financial Chair in Diabetes and the Drucker Family Chair in Diabetes Research.
Duality of Interest. G.F.L. received an investigator-initiated research grant from Merck Canada Inc. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.X. designed the study; acquired, analyzed, and interpreted the data; and wrote the manuscript. S.D. and C.M. designed the study and acquired, analyzed, and interpreted the data. B.W.P. acquired and analyzed the data. G.F.L. designed the study, interpreted the data, wrote the manuscript, and obtained funding for and supervised the study. G.F.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01600703, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-1654/-/DC1.
References
- 1.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care 2005;28:2901–2907 [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard BV, Howard WJ. Dyslipidemia in non-insulin-dependent diabetes mellitus. Endocr Rev 1994;15:263–274 [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299–308 [DOI] [PubMed] [Google Scholar]
- 5.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309–316 [DOI] [PubMed] [Google Scholar]
- 6.Xiao C, Hsieh J, Adeli K, Lewis GF. Gut-liver interaction in triglyceride-rich lipoprotein metabolism. Am J Physiol Endocrinol Metab 2011;301:E429–E446 [DOI] [PubMed] [Google Scholar]
- 7.Adeli K, Lewis GF. Intestinal lipoprotein overproduction in insulin-resistant states. Curr Opin Lipidol 2008;19:221–228 [DOI] [PubMed] [Google Scholar]
- 8.Schaefer EJ, McNamara JR, Shah PK, et al. Framingham Offspring Study Elevated remnant-like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care 2002;25:989–994 [DOI] [PubMed] [Google Scholar]
- 9.Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagné C, Couture P. Evidence of increased secretion of apolipoprotein B-48-containing lipoproteins in subjects with type 2 diabetes. J Lipid Res 2007;48:1336–1342 [DOI] [PubMed] [Google Scholar]
- 10.Xiao C, Pavlic M, Szeto L, Patterson BW, Lewis GF. Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes 2011;60:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlic M, Xiao C, Szeto L, Patterson BW, Lewis GF. Insulin acutely inhibits intestinal lipoprotein secretion in humans in part by suppressing plasma free fatty acids. Diabetes 2010;59:580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler Thromb Vasc Biol 2006;26:1357–1363 [DOI] [PubMed] [Google Scholar]
- 13.Duez H, Lamarche B, Valéro R, et al. Both intestinal and hepatic lipoprotein production are stimulated by an acute elevation of plasma free fatty acids in humans. Circulation 2008;117:2369–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes 1993;42:833–842 [DOI] [PubMed] [Google Scholar]
- 15.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest 1995;95:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao C, Dash S, Morgantini C, Lewis GF. Novel role of enteral monosaccharides in intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol 2013;33:1056–1062 [DOI] [PubMed] [Google Scholar]
- 17.Malmström R, Packard CJ, Watson TD, et al. Metabolic basis of hypotriglyceridemic effects of insulin in normal men. Arterioscler Thromb Vasc Biol 1997;17:1454–1464 [DOI] [PubMed] [Google Scholar]
- 18.Malmström R, Packard CJ, Caslake M, et al. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia 1997;40:454–462 [DOI] [PubMed] [Google Scholar]
- 19.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 20.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 2009;5:262–269 [DOI] [PubMed] [Google Scholar]
- 21.Mudaliar S, Henry RR. Incretin therapies: effects beyond glycemic control. Am J Med 2009;122(Suppl.):S25–S36 [DOI] [PubMed] [Google Scholar]
- 22.Xiao C, Dash S, Lewis GF. Mechanisms of incretin effects on plasma lipids and implications for the cardiovascular system. Cardiovasc Hematol Agents Med Chem 2012;10:289–294 [DOI] [PubMed] [Google Scholar]
- 23.Buse JB, Drucker DJ, Taylor KL, et al. DURATION-1 Study Group DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 2010;33:1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drucker DJ, Buse JB, Taylor K, et al. DURATION-1 Study Group Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 25.Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab 2011;13:366–373 [DOI] [PubMed] [Google Scholar]
- 26.Matikainen N, Mänttäri S, Schweizer A, et al. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia 2006;49:2049–2057 [DOI] [PubMed] [Google Scholar]
- 27.Meier JJ, Gethmann A, Götze O, et al. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia 2006;49:452–458 [DOI] [PubMed] [Google Scholar]
- 28.Schwartz EA, Koska J, Mullin MP, Syoufi I, Schwenke DC, Reaven PD. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis 2010;212:217–222 [DOI] [PubMed] [Google Scholar]
- 29.Xiao C, Bandsma RH, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol 2012;32:1513–1519 [DOI] [PubMed] [Google Scholar]
- 30.Hsieh J, Longuet C, Baker CL, et al. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 2010;53:552–561 [DOI] [PubMed] [Google Scholar]
- 31.Matikainen N, Taskinen MR. The effect of vildagliptin therapy on atherogenic postprandial remnant particles and LDL particle size in subjects with type 2 diabetes. Diabet Med 2013;30:756–757 [DOI] [PubMed] [Google Scholar]
- 32.Adiels M, Borén J, Caslake MJ, et al. Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler Thromb Vasc Biol 2005;25:1697–1703 [DOI] [PubMed] [Google Scholar]
- 33.Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004;117:77–88 [DOI] [PubMed] [Google Scholar]
- 34.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008;24:2943–2952 [DOI] [PubMed] [Google Scholar]
- 35.Gerich JE, Lorenzi M, Bier DM, et al. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. J Clin Invest 1976;57:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daumerie C, Henquin JC. Somatostatin and the intestinal transport of glucose and other nutrients in the anaesthetised rat. Gut 1982;23:140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chisholm C, Greenberg GR. Somatostatin-28 regulates GLP-1 secretion via somatostatin receptor subtype 5 in rat intestinal cultures. Am J Physiol Endocrinol Metab 2002;283:E311–E317 [DOI] [PubMed] [Google Scholar]
- 38.Hansen L, Hartmann B, Mineo H, Holst JJ. Glucagon-like peptide-1 secretion is influenced by perfusate glucose concentration and by a feedback mechanism involving somatostatin in isolated perfused porcine ileum. Regul Pept 2004;118:11–18 [DOI] [PubMed] [Google Scholar]
- 39.Herman GA, Bergman A, Liu F, et al. Pharmacokinetics and pharmacodynamic effects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects. J Clin Pharmacol 2006;46:876–886 [DOI] [PubMed] [Google Scholar]
- 40.Qin X, Shen H, Liu M, et al. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol 2005;288:G943–G949 [DOI] [PubMed] [Google Scholar]
- 41.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 2012;33:187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wasada T, McCorkle K, Harris V, Kawai K, Howard B, Unger RH. Effect of gastric inhibitory polypeptide on plasma levels of chylomicron triglycerides in dogs. J Clin Invest 1981;68:1106–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dash S, Xiao C, Morgantini C, Szeto L, Patterson BW, Lewis GF. Glucagon-like peptide-2 acutely stimulates chylomicron release in healthy humans. Diabetes 2013;62(Suppl. 1):A76 [Google Scholar]
- 44.Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes 2013;62:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White WB, Cannon CP, Heller SR, et al. EXAMINE Investigators Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 46.Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Investigators Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]