Abstract
The extent to which postnatal methylmercury exposure contributes to neurobehavioral delays is uncertain. Confounding may occur because the child's dietary exposure likely correlates with the mother's. This conundrum was examined in the Faroese birth cohort 1 born in 1986–1987. Exposure parameters included mercury concentrations in maternal hair at parturition, cord blood, and child blood and hair at the age-7 clinical examination (N = 923). In regression analyses, the child's current blood-mercury at age 7 (N = 694) showed only weak associations with the neuropsychological test variables, but visuospatial memory revealed a significant negative association. Mutual adjustment caused decreases of the apparent effect of the prenatal exposure. However, such adjustment may lead to underestimations due to the presence of correlated, error-prone exposure variables. In structural equation models, all methylmercury exposure parameters were instead entered into a latent exposure variable that reflected the total methylmercury load. This latent exposure showed significant associations with neurodevelopmental deficits, with prenatal exposure providing the main information. However, postnatal methylmercury exposure appeared to contribute to neurotoxic effects, in particular in regard to visuospatial processing and memory. Thus, addition in the regression analysis of exposure information obtained at a different point in time was not informative and should be avoided. Further studies with better information on exposure profiles are needed to characterize the effects of postnatal methylmercury exposure.
Keywords: Methylmercury compounds, Neuropsychological tests, Postnatal development, Prenatal exposure delayed effects, Preschool child
1. Introduction
While methylmercury is a proven developmental neurotoxicant, most of the evidence regards prenatal exposures (Grandjean et al., 2010; Karagas et al., 2012). From documented poisoning episodes, neurotoxicity is well described in children who have consumed contaminated fish (Harada, 1995; Takeuchi and Eto, 1999). Thus, adverse effects on brain development should be considered a risk associated with postnatal exposures as well. However, due to the low methylmercury concentration in human milk (Grandjean et al., 1995), any such effects would likely occur only after a weaned child has started consuming seafood. Several studies document that exposures at preschool or early school age are much lower than those associated with the mother's seafood intake during pregnancy (Debes et al., 2006; Karagas et al., 2012). Thus, the challenge is to separate possible postnatal neurotoxicity from adverse effects due to much higher prenatal exposures. As only recent exposure is reflected by mercury concentrations in hair and blood, studies of postnatal mercury exposures in young children have produced mixed results (Karagas et al., 2012; Myers et al., 2009).
Most published studies of school-age children include mercury biomarkers that indicate current exposure levels, rather than long-term exposures. In addition, confounding may occur due to prenatal methylmercury exposure and benefits from fish consumption. For example, 72 Spanish children were assessed by the McCarthy Scales of Children's Abilities; those with a hair-mercury concentration of 1 µg/g (corresponding to the US EPA Reference Dose) showed lower scores on general cognitive, memory, and verbal abilities (Freire et al., 2010). Of note, each child's fish consumption, as reflected by their hair mercury, rather than prenatal fish consumption by their mothers, was associated with the deficits. However, the lack of information on fish intake and prenatal exposure makes it difficult to draw conclusions from such evidence. In a study of Canadian Inuit children, neither prenatal nor concurrent mercury exposure was associated with child behavior ratings at 4–6 years of age (Plusquellec et al., 2010).
Using neurophysiological tests of gross and fine motor skill, current, but not prenatal, mercury was associated with increased action tremor amplitude at 4–6 years of age (Despres et al., 2005). In addition, prenatal mercury exposure was associated with longer latencies in visual evoked potentials (VEPs), whereas concurrent mercury was associated with shorter VEP latencies (Saint-Amour et al., 2006). The most recent follow-up of this population assessed performance on auditory event-related potentials (ERPs) at ages 10–13 years. Cord blood mercury was associated with both adverse and potentially beneficial effects on early auditory information processing, with increased reaction time and increased latency but fewer false alarms (i.e., false-positive errors) and greater amplitude of response on the auditory ERP task (Boucher et al., 2010). Of note, the concurrent blood mercury (median, 2.8 µg/L) was not associated with auditory ERP performance. In the Faroes, delays in transmission of auditory brain signals in 14-year-old birth cohort members were associated with each child's recent mercury exposure from fish in their own diets, not with their prenatal mercury exposures (Murata et al., 2004). Perhaps the neurophysiological outcomes are more robust than neuropsychological test results and thus more likely to reveal effects of postnatal methylmercury exposure.
The concern that postnatal methylmercury exposure might influence neurobehavioral outcomes affected by prenatal exposure has inspired some investigators to include routine adjustment for postnatal exposure in reports on prenatal methylmercury neurotoxicity (Myers et al., 2003). Similarly, when examining the possible effects of postnatal methylmercury exposure from fish consumption, the researchers adjusted for prenatal exposure in their models (Myers et al., 2009). Such adjustment may represent an imperfect solution to a common problem.
Three challenges complicate the identification of neurobehavioral effects from postnatal methylmercury exposure. The exposure during childhood may vary over time, and exposure assessment at any particular point in time may be an imprecise indicator of the postnatal exposure trajectory. Further, postnatal exposures are lower than prenatal levels (Budtz-Jorgensen et al., 2004a; Karagas et al., 2012), and a decreased postnatal susceptibility will make it difficult to identify effects associated with childhood exposures, unless particular outcome variables are more sensitive to exposures during postnatal development. Finally, postnatal exposures are likely to be associated with the prenatal levels, thereby complicating statistical adjustments. Covariates, such as essential nutrient intakes from seafood, may vary as well over time, thereby further complicating attribution of adverse effects to methylmercury exposures at specific ages. Given the substantial data base available on the first Faroese birth cohort (Budtz-Jorgensen et al., 2007; Grandjean et al., 1997, 2012), we have now applied more advanced statistical models to determine the possible significance of postnatal exposures to methylmercury in regard to neurobehavioral performance.
2. Materials and methods
2.1. Cohort establishment and exposure assessment
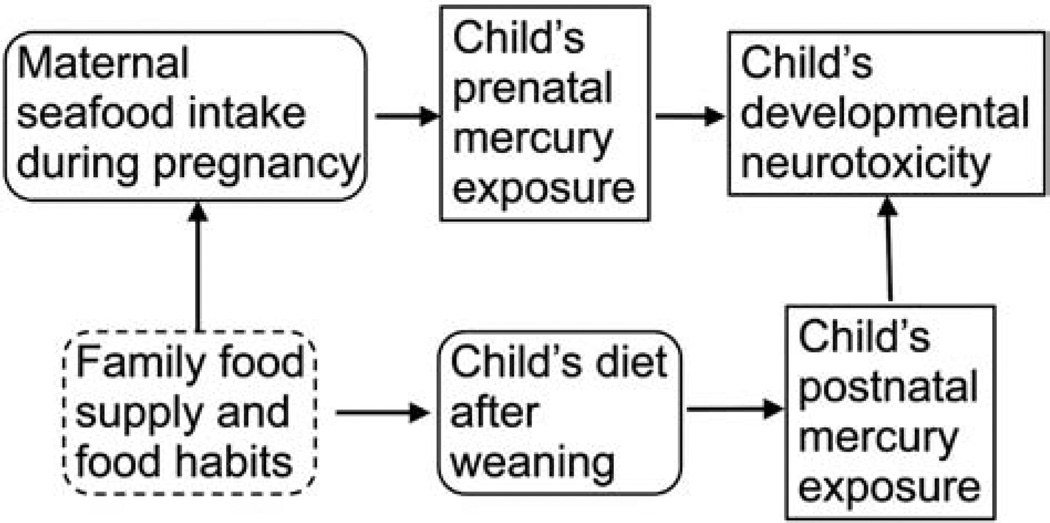
A birth cohort was generated in 1986–1987 at the three hospitals in the Faroe Islands (Grandjean et al., 1992). In connection with each singleton birth, cord blood and maternal hair were collected for subsequent analysis. At age 7 years, 923 (90%) of the cohort members participated in a thorough clinical examination with a focus on nervous system function. Methylmercury exposure at age 7 was based on analysis of the child's hair, which was obtained from almost all children, and blood-mercury concentrations were available for 672 cohort members at this age (Grandjean et al., 1999). The number of blood-mercury results has now been increased to 694 by utilizing an improved analytical method that required a smaller blood volume (Petersen et al., 2008). Quality assurance data suggest that laboratory variance was very small and contributed very little to the total imprecision (Budtz-Jorgensen et al., 2007). Both prenatal and postnatal exposures may be a reflection of household dietary habits and availability of seafood, including pilot whale meat, the main source of methylmercury in this community at the time (Fig. 1) (Weihe et al., 1996).
Fig. 1.
Directional acyclic graph showing the relationship between diet and developmental exposures to methylmercury and the possible impact of household dietary habits and seafood supply.
2.2. Neuropsychological tests
The individual tests have been previously described (Grandjean et al., 1997) and are briefly outlined here. We used the Neurobehavioral Evaluation System (NES2) Finger Tapping Test (Dahl et al., 1996; Letz and Baker, 1988), where the scores were the maximum number of taps with the preferred hand, the non-preferred hand, and both hands. In the NES2 Hand-Eye Coordination Test, the score was the average deviation from the stimulus in the best two trials. The third computer-assisted test, the NES2 Continuous Performance Test (CPT) is a 4-min attention test using a series of animal silhouettes flashed on the computer screen, where the scores were the total number of missed responses and the average reaction time during the last 3 min. Only the supervised CPT data from the first year were used. All of these outcomes were considered motor tests.
In the Bender Visual Motor Gestalt Test (Schlange et al., 1977), a visuospatial test, we scored the errors in the copying condition using the Göttingen system, while, in the recall condition, we summed the number of recognizable figures.
Among the verbally mediated tests, we used three Wechsler Intelligence Scale for Children—Revised (WISC-R) subtests (Wechsler, 1974), i.e., the Digit Spans number of correct trials in the forward condition, the Similarities raw scores, and the Block Designs scored according to WISC-R criteria based on correct design with bonus points for quick performance. We also used a Faroese translation of the California Verbal Learning Test (Children) (Delis et al., 1994), where we scored the total number of correct responses during five learning trials, the spontaneous recall after an interference list (short recall), and the spontaneous recall of the initial 20 min later (long delay) and the number correctly recognized. In the Boston Naming Test (Kaplan et al., 1983), we scored the number of objects correctly named, both spontaneously and after semantic and phonemic cueing.
2.3. Statistical analysis
We used data from subjects with complete prenatal and postnatal exposure information only. In addition to age and sex as obligatory covariates for all outcomes, we also included adjustment for the maternal score on Raven's Progressive Matrices (Raven, 1958), medical risk for neurobehavioral deficit, maternal and paternal education level, paternal employment, and day care (Grandjean et al., 1997, 2012). The computer-assisted tests were further adjusted for the child's acquaintance with computers and computer games. Because the Similarities test was administered by two different examiners, adjustment for examiner was included. While seafood nutrients may cause negative or reverse confounding of methylmercury neurotoxicity (Choi et al., 2014), we have access only to the number of fish dinners per month and did not attempt to include this parameter in the analysis (Budtz-Jorgensen et al., 2007).
Two neuropsychological outcomes were transformed for the residuals to approach a Gaussian distribution of the residuals, i.e., the logarithmic transformation of the number of missed responses on the CPT, and the square root of the WISC Block Designs score (Grandjean et al., 1997). As all exposure biomarkers were log transformed, associations were expressed as change in outcome associated with a 10-fold increase in exposure. Two-sided p-values were calculated throughout. Although calculations were carried out for all outcomes measured, we focus here on the nine major outcomes that showed an adverse effect associated with the prenatal methylmercury exposure (Grandjean et al., 1997).
Each of the neuropsychological outcome variables was considered a dependent variable in multiple regression analyses, where the postnatal mercury exposure indicator and the covariates were considered independent variables. To complement these analyses and limit the problems associated with missing data and multiple comparisons, we also used structural equation models (Bollen, 1989; Budtz-Jorgensen et al., 2002) to determine the joint effects of the postnatal exposures. In this factor analysis approach, the independent exposure variables are considered as reflections of the latent variable representing the true postnatal methylmercury exposure. We have previously used this methodology (Budtz-Jorgensen et al., 2002, 2010; Debes et al., 2006), because information from multiple exposure biomarkers can be included simultaneously, thereby affording a greater statistical power, while avoiding the need for adjustment for multiple comparisons. The goodness of fit was determined by the Root Mean Square Error of Approximation (RMSEA) and a χ2 test comparing the expected and the observed covariance (Kline, 2012). Models were considered to have an excellent fit at p values above 0.05 for the χ2 test and an upper confidence limit for the RMSEA below 0.05.
3. Results
Exposures at age 7 were much lower than those encountered prenatally (Table 1). The two biomarkers of postnatal methylmercury exposure correlated well (r = 0.83 after logarithmic transformation). Among cohort subjects with complete exposure data, the cord-blood mercury concentration, as measure of the prenatal methylmercury exposure, showed adjusted associations with the outcome variables very similar to those previously found for the complete cohort (Table 2) (Grandjean et al., 1997). In regard to the postnatal exposure as reflected by the blood-mercury at age 7, associations were in the same direction, suggesting an adverse effect, for nine of 17 outcomes (Table 2). The visuospatial memory measure showed the clearest negative association with postnatal exposure, the only one that was statistically significant.
Table 1.
Prenatal and postnatal methylmercury exposure parameters of 694 Faroese birth cohort members examined at age 7 years.
| Parameter | N | Geometric mean (interquartile range) |
|---|---|---|
| Cord-blood mercury (µg/L) | 675 | 23.3 (13.6–42.2) |
| Maternal hair-mercury (µg/g) | 692 | 4.35 (2.63–7.85) |
| Age-7 blood-mercury (µg/L) | 694 | 8.36 (4.38–18.0) |
| Age-7 hair-mercury (µg/g) | 686 | 3.03 (1.68–6.33) |
Table 2.
Change in neuropsychological outcomes at age 7 years for 10-fold increases in mercury concentration. Results are from regression analysis of data from children with known postnatal exposures after adjustment for maternal Raven score, medical risk for neurobehavioral deficit, maternal and paternal education level, paternal employment, and day care.
| Unadjusted |
Mutually adjusted |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Cord blood |
Child blood |
Cord blood |
Child blood |
|||||
| N | Beta | p | Beta | P | Beta | p | Beta | p | |
| NES2 Finger Tapping | |||||||||
| Preferred hand | 639 | −0.55 | 0.373 | 0.42a | 0.455 | −0.81 | 0.218 | 0.68a | 0.258 |
| Non-preferred hand | 641 | −0.31 | 0.599 | 1.20a | 0.024 | −0.90 | 0.153 | 1.49a | 0.009 |
| Both hands | 638 | −1.26 | 0.318 | 0.42a | 0.713 | −1.62 | 0.230 | 0.93a | 0.445 |
| NES2 Hand-Eye | |||||||||
| Error score | 641 | 0.055 | 0.061 | 0.003 | 0.911 | 0.061 | 0.050 | −0.017a | 0.552 |
| NES2 CPT | |||||||||
| Reaction time | 297 | 50.3 | 0.000 | 16.2 | 0.125 | 50.6 | 0.0001 | −0.75a | 0.946 |
| Ln total missed | 300 | 0.309 | 0.022 | 0.048 | 0.679 | 0.338 | 0.021 | −0.065a | 0.601 |
| WISC(R) | |||||||||
| Digits spans | 634 | −0.28 | 0.080 | 0.16a | 0.251 | −0.39 | 0.020 | 0.29a | 0.056 |
| Similarities | 536 | −0.17 | 0.698 | 0.31a | 0.445 | −0.34 | 0.481 | 0.42a | 0.335 |
| Sqrt. block designs | 633 | −0.19 | 0.107 | −0.12 | 0.274 | −0.17 | 0.192 | −0.06 | 0.582 |
| Bender | |||||||||
| Errors on copying | 640 | 0.83 | 0.123 | 0.57 | 0.242 | 0.70 | 0.229 | 0.34 | 0.507 |
| Reproduction | 600 | −0.25 | 0.162 | −0.38 | 0.018 | −0.11 | 0.540 | −0.34 | 0.045 |
| Boston Naming Test | |||||||||
| No cues | 618 | −1.72 | 0.002 | −0.47 | 0.348 | −1.76 | 0.003 | 0.10a | 0.844 |
| With cues | 617 | −1.91 | 0.0004 | −0.69 | 0.157 | −1.87 | 0.001 | −0.09 | 0.858 |
| CVLT-Children | |||||||||
| Learning | 629 | −1.65 | 0.077 | 1.83a | 0.027 | −2.74 | 0.006 | 2.71a | 0.002 |
| Short delay | 621 | −0.80 | 0.004 | 0.11a | 0.642 | −0.97 | 0.001 | 0.42a | 0.107 |
| Long delay | 597 | −0.72 | 0.021 | −0.09 | 0.754 | −0.79 | 0.019 | 0.17a | 0.584 |
| Recognition | 589 | −0.42 | 0.070 | −0.02 | 0.914 | −0.47 | 0.058 | 0.13a | 0.572 |
Change associated with postnatal exposure in direction opposite to prenatal exposure.
The log transformed mercury concentrations in cord blood and blood from age 7 showed a significant correlation coefficient: r = 0.37. As both prenatal and postnatal exposures may have an effect, a simple regression model was generated with both variables as possible predictors of the outcomes (Table 2). After this mutual adjustment, the postnatal regression coefficient for the reaction time changed direction. The prenatal and postnatal coefficients that were in opposite directions in the unadjusted analysis became stronger after mutual adjustment. However, the visuospatial memory measure remained significantly associated with postnatal exposure, while regression coefficients for prenatal exposure decreased after mutual adjustment. The opposite was the case in regard to the Boston Naming test, which appeared highly sensitive to prenatal exposures.
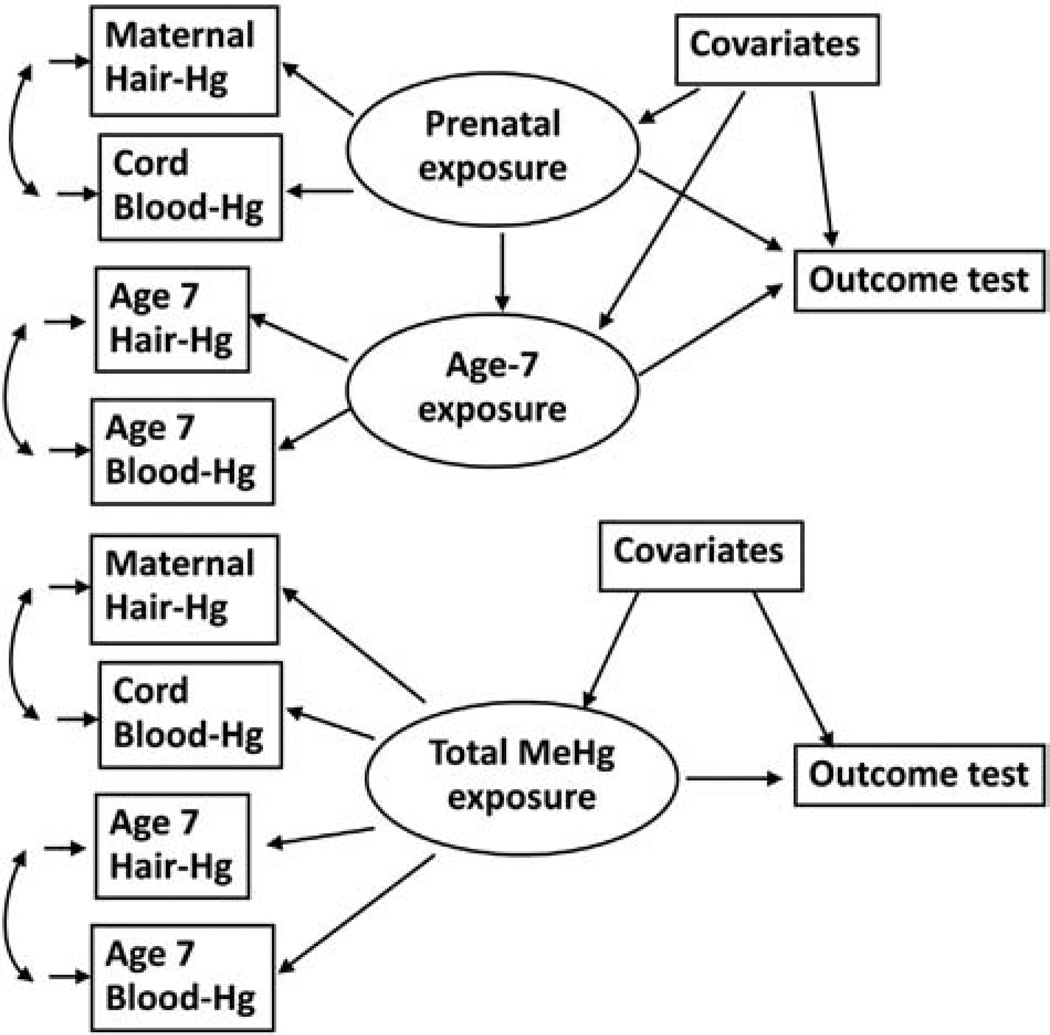
Due to the association between the two exposure variables as well as their imprecision, the mutual adjustment in the regression model may lead to biased effect estimations and a lower power to detect an effect of each exposure variable. We therefore explored the associations in structural equation models, where both hair and blood measures were included in the latent variables for prenatal and postal natal exposures (Table 3). This model included adjustment for measurement error in both exposures and would therefore lead to a less biased effect estimation (Fig. 2, top). The model is similar to the regression models in that it considers both a prenatal and a postnatal exposure and these are mutually adjusted. Still, these results rely on postnatal exposures characterized only by the samples collected at age 7 years.
Table 3.
Change in neuropsychological outcomes at age 7 years for 10-fold increases in mercury concentration. Results are from structural equation modeling (Fig. 2, top) of data from children with known postnatal exposures after adjustment for maternal Raven score, medical risk for neurobehavioral deficit, maternal and paternal education level, paternal employment, and day care.
| Mutually adjusted Prenatal |
||||
|---|---|---|---|---|
| Prenatal |
Postnatal |
|||
| Beta | p | Beta | p | |
| NES2 Finger Tapping | ||||
| Preferred hand | −2.02 | 0.006 | 0.27a | 0.658 |
| Non preferred hand | −1.37 | 0.053 | 0.46a | 0.441 |
| Both hands | −2.25 | 0.135 | −0.48 | 0.705 |
| NES2 Hand-Eye | ||||
| Error score | 0.058 | 0.087 | −0.031a | 0.274 |
| NES2 CPT | ||||
| Reaction time | 35.9 | 0.019 | 15.3 | 0.196 |
| Ln total missed | 0.302 | 0.064 | 0.074 | 0.562 |
| WISC(R) | ||||
| Digit spans | −0.29 | 0.116 | 0.25a | 0.092 |
| Similarities | −0.23 | 0.655 | 0.55a | 0.199 |
| Sqrt. block designs | −0.22 | 0.116 | −0.12 | 0.282 |
| Bender | ||||
| Errors on copying | 0.45 | 0.472 | 0.81 | 0.125 |
| Reproduction | −0.05 | 0.802 | −0.43 | 0.011 |
| Boston Naming Test | ||||
| No cues | −1.90 | 0.004 | −0.18 | 0.745 |
| Cues | −1.79 | 0.006 | −0.40 | 0.457 |
| CVLT-Children | ||||
| Learning | −1.45 | 0.189 | 1.25a | 0.172 |
| Short delay | −0.54 | 0.104 | 0.36a | 0.188 |
| Long delay | −0.63 | 0.091 | 0.19a | 0.541 |
| Recognition | −0.21 | 0.430 | 0.06a | 0.777 |
Change associated with postnatal exposure in direction opposite to prenatal exposure.
Fig. 2.
Structural equation model of a developmental methylmercury exposure as a predictor of a neuropsychological outcome variable, where available exposure biomarkers are linked to latent exposure variables at two different times (top), and where all exposure biomarker measurements are considered reflections of a single latent exposure variable (bottom).
As an alternative, we considered a model that includes both prenatal exposures and postnatal exposures to load on the latent exposure (Fig. 2, bottom). Each set of exposure markers is locally dependent, as they were measured at the same point in time. Table 4 shows the estimated mercury effects using only prenatal markers as compared to the joint effects. The two sets of regression coefficients are quite similar. Of note, the calculated adverse effect on three different measures of visuospatial performance (Block Design, and Bender errors and reproduction score) is strengthened after inclusion of postnatal exposure information, although the visuospatial memory task is not statistically significant in regard to the joint exposures.
Table 4.
Change in neuropsychological outcomes at age 7 years for 10-fold increases in mercury concentration based either on prenatal exposures only or on all exposure data. Results are from structural equation modeling (Fig. 2, bottom) of data from children with known postnatal exposures after adjustment for maternal Raven score, medical risk for neurobehavioral deficit, maternal and paternal education level, paternal employment, and day care.
| Prenatal only |
Prenatal and postnatal |
|||
|---|---|---|---|---|
| Beta | p | Beta | p | |
| NES2 Finger tapping | ||||
| Preferred hand | −1.68 | 0.008 | −2.04 | 0.006 |
| Non preferred hand | −1.03 | 0.088 | −1.19 | 0.094 |
| Both hands | −2.66 | 0.038 | −2.72 | 0.073 |
| NES2 Hand-Eye | ||||
| Error Score | 0.041 | 0.157 | 0.045 | 0.197 |
| NES2 CPT | ||||
| Reaction time | 45.5 | 0.0002 | 38.2 | 0.0005 |
| Ln total missed | 0.329 | 0.014 | 0.282 | 0.008 |
| WISC(R) | ||||
| Digit Spans | −0.21 | 0.188 | −0.15 | 0.401 |
| Similarities | 0.06 | 0.899 | 0.10 | 0.853 |
| Sqrt. Block Designs | −0.23 | 0.051 | −0.35 | 0.015 |
| Bender | ||||
| Errors on copying | 0.83 | 0.126 | 0.96 | 0.118 |
| Reproduction | −0.28 | 0.110 | −0.37 | 0.088 |
| Boston Naming Test | ||||
| No cues | −1.91 | 0.0007 | −2.50 | 0.0004 |
| Cues | −2.03 | 0.0003 | −2.42 | 0.0004 |
| CVLT-Children | ||||
| Learning | −1.28 | 0.176 | −0.77 | 0.471 |
| Short delay | −0.49 | 0.081 | −0.36 | 0.252 |
| Long delay | −0.64 | 0.043 | −0.57 | 0.116 |
| Recognition | −0.23 | 0.322 | −0.17 | 0.517 |
4. Discussion
The present study addresses the concern that postnatal methylmercury exposure from seafood intake may cause or contribute to developmental neurotoxicity. It relies on extensive data base on a Faroese birth cohort, where methylmercury exposures were characterized from both blood and hair analyses, and where a neuropsychological test battery assessed a variety of functional domains. The results for the Bender reproduction score suggest that postnatal methylmercury exposure may affect visuospatial memory, and that the effect in regard to this function may be stronger for exposures at late preschool age than for prenatal exposures. In a previous analysis based on hair-mercury analyses from age 7 (N = 907), both Block Designs and Bender Visual Motor Gestalt errors suggested exposure-dependent deficits on visuospatial processing, but neither remained after adjustment for prenatal exposure (Grandjean et al., 1997).
The results must be cautiously interpreted, as imprecise exposure assessment leads to underestimation of mercury toxicity (Budtz-Jorgensen et al., 2004b). Thus, in addition to the imprecision associated with the blood and hair sampling at a specific point in time, postnatal exposure assessment is further complicated by the fact that samples are available only at age 7 years, and the validity of exposure assessment at this age as an indication of exposures since birth is dubious. In addition, while susceptibility to methylmercury neurotoxicity may be greater during prenatal development, prenatal exposure levels are also higher, thus making assessment of any impact of postnatal exposures difficult. Assuming that the exposure assessment at age 7 is a reasonable reflection of postnatal exposure trajectories, the results of the unadjusted analyses suggest that postnatal exposures contribute little, if any, to the developmental delays for most functions.
Because prenatal and postnatal exposures are correlated, and because all exposure variables are known to be error-prone (Budtz-Jorgensen et al., 2007), mutual adjustment of the exposures may be inappropriate. Thus although some regression coefficients for the postnatal exposure were in the direction opposite to prenatal exposure, mutual adjustment resulted in an increased number of regression coefficients in opposite directions. These results are clearly unstable. In addition, both sets of exposure data may reflect a joint underlying parameter of food availability and household preferences, thus making the adjustment inappropriate.
As an alternative, structural equation models can better elucidate the effects of postnatal exposure, as relative imprecision may be taken into account. Still, the simple model with mutual adjustment shows several outcomes with regression coefficients in opposite directions. The model where all available exposure parameters are taken as reflections of a joint, overall developmental exposure may be the most appropriate choice. In this model, some regression coefficients increased in magnitude by incorporating the postnatal exposure, although others decreased.
Effects of postnatal exposure are likely domain-specific, and consideration of exposures at age 7 should rely on knowledge whether developmental vulnerability for specific functions extends into late preschool ages (Winneke, 2007). Although our knowledge on age-dependent vulnerability is incomplete, at best, visuospatial functions are known to be highly sensitive to postnatal lead exposure (Ris et al., 2004). Thus, the apparent reverse association between methylmercury at age 7 and visuospatial memory may be plausible, but needs to be confirmed by independent data, preferably with detailed postnatal exposure profiles.
The existence of developmental neurotoxicity due to postnatal exposures is supported by the previous finding of a delay in transmission of auditory brain signals in 14-year-old subjects (Murata et al., 2004). In addition, data from studies in Canada (Boucher et al., 2010) suggest that objective neurophysiological measure may be more likely to detect neurobehavioral functions affected by postnatal exposures.
The findings of this study may have implications for the interpretation of results obtained from other birth cohort studies. Thus, in a prospective study in the Seychelles, postnatal exposure was included as a mandatory covariate for the reason that it was associated with neurodevelopmental outcomes at age 5 years (Myers et al., 2003). However, associations with postnatal methylmercury exposures were both adverse and beneficial, and the authors later on recommended caution in interpreting associations with postnatal exposure (Davidson et al., 2011). Our findings support the notion that caution is needed in regard to the potential neurotoxicity from postnatal methylmercury exposure. In recent analyses of data on a new birth cohort from the Seychelles, the researchers do not adjust for postnatal exposure (Strain et al., 2012). Our findings support this decision.
5. Conclusions
The structural equation models allowed all methylmercury exposure parameters to contribute to a latent exposure variable that reflected the total methylmercury load. This latent exposure showed significant associations with neurodevelopmental deficits, with prenatal exposure contributing the most information. However, the results are also in agreement with the notion that postnatal methylmercury exposure may cause neurotoxic effects, in particular in regard to visuospatial processing and memory. Postnatal exposure levels need to be characterized in greater detail in order to properly document such effects. Because of the inherent imprecision of exposure estimates and the correlation between exposures at different developmental stages, simple mutual adjustment in regression analyses may not be appropriate to assess the relative vulnerability to methylmercury at different time windows.
Supplementary Material
Acknowledgments
This research was supported by the U.S. National Institute of Environmental Health Sciences (ES09797). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH or any other funding agency.
Footnotes
Conflict of interest statement
The authors have no competing interests to declare.
Transparency document
Transparency document associated with this article can be found, in the online version.
References
- Bollen KA. Structural equations with latent variables. New York: John Wiley; 1989. [Google Scholar]
- Boucher O, Bastien CH, Saint-Amour D, Dewailly E, Ayotte P, Jacobson JL, et al. Prenatal exposure to methylmercury and PCBs affects distinct stages of information processing: an event-related potential study with Inuit children. Neurotoxicology. 2010;31:373–384. doi: 10.1016/j.neuro.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Keiding N, Grandjean P, Weihe P. Estimation of health effects of prenatal methylmercury exposure using structural equation models. Environ Health. 2002;1:2. doi: 10.1186/1476-069X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Grandjean P, Jorgensen PJ, Weihe P, Keiding N. Association between mercury concentrations in blood and hair in methylmercury-exposed subjects at different ages. Environ Res. 2004a;95:385–393. doi: 10.1016/j.envres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Keiding N, Grandjean P. Effects of exposure imprecision on estimation of the benchmark dose. Risk Anal. 2004b;24:1689–1696. doi: 10.1111/j.0272-4332.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environ Health Perspect. 2007;115:323–327. doi: 10.1289/ehp.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Debes F, Weihe P, Grandjean P. Structural equation models for metaanalysis in environmental risk assessment. Environmetrics. 2010;21:510–527. doi: 10.1002/env.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AL, Mogensen UB, Bjerve KS, Debes F, Weihe P, Grandjean P, et al. Negative confounding by essential fatty acids in methylmercury neurotoxicity associations. Neurotoxicol Teratol. 2014;42:85–92. doi: 10.1016/j.ntt.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R, White RF, Weihe P, Sorensen N, Letz R, Hudnell HK, et al. Feasibility and validity of three computer-assisted neurobehavioral tests in 7-year-old children. Neurotoxicol Teratol. 1996;18:413–419. doi: 10.1016/0892-0362(96)00031-1. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Cory-Slechta DA, Thurston SW, Huang LS, Shamlaye CF, Gunzler D, et al. Fish consumption and prenatal methylmercury exposure: cognitive and behavioral outcomes in the main cohort at 17 years from the Seychelles child development study. Neurotoxicology. 2011;32:711–717. doi: 10.1016/j.neuro.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test—Children's Version (CVLT-C) San Antonio: Psychological Corporation; 1994. [Google Scholar]
- Despres C, Beuter A, Richer F, Poitras K, Veilleux A, Ayotte P, et al. Neuromotor functions in Inuit preschool children exposed to Pb, PCBs, and Hg. Neurotoxicol Teratol. 2005;27:245–257. doi: 10.1016/j.ntt.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Freire C, Ramos R, Lopez-Espinosa MJ, Diez S, Vioque J, Ballester F, et al. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ Res. 2010;110:96–104. doi: 10.1016/j.envres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Jorgensen PJ, Clarkson T, Cernichiari E, Videro T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch Environ Health. 1992;47:185–195. doi: 10.1080/00039896.1992.9938348. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Needham LL, Burse VW, Patterson DG, Jr, Sampson EJ, et al. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ Res. 1995;71:29–38. doi: 10.1006/enrs.1995.1064. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jorgensen E, White RF, Jorgensen PJ, Weihe P, Debes F, et al. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am J Epidemiol. 1999;150:301–305. doi: 10.1093/oxfordjournals.aje.a010002. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Satoh H, Murata K, Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect. 2010;118:1137–1145. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Nielsen F, Heinzow B, Debes F, Budtz-Jorgensen E. Neurobehavioral deficits at age 7 years associated with prenatal exposure to toxicants from maternal seafood diet. Neurotoxicol Teratol. 2012;34:466–472. doi: 10.1016/j.ntt.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120:799–806. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling third edition. 3rd ed. New York: Guilford; 2012. [Google Scholar]
- Letz R, Baker EL. NES2, neurobehavioral evaluation system manual. 4th ed. Winchester, MA: Neurobehavioral Systems, Inc.; 1988. [Google Scholar]
- Murata K, Weihe P, Budtz-Jorgensen E, Jorgensen PJ, Grandjean P. Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. J Pediatr. 2004;144:177–183. doi: 10.1016/j.jpeds.2003.10.059. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Thurston SW, Pearson AT, Davidson PW, Cox C, Shamlaye CF, et al. Postnatal exposure to methyl mercury from fish consumption: a review and new data from the Seychelles Child Development Study. Neurotoxicology. 2009;30:338–349. doi: 10.1016/j.neuro.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MS, Halling J, Bech S, Wermuth L, Weihe P, Nielsen F, et al. Impact of dietary exposure to food contaminants on the risk of Parkinson's disease. Neurotoxicology. 2008;29:584–590. doi: 10.1016/j.neuro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Plusquellec P, Muckle G, Dewailly E, Ayotte P, Bégin G, Desrosiers C, et al. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology. 2010;31:17–25. doi: 10.1016/j.neuro.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Raven J. Standard progressive matrices. London: H. K. Lewis; 1958. [Google Scholar]
- Ris MD, Dietrich KN, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and neuropsychological outcome in adolescence. J Int Neuropsychol Soc. 2004;10:261–270. doi: 10.1017/S1355617704102154. [DOI] [PubMed] [Google Scholar]
- Saint-Amour D, Roy MS, Bastien C, Ayotte P, Dewailly E, Despres C, et al. Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. Neurotoxicology. 2006;27:567–578. doi: 10.1016/j.neuro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Schlange H, Stein B, von Boetticher I, Taneli S. Göttinger Formreproduktions-test. Göttingen: Verlag für Psychologie; 1977. [Google Scholar]
- Strain JJ, Davidson PW, Thurston SW, Harrington D, Mulhern MS, McAfee AJ, et al. Maternal PUFA status but not prenatal methylmercury exposure is associated with children's language functions at age five years in the Seychelles. J Nutr. 2012;142:1943–1949. doi: 10.3945/jn.112.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Eto K. The pathology of Minamata disease. A tragic story of water pollution. Fukuoka: Kyushu University Press; 1999. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children revised. New York: Psychological Corp.; 1974. [Google Scholar]
- Weihe P, Grandjean P, Debes F, White R. Health implications for Faroe islanders of heavy metals and PCBs from pilot whales. Sci Total Environ. 1996;186:141–148. doi: 10.1016/0048-9697(96)05094-2. [DOI] [PubMed] [Google Scholar]
- Winneke G. Appraisal of neurobehavioral methods in environmental health research: the developing brain as a target for neurotoxic chemicals. Int J Hyg Environ Health. 2007;210:601–609. doi: 10.1016/j.ijheh.2007.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.