Abstract
It is the precise connectivity between skeletal muscles and their corresponding tendon cells to form a functional myotendinous junction (MTJ) that allows for the force generation required for muscle contraction and organismal movement. The Drosophila MTJ is comprised of secreted extracellular matrix (ECM) proteins deposited between integrin-mediated hemi-adherens junctions on the surface of muscle and tendon cells. In this paper, we have identified a novel, cytoplasmic role for the canonical nuclear import protein Moleskin (Msk) in Drosophila embryonic somatic muscle attachment. Msk protein is enriched at muscle attachment sites in late embryogenesis and msk mutant embryos exhibit a failure in muscle-tendon cell attachment. Although the muscle-tendon attachment sites are reduced in size, components of the integrin complexes and ECM proteins are properly localized in msk mutant embryos. However, msk mutants fail to localize phosphorylated Focal adhesion kinase (pFAK) to the sites of muscle-tendon cell junctions. In addition, the tendon cell specific proteins Stripe (Sr) and activated mitogen-activated protein kinase (MAPK) are reduced in msk mutant embryos. Our rescue experiments demonstrate that Msk is required in the muscle cell, but not in the tendon cells. Moreover, muscle attachment defects due to loss of Msk are rescued by an activated form of MAPK or the secreted Epidermal Growth Factor receptor (Egfr) ligand Vein. Taken together, these findings provide strong evidence that Msk signals non-autonomously through the Vein-Egfr signaling pathway for late tendon cell late differentiation and/or maintenance.
Keywords: Drosophila, Muscle development, Moleskin; Myotendinous junction, MAPK
INTRODUCTION
Muscle development proceeds through an exquisitely regulated series of myoblast fusion and myotube guidance events, which must be followed by establishment of a stable myotendinous junction (MTJ). The MTJ is the primary site for force transmission from the interior of the muscle cell, across its membrane, to the extracellular matrix (ECM). In healthy muscle tissue, the MTJ provides resistance against the mechanical stress generated during muscle contraction and allows for organismal movement. It is the precise connectivity between skeletal muscles and their corresponding target tendon cells that allow for the proper formation of the MTJ at muscle-tendon attachment sites. While many features concerning MTJ formation, structure, and function are conserved between both vertebrates and invertebrates, studies in the genetically tractable organism Drosophila melanogaster have proven instrumental in delineating the sequence of events in MTJ assembly and function.
Early tendon cell induction occurs around the same time as FC specification, but is muscle-independent (Becker et al., 1997). The initial pattern of tendon cell precursors are determined by signaling pathways that pattern the epidermis, including the Wingless, Hedgehog, Notch and Egf receptor pathways (Hatini and DiNardo, 2001). This combinatorial network results in expression of the stripe gene, which encodes for two isoforms of the epidermal growth factor (Egf)-like transcription factor, SrA and SrB (Frommer et al., 1996; Volk and VijayRaghavan, 1994). SrB positively maintains its own expression and turns ectodermal cells into tendon cell precursors. In these early differentiated tendon cells, Stripe induces the expression of genes required the initial steps in myotube migration. For example, Slit is expressed in a subset of tendon cell precursors and is an attractant for muscles that express Robo (Kramer et al., 2001). Other guidance molecules required for extension of the ventral longitudinal muscles include the muscle-expressed Perdido/Kon-tiki, Dgrip, and Echinoid proteins (Estrada et al., 2007; Schnorrer et al., 2007; Swan et al., 2006; Swan et al., 2004).
The differentiation of progenitor tendon cells into mature tendon cells requires signaling between the newly migrated muscle cell and corresponding target tendon cell. As myotubes approach their target tendon cells, αPS2 integrin begins to accumulate at the ends of the muscle (Bogaert et al., 1987; Leptin et al., 1989). This integrin-mediated adhesion is required for the localization and/or accumulation of Vein, a neuregulin-like secreted ligand of the Egfr pathway (Bogaert et al., 1987; Leptin et al., 1989; Martin-Bermudo, 2000; Yarnitzky et al., 1997). The Egfr receptor is expressed on the surface of tendon cells and is thought to signal through activated MAPK in conjunction with SrA to maintain the expression of genes necessary for late tendon cell differentiation, including Short stop (Shot), Delilah (Dei) and β1 Tubulin (β1 Tub).
The mature MTJ is formed when fully differentiated tendon cells are physically connected to muscles cells by ECM components, which provide strength and elasticity for subsequent muscle contractions. Mature tendon cells secrete the ECM proteins Thrombospondin (Tsp) and Laminin (Lam), while muscle cells make the secreted protein Tiggrin (Tig) (Bunch et al., 1998; Chanana et al., 2007; Fogerty et al., 1994; Martin et al., 1999; Subramanian et al., 2007). It is the association of the αPS2βPS integrin complex to the ECM components Thrombospondin (Tsp) and Tiggrin (Tig) that participate in the muscle-ECM adherens junctions at the MTJ. The tendon-ECM adherens junctions that comprise the MTJ are mediated by the heterodimers αPS1βPS that bind the ECM component Laminin. On the cytoplasmic face of the integrin adherens junctions, Talin, Integrin-linked kinase (Ilk), and PINCH function to link the membrane-bound integrin heterodimers to the internal actin cytoskeleton (Brown et al., 2002; Clark et al., 2003; Zervas et al., 2001). It is the formation of this MTJ that is essential for withstanding force contraction within a muscle cell, as mutations in the genes that encode for the integrins, ECM components, and cytoplasmic linker proteins Talin, and Ilk all result in muscles that detach from the MTJs (Schweitzer et al., 2010).
We have identified Moleskin (Msk), or Drosophila Importin-7 (DIM-7, also known as RanBP7), as an essential protein for the attachment of the embryonic somatic musculature. Msk is conserved in Xenopus, Drosophila and humans, and has been well-studied for its role in the nuclear import of proteins (Mason and Goldfarb, 2009). Importin-7 (Imp-7) was originally identified in Xenopus where it co-purifies with Imp-β and binds the small GTPase Ran (Gorlich et al., 1997). Imp-7 is a unique member of the Imp-β superfamily of proteins. While the canonical Imp-β proteins facilitate nuclear import by binding to both Importin-α and to the nuclear pore complex, Imp-7 can function with and without Imp-β. For example, histone H1 requires the Imp-β/Imp-7 heterodimer for proper nuclear import (Jakel et al., 1999). In contrast, Imp-7 alone can directly facilitate import of ribosomal proteins and the glucocorticoid receptor (Freedman and Yamamoto, 2004; Jakel and Gorlich, 1998). However, as described below, Msk also has functions that are independent of its role in nuclear import.
In flies, Msk/DIM-7 was identified in a yeast 2-hybrid screen as an interacting partner of Corkscrew (Csw)/SHP2, a component of the receptor tyrosine kinase (RTK) signaling pathway (Lorenzen et al., 2001). Consistent with a role in the RTK signaling pathway, Msk can bind to and transport the doubly phosphorylated, activated form of D-ERK (dp-ERK, or MAP kinase, hereafter referred to as MAPK) into the nucleus (James et al., 2007; Lorenzen et al., 2001). Since activated MAPK can function in both the cytoplasm and nucleus to activate downstream targets, the nucleocytoplasmic distribution of activated MAPK is important for determining downstream phenotypic outputs of the RTK pathway. For example, cytoplasmic MAPK is required for wing vein development, while nuclear MAPK regulates cell proliferation (Marenda et al., 2006). In the developing eye, activated MAPK in the cytoplasm is required for neuronal cell fate decisions, while activated MAPK in the nucleus controls ommatididal rotation (Vrailas et al., 2006). In both of these developmental pathways, the availability of activated MAPK in the cytoplasm and/or nucleus is regulated by Msk binding to activated MAPK.
The integrin signaling pathway plays an important role in regulating Msk-dependent MAPK activation. Alleles of msk were identified in a screen for dominant suppressors of an activated integrin αPS2 wing phenotype called Blistermaker (Baker et al., 2002). Consistent with this, Msk protein is found at the cell cortex in S2 cells where it colocalizes with integrins in a Csw-dependent manner (James et al., 2007). Furthermore, the nuclear accumulation of MAPK is compromised if there is a reduction in Msk protein levels or decrease in integrin function. Taken together, current models suggest that Msk may be a downstream link to integrate signals between the integrin and RTK pathways. Msk is hypothesized to be sequestered by an integrin/Csw complex at the cell periphery. Upon activation of integrin or RTK signaling, Msk can be phosphorylated and released from the cell cortex to then bind dp-ERK for nuclear import (James et al., 2007; Mason and Goldfarb, 2009).
Herein, we describe a novel role for Msk in the attachment of the somatic musculature in Drosophila embryonic myogenesis. Embryos mutant for msk exhibit defects in muscle attachment to tendon cells. Consistent with this, Msk protein localizes to the sites of muscle-tendon cell attachment. Intracellular proteins required for the formation and stable adhesion of the MTJ, such as βPS int and Ilk, are properly localized in msk mutants. Furthermore, the integrin-interacting ECM proteins, Tsp and Tig, are secreted normally. Msk mutants show a loss of phosphorylated FAK in the muscle and a loss of Sr and activated MAPK in the tendon cells. Reintroduction of Msk in the muscle or activated MAPK in the tendon cells in a msk mutant background restores muscle-tendon attachment. We also found that Vein functions downstream of Msk to activate the Egfr pathway in the tendon cells. We propose a model where Msk is essential for cell signaling from the muscle to the tendon cell to mediate proper muscle-tendon attachment and possibly MTJ maintenance.
MATERIALS AND METHODS
Genetics
Fly stocks were raised on standard cornmeal medium at 25°C unless otherwise indicated. Oregon R or y,w was used as the wild-type strain. The following fly stocks and/or alleles were used in this study: msk2/TM3-lacZ and msk4/TM3-lacZ [a gift from Lizabeth Perkins; (Baker et al., 2002)]; Ilk-GFP [a gift from Julie Kadmras; (Clark et al., 2003)]; mef2-GAL4 [(Geisbrecht et al., 2008)]; UAS-MAPKWT and UAS-MAPKSEM [a gift from Jessica Triesman; (Roignant and Treisman, 2010)]; and UAS-Vein [a gift from Talia Volk; (Yarnitzky et al., 1997)]. The following stocks were obtained from the Bloomington Stock Center: w; msk5 P{neoFRT}80B/TM3, P{ftz-lacZ.ry+}TM3, Sb1 (BL-23879), Df(3L)66c-28/TM3, Sb (BL-1541); MTD-GAL4; sr-GAL4/TM6 (BL-26663); en-GAL4 (BL-6356); sim-GAL4 (BL-9150). The following stocks were generated by standard meiotic recombination and verified by complementation and/or PCR: msk5, UAS-msk (for rescue); mef2-GAL4; msk4 (for rescue); sr-GAL4; msk4 (for rescue), ilk-GFP, msk4; UAS-MAPKWT, msk5 (for rescue); UAS-MAPKWT, msk4 (for rescue); UAS-MAPKSEM, msk5 (for rescue); UAS-MAPKSEM, msk4 (for rescue); UAS-Vein, msk4 (for rescue).
In situ hybridization and Immunostaining
Embryos were collected on agar-apple juice plates and aged at 25°C. For immunostaining, embryos were fixed and stained as described (Geisbrecht et al., 2008). Primary antibodies used were: anti-MHC (1:500, Susan Abmayr); anti-Msk [1:1000, (Lorenzen et al., 2001)]; anti-TM (1:50, The Babraham Institute, Cambridge, UK); anti-Mef2 [1:1000, (Bour et al., 1995)]; βPS-integrin (1:50, Developmental Studies Hybridoma Bank (DSHB), University of Iowa); anti-Talin E16B (1:20, DSHB, University of Iowa); anti Tig [1:1000, (Fogerty et al., 1994)]; anti-Tsp [1:500, (Subramanian et al., 2007)]; anti-GIT1 [1:500, (Bahri et al., 2009)]; anti-FAK[pY397] (1:1000, Invitrogen); anti-dp-ERK/MAPK (1:100, Sigma); anti-Sr [1:200; (Frommer et al., 1996)]. Secondary antibody used for immunohistochemistry was goat anti-mouse-HRP (1:200, Jackson). Secondary antibodies for fluorescent immunostaining were Alexa Fluor 488 or Alexa Fluor 546 (1:400, Molecular Probes, Carlsbad, CA). Phalloidin 546 was used for F-actin labeling. Tyramide enhancement was used to improve signals for anti-Msk, anti-GIT1, and anti-dp-ERK-MAPK (Invitrogen, Carlsbad, CA). Fluorescent images were collected on Olympus Fluoview300 and processed using Photoshop.
Molecular Biology
The ORF of the msk cDNA was cloned into the entry vector (Drosophila GATEWAY™ cloning system, Invitrogen) in the proper reading frame. Msk-YFP fusions were generated using the pTVW and pTWV gateway destination vectors (pUAST-based vector, EYFP fusions at either end) using the Drosophila Gateway Cloning System according to the instruction manual and injected via Genetic Services, Inc.
RESULTS
Drosophila moleskin mutants exhibit defects in embryonic muscle attachment
We undertook a proteomics approach to identify new proteins that may be required in the development of the Drosophila embryonic somatic musculature (Geisbrecht et al., 2008). One protein that was uncovered using this approach was Drosophila Importin-7 (DIM-7), also known as Moleskin (Msk). Other proteins identified in this screen will be published elsewhere.
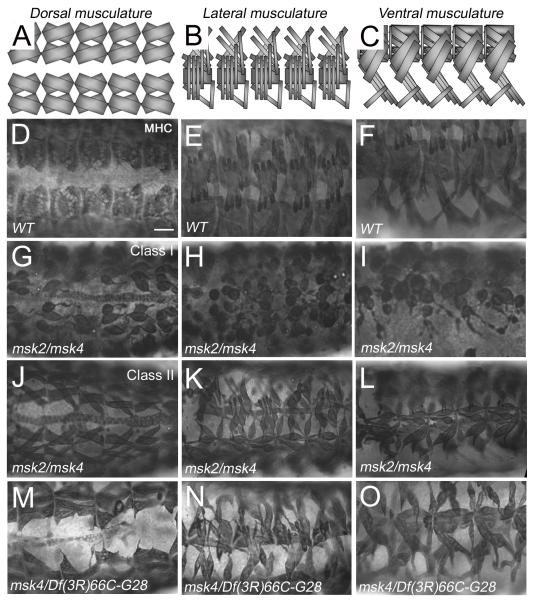
To determine whether there is a significant role for Msk in the development of the somatic musculature, we examined the muscle phenotypes of trans-heterozygous combinations of available msk alleles (Baker et al., 2002). After stage 16, msk2/msk4 mutant embryos stained with anti-Myosin heavy chain (MHC) exhibited an aberrant muscle pattern, predominantly characterized by missing muscles (12.1%, n=33) and/or muscles that were not positioned correctly (57.6%). Upon closer examination, the apparent muscle positioning phenotypes fell into two classes: either severe (Class I) or moderate (Class II) muscle attachment defects. A representative example of the Class I severe phenotype is shown in Figure 1G-I, where 12.1 % of the embryos of this genotype had muscles that were completely detached from the epidermis and “balled” up. 45.5% of the homozygous msk2/msk4 embryos (termed Class II) showed muscles that were for the most part correctly positioned, but at least one end of the muscle failed to attach correctly to the corresponding epidermal attachment site (Fig. 1J-L). Similar phenotypes were observed in msk4/msk5 mutant embryos, where 12.5% of the embryos exhibited the weaker attachment defects, while 44.6% revealed severe attachment defects (n=56). These muscle defects affected all muscle subsets, as the same phenotypes were observed in the dorsal (Fig. 1G, J, M), lateral (Fig. 1H, K, N) and ventral (Fig. 1I, L, O) muscle groups. All msk alleles we analyzed contain mutations that result in Msk protein truncations and are predicted to be genetically null alleles (Baker et al., 2002). Our data supports this conclusion. The severity of muscle attachment phenotypes did not increase in embryos trans-heterozygous for either msk5 (data not shown) or msk4 and the deficiency line, Df(3L)66c-28 (Fig. 1M-O), which removes the entire msk locus.
Figure 1. Msk mutant embryos exhibit defects in somatic muscle attachment.
(A-C) Schematic illustration of the WT muscle pattern in the dorsal (A), lateral (B) and ventral (C) musculature. (D-O) The dorsal (D, G, J, M), lateral (E, H, K, N), and ventral (F, I, L, O) musculature in stage 17 embryos visualized immunohistochemically with MHC antibody. (D-F) Wild-type embryos exhibit a stereotypical repeating pattern of muscles in each hemisegment. (G-I) Embryos trans-heterozygous for the msk2 and msk4 alleles exhibit severe muscle attachment defects (Class I) that affect all muscle subsets (12.1%; n=33). (J-L) The trans-heterozygous combination msk2/msk4 shows variable penetrance as 45.5% of the embryos show moderate defects in muscle attachment (Class II). (M-O) msk4 over a deficiency that removes the msk locus also exhibits muscle attachment defects with variable penetrance. In all embryos, anterior is to the left. Scale bar: 20 μm.
The residual muscle-tendon association observed in the Class II embryos is likely due to the presence of maternal Msk. Msk transcript and protein are observed in early Drosophila 0-2 h embryos before zygotic transcription has begun (data not shown). Consistent with the presence of maternal Msk, the formation of ventral acute muscle 2 (VA2) occurs normally in msk homozygous embryos (Johnson Hamlet and Perkins, 2001). To assess if removal of maternal Msk increased the severity of the muscle detachment phenotypes, we utilized germline clone analysis (GLC). GLC analysis of the null msk5 allele does not result in viable eggs as Msk is required for cell growth (Baker et al., 2002). Therefore, we generated flies of the genotype FRT2A, msk1 for GLC analysis with the expectation that the predicted hypomorphic msk1 allele may be sufficient to knockdown msk function, yet allow the animals to survive until late embryogenesis when the musculature could be examined. However, female flies from these experiments did not produce embryos and subsequent dissection of the resulting ovaries showed severe atrophy (data not shown). This result suggests Msk is required in oogenesis, and/or that the predicted msk1 allele may be stronger than a weak allele.
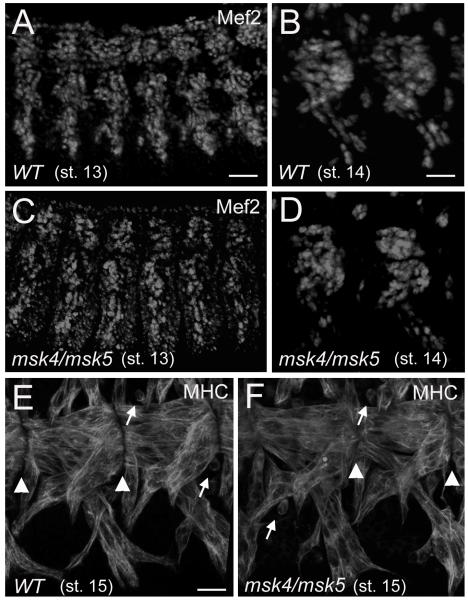
Our phenotypic analysis indicates that msk mutants exhibit pleiotropic defects in muscle development, including muscle patterning and muscle detachment phenotypes. Therefore, we sought to determine whether these defects may be due to an earlier role in muscle cell fate commitment or the migration of myotubes to their target sites of attachment. Mef2 is a transcription factor expressed in the early mesoderm and its expression is maintained throughout embryogenesis in different muscle cell lineages. Consistent with a central role in muscle development, both genetic analysis of mef2 mutants and enhancer studies show that Mef2 is required for multiple myogenic events, including myoblast fusion and the regulation of muscle identity genes (Potthoff and Olson, 2007; Sandmann et al., 2006). In wild-type embryos, Mef2 protein is apparent in the nuclei of developing muscles in stage 13 and 14 embryos (Figure 2A, B). Similar numbers of Mef2-positive muscle cells were observed in msk mutants (Figure 2C, D). No particular muscle or muscle group is consistently missing in the small percentage of msk mutant embryos that exhibit patterning defects, or missing muscles (12.1%), suggesting that Msk affects all muscles. By stage 15 in myogenesis, the wild-type muscles that reach their corresponding muscle-tendon-attachment site are positioned properly for MTJ assembly (Figure 2E). This process is not affected in msk mutants as elongated myotubes are observed at the segment borders of the ventral longitudinal muscles in stage 15 embryos (Figure 2F). These data taken together strongly suggest the muscle attachment defects in msk mutants are not due to earlier defects in cell fate specification or migration of the muscles to their target tendon cells.
Figure 2. Muscle cell fate and myotube migration are not affected in msk mutant embryos.
(A-D) Immunofluorescent stainings of stage 13 (A, C) and stage 14 (B, D) embryos. (A-D) Similar numbers of Mef2-expressing myoblasts are observed in WT (A, B) and msk4/msk5 homozyogous embryos (C, D) in both the lateral (A, C) and ventral longitudinal muscles (B, D). (E, F) Confocal micrographs of the ventral musculature in stage 15 embryos stained with a monoclonal antibody to MHC. (E) By stage 15 in WT embryos, the ventral longitudinal muscles have migrated to the site of muscle-tendon attachment. (F) The ability of the ventral muscles to find their proper attachment sites is not affected in msk mutants. Arrowheads denote muscle-tendon attachment sites and arrows point to unfused myoblasts still present in stage 15 embryos. In all embryos, anterior is to the left and dorsal is up. Scale bars: 50 μm in A, C; 20 μm in B, D; 10 μm in E, F.
Msk is required in muscles cells, but not the tendon cells, for proper muscle-tendon attachment
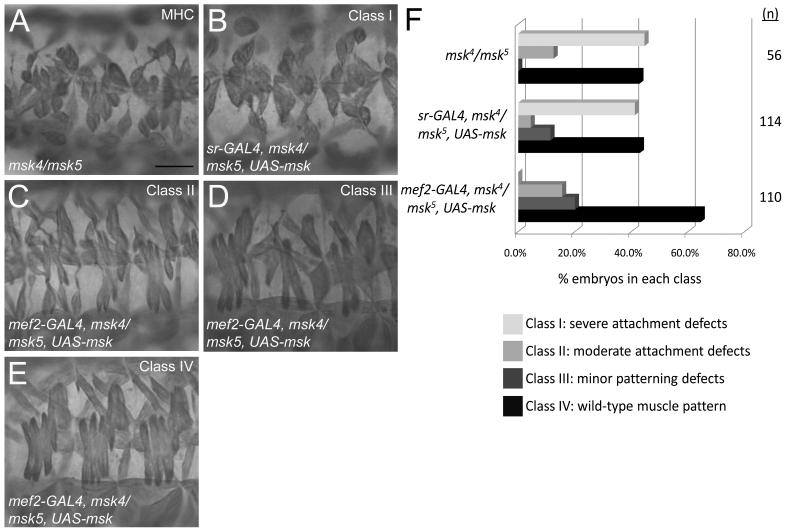
Cell-cell signaling between the muscle cells and their corresponding tendon cells is critical for proper muscle-tendon attachment (Schweitzer et al., 2010). To identify whether Msk is required in the muscle and/or tendon cells for proper muscle attachment, we performed tissue-specific rescue experiments using the GAL4/UAS system. We expressed UAS-msk in the tendon cells of msk trans-heterozygous embryos using the sr-GAL4 driver. No rescue of the muscle attachment defects in msk4/msk5 embryos were observed (compare Fig. 3A to 3B). Next, we used the mef2-GAL4 driver to express UAS-msk in all muscle cells. The resulting phenotypes were divided into four classes, based upon the severity of the muscle defects (Fig 3F). Class I embryos were characterized by severe muscle detachment phenotypes, including embryos of the genotype msk4/msk5 that failed to be rescued by the expression of sr-GAL4 in the tendon cells (Fig. 3A, B). However, expression of UAS-msk by mef2-GAL4 resulted in either partial rescue of attachment defects (Fig. 3C; Class II), or complete rescue to a WT muscle pattern (Fig. 3E; Class IV). Some of the embryos rescued by mef2-GAL4::UAS-msk in a msk4/msk5 mutant background exhibited minor patterning defects, but no muscle attachment defects (Fig. 3D; Class III). These experiments suggest the muscle-specific expression of Msk is essential for its function.
Figure 3. Msk is required in muscle cells for proper muscle-tendon attachment.
(A-E) MHC staining of 3 hemisegments of the lateral musculature in stage 17 embryos. (A) A representative embryo of the genotype msk4/msk5 shows severe defects in muscle attachment where muscles have pulled away from attachment sites and are found in a “ball.” (B-E) Expression of Msk in either the tendon cells (B) or the muscle cells (C-E) in msk4/msk5 embryos using the GAL4/UAS system. (B) Expression of UAS-msk in the tendon cells using sr-GAL4 does not rescue the muscle attachment defects (Class I). (C-E) Expression of UAS-msk in the muscle under control of the mef2 promoter results in minor attachment defects (C; Class II), minor patterning defects (D; Class III), or rescue to a WT muscle pattern (E; Class IV). Scale bar: 20 μm (F) A bar graph quantitating the ability of mef2-GAL to either partially (Class II; 15.5%) or fully (Class IV; 64.5%) rescue the muscle attachment defects in msk4/msk5 embryos (n=110). In contrast, embryos with sr-GAL4 are not rescued by expression of Msk in the tendon cells and exhibit phenotypes quantitatively similar to that of msk4/msk5 mutants only. Scale bar: 20 μm.
Msk protein is enriched at muscle attachment sites
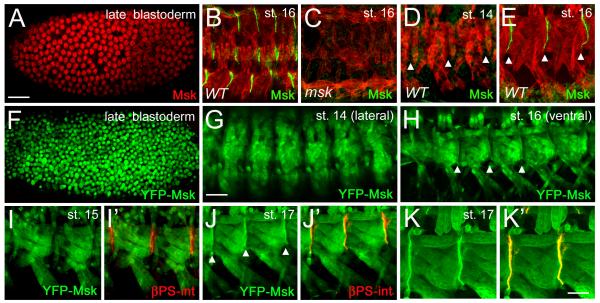
Our phenotypic analysis suggests that Msk functions in muscle tissue during the later stages of myogenesis to mediate muscle-tendon cell attachment. We examined the distribution of Msk protein throughout embryogenesis to gain a better understanding of the role of Msk in this process. Consistent with previous results (Lorenzen et al., 2001), Msk is dynamically expressed in early to mid embryonic development and can be found in either the nucleus or cytoplasm (Fig. 4A; data not shown). Immunostaining with antisera generated against the Msk protein (Lorenzen et al., 2001) in stage 16 embryos showed a strong accumulation of Msk protein at the segment borders where muscles connect to tendon cells (Fig. 4B). This staining pattern was present in the dorsal, lateral and ventral muscle groups and was not detected in msk4/msk5 mutant embryos (Fig. 4C). To confirm and extend our observation that Msk protein is enriched at sites of muscle-tendon attachment, we generated an N-terminal YFP-Msk fusion protein under control of the GAL4/UAS system. Using the early MTD-GAL4 driver, YFP-Msk was found in the nucleus in late blastoderm stage embryos (Fig. 4F). This localization nicely recapitulated the nuclear staining pattern observed with Msk antisera in blastoderm embryos (Fig. 4A)(Lorenzen et al., 2001). In addition, the subcellular distribution of YFP-Msk in Drosophila S2 cells appeared identical to Msk protein localization using antisera raised against Msk protein (Supp Fig. 1A-C)(James et al., 2007). Coincident with the process of myoblast fusion in stage 14 embryos, YFP-Msk expression was observed in both the nucleus and cytoplasm of the developing myotubes (Fig. 4G), although the significance of the nuclear expression is unclear. In the somatic musculature by stage 14, βPS integrin (βPS int) accumulation can be observed at the muscle attachment sites (Bogaert et al., 1987). However, we could not detect Msk protein by antibody staining (Fig. 4D) or YFP-Msk fusion protein enrichment at the site of the future MTJs, where βPS int is clearly present (Fig. 4I-I’). Consistent with our genetic data that Msk is required after stage 15 for muscle attachment, we first observed Msk protein at muscle attachment sites in stage 16 embryos (Fig. 4E). This localization persisted in stage 17 embryos, where colocalization with βPS int was apparent (Fig. 4J-K”). The appearance of Msk protein at the muscle-tendon attachment sites at the beginning of stage 16 in myogenesis strongly suggests Msk is required for the differentiation and/or maintenance, but not initiation of MTJ formation.
Figure 4. Msk protein localizes to muscle-tendon attachment sites in late stage embryos.
(A-E) Msk protein expression and localization in embryogenesis visualized using anti-Msk antisera. (A) Msk protein is nuclear in late blastoderm embryos. (B-E) Msk protein is in green and muscle is labeled in red using anti-Tropomyosin (TM). (B) Msk becomes enriched at the dorsal, lateral and ventral muscle-tendon attachment sites in stage 16 embryos. (C) Msk attachment site staining is absent in msk4/msk5 mutant embryos. (D, E) Msk protein is not observed at the ventral muscle-attachment sites in stage 14 embryos (D), but is observed after stage 15 (E). (F-K’) Msk protein expression visualized using the fusion protein Msk-YFP (green). Expression of MTD-GAL4::Msk-YFP in late blastoderm embryos (F) and mef2-GAL4::Msk-YFP in stage 16 embryos (G-K’). The Msk-YFP protein expression and localization (F) mimics the nuclear Msk protein expression (A). YFP-Msk protein is detected in both the nucleus and cytoplasm in stage 14 (G) and stage 16 (H) embryos, but becomes enriched at the ends of muscles in the latter stage (arrowhead in H). (I’, J’, K’) Double labeling with βPS-integrin (red) shows the location of the muscle attachment site in the ventral musculature. YFP-Msk expression occurs throughout the muscle, but is not enriched near βPS-integrin in stage 15 embryos (Fig. 4I, I’). In stage 17 embryos, YFP-Msk protein is enriched at the sites of muscle attachment and colocalizes with βPS-int (Fig. 4J’, K’; arrowheads). Scale bars: 50 μm in A-D; 20 μm in E-H’; 10 μm in I-I’.
Integrin and ECM components are properly localized in msk mutant embryos
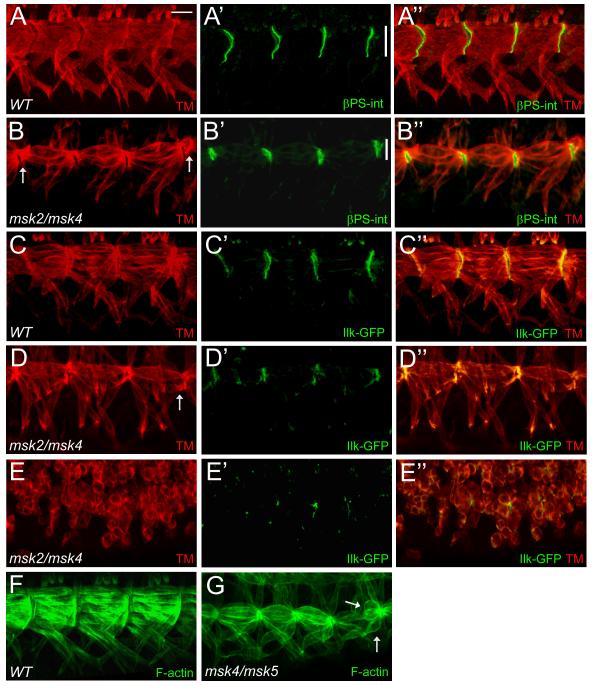
The enrichment of Msk protein at the sites of muscle-tendon cell attachments and the detachment of the somatic muscles in msk mutant embryos raised the possibility that integrin-mediated muscle-tendon adhesion is defective in msk mutant embryos. To examine this in more detail, we focused our analysis on the ventral longitudinal muscles, where the MTJ sites are large and easily observed (Fig. 1C). Here, βPS integrins are expressed at the ends of the ventral muscles and the basal side of the tendon cells to make contact with ECM proteins deposited between the muscle and tendon cells (Fig. 5A-A”). In msk mutants, βPS int was still localized to the correct positions at the segment borders of the ventral muscles (Fig. 5B-B”). We never observed βPS int expression at the ends of muscles that had already detached (arrows). However, the domain of βPS int expression was reduced in size compared to WT embryos (compare line in Fig. 5A’ to Fig. 5B’). To further elucidate the role of Msk in muscle attachment at the MTJ, we examined the distribution of the cytoskeletal proteins ILK and Talin. Both of these proteins function to link the cytoplasmic domain of integrins to the actin cytoskeleton within the muscle cell. Like βPS int, ILK was still expressed and localized to the correct location in msk mutants that exhibited either moderate (Fig. 5D-D”) or severe (Fig. 5E-E”) attachment defects. Consistent with the results obtained for both βPS int and ILK, immunostaining against Talin revealed it was also correctly localized (Supp. Fig. 2A-B’). Mutations in mys (βPS int) or rhea (Talin) show adhesion defects that result from detachment of the cell membrane from the ECM, while Ilk, PINCH, or partial loss-of-function αPS2 int mutants are characterized by detachment of the actin cytoskeleton from the muscle cell membrane (Brown, 1994; Clark et al., 2003; Leptin et al., 1989; Newman and Wright, 1981; Zervas et al., 2001). To investigate if a defect in actin tethering to the muscle cell membrane is responsible for the detachment phenotype observed in msk mutants, we labeled the mutants with phalloidin to visualize F-actin filament organization. Both WT (Fig. 5F) and msk4/msk5 mutants (Fig. 5G) stained strongly for actin. In addition, numerous F-actin based structures extended across the entire muscle filament and accumulated normally at the sites of attachment in both genotypes.
Figure 5. Components of integrin-mediated adherens junctions localize to the correct positions in msk mutant embryos.
(A-G) Immunofluorescent stainings of stage 17 embryos. Ventral musculature with anterior to the left and dorsal up. (A-B”) βPS-integrin staining in WT embryos localizes to the ends of muscles where they form contacts with tendon cells (A-A”). Msk mutants have correctly positioned, but smaller muscle attachment sites (compare vertical white bars) as demonstrated by βPS-int staining (B’-B”). (C-D”) Ilk-GFP also localizes to muscle attachment sites in WT (C’-C”). In msk mutants, Ilk-GFP is present at the correct place, although altered in size, in embryos that exhibit mild (D’-D”) or severe (E’-E”) muscle attachment defects. (F, G) Phalloidin labeling reveals F-actin filaments that span each muscle and accumulate at the ends of muscles in both WT (F) and msk (G) mutants. Arrows point out muscles that have detached. Scale bar: 20 μm.
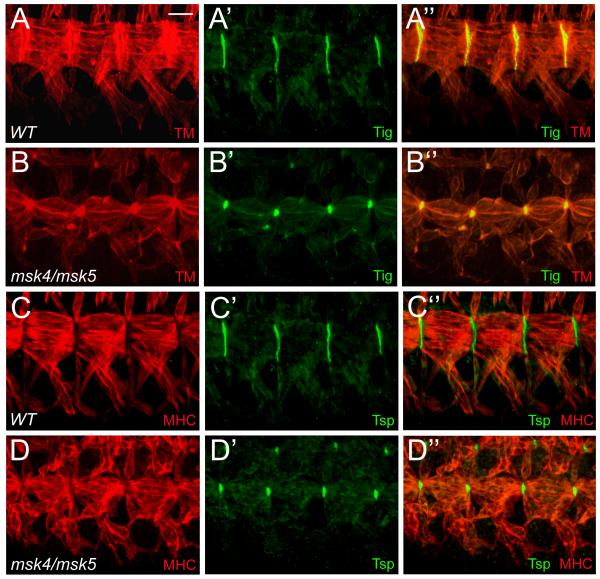
The muscle-specific αPS2βPS integrin heterodimer that localizes to the ends of the muscles interacts with the ECM proteins Tsp and Tig, secreted from the tendon cells and muscles, respectively (Bunch et al., 1998; Chanana et al., 2007; Fogerty et al., 1994; Subramanian et al., 2007). To determine whether the observed muscle defects are a result of improper ECM secretion and/or localization, we examined msk mutant embryos for Tsp and Tig protein distribution. In WT embryos, Tig (Fig. 6A-A”) and Tsp (Fig. 6C-C”) expression and localization are similar to that of βPS-integrin at the MTJ. In msk mutants, both Tig (Fig. 6B-B”) and Tsp (Fig. 6D-D”) are still present, although reduced in size. These results taken together suggest the defects underlying the muscle detachment phenotype observed in msk mutants are not due to general defects in either the extracellular or cytoplasmic face of integrin-mediated adherens junctions.
Figure 6. The ECM proteins Tig and Tsp are secreted and localized to muscle attachment sites.
(A-D”) Ventral musculature with anterior to the left and dorsal up. (A-D”) The ventral musculature (red) in stage 16 embryos stained with ECM proteins (green). (A-B”) Tig is enriched at muscle-muscle attachment sites in wild-type embryos (A-A”). The localization and expression of Tig is still present in msk mutant embryos, although the size of the attachment site is reduced (B-B”). (C-D”) Tsp is enriched at muscle-tendon attachment sites in both wild-type (C-C”) and msk mutant embryos (D-D”). As with Tig, there is a reduction in the size of the attachment sites. Scale bar: 20 μm.
Msk is required for pFAK localization to the muscle attachment sites
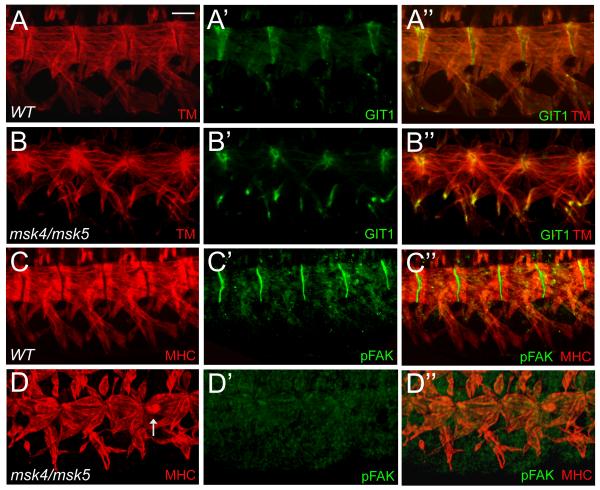
GIT1 is an adapter protein in mammals that associates with com ponents of focal adhesion complexes to modulate focal adhesion disassembly and cell motility (Bahri et al., 2009). In late stage embryos, GIT1 localizes to muscle attachment sites and can be visualized at the tips of myotubes (Fig. 7A’-A”). In msk mutants, GIT1 expression was still present and properly localized to muscle attachment sites (Fig. 7B’-B”). Interestingly, GIT1 expression was also upregulated at the ends of the ventral muscles, although the significance of this is yet unclear. In response to integrin activation, the vertebrate non-receptor tyrosine kinase Focal adhesion kinase (FAK) binds to the cytoplasmic tail of integrins (Grabbe et al., 2004). FAK becomes activated upon phosphorylation and can then interact with GIT1 in mammalian systems (Zhao et al., 2000). An antibody generated against the mammalian phosphorylated FAK (pFAKY397) protein cross-reacts with the phosphorylated form of Drosophila FAK (pFAK) and is strongly localized to embryonic muscle attachment sites (Fig. 7C’-C”), (Grabbe et al., 2004). Surprisingly, pFAK expression was undetectable in all msk mutant embryos examined (Fig. 7D’-D”). However, no increase and/or abnormal localization of pFAK is observed in embryos overexpressing Msk in the musculature (data not shown), suggesting that Msk is not sufficient for pFAK localization.
Figure 7. Msk is required for pFAK localization.
(A-D”) Immunofluorescent stainings of stage 17 embryos. A-D” are views of the ventral musculature with anterior to the left and dorsal up. (A-B”) GIT1 properly localizes to muscle attachment sites in both WT (A’-A”) and msk mutant embryos (B’-B”). Phosphorylated FAK, normally present at the sites of muscle-tendon attachments (C’-C”), is absent in all msk mutants examined (D’-D”). Arrows indicates detached muscle. Scale bar: 20 μm.
Msk is necessary, but not sufficient for tendon cell induction
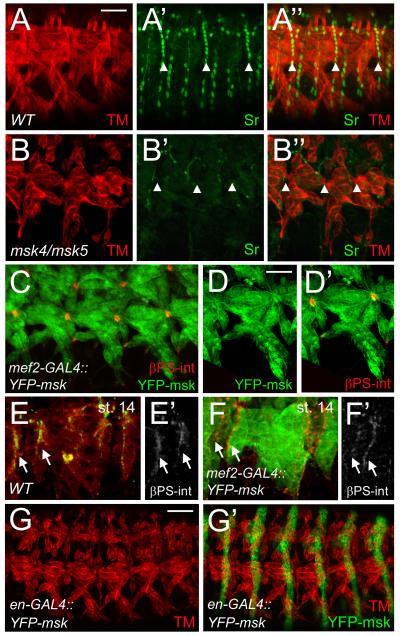
Muscle attachment is complete when the muscle-specific αPS2βPS integrin heterodimer and tendon-specific αPS1βPS integrin heterodimer form a mature MTJ with ECM proteins deposited between the two cells types (Schweitzer et al., 2010). After initial tendon cell induction by SrB in stage 11-12 embryos, SrA is activated at a later developmental stage to turn on transcription of genes required for muscle targeting to tendon cells, including slit and Tsp. The smaller attachment sites observed with Tsp immunolocalization in msk mutant embryos is phenotypically similar to that Tsp staining in sr155/srDG4 embryos (Subramanian et al., 2007). To examine if the aberrant muscle attachment defects in msk mutants could be due to aberrant tendon cell function, we examined the expression of Sr, a transcription factor that is required for the induction and maintenance of tendon cell fate. Sr is normally expressed in all tendon cells in late stage WT embryos (Fig. 8A-A”). In msk mutants, Sr expression is severely reduced and observed occasionally in only a few tendon cells (Fig. 8B-B”).
Figure 8. Msk is necessary, but not sufficient for tendon cell induction.
Immunofluorescent stainings. Ventral musculature with anterior to the left and dorsal up. (A-B”) Sr protein is found in all mature tendon cells in stage 16 embryos (A’-A”). A decrease in Msk levels results in a dramatic decrease in Sr protein (B’-B”). Muscle is shown in red. (C-D”) Expression of YFP-Msk in the musculature with mef2-GAL4 results in either a WT muscle pattern (Figure 4H) or muscles that appear pointed at the ends and have smaller attachment sites as visualized by βPS-integrin staining (C-D”). (E-F’) βPS-int is present at the ends of muscles in stage 14 embryos in WT (E, E’) and in muscles that are over-expressing Msk (F, F’). (G-G ’) Expression of YFP-Msk in all epidermal cells under control of the engrailed promoter shows muscles (red) with aberrant muscle attachments. In all examples, anterior is left and dorsal is up. All panels are representative embryos of ventral muscles. Scale bars: 20 μm in A-C; 10 μm in D-D’; 50 μm in G-G’.
Ectopic expression of Sr in a WT background results in mild aberrations of the normal muscle pattern. When Sr is induced by engrailed-GAL4 (en-GAL4) or patched-GAL4 (ptc-GAL4), the lateral muscles are either missing or connected to cells ectopically expressing Sr (Frommer et al., 1996; Volk and VijayRaghavan, 1994). To examine if ectopic expression of Msk in either the muscle or epidermis could phenocopy that of ectopic Sr induction, we expressed UAS-YFP-Msk or UAS-Msk (data not shown) in either tissue. 73.6% of the embryos of the genotype mef2-GAL4::UAS-YFP-Msk (n=19) showed a WT muscle pattern (Fig. 4H). In the other 26.3% of the mef2-GAL4::UAS-YFP-Msk embryos, the ventral longitudinal muscles were attached at the correct locations, but exhibited abnormal attachment sites. (Fig. 8C). However, in these embryos, Sr expression was not significantly different from WT (Supp Fig. 3A-A”) and was not induced or upregulated at earlier stages (data not shown). High magnification views of representative embryos show the muscles appear pointed at the muscle ends and result in small, round attachment sites as visualized by colocalization with βPS-integrin (Fig. 8D, D’). This phenotype is reminiscent of embryos that have mutations in the slowdown (slow) gene, which encodes a secreted protein essential for MTJ assembly. In slow mutants, βPS-integrin, Talin, and Tsp accumulate prematurely at the ends of migrating muscles (Gilsohn and Volk). Therefore, we examined if the premature accumulation and/or localization of βPS int could be one mechanism for the pointed muscles that end in small attachment sites in embryos that over-express Msk. We analyzed stage 14 embryos expressing YFP-Msk and found that the accumulation of βPS int at the ends of migrating muscles was similar to that in WT embryos (Fig. 8E-F’). Surprisingly, expression of YFP-Msk by en-GAL4 in the epidermis also resulted in muscle attachment defects (Fig. 8E-E’; 42.4%; n=33). However, as in en-GAL4::UAS-Sr embryos (Frommer et al., 1996; Volk and VijayRaghavan, 1994), we never observed mis-migration of the muscles or aberrant attachment to ectopic Msk-expressing cells. We were not able to induce abnormal migration of muscles to either the salivary glands (Supp. Fig. 3B-B”) or towards the CNS (Supp. Fig. 3C-C”) by ectopic expression of YFP-Msk using sim-GAL4. These results taken together support a model where Msk may be necessary for tendon cell differentiation, but is not sufficient to induce Sr expression. Our data suggests that the detached muscles and smaller attachment sites present in embryos that over-express Msk do not result from the early accumulation of Integrin complexes or abnormal upregulation of Sr expression.
Msk signals to the tendon cells for normal MAPK function
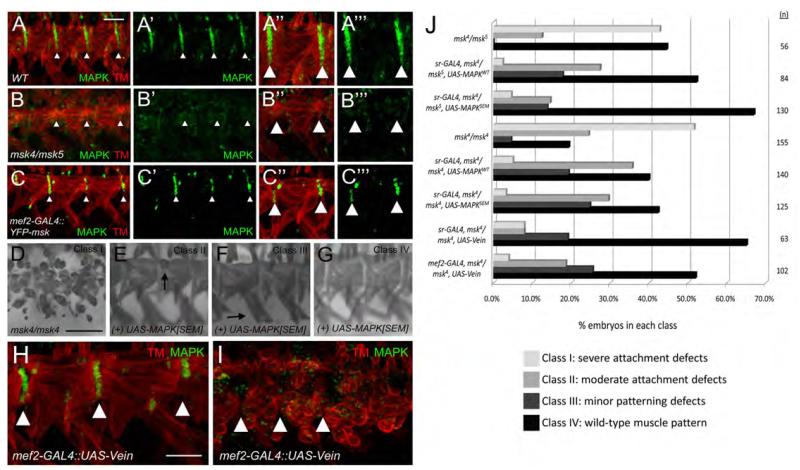
The Egfr pathway signals through the activated form of MAPK in tendon cells (Martin-Bermudo, 2000). To test if Msk affects the activation of MAPK in muscle-tendon attachment, we used an antibody that recognizes the activated form of MAPK (Gabay et al., 1997). In WT embryos, we observed MAPK in the tendon cells (Fig. 9A-A’”). In contrast, we could not detect activated MAPK in the tendon cells of msk mutant embryos (Fig. 9B-B’”). Furthermore, expression of excess Msk in the muscle resulted in a reduction in the number of MAPK-expressing tendon cells (Fig. 9C-C’”). This suggests that proper levels of Msk are necessary to regulate activated MAPK in the tendon cells. To test if MAPK functions downstream of Msk, we expressed both the wild-type and activated (SEM) forms of MAPK in a msk mutant background. Expression of both forms of the MAPK protein in tendon cells using sr-GAL4 rescued the msk muscle attachment defects (Fig. 9J). The severe muscle attachment phenotype observed in msk4/msk5 mutant embryos (42.8%; n=56) was reduced to only 2.4% of the embryos when UAS-MAPKWT was reintroduced into the tendon cells (n=84). The activated form of MAPK, (UAS-MAPKSEM) resulted in stronger rescue of the attachment phenotype, where 67.0% of the embryos were restored to WT (n=130) compared to only 42.3% of WT embryos in the control genotype (msk4/msk5; n=56). We wanted to verify the ability of MAPK to rescue msk mutants in a different genetic background. We chose to rescue msk4 homozygous mutant embryos, as the muscle attachment defects in these embryos were more severe, where 51.6% of the embryos showed a severe attachment phenotype (Fig. 9D). Similar rescue trends were observed when we expressed UAS-MAPKWT or UAS-MAPKSEM in the msk4 background. The percentage of embryos that exhibited severe muscle attachment defects were to reduced 5.0% and 3.2% upon tendon-cell specific expression of MAPKWT or MAPKSEM, respectively (Fig. 9J).
Figure 9. MAPK and Vein act downstream of Msk to mediate muscle-tendon attachment.
(A-C’”) Immunofluorescent stainings of muscle (red) and activated MAPK (green). (A-A’”) In WT embryos, MAPK is present in the tendon cells and extends the length of the ventral muscles at the segment borders. (B-B’”) In msk mutants, activated MAPK cannot be detected at the muscle-tendon attachment sites. (C-C’”) The number of tendon cells that show activated MAPK staining is reduced in embryos that over-express Msk in the musculature. (D-G) MHC staining. (D) Severe attachment defects are observed in msk4/msk4 embryos (Class I). Expression of activated MAPK (MAPKSEM) in the tendon cells using sr-GAL4 results in muscles with mild attachment defects (E; Class II), patterning defects (F; Class III), or complete rescue to a WT muscle pattern (G; Class IV). (H, I) Expression of Vein in the musculature results in smaller attachment sites (compare to Figure 9A-A’”) and reduced MAPK staining (H) or severe attachment defect and a complete loss of activated MAPK at the attachment sites (I). (J) Bar graph that quantitates the rescue of muscle attachment defects in msk4/msk5 or msk4/msk4 mutant embryos upon over-expression of WT MAPK, activated MAPK, or Vein. In all examples, anterior is left and dorsal is up. All panels are representative embryos of ventral muscles. Scale bars: 20 μm in A-G; 10 μm in H-I.
Msk is postulated to integrate downstream signals from both the Egfr and integrin signaling pathways to regulate the subcellular localization of MAPK (James et al., 2007; Mason and Goldfarb, 2009). Integrins are required to promote the proper localization and/or accumulation of the secreted Egfr ligand, Vein (Martin-Bermudo, 2000). We therefore tested if Vein signaling could be one mechanism by which the muscle-expressed Msk maintains tendon cell function. As previously reported (Yarnitzky et al., 1997), we observed that over-expression of Vein in the muscle occasionally resulted in ectopic tendon cells. But we also found embryos with smaller attachment sites and mild muscle attachment defects (39.3%; n=61). In these same embryos, there was a reduction in the number of tendon cells with activated MAPK (Fig. 9H). This phenotype was similar to that observed with the over-expression of Msk in the muscle (Fig. 9C-C’”). Furthermore, severe muscle attachment defects and a loss of activated MAPK was seen in 21.3% of the embryos (Fig. 9I; n=61). We were able to rescue the severe msk4/ msk4 muscle attachment defects by expressing UAS-Vein in either the muscle or tendon cells (Fig. 9J). Tendon cell expressed-Vein rescued better than muscle-expressed Vein, presumably because secreted Vein was in closer proximity to the tendon cells expressing its Egf receptor. Collectively, these data suggest a model where Msk signals to the tendon cell via the secreted Egfr ligand Vein to influence activated MAPK signaling in the tendon cells. A loss of this signaling results in fewer tendon cells with activated MAPK activity, smaller attachment sites, and the inability of tendon cells to maintain proper muscle-tendon attachment.
DISCUSSION
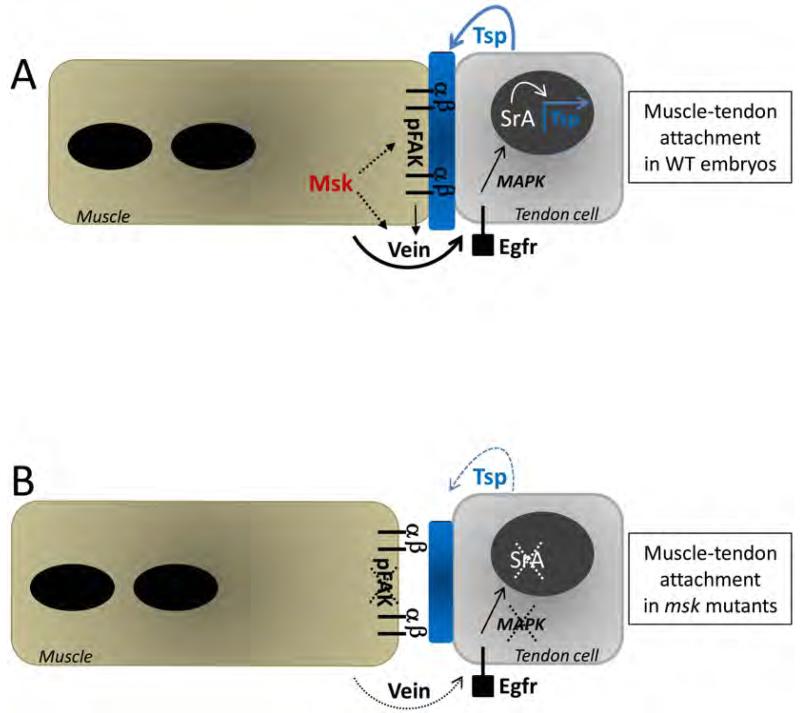
In Drosophila, the formation of a stable myotendinous junction is essential to withstand the force of muscle contraction required for larval hatching. Proper formation of the MTJ requires proper integrin heterodimer formation at the junctions between both muscle cells and tendon cells for a permanent linkage to the ECM proteins deposited between these two cell types. The precise mechanism by which the muscle cells signal to the tendon cells to form and maintain the semi-adherens junctions that comprise the stable MTJ is still being elucidated. Here, we show that the canonical nuclear import protein Msk is essential for Drosophila somatic muscle attachment. Moreover, we provide a model explaining how Msk may function non-cell autonomously from the muscle to the tendon cell for proper MTJ maintenance. Though vein mRNA is produced in the myotubes, Vein protein is secreted and is restricted to the junctions at muscle-tendon attachment sites (Yarnitzky et al., 1997). As shown in Figure 10, Msk signals through the secreted Egfr ligand Vein to mediate cross-talk between the muscle and tendon cells. The binding of Vein to the tendon-expressed Egfr activates a signaling cascade through activated MAPK. Activated MAPK translocates to the nucleus and with SrA, activates downstream genes to induce terminal differentiation in the tendon cells. An inability of the tendon cells to maintain activated MAPK and Sr activity would affect the amounts of target proteins required to maintain stable muscle-tendon adhesion. For example, a decrease in Tsp deposition into the ECM would result in smaller attachment sites and an inability to maintain a tight integrin-ECM association.
Figure 10. Model of Msk function at the muscle-tendon attachment site.
(A) In WT embryos, mature muscles associate with their corresponding tendon cells via an ECM-rich matrix (shown in blue) between the cells. Msk is required in the muscle and functions via secreted Vein to affect tendon cell function. Binding of Vein to the tendon-expressed Egfr activates a signaling cascade through activated MAPK. Activated MAPK translocates to the nucleus and with SrA, activates downstream genes to induce terminal differentiation in the tendon cells. Tight association of the muscle-expressed αPS2βPS integrin heterodimer to ECM proteins (such as the SrA target Tsp) is essential to withstand the force of muscle contraction. Msk may directly affect Vein secretion, accumulation and/or localization at the site of the muscle-tendon attachment site. Alternatively, Msk may affect Vein activity indirectly by modulating integrin-mediated adhesion. This adhesive function is required for Vein to properly activate the Egfr signaling pathway in the tendon cells. The effect of Msk on integrin-mediated adhesion may be direct or occur via phosphoproteins such as pFAK. Solid arrows denote previously published studies. Dotted arrows represent potential role(s) of Msk based upon our results. (B) In msk mutants, the ECM matrix (blue) is reduced in size and the muscle is no longer attached at the muscle-tendon attachment site. Terminal tendon cell differentiation does not occur as Sr and activated MAPK are dramatically reduced. Subsequently, Tsp expression and secretion is reduced, presumably resulting in smaller attachment sites. Within the muscle cells, pFAK is absent. Here, dotted lines and arrows denote a loss of protein and/or function due to a decrease in Msk protein.
Msk plays a general role in myogenesis as defects in msk mutant embryos were observed in all hemisegments and affected all muscle groups. The variable penetrance, which we classified as either major (Class I) or moderate (Class II) muscle detachment phenotypes, present in msk mutant embryos is likely due to the presence of maternal msk transcript. Attempts to further knockdown Msk levels by removal of maternal load resulted in non-viable egg chambers, consistent with a requirement for Msk function in cell viability (Johnson Hamlet and Perkins, 2001). It is possible that a further decrease, but not complete loss in Msk function, could result in earlier defects in myogenesis as we observed muscle patterning defects, predominantly characterized by missing muscles, in a subset of msk mutant embryos (12.1% in msk2/msk4 and 12.5% in msk4/msk5 trans-heterozygous combinations).
Late tendon cell differentiation and/or maintenance are affected in msk mutants
After cell fate determination is established in somatic muscle development, myoblast fusion and myotube migration begin to proceed simultaneously in stage 13 embryos until the final muscle pattern is completed (Schnorrer and Dickson, 2004). As the migrating myotubes approach their target tendon cells, the αPS2βPS integrin heterodimer begins to accumulate at the leading edge of the muscle. This integrin complex is required for at least two separate events: (1) to serve as a transmembrane link between the internal actin cytoskeleton and the ECM components Tsp and Tig (Schweitzer et al., 2010); and (2) for the proper localization and/or accumulation of Vein (Yarnitzky et al., 1997). The accumulation of Vein at the sites of muscle-tendon interactions is necessary for activation of the Egfr pathway and subsequent late tendon cell differentiation. In these mature, muscle-linked tendon cells, SrB expression is positively regulated. SrB also turns on the downstream transcriptional target Tsp, resulting in more Tsp secretion and subsequent strengthening of the MTJ through integrin binding (Schweitzer et al., 2010).
Our results suggest that Msk affects the later stages of tendon cell maturation and MTJ formation and/or maintenance. First, we did not observe any obvious defects in myoblast fusion or the guidance of muscles to their correct target tendon cell. Furthermore, Msk protein expression, visualized by both antibody immunolocalization and a fluoresecently-tagged Msk fusion protein, demonstrates that enrichment of Msk protein at the future muscle-tendon attachment sites occurs after stage 15. The appearance of Msk protein localization corresponds to the timing of MTJ junction formation, but it does not rule out the possibility that Msk has a role in earlier myogenic events. Third, the muscle detachment phenotypes observed in msk mutant embryos are consistent with the myospheroid phenotype observed for other genes, including myospheroid (βPS int), inflated (αPS2 int), and rhea (Talin), which are well-characterized for their role in embryonic muscle attachment (Brown, 1994; Brown et al., 2002; Leptin et al., 1989; Newman and Wright, 1981). Finally, in all msk mutants examined, regardless of the severity of the muscle detachment phenotypes, MTJ formation occurred in the correct location. Although the muscle attachment sites were smaller in msk mutants than in WT embryos, they were initially formed correctly, but not capable of reaching their mature size. These data taken together indicate that the muscle-specific αPS2βPS integrin complex initially forms an attachment to the ECM proteins Tig and Tsp. However, as mature tendon cell induction is compromised in msk mutant embryos, whereby the tendon cells are not able to produce and secrete proper levels of Tsp protein. Thus, the size of the mature MTJ is reduced and results in a decreased affinity at the muscle-tendon junctions.
Sr is a key factor in tendon cell differentiation in the embryo and fly thorax (Fernandes et al., 1996; Frommer et al., 1996; Volk and VijayRaghavan, 1994). In the embryo, SrB is essential for early tendon cell induction and SrA is activated for later tendon cell maturation (Volohonsky et al., 2007). Furthermore, ectopic expression of Sr can act as a guidance cue for migrating myotubes as ectopic expression of Sr in epidermal cells, the salivary glands, or the CNS results in muscle patterning defects where muscles take the incorrect route and/or become attached to ectopic cells expressing Sr (Becker et al., 1997; Vorbruggen and Jackle, 1997). Even though our data shows that Msk is required for nuclear Sr in the tendon cells, ectopic expression of Msk is not sufficient for tendon cell induction based upon three lines of experimentation. First, ectopic Msk expression in either the muscle or epidermis resulted in aberrant muscle attachment, but not misguided myotubes. All muscles were found to be in the correct position, regardless of Msk expression in domains outside of the normal hemisegments. Second, ectopic Msk expression was not sufficient to induce either early or elevated Sr levels. Third, expression of Msk in the salivary glands or CNS did not result in misguided muscles towards these locations.
Role of Msk in FAK-mediated Integrin adhesion and tendon cell signaling
Our tissue-specific rescue experiments show that Msk is required in the muscle cell for proper muscle-tendon attachment to occur. Thus, we propose two mechanisms, which are not mutually exclusive, by which Msk may be functioning in the muscle cells to exert its effect on tendon cell maturation (Fig. 10). First, Msk may be required directly and/or indirectly for the localization and/or accumulation of secreted Vein. As antibodies against Vein were not available for us to test this possibility, we showed that reintroducing Vein in msk mutants could rescue muscle attachment defects. Second, Msk may act via an integrin-dependent mechanism to modulate adhesion, which is explained in detail below.
From our studies, Msk localizes to the ends of muscles at the sites of muscle attachment. We propose that this localization of Msk recruits other proteins to the sites of muscle attachment to sequester proteins near the cell periphery and/or to modulate integrin affinity at the muscle attachment site. First, the absence of pFAK localization in msk mutants strongly suggests that Msk is essential for pFAK localization to the muscle-tendon attachment site. Surprisingly, mutations in FAK do not result in embryonic muscle attachment defects (Grabbe et al., 2004). However, pFAK localization is also lost in integrin mutants, suggesting that pFAK is involved in undefined events in myogenesis. If Msk serves as a scaffold protein to localize pFAK and/or other molecules to the sites of muscle-tendon cell attachment, these proteins may play an accessory role in integrin-mediated adhesion. One idea is that a Msk-pFAK complex may serve to limit the signaling function of integrins so its adhesive role predominates in MTJ formation. It is well-established that integrins play both adhesive and signaling roles in cell migration and development (Brower, 2003; Pinon and Wehrle-Haller, 2010). Clustering of the cytoplasmic tails of βPS-integrins initiates a downstream signaling pathway that regulates gene expression in the Drosophila midgut, but is not sufficient to induce tendon cell differentiation in formation of the MTJ (Martin-Bermudo et al., 1999). This suggests that integrin-mediated adhesion is required to assemble ECM components and influence the ability of Vein to activate the Egfr pathway. While loss of FAK activity does not result in somatic muscle defects, overexpression of FAK does. Muscles that have detached from the epidermis as a result of FAK overexpression still retain αPS2 integrins at the muscle ends (Grabbe et al., 2004). As observed in mammalian systems, this raises the possibility that pFAK may play a role in integrin complex disassembly. Excess pFAK may either displace proteins that bind to the cytoplasmic domain of integrins or excessively phosphorylate proteins resulting in integrin complex turnover and a decrease in stable adhesion. Alternatively, pFAK may exhibit redundancy with another protein at the attachment sites. There is precedence for this in the fly as pFAK functions redundantly with the tyrosine kinase Src downstream of integrins in the larval neuromuscular junctions (NMJs) to restrict NMJ growth (Tsai et al., 2008). Futhermore, in FAK mutants, phosphotyrosine signal is still observed at the sites of muscle attachment (Grabbe et al., 2004), supporting the idea that another tyrosine kinase is functional. A viable candidate may be Src42A, as it is also expressed at the sites of muscle attachment in the embryo (Takahashi et al., 2005).
The canonical role for Msk is to import proteins, such as activated MAPK into the nucleus. However, we did not detect activated MAPK or nuclear Msk in the nuclei of muscle cells. As we showed in Fig. 4, we detected strong Msk immunolocalization at the MTJ in stage 16 embryos, but not in the nuclei of developing muscles in stages 13-15. We cannot rule out the possibility that Msk is present at low levels in the muscle and we were not able to detect it. It is true that we can see our YFP-Msk fusion protein in the nucleus (Fig. 4G, H), but this may be due to the over-expression of our protein. While it is possible that nuclear Msk and MAPK in muscles are required for some aspects of myogenesis, this requires further analysis.
Future experiments will determine the precise mechanism by which Msk influences Vein secretion, localization, and/or accumulation at muscle-tendon cell attachment sites. Is it mediated through integrins? Is phosphorylation of Msk essential for activity? Msk is tyrosine phosphorylated in response to insulin and PS integrins, although the kinase remains unknown. FAK may be an example of a kinase that can phosphorylate Msk at the muscle attachment sites. In mammalian studies, the canonical role for Importin-7 is in nuclear import. To our knowledge, other roles for cytoplasmic Importin-7 have not been examined. Thus, it will be interesting to uncover new roles for Importin-7, specifically in vertebrate muscle development.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Liz Perkins, Hanh Nguyen, Julie Kadmras, Talila Volk, John Fessler, Xiaohang Yang, and Jessica Treisman for providing fly stocks and reagents. We thank Bridget Biersmith and Zong-Heng Wang for critical reading of the manuscript. We thank the Developmental Studies Hybridoma Bank developed under NICHD for antibodies and the Bloomington Stock Center for flies. The proteomics screen was performed in Susan Abmayr’s lab (NIH AR044274). E.R.G. is supported by National Institutes of Health grant AR059311.
REFERENCES
- Bahri SM, Choy JM, Manser E, Lim L, Yang X. The Drosophila homologue of Arf-GAP GIT1, dGIT, is required for proper muscle morphogenesis and guidance during embryogenesis. Dev Biol. 2009;325:15–23. doi: 10.1016/j.ydbio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Baker SE, Lorenzen JA, Miller SW, Bunch TA, Jannuzi AL, Ginsberg MH, Perkins LA, Brower DL. Genetic interaction between integrins and moleskin, a gene encoding a Drosophila homolog of importin-7. Genetics. 2002;162:285–96. doi: 10.1093/genetics/162.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Pasca G, Strumpf D, Min L, Volk T. Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development. 1997;124:2615–22. doi: 10.1242/dev.124.13.2615. [DOI] [PubMed] [Google Scholar]
- Bogaert T, Brown N, Wilcox M. The Drosophila PS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell. 1987;51:929–40. doi: 10.1016/0092-8674(87)90580-0. [DOI] [PubMed] [Google Scholar]
- Bour BA, O’Brien MA, Lockwood WL, Goldstein ES, Bodmer R, Taghert PH, Abmayr SM, Nguyen HT. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;9:730–41. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- Brower DL. Platelets with wings: the maturation of Drosophila integrin biology. Curr Opin Cell Biol. 2003;15:607–13. doi: 10.1016/s0955-0674(03)00102-9. [DOI] [PubMed] [Google Scholar]
- Brown NH. Null mutations in the alpha PS2 and beta PS integrin subunit genes have distinct phenotypes. Development. 1994;120:1221–31. doi: 10.1242/dev.120.5.1221. [DOI] [PubMed] [Google Scholar]
- Brown NH, Gregory SL, Rickoll WL, Fessler LI, Prout M, White RA, Fristrom JW. Talin is essential for integrin function in Drosophila. Dev Cell. 2002;3:569–79. doi: 10.1016/s1534-5807(02)00290-3. [DOI] [PubMed] [Google Scholar]
- Bunch TA, Graner MW, Fessler LI, Fessler JH, Schneider KD, Kerschen A, Choy LP, Burgess BW, Brower DL. The PS2 integrin ligand tiggrin is required for proper muscle function in Drosophila. Development. 1998;125:1679–89. doi: 10.1242/dev.125.9.1679. [DOI] [PubMed] [Google Scholar]
- Chanana B, Graf R, Koledachkina T, Pflanz R, Vorbruggen G. AlphaPS2 integrin-mediated muscle attachment in Drosophila requires the ECM protein Thrombospondin. Mech Dev. 2007;124:463–75. doi: 10.1016/j.mod.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Clark KA, McGrail M, Beckerle MC. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development. 2003;130:2611–21. doi: 10.1242/dev.00492. [DOI] [PubMed] [Google Scholar]
- Estrada B, Gisselbrecht SS, Michelson AM. The transmembrane protein Perdido interacts with Grip and integrins to mediate myotube projection and attachment in the Drosophila embryo. Development. 2007;134:4469–78. doi: 10.1242/dev.014027. [DOI] [PubMed] [Google Scholar]
- Fernandes JJ, Celniker SE, VijayRaghavan K. Development of the indirect flight muscle attachment sites in Drosophila: role of the PS integrins and the stripe gene. Dev Biol. 1996;176:166–84. doi: 10.1006/dbio.1996.0125. [DOI] [PubMed] [Google Scholar]
- Fogerty FJ, Fessler LI, Bunch TA, Yaron Y, Parker CG, Nelson RE, Brower DL, Gullberg D, Fessler JH. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development. 1994;120:1747–58. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- Freedman ND, Yamamoto KR. Importin 7 and importin alpha/importin beta are nuclear import receptors for the glucocorticoid receptor. Mol Biol Cell. 2004;15:2276–86. doi: 10.1091/mbc.E03-11-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer G, Vorbruggen G, Pasca G, Jackle H, Volk T. Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J. 1996;15:1642–9. [PMC free article] [PubMed] [Google Scholar]
- Gabay L, Seger R, Shilo BZ. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development. 1997;124:3535–41. doi: 10.1242/dev.124.18.3535. [DOI] [PubMed] [Google Scholar]
- Geisbrecht ER, Haralalka S, Swanson SK, Florens L, Washburn MP, Abmayr SM. Drosophila ELMO/CED-12 interacts with Myoblast city to direct myoblast fusion and ommatidial organization. Dev Biol. 2008;314:137–49. doi: 10.1016/j.ydbio.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsohn E, Volk T. Slowdown promotes muscle integrity by modulating integrin-mediated adhesion at the myotendinous junction. Development. 137:785–94. doi: 10.1242/dev.043703. [DOI] [PubMed] [Google Scholar]
- Gorlich D, Dabrowski M, Bischoff FR, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. J Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe C, Zervas CG, Hunter T, Brown NH, Palmer RH. Focal adhesion kinase is not required for integrin function or viability in Drosophila. Development. 2004;131:5795–805. doi: 10.1242/dev.01462. [DOI] [PubMed] [Google Scholar]
- Hatini V, DiNardo S. Divide and conquer: pattern formation in Drosophila embryonic epidermis. Trends Genet. 2001;17:574–9. doi: 10.1016/s0168-9525(01)02448-9. [DOI] [PubMed] [Google Scholar]
- Jakel S, Albig W, Kutay U, Bischoff FR, Schwamborn K, Doenecke D, Gorlich D. The importin beta/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J. 1999;18:2411–23. doi: 10.1093/emboj/18.9.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel S, Gorlich D. Importin beta, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 1998;17:4491–502. doi: 10.1093/emboj/17.15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BP, Bunch TA, Krishnamoorthy S, Perkins LA, Brower DL. Nuclear localization of the ERK MAP kinase mediated by Drosophila alphaPS2betaPS integrin and importin-7. Mol Biol Cell. 2007;18:4190–9. doi: 10.1091/mbc.E06-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Hamlet MR, Perkins LA. Analysis of corkscrew signaling in the Drosophila epidermal growth factor receptor pathway during myogenesis. Genetics. 2001;159:1073–87. doi: 10.1093/genetics/159.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer SG, Kidd T, Simpson JH, Goodman CS. Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science. 2001;292:737–40. doi: 10.1126/science.1058766. [DOI] [PubMed] [Google Scholar]
- Leptin M, Bogaert T, Lehmann R, Wilcox M. The function of PS integrins during Drosophila embryogenesis. Cell. 1989;56:401–8. doi: 10.1016/0092-8674(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Lorenzen JA, Baker SE, Denhez F, Melnick MB, Brower DL, Perkins LA. Nuclear import of activated D-ERK by DIM-7, an importin family member encoded by the gene moleskin. Development. 2001;128:1403–14. doi: 10.1242/dev.128.8.1403. [DOI] [PubMed] [Google Scholar]
- Marenda DR, Vrailas AD, Rodrigues AB, Cook S, Powers MA, Lorenzen JA, Perkins LA, Moses K. MAP kinase subcellular localization controls both pattern and proliferation in the developing Drosophila wing. Development. 2006;133:43–51. doi: 10.1242/dev.02168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bermudo MD. Integrins modulate the Egfr signaling pathway to regulate tendon cell differentiation in the Drosophila embryo. Development. 2000;127:2607–15. doi: 10.1242/dev.127.12.2607. [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo MD, Alvarez-Garcia I, Brown NH. Migration of the Drosophila primordial midgut cells requires coordination of diverse PS integrin functions. Development. 1999;126:5161–9. doi: 10.1242/dev.126.22.5161. [DOI] [PubMed] [Google Scholar]
- Martin D, Zusman S, Li X, Williams EL, Khare N, DaRocha S, Chiquet-Ehrismann R, Baumgartner S. wing blister, a new Drosophila laminin alpha chain required for cell adhesion and migration during embryonic and imaginal development. J Cell Biol. 1999;145:191–201. doi: 10.1083/jcb.145.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason DA, Goldfarb DS. The nuclear transport machinery as a regulator of Drosophila development. Semin Cell Dev Biol. 2009;20:582–9. doi: 10.1016/j.semcdb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Newman SM, Jr., Wright TR. A histological and ultrastructural analysis of developmental defects produced by the mutation, lethal(1)myospheroid, in Drosophila melanogaster. Dev Biol. 1981;86:393–402. doi: 10.1016/0012-1606(81)90197-4. [DOI] [PubMed] [Google Scholar]
- Pinon P, Wehrle-Haller B. Integrins: versatile receptors controlling melanocyte adhesion, migration and proliferation. Pigment Cell Melanoma Res. 2010 doi: 10.1111/j.1755-148X.2010.00806.x. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134:4131–40. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- Roignant JY, Treisman JE. Exon junction complex subunits are required to splice Drosophila MAP kinase, a large heterochromatic gene. Cell. 2010;143:238–50. doi: 10.1016/j.cell.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann T, Jensen LJ, Jakobsen JS, Karzynski MM, Eichenlaub MP, Bork P, Furlong EE. A temporal map of transcription factor activity: mef2 directly regulates target genes at all stages of muscle development. Dev Cell. 2006;10:797–807. doi: 10.1016/j.devcel.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Schnorrer F, Dickson BJ. Muscle building; mechanisms of myotube guidance and attachment site selection. Dev Cell. 2004;7:9–20. doi: 10.1016/j.devcel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Schnorrer F, Kalchhauser I, Dickson BJ. The transmembrane protein Kon-tiki couples to Dgrip to mediate myotube targeting in Drosophila. Dev Cell. 2007;12:751–66. doi: 10.1016/j.devcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Zelzer E, Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development. 2010;137:2807–17. doi: 10.1242/dev.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Wayburn B, Bunch T, Volk T. Thrombospondin-mediated adhesion is essential for the formation of the myotendinous junction in Drosophila. Development. 2007;134:1269–78. doi: 10.1242/dev.000406. [DOI] [PubMed] [Google Scholar]
- Swan LE, Schmidt M, Schwarz T, Ponimaskin E, Prange U, Boeckers T, Thomas U, Sigrist SJ. Complex interaction of Drosophila GRIP PDZ domains and Echinoid during muscle morphogenesis. EMBO J. 2006;25:3640–51. doi: 10.1038/sj.emboj.7601216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan LE, Wichmann C, Prange U, Schmid A, Schmidt M, Schwarz T, Ponimaskin E, Madeo F, Vorbruggen G, Sigrist SJ. A glutamate receptor-interacting protein homolog organizes muscle guidance in Drosophila. Genes Dev. 2004;18:223–37. doi: 10.1101/gad.287604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Takahashi F, Ui-Tei K, Kojima T, Saigo K. Requirements of genetic interactions between Src42A, armadillo and shotgun, a gene encoding E-cadherin, for normal development in Drosophila. Development. 2005;132:2547–59. doi: 10.1242/dev.01850. [DOI] [PubMed] [Google Scholar]
- Tsai PI, Kao HH, Grabbe C, Lee YT, Ghose A, Lai TT, Peng KP, Van Vactor D, Palmer RH, Chen RH, Yeh SR, Chien CT. Fak56 functions downstream of integrin alphaPS3betanu and suppresses MAPK activation in neuromuscular junction growth. Neural Dev. 2008;3:26. doi: 10.1186/1749-8104-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T, VijayRaghavan K. A central role for epidermal segment border cells in the induction of muscle patterning in the Drosophila embryo. Development. 1994;120:59–70. doi: 10.1242/dev.120.1.59. [DOI] [PubMed] [Google Scholar]
- Volohonsky G, Edenfeld G, Klambt C, Volk T. Muscle-dependent maturation of tendon cells is induced by post-transcriptional regulation of stripeA. Development. 2007;134:347–56. doi: 10.1242/dev.02735. [DOI] [PubMed] [Google Scholar]
- Vorbruggen G, Jackle H. Epidermal muscle attachment site-specific target gene expression and interference with myotube guidance in response to ectopic stripe expression in the developing Drosophila epidermis. Proc Natl Acad Sci U S A. 1997;94:8606–11. doi: 10.1073/pnas.94.16.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrailas AD, Marenda DR, Cook SE, Powers MA, Lorenzen JA, Perkins LA, Moses K. smoothened and thickveins regulate Moleskin/Importin 7-mediated MAP kinase signaling in the developing Drosophila eye. Development. 2006;133:1485–94. doi: 10.1242/dev.02334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitzky T, Min L, Volk T. The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells. Genes Dev. 1997;11:2691–700. doi: 10.1101/gad.11.20.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol. 2001;152:1007–18. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol. 2000;20:6354–63. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.