Abstract
Background/Objectives
Vitamin D may modify the risk of type 2 diabetes mellitus. The aim of this review was to examine the association between vitamin D status and incident type 2 diabetes, and the effect of vitamin D supplementation on glycemic outcomes.
Methods
We performed a systematic review of English-language studies using MEDLINE through February 2011. Longitudinal cohort studies reporting associations between vitamin D status and incident type 2 diabetes, and randomized controlled trials (RCTs) of vitamin D supplementation, were included. Study characteristics and results were extracted, and study quality was assessed.
Results
A total of 8 observational cohort studies and 11 RCTs were included. In meta-analyses of observational studies, vitamin D intake > 500 international units (IU)/day decreased the risk of type 2 diabetes by 13% compared with vitamin D intake < 200 IU/day. Individuals with the highest vitamin D status (> 25 ng/ml) had a 43% lower risk of developing type 2 diabetes (95% confidence interval 24, 57%) compared with those in the lowest group (< 14 ng/ml). In post hoc analyses from eight trials among participants with normal glucose tolerance at baseline and in three small underpowered (n = 32–62) trials of patients with established type 2 diabetes, there was no effect of vitamin D supplementation on glycemic outcomes. In two trials among patients with baseline glucose intolerance, vitamin D supplementation improved insulin resistance.
Conclusions
Vitamin D may play a role in type 2 diabetes; however, to better define the role of vitamin D in the development and progression of type 2 diabetes, high-quality observational studies and RCTs that measure blood 25-hydroxyvitamin D concentration and clinically relevant glycemic outcomes are needed.
Keywords: vitamin D, type 2 diabetes mellitus, systematic review, meta-analysis
Introduction
Type 2 diabetes has become a significant global health care problem. In the United States alone, total prevalence is expected to more than double in the next few decades from 6% of the population in 2005 (16 million) to 12% in 2050 (48 million; Narayan et al., 2006). From a worldwide perspective, the total number of people with diabetes is expected to rise from 171 million in 2000 to 366 million by 2030 (Wild et al., 2004). Type 2 diabetes is associated with serious morbidity and increased mortality. Although therapies for type 2 diabetes and its complications have improved over the last few decades, the increasing burden of type 2 diabetes highlights the need for innovative approaches for the prevention and management of the disease.
There is mounting evidence suggesting that vitamin D may influence several non-skeletal medical conditions, including cardiovascular disease, cancer, autoimmune disorders and type 2 diabetes. A potential role of vitamin D in type 2 diabetes is suggested by a reported seasonal variation in the control of glycemia in patients with type 2 diabetes, being worse in the winter when hypovitaminosis D is more prevalent (Campbell et al., 1975). Additional evidence for a role of vitamin D in type 2 diabetes comes from a large number of cross-sectional studies, which have generally reported an inverse association between vitamin D status and prevalent hyperglycemia. Recently, longitudinal observational studies and intervention studies have also been published on the relationship between vitamin D and type 2 diabetes. We performed a systematic review of longitudinal observational studies of vitamin D status and trials of vitamin D supplementation on glycemic outcomes.
Materials and methods
Data sources and study selection
We conducted a literature search for longitudinal observational studies of vitamin D status and development of type 2 diabetes, and randomized controlled trials (RCTs) evaluating the effect of vitamin D supplementation (cholecalciferol (D3) or ergocalciferol) with or without calcium supplementation on glycemic parameters.
Two authors (JM and MM) independently performed a search of MEDLINE and the Cochrane Database of Systematic Reviews through February 2011. Search terms were vitamin D and diabetes mellitus, and the search was limited to publications in English. The search included studies in which vitamin D status was assessed by either self-reported vitamin D intake, blood 25-hydroxyvitamin D (25(OH)D) concentration or 25(OH)D score predicted from self-reported data. Diabetes-related outcomes included incident type 2 diabetes and, in the context of RCTs only, change in glycemia (fasting plasma glucose, 2-h glucose level after an oral glucose tolerance test or hemoglobin A1c). Titles and abstracts of the resulting articles were examined, and full-text articles were retrieved after excluding non-eligible ones. Bibliographic references of recovered articles were examined to look for additional studies. We excluded animal studies, cross-sectional or retrospective case–control studies, and short-term (< 1 month) randomized trials. Nested case– control studies in which data on vitamin D status was collected before outcome assessment were included. We excluded studies on type 1 diabetes because its pathophysiology is different from type 2 diabetes. We excluded studies in children, pregnant women and patients with conditions that affect vitamin D metabolism, such as chronic kidney disease stage 3 or higher or hyperparathyroidism, and trials that used a vitamin D preparation other than D3, ergocalciferol or non-oral routes of vitamin D administration.
Data extraction and quality assessment
Data from each study were extracted by one of the reviewers and confirmed by the other. The extracted data included: study design; participant characteristics; cohort source; longest reported follow-up period; method of assessing vitamin D status or details of vitamin D supplementation; association between vitamin D (status or supplementation) and outcome; potential confounding variables adjusted for, with particular emphasis on age, race, weight and variables related to sun exposure (for example, season and location); method of ascertaining glycemia outcome, and statistical analysis.
Study quality was determined by the primary data extractor and confirmed by at least one other co-author. To inform the assessment of study quality, we considered factors in the Strengthening the Reporting of Observational Studies in Epidemiology statement for observational studies (von Elm et al., 2007), nutrition-specific items from a critical appraisal of micronutrient systematic reviews (Lichtenstein et al., 2008) and the Consolidated Standards of Reporting Trials statement for reporting clinical trials (Moher et al., 2001). We used a three-category grading system for both observational studies and RCTs. Good quality studies adhere most closely to the commonly held concepts of high quality, including clear descriptions of the population and setting; unbiased assessments of vitamin D status and outcomes; appropriate statistical analysis including multivariate analysis adjusting for age, race, weight, and/or sun exposure and intention-to-treat analysis; no obvious reporting omissions or errors; and < 20% dropouts. Fair quality studies have some deficiencies in the above criteria, but these are unlikely to result in major biases. Observational studies that measured vitamin D intake as the key predictor variable were rated fair. Poor quality studies have major deficiencies in design, analyses or reporting; hence, major bias could not be excluded.
Data synthesis and analysis
Data were combined, whenever possible, for meta-analysis. The random effects model was used when at least three studies with a homogeneous predictor and outcome were available (DerSimonian and Laird, 1986). For observational studies, we synthesized relative risks, or hazard or odds ratios comparing the extreme categories of vitamin D status (highest versus lowest, as defined within each study), provided that the categories corresponded to similar levels of vitamin D intake or blood 25(OH)D concentration across studies. We tested between-study heterogeneity with the Q statistic (significant when P < 0.10) and quantified its extent with I2 (0–100%), expressing the fraction of between-study variability attributable to heterogeneity rather than chance (> 50% suggests high heterogeneity; Higgins et al., 2003). RCTs were evaluated based on their characteristics, and meta-analyses were attempted whenever possible to pool the results. All calculations and graphs were obtained using Comprehensive Meta-Analysis version 2.2.050 (Biostat, Englewood, NJ, USA).
Results
Longitudinal observational cohort studies
Our search identified seven manuscripts that reported the association between vitamin D status and risk of developing type 2 diabetes using data from eight different cohort studies (Liu et al., 2005, 2010; Pittas et al., 2006, 2010; Knekt et al., 2008; Kirii et al., 2009; Anderson et al., 2010; Table 1). The studies included 238 423 participants, age range 30–75 years, the majority of whom were white (at least 80%) and who were followed for upward of 22 years for the development of incident type 2 diabetes. All studies assessed the exposure, vitamin D status, only once at baseline. Three studies did so by self-reported total vitamin D intake (Liu et al., 2005; Pittas et al., 2006; Kirii et al., 2009); one study calculated a predicted 25(OH)D score (Liu et al., 2010) and three studies (in four cohorts) measured blood 25(OH)D concentration (Knekt et al., 2008; Anderson et al., 2010; Pittas et al., 2010). Ascertainment of the outcome, type 2 diabetes, was by validated self-report (Liu et al., 2005; Pittas et al., 2006, 2010; Kirii et al., 2009), by a combination of self-report and laboratory measurement (Liu et al., 2010) or by medical record data (Knekt et al., 2008; Anderson et al., 2010). Six studies reported multivariate adjusted results (Pittas et al., 2006, 2010; Knekt et al., 2008; Kirii et al., 2009; Anderson et al., 2010; Liu et al., 2010); one study adjusted only for age (Liu et al., 2005).
Table 1.
Observational longitudinal cohort studies of vitamin D status (plasma or serum 25[OH]D concentration, predicted 25[OH]D concentration or self-reported vitamin D intake) and incident type 2 diabetes
| Study, year (reference), cohort (country) |
Male, % | Mean baseline age (range), years |
White, % | n/N (incidence, %)a | Vitamin D measure; comparisonb |
Mean follow-up, years (start–end) | Results, adjusted RR, OR or HR (95% CI) P-value for trend |
Outcome (ascertainment method) |
Adjustments | Study Qualityc |
|---|---|---|---|---|---|---|---|---|---|---|
| Liu et al., 2005 Women’s Health Study (USA) | 0 | 52 (45–75) | 95 | 805/10 066 (8.0) | Vitamin D intake (total); ≥511 vs ≤159 IU/day | 9 (ND) | 0.73 (0.54, 0.99) P = 0.02d | Type 2 diabetes (validated self-report) | Age | Fair |
| Pittas et al., 2006 Nurses’ Health Study (USA) | 0 | 46 (30–55) | 98 | 4843/83 779 (5.8) | Vitamin D intake (total); >800 vs ≤200 IU/day | 20 (1980–2000) | 0.87 (0.69, 1.09) P = 0.67 | Type 2 diabetes (validated self-report) | Age, BMI, exercise, residence, otherse | Fair |
| Knekt et al., 2008 Finnish Mobile Clinic Health Examination Survey (Finland) | 100 | ND (40–74) | 100 | 105/1628 (6.4); nested case–control study with 206 control participants | 25(OH)D concentration; 30 vs 10 ng/ml (mean) | 22 (1973–1994) | 0.49 (0.15, 1.64) P = 0.06 | Type 2 diabetes (medication treated, registry based) | Age, BMI, exercise, season, othersf | Good |
| 0 | ND (40–74) | 100 | 125/1699 (7.4); nested case–control study with 246 control participants | 25(OH)D concentration; 25 vs 9 ng/ml (mean) | 0.91 (0.37, 2.23) P = 0.66 | Age, BMI, exercise, season, othersf | ||||
| Knekt et al., 2008 Mini-Finland Health Survey (Finland) | 100 | 53 (40–69) | 100 | 83/1948 (4.3); nested case–control study with 245 control participants | 25(OH)D concentration; 31 vs 9 ng/ml (mean) | 17 (1978–1994) | 0.17 (0.05, 0.52) P <0.001 | Type 2 diabetes (medication treated, registry based) | Age, BMI, exercise, season, othersf | Good |
| 0 | ND (40–69) | 100 | 99/2228 (4.4); nested case–control study with 289 control participants | 25(OH)D concentration; 25 vs 8 ng/ml (mean) | 1.45 (0.58, 3.62) P = 0.83 | Age, BMI, exercise, season, othersf | ||||
| Kirii et al., 2009 Japan Public Health Center-based Prospective Study (Japan) | 100 | 57 (40–69) | ND (~ 100% Japanese) | 634/25 877 (2.4) | Vitamin D intake (total); 720 vs 188 IU/day (mean) | 5 (1990–1998) | 0.96 (0.74, 1.23) P = 0.35 | Type 2 diabetes (validated self-report) | Age, BMI, exercise, othersg | Fair |
| 0 | 57 (40–69) | ND (~ 100% Japanese) | 480/33 919 (1.4) | Vitamin D intake (total); 696 vs 192 IU/day (mean) | 5 (1990–1998) | 0.88 (0.67, 1.16) P = 0.67 | Type 2 diabetes (validated self-report) | |||
| Liu et al., 2010 Framingham Offspring Study (USA | 54 | 60 | ~ 100 | 133/2956 (4.4) | Predicted 25(OH)D score; 22 vs 17 ng/ml (median) | 7 (1991–2001) | 0.60 (0.37, 0.97) P = 0.03 | Type 2 diabetes (medication treated, laboratory based) | Age, sex, waist circumferenceh | Fair |
| Pittas et al., 2010 Nurses’ Health Study (USA) | 0 | 46 (30–55) | 98 | 608/32 826 (1.8); nested case–control study with 569 control participants | 25(OH)D concentration; 33 vs 14 ng/ml (median) | 14 (1990–2004) | 0.52 (0.33, 0.83) P = 0.008 | Type 2 diabetes (validated self-report) | Age, BMI, exercise, season, race, othersi | Good |
| Anderson et al., 2010 Intermountain Healthcare system (USA) | 25 | 55 | ND | NR/41 497 (NR) | 25(OH)D concentration; ≤15 vs >30 ng/ml (median) | 1.3 (2000 –2009) | 1.89 (1.54, 2.33) P <0.001 | Diabetes (physician diagnosed based on International Classification of Disease, 9th edition code) | Age, gender, othersj | Poor |
Abbreviations: 25(OH)D, plasma or serum 25-hydroxyvitamin D; BMI, body mass index; HDL, high-density lipoprotein; HR, hazard ratio; IU, international unit; ND, no data; OR, odds ratio; RR, relative risk.
Number of cases if nested case–control study.
Highest/lowest-risk category versus reference category.
Study quality is determined based on a three-category grading system, as described in the text.
Estimated from reported data.
Family history of diabetes, hypertension, calcium intake, smoking, alcohol, coffee and other diet.
Smoking, education and medications.
Family history of diabetes, smoking, diet (alcohol, coffee, magnesium and total energy) and hypertension.
Family history of diabetes, hypertension, low HDL cholesterol, high triglycerides, impaired fasting glucose and diet.
Fasting status, latitude, hypercholesterolemia, hypertension, family history of diabetes, smoking, physical activity, alcohol, multivitamin use and diet.
Hypertension, hyperlipidemia, heart failure, infection, depression and renal failure.
To convert 25(OH)D concentration from ng/ml to nmol/l, multiply by 2.459.
In the Women’s Health Study, an intake of > 511 international units (IU)/day of vitamin D was associated with a 27% lower risk of developing type 2 diabetes compared with an intake of < 159 IU/day (Liu et al., 2005). However, this analysis was limited because it did not adjust for any risk factors for type 2 diabetes other than age. In the Nurses’ Health Study, after multivariate adjustment for age, body mass index (BMI) and non-dietary covariates, women who reported consumption of > 800 IU/day of vitamin D had a 23% lower risk of developing incident type 2 diabetes, by validated self-report, compared with women who reported consumption of < 200 IU/day (Pittas et al., 2006). The association, however, was attenuated and became non-significant after adjusting for dietary factors (Table 1). The dietary variables responsible for attenuation of the results were magnesium and calcium, which share dietary sources with vitamin D. In the same study, women who reported the highest calcium (> 1200 mg/day) and vitamin D (> 800 IU/day) intakes (1.3% of the cohort) had a statistically significant 33% lower risk of type 2 diabetes compared with women with the lowest calcium (< 600 mg/day) and vitamin D (< 400 IU/day) intakes. In a Japanese cohort of men and women aged 40–70 years, over a 5-year follow-up period, there was no statistically significant association between higher (~ 700 IU/day) versus lower (~ 200 IU/day) self-reported intake of vitamin D intake, or higher (~ 600 to 800 mg/day) versus lower (~ 250 to 350 mg/day), calcium intake and incident type 2 diabetes (Kirii et al., 2009). However, men and women who self-reported higher consumption of both vitamin D (> 400 IU/day) and calcium (~ 600 to 800 mg/day) had an ~ 40% lower risk of type 2 diabetes. In the Framingham Offspring Study (men and women), investigators used a subsample of the cohort to develop a regression model to predict blood 25(OH)D concentrations from age, sex, BMI, month of blood sampling, total self-reported vitamin D intake, smoking status and total energy intake (Liu et al., 2010). Using this model, a predicted 25(OH)D score was calculated for each non-diabetic participant. The association between the predicted 25(OH)D score and incidence of type 2 diabetes, defined as the use of diabetes medication or laboratory values diagnostic for diabetes, was assessed during a mean average follow-up of 6 years. Compared with participants in the lowest tertile of the predicted 25(OH)D score, those in the highest tertile had a 40% lower risk of type 2 diabetes after multivariate adjustment.
There are three longitudinal observational studies that have reported the association between measured blood 25(OH)D concentration and incident type 2 diabetes (Knekt et al., 2008; Anderson et al., 2010; Pittas et al., 2010); two were case–control studies nested within prospective cohorts (Knekt et al., 2008; Pittas et al., 2010) and one study used electronic medical records data captured during routine medical care (Anderson et al., 2010). In the nested case–control studies, after a specified period of follow-up, blood samples from participants who developed type 2 diabetes and matched non-diabetic controls were retrieved from stored samples, and blood 25(OH)D was measured at a time when all participants were free from type 2 diabetes and blood concentrations of 25(OH)D concentration were compared between cases and controls. The study by Knekt et al. (2008), which pooled data from two cohorts in Finland (total of 7503 available participants) included 412 cases who developed type 2 diabetes during the follow-up period and 986 matched controls (partial results were published in an earlier manuscript by Mattila et al. (2007)). After multivariate adjustment including BMI, participants who were in the highest quartile of serum 25(OH)D levels at baseline (mean 25[OH]D 27.6 ng/ml), compared with those in the lowest quartile (mean 25[OH]D 8.9 ng/ml), had a 40% lower risk of developing incident type 2 diabetes. Men in the highest quartile (mean 25[OH]D 30 ng/ml) had a 72% lower risk of developing type 2 diabetes compared with men in the lowest quartile (mean 25[OH]D 10 ng/ml); there was no significant association among women. The second nested case–control study was conducted using data from 608 women with newly diagnosed type 2 diabetes and 559 controls who were enrolled in the Nurses’ Health Study (Pittas et al., 2010). After adjusting for matching variables and risk factors for diabetes including BMI, higher levels of plasma 25(OH)D were associated with a lower risk for type 2 diabetes. The odds ratio for incident type 2 diabetes in the top (median 25[OH]D 33.4 ng/ml) versus the bottom (median 25[OH]D 14.4 ng/ml) quartile was 0.52 (95% confidence interval (CI) 0.33–0.83). The association was consistent across subgroups of baseline BMI, age and calcium intake, although the association appeared to be stronger among overweight/obese versus normal weight women (odds ratio 0.46 versus 0.63, respectively). In this study, spline regression models showed no apparent threshold and no deviation from linearity for the relation between 25(OH)D and risk of incident type 2 diabetes, although the shape of the figure suggested a stronger decrease in risk within the higher range of plasma 25(OH)D concentrations above 30–35 ng/ml. The apparently inconsistent results among women in the Finnish and American cohorts may be explained by the higher baseline 25(OH)D levels in women in the Nurses’ Health Study compared with the Finnish cohort (23 versus 15 ng/ml, respectively), which suggests that a potential threshold for 25(OH)D levels exists, above which the risk of type 2 diabetes declines. Anderson et al. (2010), using information from a large electronic database that contained 41 504 patient records with at least one measured 25(OH)D level, determined the relation between vitamin D levels and incident cardiometabolic diseases. After multivariate adjustment for various risk factors, compared with patients with 25(OH)D > 30 ng/ml, those with 25(OHD) < 15 ng/ml had an 89% increase in the risk of developing type 2 diabetes.
Another longitudinal observational study has also reported inverse associations between baseline 25(OH)D concentration and future glycemia and insulin resistance, but it did not report results on incident diabetes (Forouhi et al., 2008).
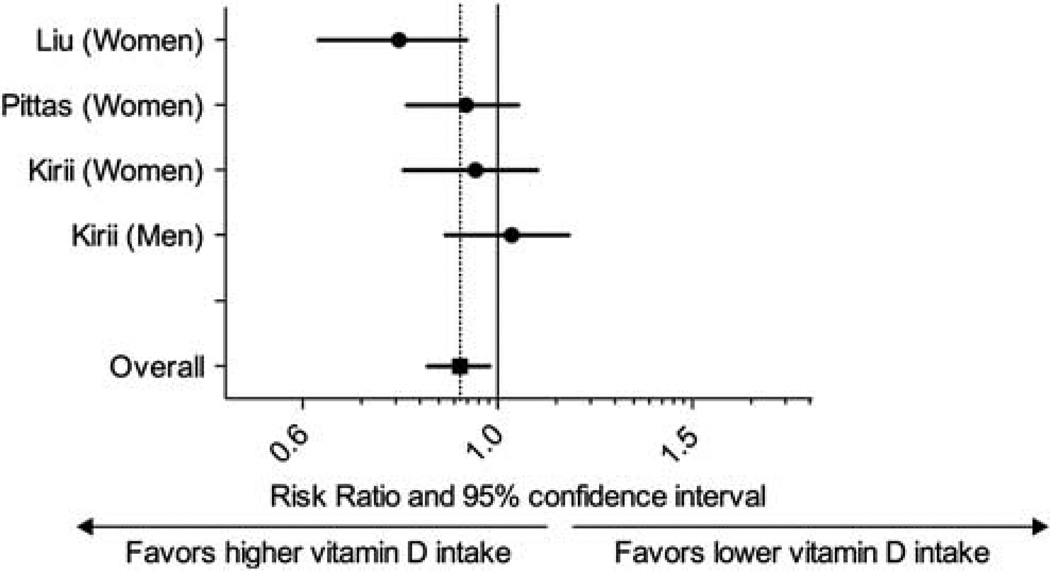
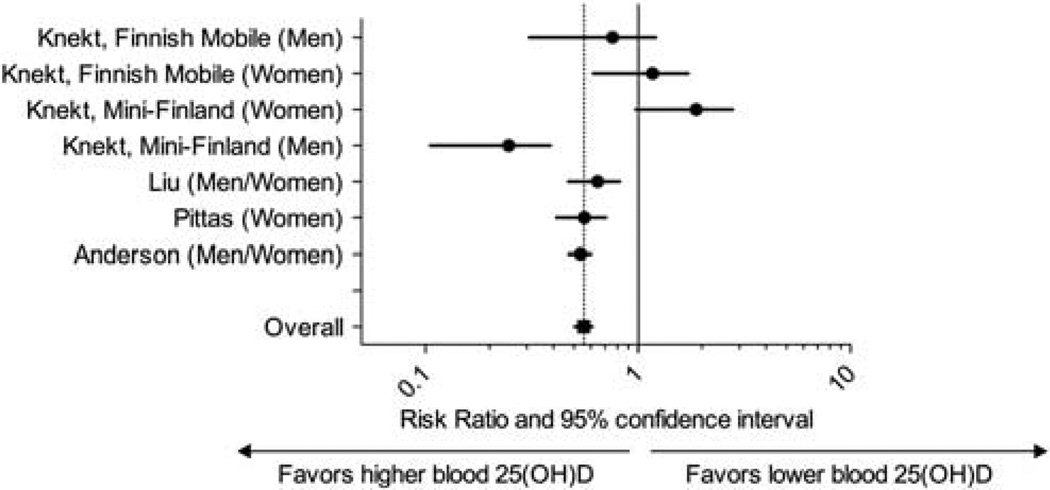
After combining the data from three studies that assessed vitamin D status by vitamin D intake, there was a statistically significant association between vitamin D intake > 500 IU/day with the development of newly diagnosed type 2 diabetes (relative risk 0.87, 95% CI, 0.76–0.99) versus vitamin D intake < 200 IU/day (Figure 1). There was no heterogeneity among studies (Q = 1.87, P = 0.6, I2 = 0%). After combining the data from four studies (five cohorts) that measured 25(OH)D as the predictor, participants with 25(OH)D > 25 ng/ml had a 43% lower risk of developing type 2 diabetes (95% CI, 24%, 57%) when compared with individuals with 25(OH)D < 14 ng/ml (Figure 2), with only small degree of heterogeneity (Q = 9.7, P = 0.14, I2 = 38%).
Figure 1.
Meta-analysis of the association between self-reported vitamin D intake and incident type 2 diabetes.
Figure 2.
Meta-analysis of the association between baseline blood 25(OH)D concentration and incident type 2 diabetes.
Randomized trials of vitamin D and glycemic outcomes
The search identified 11 RCTs, which have reported the effects of vitamin D (ergocalciferol or D3) supplementation (with or without calcium) on measures of glycemia (Nilas and Christiansen, 1984; Hsia et al., 2007; Pittas et al., 2007a; Sugden et al., 2008; de Boer et al., 2008; Avenell et al., 2009; Nagpal et al., 2009; Zittermann et al., 2009; Witham et al., 2010; von Hurst et al., 2010; Jorde et al., 2010a) or incident diabetes by self-report (de Boer et al., 2008; Avenell et al., 2009; Table 2). Eight trials have included participants with normal glucose tolerance (Nilas and Christiansen, 1984; Hsia et al., 2007; Pittas et al., 2007a; de Boer et al., 2008; Avenell et al., 2009; Zittermann et al., 2009; von Hurst et al., 2010; Jorde et al., 2010a), and three trials included participants with stable type 2 diabetes (Sugden et al., 2008; Jorde and Figenschau, 2009; Witham et al., 2010). Study duration varied from 6 weeks to 9 years. Doses of vitamin D ranged from 400 to 8571 IU/day; however, several trials provided supplementation with large infrequent doses of vitamin D (Sugden et al., 2008; Nagpal et al., 2009; Witham et al., 2010; Jorde et al., 2010a, b). In three studies, vitamin D3 was combined with calcium (Pittas et al., 2007a; de Boer et al., 2008; Jorde et al., 2010a). Only the Women’s Health Initiative and the Randomized Evaluation of Calcium and/OR vitamin D Trials were considered good quality studies (Hsia et al., 2007; de Boer et al., 2008; Avenell et al., 2009). The trials were too heterogeneous to perform a meta-analysis.
Table 2.
Randomized controlled trials of the effect of vitamin D (cholecalciferol or ergocalciferol) supplementation (with or without calcium) on glucose tolerance
| Study, first author, year (country) |
Men, % | Mean baseline age, years (range) |
BMI, kg/m2 |
Participants | Mean baseline 25(OH)D concentration and calcium intake |
Interventions (number of participants) |
Study duration |
Effect of vitamin D vs placebo (P-value) |
Study quality (justification) |
|---|---|---|---|---|---|---|---|---|---|
| Nilas and Christiansen, 1984 (Denmark) | 0 | ND (45–54) | ND | Postmenopausal; healthy; n = 151 | ND | D3 2000 IU/day (n = 25) vs 1(OH)2D3 0.25 mcg/day (n = 23) vs placebo (n = 103). All received calcium, 500mg/day | 2 years | ↔FPG change, 2.16 vs −5.94 vs 2.34 mg/dl P = 0.97) | Fair (post hoc analyses) |
| Pittas et al., 2007a (USA) | 38 | 71 (≥ 65) | 27 | Normal fasting glucose; n = 222 | 30 ng/ml calcium intake, 750mg/day | D3 700 IU/day plus calcium citrate 500 mg/day (n = 108) vs placebo (n = 114) | 3 years | ↔FPG change, 2.70 vs 2.16 mg/dl (P = 0.55) ↔IRHOMA | Fair (post hoc analysis) |
| 52 | 71 (ND) | Impaired fasting glucose; n = 92 | 30 ng/ml calcium intake, 680mg/day | D3 700 IU/day plus calcium citrate 500 mg/day (n = 45) vs placebo (n = 47) | 3 years | ↓FPG change, 0.36 vs 6.13 mg/dl (P = 0.042) ↓IRHOMA | |||
| de Boer et al., 2008 (USA) | 0 | 62 (50–79) | ND | Postmenopausal without diabetes | <32 ng/ml (for 89% of participants) | D3 400 IU/day plus calcium carbonate 1000mg/day (n = 16 999) vs placebo (n = 16 952) | 7 years | ↔Incidence of diabetes (self-reported); HR 1.01 (0.94–1.10; P = 0.95) ↔Insulin secretion ↔IRHOMA | Fair (post hoc analysis) |
| 0 | ND (50–79) | Normal fasting glucose; n = 1637 | D3 400 IU/day plus calcium carbonate 1000mg/day (n = 866) vs placebo (n = 771) | 6 years | ↔FPG change, 3.80 vs 4.61 mg/dl (P = 0.32) | ||||
| 0 | ND (50–79) | Impaired fasting glucose; n = 1457 | D3 400 IU/day plus calcium carbonate 1000mg/day (n = 718) vs placebo (n = 739) | 6 years | ↔FPG change, 3.93 vs 4.69 mg/dl (P = 0.79) | ||||
| Sugden et al., 2008 (UK) | 53 | 64 (ND) | 31 | Stable type 2 diabetes; 25(OH)D < 20 ng/ml; n = 34 | 15 ng/ml | D2 100 000 IU once (equivalent to 1785 IU/day; n = 17) vs placebo (n = 17) | 8 weeks | ↔Hemoglobin A1c change, 0.01% vs −0.05% (P = 0.74) ↔IRHOMA change, −39.7 vs −25.6 (P = 0.72) IRHOMA significantly improved if 25OHD rise> 5 ng/ml | Fair (completers only analysis; 20% dropout) |
| Avenell et al., 2009 (UK) | 15 | 77 (≥ 70) | ND | History of fracture | ND | D3 800 IU/day (n = 2649) vs placebo (n = 2643; 2 × 2 factorial design with calcium carbonate 1000mg/day) | 2–5 years | ↔Incidence of diabetes (self-reported), intention-to-treat HR 1.11 (0.77–1.62; P = 0.57) ↔Incidence of diabetes (self-reported), compliant HR 0.68 (0.40–1.16; P = 0.16) | Good (self-report; no data on baseline glucose control) |
| Nagpal et al., 2009 (India) | 100 | 43 (> 35) | 26 | Healthy; central obesity; n = 71 | 15 ng/ml | D3 120 000 IU three times (equivalent to 8571 IU/day; n = 35) vs placebo (n = 36) | 6 weeks | ↑OGIS (change from baseline, ml/min*kg) 21.17 vs −8.89 (P = 0.055) ↔IRHOMA change from baseline, 0.14 vs 0.16 (P = 0.95) ↔QUICKI 0 vs 0 (P = 0.9) | Fair (25% dropout; no power; no primary outcome) |
| Zittermann et al., 2009 (Germany) | 33 | 48 (18–70) | 33 | Healthy; BMI > 27 kg/m2; n = 200 | 12 ng/ml | D3 3332 IU/day (n = 82) vs placebo (n = 83). All received weight reduction advice for 24 weeks | 1 year | ↔Hemoglobin A1c change, −0.25% vs −0.25% (P = 0.96) ↔FPG change, −3.8 vs −4.9 mg/dl (P = 0.39) | Fair (20% dropout) |
| Jorde and Figenschau, 2009 (Norway) | 50 | 56 (21–75) | Stable type 2 diabetes; n = 32 | 24 ng/ml | D3 40 000 IU/week (equivalent to 5714 IU/day; n = 16) vs placebo (n = 16) | 26 weeks | ↔Hemoglobin A1c change, 0.2% vs −0.2% (P = 0.90) ↔FPG change, −3.6 vs 7.2 mg/dl (P = 0.43) ↔IRHOMA 0.3 vs –0.2 (P = 0.58) | Fair (insulin allowed) | |
| von Hurst et al., 2010 (New Zealand) | 0 | 42 (23–68) | 27.5 | Insulin resistance (IRHOMA > 1.93 and TG/HDL≥ 3); no diabetes; 25(OH)D < 20 ng/ml (50 nmol/l); n = 81 | 8 ng/ml | D3 4000 IU/day (n = 42) vs placebo (n = 39) | 26 weeks | ↔FPG change, 1.8 vs 1.8 mg/dl (P = 0.82) ↓IRHOMA change from baseline, −0.2 vs 0.2 (P = 0.02; highest effect seen when final 25OHD> 36 ng/ml ↓Insulin ↔HOMA 2% B; C-peptide | Fair (23% dropout) |
| Jorde et al., 2010a (Norway) | 36 | 38 (21–70) | 35 | Overweight/obese; no diabetes; n = 438 | 23 ng/ml | D3 40 000 IU/week (equivalent to 5714 IU/day; n = 150) vs D3 20 000 IU/week (equivalent to 2857 IU/day; n = 139) vs placebo (n = 149). All received calcium, 500 mg/day | 1 year | ↔Hemoglobin A1c change, 0.09% vs 0.11% vs 0.09% (P = NS) ↔FPG change, 0.02 vs 0.08 vs 0.08mmol/l (P = NS) ↔2hPG change, 2.7 vs 6.5 vs −0.4mg/day (P = NS) ↔IRHOMA, 0.23 vs –0.05 vs 0.36 (P = NS) ↔QUICKI | Fair (post hoc analyses; 25% dropout; completes only analysis; no power calculations) |
| Witham et al., 2010 (UK) | ND | 65 (> 18) | 31 | Type 2 diabetes; 25(OH)D <40 ng/ml (100 nmol/l); n = 61 | 18 ng/ml | D3 100 000 IU orally once (equivalent to 892 IU/day; n = 19) vs D3 200 000 IU orally once (equivalent to 1785 IU/day; n = 20) vs placebo (n = 22) | 16 weeks | ↔Hemoglobin A1c change, ‘no change’ (data NR) ↔FPG change, ‘no change’ (data NR) ↔IRHOMA change, ‘no change’ (data NR) | Fair (underpowered) |
Abbreviations: 2hPG, plasma glucose 2 h after 75 g glucose load; 25(OH)D, plasma or serum 25-hydroxyvitamin D; BMI, body mass index; D2, ergocalciferol; D3, cholecalciferol; FPG, fasting plasma glucose; HDL, high-density lipoprotein; HR, hazard ratio; IRHOMA, insulin resistance by homeostasis model assessment; IU, international unit; ND, no data; NS, not significant; OGIS, oral glucose insulin sensitivity; TG, triglyceride; QUICKI, quantitative insulin-sensitivity check index.
↓, decreased (statistically significant);↑, increased (statistically significant);↔, no difference (no statistical significance).
To convert 25(OH)D concentration from ng/ml to nmol/l, multiply by 2.459 and to convert FPG form mg/dl to mmol/l, multiply by 0.0555
Trials in participants with normal glucose tolerance
There was no effect of vitamin D supplementation on measures of glycemia including fasting plasma glucose or hemoglobin A1c and insulin resistance measured by homeostatic model assessment (HOMA) in participants with normal glucose tolerance at baseline in seven trials (Nilas and Christiansen, 1984; Hsia et al., 2007; Pittas et al., 2007a; de Boer et al., 2008; Avenell et al., 2009; Zittermann et al., 2009; von Hurst et al., 2010; Jorde et al., 2010a). Similarly, vitamin D supplementation had no effect on incident type 2 diabetes in individuals with normal glucose tolerance (de Boer et al., 2008; Avenell et al., 2009). However, five of the studies were designed for non-glycemic outcomes, and the analyses on vitamin D and type 2 diabetes were post hoc (Nilas and Christiansen, 1984; Pittas et al., 2007a; de Boer et al., 2008; Avenell et al., 2009; Jorde et al., 2010a). Furthermore, all trials with the exception of the Women’s Health Initiative Trial (de Boer et al., 2008) and the Randomized Evaluation of Calcium and/OR vitamin D Trial (Avenell et al., 2009) were underpowered for glycemic outcomes. In several trials, including the two largest ones (de Boer et al., 2008; Avenell et al., 2009), adherence with supplementation was suboptimal, which may have limited drawing any conclusions. For example, in a post hoc analysis of the Randomized Evaluation of Calcium and/OR vitamin D Study, a community-based effectiveness trial designed for bone outcomes (Avenell et al., 2009), supplementation with 800 IU/day of vitamin D3 (given in a 2 × 2 factorial design with calcium carbonate) did not change the risk of self-reported type 2 diabetes; however, among study participants who were highly compliant with supplementation, there was a notable trend toward reduction in type 2 diabetes risk with vitamin D3 (odds ratio 0.68; 95% CI 0.40–1.16).
Trials in participants with type 2 diabetes
There were three small underpowered RCTs in which participants with established type 2 diabetes were randomized to high infrequent doses of ergocalciferol (100 000 IU given once; n = 34; Sugden et al., 2008) or vitamin D3 (40 000 IU/week for 26 weeks; n = 36; Jorde and Figenschau, 2009 or 100 000 or 200 000 IU given once; n = 61; Witham et al., 2010; Table 2). In these trials, there was no change in hemoglobin A1c; (Sugden et al., 2008; Jorde and Figenschau, 2009;Witham et al., 2010) fasting plasma glucose; (Jorde and Figenschau, 2009; Witham et al., 2010) or insulin resistance (Jorde and Figenschau, 2009; Witham et al., 2010) with vitamin D supplementation after a follow-up period of 8–26 weeks.
Insulin resistance
Among five trials in participants with normal glucose tolerance that reported insulin resistance as an outcome, four studies showed no effect of vitamin D supplementation (Pittas et al., 2007a; de Boer et al., 2008; Nagpal et al., 2009; Jorde et al., 2010a). In contrast, the study by Von Hurst et al. randomized non-diabetic insulin-resistant vitamin D-deficient (25(OH)D < 20 mg/dl) South Asian women, 23- to 68-years old, to 4000 IU/day of vitamin D3 (n = 42) or placebo (n = 39) for 6 months. Significant improvement in insulin resistance, assessed by homeostasis model assessment (IRHOMA), was seen with vitamin D supplementation compared with placebo, which was more evident when the end point blood 25(OH)D concentration reached 32 mg/dl. Similarly, in a post hoc analysis of a trial designed to assess bone-related outcomes, the combined daily supplementation with 700 IU of vitamin D3 and 500mg of calcium as carbonate improved IRHOMA, only among those with glucose intolerance at baseline but had no effect among those with normal glucose tolerance (Pittas et al., 2007a).
Combined vitamin D and calcium
Two trials have reported the effect of combined vitamin D3 and calcium supplementation versus placebo on type 2 diabetes risk; both analyses were post hoc. In the Women’s Health Initiative Trial, vitamin D3 (400 IU/day) and calcium supplementation (1000 mg/day) failed to reduce the risk of developing self-reported type 2 diabetes over a 7-year period and there was also no significant effect of treatment on simple indices of insulin resistance (de Boer et al., 2008). The Women’s Health Initiative Trial used a low dose of vitamin D and allowed all participants to take vitamin D supplements on their own during the trial. Although the effect of supplementation on 25(OH)D concentration was not reported, based on dose and compliance, it has been estimated to be only 2 ng/ml (Lappe et al., 2007), which is a very unlikely increment to be associated with any change in risk of cardiometabolic disease, based on data from observational studies. In the trial by Pittas et al. (2007a), combined supplementation with vitamin D3 and calcium carbonate did not have an effect on fasting plasma glucose levels. However, there was an interaction between baseline glycemia and supplementation on changes in glycemia. In a subgroup analysis, among participants with impaired fasting glucose at baseline, combined vitamin D3 and calcium carbonate supplementation attenuated the increase in fasting glycemia that occurs over time in this population, but it had no effect on glycemia among subjects with normal glucose tolerance at baseline (Pittas et al., 2007a), suggesting that vitamin D may benefit only individuals at high risk for type 2 diabetes.
Discussion
Overall, observational longitudinal studies have shown an inverse association between the vitamin D status (25(OH)D or self-reported vitamin D intake) and the development of type 2 diabetes. In RCTs, vitamin D supplementation did not show any beneficial effects on glycemic measures among persons with normal glucose tolerance but there were beneficial effects among patients with glucose intolerance or insulin resistance at baseline.
From a biological perspective, the presence of the vitamin D receptor in many cell types and organs, and the local production of 1, 25(OH)2D in several extrarenal organs, including β-pancreatic cells, supports potential broad-ranging effects of vitamin D outside of skeletal health, including type 2 diabetes. Vitamin D is thought to have both direct (by the activation of the vitamin D receptor) and indirect (by the regulation of calcium homeostasis) effects on various mechanisms related to the pathophysiology of type 2 diabetes, including impaired pancreatic-β cell function and insulin resistance (Pittas et al., 2007b).
Several possible reasons may account for the attenuation of the association between vitamin D and type 2 diabetes seen in observational studies and published RCTs. The inverse association between vitamin D status and glycemic outcomes may be confounded by a variety of factors. Vitamin D status is an excellent marker of ‘good’ health, including positive associations with young age, normal body weight and a healthy lifestyle (Prentice et al., 2008). A lower vitamin D status may be a reflection of chronic non-specific illness; therefore, the inverse association seen in observational studies, especially cross-sectional studies, may be due to reverse causation. Moreover, additional components in foods rich in vitamin D may have direct effects on glycemic outcomes or, alternatively, foods rich in vitamin D may replace other foods that increase risk of type 2 diabetes. Observational studies have also used single measurement of blood 25(OH)D concentration to define vitamin D status, which may not reflect vitamin D status over long periods, as risk factors for vitamin D deficiency increase with time (for example, aging, declining physical activity, changes in dietary habits, change in body weight or appearance of comorbidities). Therefore, inaccurate assessment of the exposure, especially when vitamin D status is assessed by vitamin D intake (Holden and Lemar, 2008), and uncontrolled or residual confounding may explain the results of the observational studies. Finally, the ascertainment of the outcome variable in observational studies is often done based on self-report or existing medical records, which may introduce additional unintended bias. Analyses within longitudinal observational studies in which the exposure is quantified by repeated measurements of 25(OH)D over time to accurately estimate vitamin D status over long periods and the outcomes are objectively assessed by use of laboratory measures will improve the validity of observational studies.
RCTs are less likely to be biased and/or affected by confounding, and therefore are considered more definitive type of studies. In published trials, there does not appear to be an effect of vitamin D supplementation on glycemia among populations with normal glucose tolerance at baseline; however, firm conclusions cannot be drawn because most studies were either small or post hoc analyses of completed trials. For example, theWomen’s Health Initiative Trial, which is the largest trial on vitamin D and calcium supplementation to date, reported no statistically significant effect on type 2 diabetes outcome. Potential explanations for the null finding of this trial include the low dose of vitamin D, which is also suggested by the lack of effect of supplementation on fractures, difficulties with medications adherence as < 60% of participants were compliant by the end of the trial and the fact that the participants in both groups were allowed to take supplements in addition to the study medication. The study by Pittas et al. (2007a) showed a beneficial effect of vitamin D and calcium supplementation on fasting blood glucose and insulin resistance only in the group that had impaired fasting glucose at baseline. In this study, the effect size of combined vitamin D and calcium supplementation on fasting glycemia in the high-risk subgroup was similar to the effect size seen in the Diabetes Prevention Program Trial with intensive lifestyle or metformin therapy (Knowler et al., 2002). However, in a similar subgroup analysis in the Women’s Health Initiative Trial and in a smaller 1-year trial with weekly high-dose vitamin D supplementation, there was no benefit of supplementation with vitamin D3 among those with glucose intolerance (de Boer et al., 2008; Jorde et al., 2010a). In the trial by Avenell et al. (2009), a community-based effectiveness trial designed for bone outcomes, supplementation with vitamin did not change the risk of self-reported type 2 diabetes; however, among study participants who were highly compliant with supplementation, there was a notable trend toward reduction in type 2 diabetes risk with vitamin D, which highlights the importance of efficacy versus effectiveness in community-based trials. In efficacy trials, internal validity (for example, did participants follow the intervention? Is there any proof that they did so? Is outcome measured in an objective way?) is emphasized and these trials are better at evaluating causality. In effectiveness trials, external validity (for example, how does the intervention do in ‘real-life’ situations?) is emphasized and these studies are better at informing policy changes. Additionally, several trials provided supplementation using large infrequent doses of vitamin D (Sugden et al., 2008; Nagpal et al., 2009; Witham et al., 2010; Jorde et al., 2010a, b), a commonly used clinical approach, the benefit of which has been questioned lately in the setting of studies showing increased risk of adverse outcomes (Dawson-Hughes and Harris, 2010; Sanders et al., 2010). Finally, any trials with vitamin D may be difficult to interpret because (1) ultraviolet radiation contributes to serum 25(OH)D levels, (2) the level of oral intake of vitamin D is poorly assessed and varies between participants and (3) many factors such as genetics, BMI and diet have a role in the response to vitamin D intake and (4) participants start with a wide range in vitamin D levels at baseline.
Similarly, no conclusions can be drawn from the three trials that have included patients with type 2 diabetes because of several limitations: (1) all studies were underpowered (only 16–20 participants per arm); (2) only one study (Jorde and Figenschau, 2009) was designed for glycemic outcomes; however, it recruited only about half of the planned cohort; (3) only one study (Sugden et al., 2008) reported baseline use of diabetes medications; however, in that study metformin use rate was much higher in the vitamin D arm; (4) changes in diabetes medications during the study duration were not reported and were not taken into account in the analyses; and (5) all three trials used large infrequent doses of vitamin D.
In conclusion, there is a biological plausibility of an important role of vitamin D in type 2 diabetes, and lower vitamin D status and intake are associated with higher risk of incident type 2 diabetes in observational studies; however, the effect of vitamin D supplementation on glycemic outcomes was not evident in small underpowered trials or post hoc analyses of larger trials. Overall, the available data are currently insufficient to support the contention that type 2 diabetes can be improved by raising 25(OH)D concentration. Confirmation of a potential beneficial effect of vitamin D on type 2 diabetes is needed in large trials conducted in well-defined populations (for example, pre-diabetes, early type 2 diabetes and whites versus non-whites) specifically designed to test the hypothesis that vitamin D status is a direct contributor to type 2 diabetes pathogenesis. Such an intervention, if proven effective, could have substantial public health implications.
Acknowledgements
This study was supported by NIH research grants R01DK76092 and R01DK79003 (to AGP; by the National Institute of Diabetes and Digestive and Kidney Disease, the Office Of The Director-National Institutes of Health and the National Institutes of Health Office of Dietary Supplements) and UL1 RR025752 (to Tufts Medical Center; by the National Center for Research Resources) and the Endocrine Fellows Foundation Marilyn Fishman Grant for Diabetes Research grant (to JM). We thank Dr Robert Goldberg for helpful comments and suggestions.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Avenell A, Cook JA, MacLennan GS, McPherson GC. Vitamin D supplementation and type 2 diabetes: a substudy of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438) Age Ageing. 2009;2009;38:606–609. doi: 10.1093/ageing/afp109. [DOI] [PubMed] [Google Scholar]
- Campbell IT, Jarrett RJ, Keen H. Diurnal and seasonal variation in oral glucose tolerance: studies in the Antarctic. Diabetologia. 1975;1975;11:139–145. doi: 10.1007/BF00429838. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS. High-dose vitamin D supplementation: too much of a good thing? Jama. 2010;2010;303:1861–1862. doi: 10.1001/jama.2010.598. [DOI] [PubMed] [Google Scholar]
- de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;2008;31:701–707. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990–2000. Diabetes. 2008;2008;57:2619–2625. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JM, Lemar LE. Assessing vitamin D contents in foods and supplements: challenges and needs. Am J Clin Nutr. 2008;2008;88:551S–553S. doi: 10.1093/ajcn/88.2.551S. [DOI] [PubMed] [Google Scholar]
- Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;2007;115:846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. 2009;2009;48:349–354. doi: 10.1007/s00394-009-0020-3. [DOI] [PubMed] [Google Scholar]
- Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. 2010a;267:462–472. doi: 10.1111/j.1365-2796.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- Jorde R, Sneve M, Torjesen PA, Figenschau Y, Goransson LG, Omdal R. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. 2010b;50:175–180. doi: 10.1016/j.cyto.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Kirii K, Mizoue T, Iso H, Takahashi Y, Kato M, Inoue M, et al. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia. 2009;52:2542–2550. doi: 10.1007/s00125-009-1554-x. [DOI] [PubMed] [Google Scholar]
- Knekt P, Laaksonen M, Mattila C, Harkanen T, Marniemi J, Heliovaara M, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–671. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr. 2008;138:2297–2306. doi: 10.3945/jn.108.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Meigs JB, Pittas AG, Economos CD, McKeown NM, Booth SL, et al. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am J Clin Nutr. 2010;91:1627–1633. doi: 10.3945/ajcn.2009.28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin, D and the prevalence of metabolic syndrome in middle-aged and older US women. Diabetes Care. 2005;28:2926–2932. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- Mattila C, Knekt P, Mannisto S, Rissanen H, Laaksonen MA, Montonen J, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–2570. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Jama. 2001;285:1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: US. Diabetes Care. 2006;2006;29:2114–2116. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- Nilas L, Christiansen C. Treatment with vitamin D or its analogues does not change body weight or blood glucose level in postmenopausal women. Int J Obes. 1984;8:407–411. [PubMed] [Google Scholar]
- Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007a;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007b;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33:2021–2023. doi: 10.2337/dc10-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88:500S–506S. doi: 10.1093/ajcn/88.2.500S. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. Jama. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vanden-broucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53:2112–2119. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–1327. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]