Abstract
Extracellular cesium causes synchronous, interictal-like bursting and prevents maintenance of long-term depression (LTD) in the CA1 hippocampal region. We have investigated the cellular mechanisms underlying cesium actions. Whole-cell recordings showed that brief (2 min) bath exposures to cesium caused pyramidal cell hyperpolarization associated with decreased membrane conductance attributable to blockade of an inward h-type current. After prolonged (>2 min) exposures, a late depolarizing response was observed; this effect was not associated with changes in cell membrane conductance. Recordings from interneurons revealed that Ih is expressed in a subpopulation of cells and that cesium effects on interneurons expressing Ih are comparable to those observed in pyramidal cells. Consistent with this effect, cesium decreased the early component of the IPSP recorded in pyramidal cells. Interneurons lacking Ih were not affected by cesium but developed a depolarizing response when drug applications were paired to orthodromic stimulation. We concluded that cesium actions on LTD and cesium-induced epileptiform activity were not attributable exclusively to its direct effects on neurons. Recordings from hippocampal slice astrocytes revealed that cesium interfered with glial electrical responses during LTD induction. Cesium blocked glial inwardly rectifying potassium channels and increased the amplitude and duration of stimulation-evoked [K+]outincreases. Thus, the effects of cesium on CA1 synchronization and synaptic plasticity appear to be mediated predominantly by blockade of glial voltage-dependent potassium uptake.
Keywords: spatial buffering, epilepsy, synchronization, astrocyte, potassium, extracellular space
Hippocampal excitability is regulated by an interaction of excitatory and inhibitory potentials (Schwartzkroin and Prince, 1980; Schwartzkroin and Wyler, 1980; Schwartzkroin, 1986;Kriegstein et al., 1987). In CA1 neurons depolarizing ion conductances are regulated primarily by the voltage-dependent activation/inactivation properties of Na+ and Ca2+ channels. Na+ and Ca2+currents also underlie the generation of EPSPs. Termination of depolarizing potentials occurs by the voltage- and calcium-dependent activation of intrinsic potassium conductances and by activation of inhibitory interneurons releasing GABA (Lacaille et al., 1987; Janigro and Schwartzkroin, 1988a,b). The latter are responsible for the postsynaptic activation of chloride and potassium currents. Whereas INa, ICa and IEPSP are, under physiological conditions, relatively independent from modest changes in the driving force for the permeant ions (because ENa and ECa are far from cell resting potential), both repolarizing potassium and IPSP currents are affected by even modest changes in resting potential (RMP), [K+]out, or [Cl−]in (Somjen, 1979; Walz and Hertz, 1983;Ballanyi et al., 1987; Dietzel et al., 1989; Ballanyi et al., 1993).
Because neuronal RMP depends, albeit not exclusively, on [K+]out, the maintenance of a homeostatic control for [K+]out plays a crucial role in the regulation of neuronal firing. [K+]out is controlled by neuronal and glial mechanisms (Hagland and Schwartzkroin, 1990; Brines and Robbins, 1993). In the CNS, the potassium spatial buffering hypothesis (Orkand, 1966) has attempted to describe some of the features involved in the glial regulation of [K+]out (Ballanyi et al., 1987; Casullo and Krnjevic, 1987).
The functional properties of neuronal voltage-dependent ion currents are routinely investigated by using pharmacological tools aimed at ion channel blockade. In particular, monovalent cations such as cesium (1–3 mm) have been shown to cause voltage-dependent block (as well as activation; see DiFrancesco, 1982) of both inwardly rectifying potassium (IIR) and mixed cation currents (Ih) (Spain et al., 1987a; Maccaferri et al., 1993). Only Ih is expressed in hippocampal neurons (Maccaferri et al., 1993). However, both of these cesium-sensitive conductances (IIR and Ih) have also been described in hippocampal and cortical astrocytes (Sontheimer and Waxman, 1993;Bayliss et al., 1994; Guatteo et al., 1996). Whereas neuronal Ih is responsible for control of resting membrane potential, glial mixed cation, hyperpolarization-activated (Iha), and potassium currents are thought to be involved in buffering extracellular potassium ions.
We have shown recently that extracellularly applied cesium can abolish maintenance of long-term depression in the hippocampus (Maccaferri et al., 1994). This effect was associated with epileptiform activity characterized by synchronous burst discharges of CA1 pyramidal cells. Similar observations of synchronous activity have been reported for neocortical slices treated with cesium and with the convulsant bicuculline (Hwa and Avoli, 1991). Because the consequences of cesium application in the CA1 region were difficult to explain on the basis of a purely neuronal effect on Ih, which would hyperpolarize neurons, we decided to investigate further the electrophysiological properties of CA1 neurons and glial cells exposed to millimolar concentrations of cesium. We hypothesized that the neuronal synchronization and abolishment of LTD were attributable to cesium-mediated blockade of K+ uptake into hippocampal glia.
MATERIALS AND METHODS
Slice preparation. Hippocampal slices were prepared from young male Wistar rats (16–18 d) (Maccaferri et al., 1993, 1994). Briefly, ether-anesthetized rats were decapitated and the heads were kept in ice-cold, oxygenated, modified, artificial CSF (ACSF) composed of (in mm): 120 NaCl, 3.1 KCl, 4 MgCl2, 1 CaCl2, 1.25 KH2PO4, 26 NaHC03, 10 dextrose. The whole brain was rapidly dissected and glued on the stage of a vibratome, and 400-μm-thick slices were cut perpendicular to the longitudinal axis of the hippocampus. Slices were then stored at 32°C in a recovery chamber containing the following oxygenated saline solution (in mm): 120 NaCl, 3.1 KCl, 1 MgCl2, 2 CaCl2, 1.25 KH2PO4, 26 NaHCO3, 10 dextrose at 32°C. Both solutions were equilibrated with 95% O2/5% CO2 to a final pH of 7.4.
Hippocampal patch-clamp recording. After at least 1 hr spent in the holding chamber, slices were gently transferred to a submersion recording chamber where they were continuously perfused at a rate of 4–5 ml/min with freshly oxygenated ACSF. In some experiments (for example, Fig. 3), a modified perfusion apparatus consisting of a perfusion pipette (200 μm diameter) was used. The perfusion outlet was placed either in the recording chamber 5 mm from the slice or directly above the CA1 region (see below). The latter method allowed solution exchange within 5 sec. Patch-clamp recordings were carried out at room temperature (range 22–25°C) in the whole-cell configuration using an Axopatch 200A (Axon Instruments, Foster City, CA); temperature fluctuations allowed within the same experiment were <1°C. Seal formation was established under visual control, maintaining positive pressure in the patch electrode when entering into the slice. Field potentials were recorded with an extracellular pipette filled with normal ACSF equilibrated with 95% O2/5% CO2. Patch pipettes were filled with (mm): 140 K-gluconate, 1 MgCl2, 2 Na2ATP, 0.3 NaGTP, 10 HEPES, 0.5 EGTA, final pH of 7.2. Pipettes had a resistance of ∼4 MΩ. Cell and pipette capacitance compensation, signal filtering, and series resistance compensation were performed as described previously (Maccaferri et al., 1993, 1994).
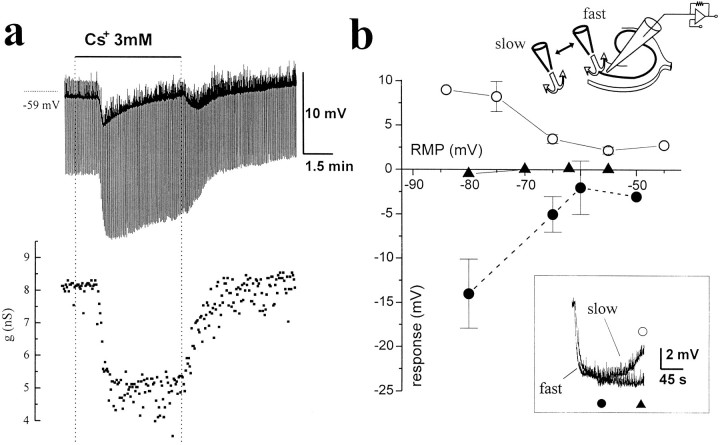
Fig. 3.
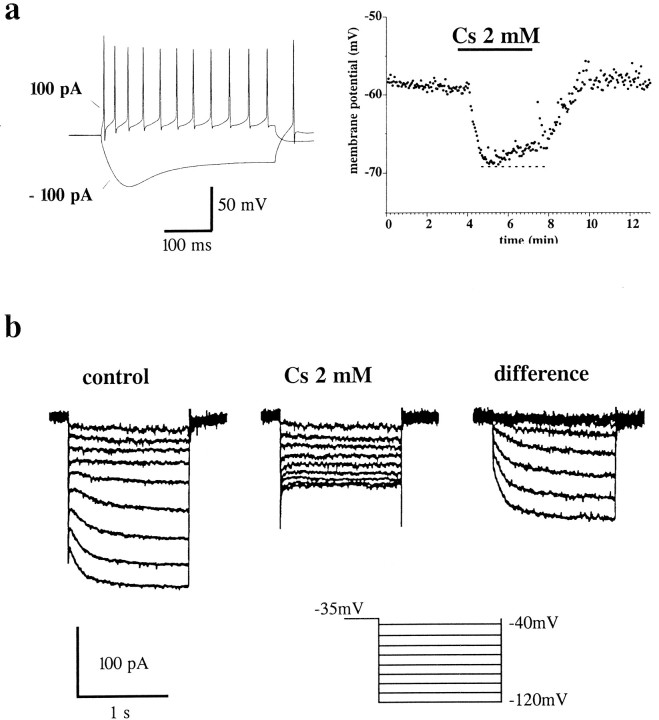
Complex actions of cesium on CA1 pyramidal cell RMP and RIN. a, Top, Voltage recording from a pyramidal cell (initial RMP = −59 mV).Bottom, Cell membrane conductance changes. Note the hyperpolarization induced by “slow” application andremoval of cesium (3 mm). After the early hyperpolarizing response induced by cesium, the cell progressively depolarized. This response was not associated with a change in cell RIN(a, bottom). The current pulses injected were of 50 pA amplitude and 300 msec duration. Note that the frequency of spontaneous EPSPs increased after cesium perfusion and was even higher after cesium washout. b, Correlation between the initial holding potential of pyramidal cells in current clamp (abscissa) and the voltage changes attributed to a local (“fast”) or bath (“slow”) application of cesium. The depolarizing phase, as measured relative to the maximal hyperpolarized level, was observed after slow perfusion (open circles), but not after fast perfusion (filled triangles). Filled circles refer to the maximal hyperpolarization obtained after either fast or slow application of Cs+. Theinset in b shows the superimposition of raw data from one of these experiments to compare the different time course of membrane potential with different perfusion rates.
Orthodromic activation of the afferent pathway was accomplished by a constant current stimulator WPI A365 (World Precision Instruments). The stimulation was carried out by a bipolar concentric tungsten electrode placed in the stratum radiatum of the CA1 region to stimulate Schaffer collaterals. Stimulation rate was set at 0.1, 0.25, or 1 Hz, and pulse duration was fixed at 50 μsec. In protocols used to elicit LTD, stimulus intensity was fixed at two-thirds of the maximally effective stimulus. In protocols designed to test changes in RMP during 1 Hz stimulation, stimulus intensity was set above the firing threshold. Postsynaptic field potentials (fEPSP) recorded in stratum radiatum of CA1 were quantified by measuring the slope of the initial phase of the response. Current or voltage traces were recorded on a digital tape recorder (BioLogic) digitized at 48 kHz on DAT tapes for off-line analysis.
Variable lengths of application of cesium were used to evoke Ih-dependent and -independent responses (see Results). Perfusions lasting 2 min are referred to as “brief,” whereas longer perfusion times (6 min) are designated as “prolonged.” In addition, two slice perfusion methods were used to control the rate of solution exchange within the slice. “Slow” perfusion was achieved by placing a 200 μm internal diameter perfusion pipette ∼5 mm from the slice. To increase the rate of drug application and clearance from the slice (“fast” perfusion), the same pipette was positioned immediately above the recording electrode.
Perforated whole-cell recording from glial cells. The experiments carried out to elucidate the electrical behavior ofin situ glial cells were performed with the perforated-patch technique from visually localized cells. The antibiotic gramicidin was used at a concentration of 15 μg/ml in a solution containing (mm): 35 HEPES, 70 KCl, 70 KF, 10 NaCl, 1 EGTA. We routinely used KF to monitor patch-rupturing events. Accidental rupture of the seal was characterized by a large and sudden depolarization attributable to the blocking action of intracellular KF on potassium currents. Series resistance was ∼70 MΩ and was compensated at 70%.
Extracellular potassium measurements by ion-selective microelectrodes. Double-barreled borosilicate capillaries were treated with sulfuric acid dissolved in 30% H2O2, carefully washed, and treated with increasing concentrations of acetone to displace water and improve drying. Pipettes were dried at 100°C and pulled by a Narishige PB-7 vertical puller to obtain microelectrodes with tip diameters of ∼2.0–2.5 μm. The back of one barrel was plugged by wax, and the whole microelectrode was exposed overnight to thrymethyl-chloro-sylane (1 ml in 1 l volume whole glass chamber). The tip of the sylanized barrel was back-filled with the potassium selective resin (Fluka Cocktail “B”), and the rest of the barrel was filled with KCl (140 mm). The reference barrel was filled with ACSF. A WPI high-impedance dual-differential electrometer (WPI FD223) was used for both [K+]out and simultaneous field potential recordings. Both signals were digitized and stored on DAT tape. The potential measured by ion-selective microelectrodes (ISMs) is the sum of the field potential and the electromotive force (EMF) generated by the activity of the ion to which the electrode is sensitive. The field potential was subtracted analogically from the ion measurement to dissect the contribution attributable to [K+]out changes.
A set of microelectrodes was prepared the day before the experiments. Completed electrodes were stored overnight and calibrated just before use. We routinely performed tests for the selectivity of the electrode when the potassium channel blocker cesium was to be used during [K+]out measurements. A complete description of the methods used to compensate for the interfering ion can be found in references (Nicolsky, 1937; Eisenman, 1967; Ammann, 1986). Briefly, the EMF produced by the potassium selective electrode in the presence of a constant concentration of cesium can be described by the Nicolsky–Eisenman equation: EMF =E0 + s log[aK+ ∑KK,CsaCs]
where Eo is the reference potential (compensated by the amplifier), s is the Nernstian slope (59 mV/decade at room temperature), aK andaCs are the activities for potassium and the interfering ion, respectively, and KK,Csrepresents the potentiometric selectivity factor and is the parameter that considers the interfering action of cesium.KK,Cs was estimated according to the fixed interference method (Ammann, 1986). The relationship between EMF read by the electrometer and the corresponding [K+] was obtained by fitting the Nicolsky–Eisenman equation to the experimental calibration points. The calibration was performed using ACSF where increasing [K+] was compensated for by removal of isomolar [Na+]. Potassium concentrations of 3, 4.35, 8, 12, and 43.5 mm, with or without CsCl 3 mm, were used. Data are presented as mean ± SEM.
RESULTS
Effects of Cs+ on synaptic plasticity and CA1 excitability
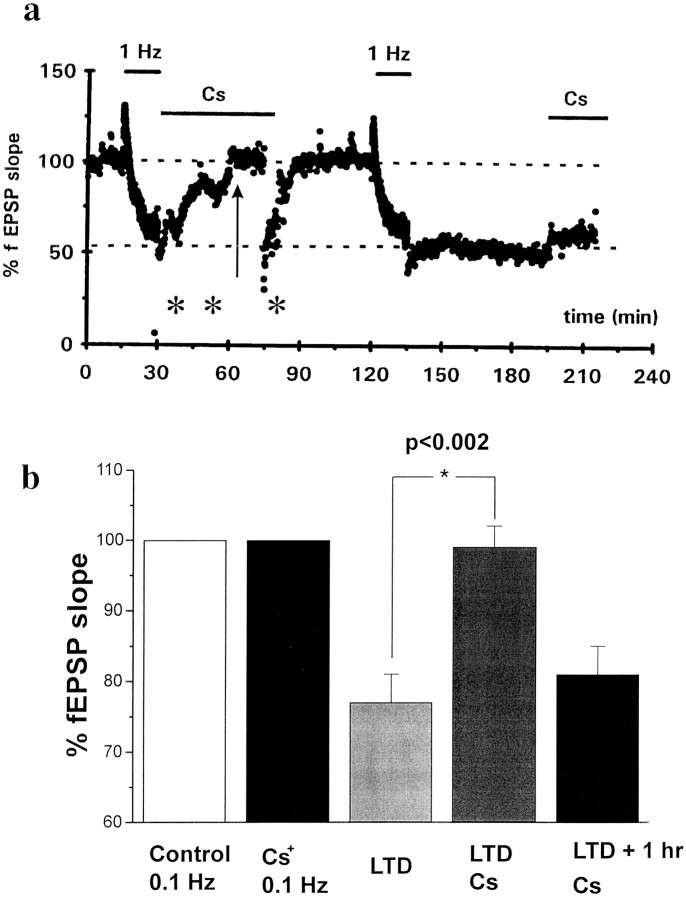
After establishing a 30 min baseline orthodromically stimulating the Schaffer collaterals at 0.1 Hz, LTD of synaptic potentials at the CA3–CA1 synapse was induced by stimulation at 1 Hz for 15 min (Dudek and Bear, 1992; Mulkey and Malenka, 1992; Christie et al., 1994;Maccaferri et al., 1994). In control experiments, LTD induction was characterized by a depression of the fEPSP slope (25 ± 3%,n = 12; Fig. 1B). Perfusion with 2 mm Cs+ for up to 15 min had no effect on fEPSP slope during 0.1 Hz stimulation. In our previous study, we tested the effect of Cs+ pretreatment on LTD maintenance. In this study, we extended our observations by applying Cs+ during (n = 9) or immediately after (n = 9) the induction of LTD. Both protocols caused reversal of long-term depression. The effect of cesium was reversible upon prolonged washout (>45 min), because after removal of the drug the same slices were able to undergo additional LTD (Fig. 1). Cesium application 60 min after LTD induction failed to elicit reversal of LTD (Fig. 1A,B, n = 5). The spontaneous activity/hyperexcitability caused by cesium (for example, Fig. 2) resulted in an apparent scatter of the fEPSP data. This does not reflect either depression or potentiation of the fEPSP but relates rather to unstimulated field potential changes (see, for example, Fig. 1).
Fig. 1.
Cesium prevents maintenance of long-term depression. LTD was induced by orthodromic stimulation of the Schaffer collaterals at 1 Hz for 15 min. Field excitatory postsynaptic potentials were evoked and recorded by the extracellular pipette. Note the marked decrease in fEPSP slope that occurred during 1 Hz stimulation (a). At the end of the first 15 min of 1 Hz stimulation, cesium (2 mm) was added to the bath solution. This procedure reduced the synaptic depression. Asterisksindicate increased scatter of the fEPSP slope values recorded after the first train of 1 Hz and during perfusion of cesium. This scatter is a reflection of the increased excitability of the tissue (see text), which caused spontaneous fEPSP and/or CA1 neuronal bursting activity. Note that the effects of cesium on spontaneous activity were not reversed by cesium washout. After washout of cesium, LTD could be induced and maintained in the same slice. Cesium application after LTD induction effectively reduced the synaptic depression only if cesium were applied immediately after the end of 1 Hz stimulation. Application of cesium 1 hr after LTD failed to induce any significant change in LTD maintenance or cause neuronal synchronization. The lack of effect was not attributable to the shorter exposure time to cesium, because similar results were obtained after prolonged perfusions.b, Graph showing the cumulative response of 18 slices. The “LTD” and “LTD+Cs” data refer to the amplitude of the response at 30 min after 1 Hz stimulation (arrow inA) and have been normalized by baseline values (Control 0.1 Hz). Cesium had no effects on fEPSP slope in slices treated only with 0.1 Hz stimulation (Cs+ 0.1 Hz). Similarly, Cs+ did not cause reversal of LTD when applied 1 hr after LTD induction (LTD+1 hr)
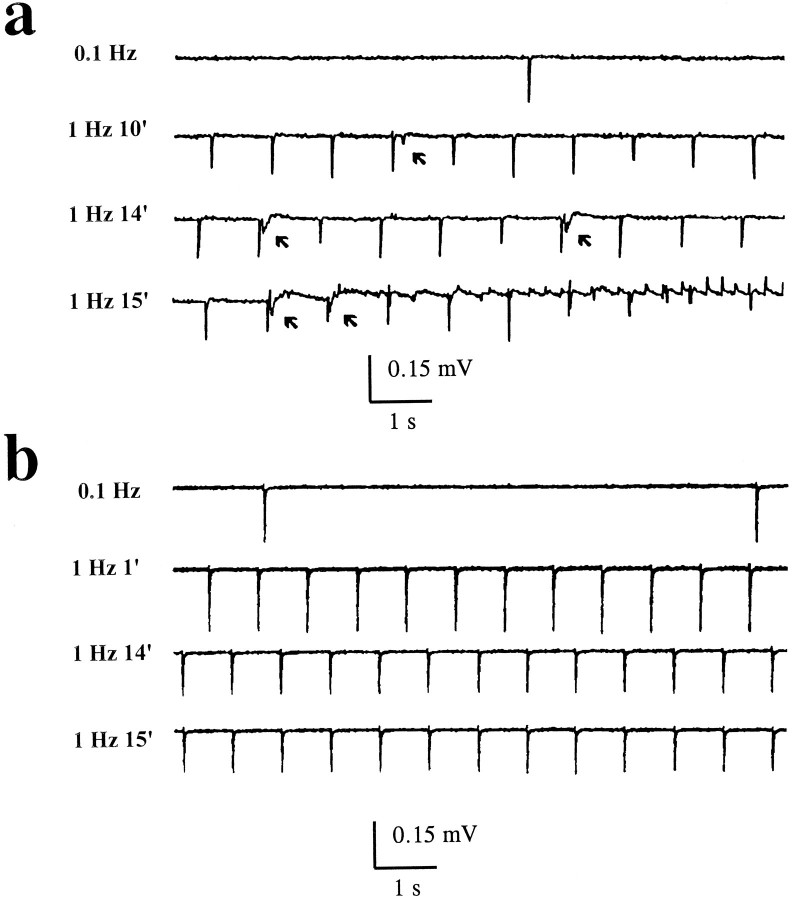
Fig. 2.
The effects of cesium on LTD are not mediated by blockade of Ih. Effects on field potentials of cesium and Zeneca ZD-7288 applications paired to 1 Hz stimulation. Both stimulating and recording electrodes were placed in stratum radiatum. a, At low (0.1 Hz) stimulation frequency, cesium (3 mm) failed to induce any effects on the evoked fEPSP even after prolonged perfusion (top trace, 15 min after Cs+ + 0.1 Hz stimuli). Similarly, brief episodes of stimulation at 1 Hz also left the fEPSP response unchanged. However, after protracted (10 min) exposure to cesium while stimulating at 1 Hz, afterdischarge activity became evident (arrows). The latter outlasted the washout of cesium (data not shown). b, In contrast to cesium, the Ih-specific blocker Zeneca ZD-7288 (10 μm) did not cause the appareance of spontaneous afterdischarge-like activity during 15 min of orthodromic stimulation used to elicit LTD.
When LTD protocols were paired to co-application of cesium (2 or 3 mm), or when cesium application closely followed LTD induction, large spontaneous field potentials were recorded (experiments from 18 slices). These field potentials reflected synchronous burst discharges of CA1 pyramidal cells as revealed by intracellular recordings (data not shown) and were never observed when application of cesium was paired to low-frequency (0.1 Hz) stimulation alone. LTD induction per se never caused spontaneous population discharges or resulted in synchronous field bursting (n= 20).
The cesium-induced epileptiform activity during 1 Hz stimulation developed over time; the appearance of spontaneous events was preceded by afterdischarges after orthodromic stimuli (Fig.2A, arrows; n = 9 slices). Subsequently, spontaneous rhythmic events became the predominant feature of the field potential (see recordings at 15 min in Fig.2A). The effects of cesium on neuronal synchronization and hyperexcitability typically outlasted the application of the drug itself and were independent of experimental stimulation of the afferent pathways. In fact, spontaneous activity persisted after cessation of orthodromic 1 Hz stimulation.
Because these actions of cesium could be attributed to an effect on neuronal Ih or on IIR expressed in glia (or both), we used a pharmacological manipulation to dissect out the relative contribution of these two mechanisms. In contrast to cesium, the Ih-specific ion channel blocker Zeneca ZD-7288 (10 μm) (BoSmith et al., 1993) failed to affect LTD maintenance and did not cause spontaneous field activity (Fig.2B, n = 5 slices).
Cs+ increases CA1 pyramidal cell RIN and causes a biphasic change in RMP
In current-clamp experiments, brief applications of cesium (2–3 mm) at cell resting potential (−59 ± 0.5 mV, RIN 260 ± 16 MΩ; n = 20) invariably caused hyperpolarization of CA1 pyramidal cells (by −3.5 ± 0.3mV, Cs+ 2 mm, n = 15; by −5.6 ± 0.6 mV, Cs+ 3 mm,n = 5) associated with a large increase in cell input resistance (50% ± 8%, Cs+ 3 mm,n = 5). Cesium caused the disappearance of the typical sag potential recorded in these cells during brief hyperpolarizing current injections, suggesting that the main action of cesium on CA1 cells was attributable to blockade of Ih (Maccaferri et al., 1993). These effects of brief bath perfusion of cesium were mimicked by the h-current-specific blocker Zeneca ZD-7288 (BoSmith et al., 1993; Gasparini and DiFrancesco, in press).
During prolonged bath applications of Cs+ (Fig.3), the early hyperpolarizing response was followed by a late depolarization. To understand the nature of these responses, hyperpolarizing steps (300 msec duration, 50 pA amplitude) were applied to current-clamped pyramidal cells and cell input resistance changes were monitored (Fig. 3A). This procedure revealed that although the cesium-induced hyperpolarization was associated with a marked increase in RIN, the depolarizing response (3.8 ± 0.8 mV after 6 min in Cs+ 3 mm,n = 5) was not accompanied by any further detectable membrane conductance change. Removal of cesium caused a transient membrane hyperpolarization (2.4 ± 0.8 mV, Cs+ 3 mm, n = 5) at a time when the input resistance of the cells recovered (i.e., decreased) to pre-cesium values. In addition to the effects on RMP and RIN, cesium application was also characterized by a flurry of postsynaptic potential activity that outlasted the application of the blocker (Fig. 3A). Qualitatively similar results were obtained from cells bathed in 1, 2, and 5 mm cesium.
These results could be explained by an abnormal accumulation of K+ in the extracellular space. A consequent prediction is that increasing the rate of clearance of K+ would prevent the Cs-induced after-depolarization. To test this hypothesis, the perfusate was applied by a micropipette positioned above the CA1 region in close proximity to the cells that we were recording in order to increase the rate of K+ clearance. In Figure 3B, we compare results obtained using “fast” and “slow” perfusion protocols.
With slow perfusion, cesium caused a typical biphasic hyperpolarization/depolarization response (Fig. 3B, • and ○, respectively). In contrast, when the pipette was positioned near the recorded cell, only cesium-induced hyperpolarizations were observed. The graph in Figure 3B depicts the voltage dependency of these responses, obtained from four separate experiments; filled triangles refer to the size of the depolarizing response observed after fast perfusion with cesium. Depolarizing shifts are referred to the maximal hyperpolarized level recorded during cesium perfusion. These results are consistent with the hypothesis that cesium-induced depolarizations of pyramidal cells are attributable to accumulation of K+ in the extracellular space.
Cs+ reduces IPSPs but decreases CA1 pyramidal excitability
Because experimental epileptogenesis is, in some cases, attributable to a failure of synaptic inhibition, and because Cs+ application led to hyperpolarization, we studied the effects of Cs+ on IPSP generation (Fig. 4). In control solutions, orthodromic activation of Schaffer collaterals elicited a typical EPSP/IPSP sequence in pyramidal cells (Fig.4A). Because brief application of cesium hyperpolarized CA1 pyramidal cells, we compared the voltage-independent component of the actions of cesium by DC current injection to the pre-Cs+ RMP (Fig. 4A). After compensation of the DC potential change, the main effect of cesium was invariably a depression of the early component of the IPSP, whereas the late IPSP component was modified little (Fig. 4A;n = 5). In addition, a small but reproducible increase in the size of the EPSP was also recorded. These results are emphasized in the bottom trace of Figure 4A, in which the difference of the records obtained before and after exposure to cesium is shown.
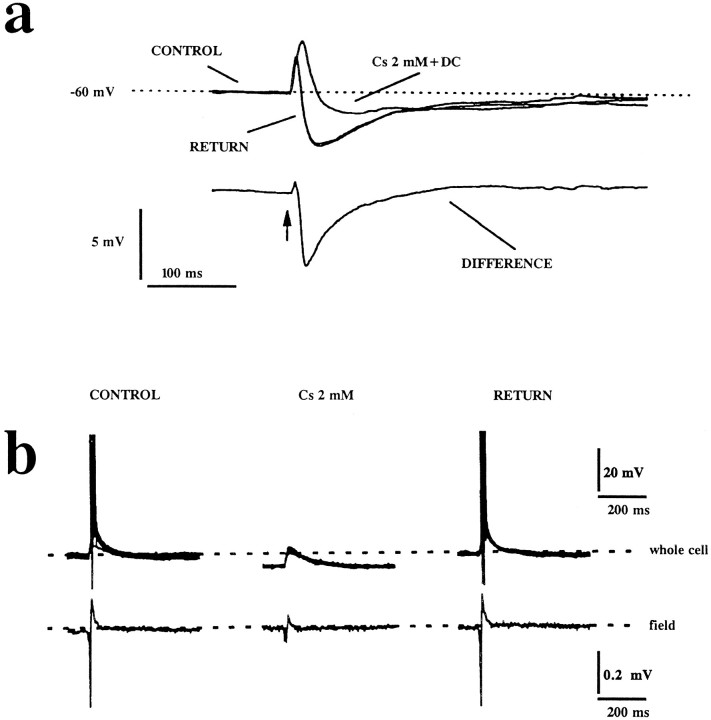
Fig. 4.
Effects of brief bath applications of cesium on evoked postsynaptic potentials. a, Orthodromic stimulation (arrow) induced EPSPs/IPSPs in stereotyped sequence under control recording conditions. Cesium (2 mm) caused a dramatic reduction of the size of the early IPSP, while leaving the late IPSP unchanged. This was evaluated at the same membrane potential, compensated for by DC injection. The bottom trace shows the potential profile obtained by subtracting the trace obtained in control from that in cesium. b, Cesium-induced hyperpolarizations depress CA1 pyramidal cell excitability. Simultaneous recordings from a CA1 pyramidal cell and stratum pyramidale field potential before, during, and after application of cesium during orthodromic, above-threshold stimulation are shown. Note that cesium caused a depression of the population spike (53 ± 18%, n = 5). Each whole-cell recording panel shows 10 superimposed traces. The extracellular field potentials are shown as averages of 10 trials at a stimulation frequency of 0.1 Hz.
Despite the concomitant reduction of the IPSPs, CA1 pyramidal cells were less excitable after a brief application of cesium because of the simultaneous hyperpolarizations induced by the drug. Schaffer collaterals were stimulated during simultaneous recording of population spikes and whole-cell potentials (Fig.4B). Stimulating current was chosen to elicit EPSPs above firing threshold. Cesium hyperpolarized pyramidal cells below firing threshold, an effect that was reversible upon washout (Fig.4B; n = 5). During hyperpolarization of pyramidal cells, a decrease of population spike amplitude by 53 ± 18% (n = 5) was observed.
Cs+ hyperpolarizes a subpopulation of interneurons
The observed effects of cesium on IPSPs were consistent with a direct effect on inhibitory interneurons. To test the hypothesis that Cs+ hyperpolarizes interneurons, recordings were obtained from visually identified cells in CA1 stratum radiatum or lacunosum moleculare (n = 10). These cells were identified as interneurons by fast action potentials and pronounced afterspike hyperpolarizations (Lacaille et al., 1987; Lacaille and Schwartzkroin, 1988). In nonstimulated slices, bath application of cesium either caused cell hyperpolarization (Fig. 5A,right; n = 5) or had no effect (n = 5), suggesting that a percentage of the cells did not express any cesium-sensitive conductances at RMP. Cells that responded to cesium with hyperpolarization were found to express h-currents similar to those of pyramidal cells (Fig. 5B). This is in agreement with the observation that a sag was recorded upon injection of hyperpolarizing current (Fig. 5A,left). Thus, brief cesium application hyperpolarized the cells (by 3.4 ± 1.1 mV), whereas prolonged applications resulted in a biphasic hyperpolarization/depolarization. Cesium-resistant cells, on the other hand, did not express any appreciable hyperpolarization-activated h-currents. These findings suggest that the action of brief applications of cesium on the early postsynaptic inhibitory potential (Fig. 4A) recorded from CA1 pyramidal cells was attributable to an indirect effect, for example, hyperpolarization of inhibitory interneurons after Ihblockade. However, the effect on interneurons failed to explain the synchronicity/hyperexcitability observed after application of cesium during LTD induction because, despite this disinhibitory effect, CA1 synaptic responses were decreased because of pyramidal cell hyperpolarization (for example, Fig. 4B).
Fig. 5.
Cesium-sensitive Ih currents are expressed in interneurons. Effects of cesium on interneuron membrane potentials and ion currents in a stratum radiatum interneuron expressing Ih. a, Left, Current-clamp recording from a resting potential of −54 mV. Note the pronounced voltage “sag” elicited by hyperpolarizing current injection. Right, Resting potential changes during application of 2 mm cesium. An early hyperpolarizing response was followed by a steady depolarization. Washout of cesium restored the original RMP. The dashed line shows the maximal hyperpolarization potential reached after application of the drug. b, Voltage-clamp recording in a different stratum radiatum interneuron from a holding potential of −35 to −120 mV in control and in the presence of cesium (2 mm). Atright is the digitally subtracted Cs+-sensitive current Ih.
Pairing application of Cs+ to 1 Hz orthodromic stimulation causes depolarization of interneurons
Although it was possible to record from only five interneurons lacking Ih, we were able to accomplish an important analysis of the effect of cesium on neuronal membrane potential because cesium-induced depolarizations could be studied in isolation from cesium-induced hyperpolarizations (Fig. 6). Under current-clamp conditions and at cell resting potential (between −55 and −58 mV), these cells did not display any appreciable response to brief perfusion of external cesium [Fig. 6B(dotted line), 6C1]. Voltage-clamp analysis revealed the presence of inward currents sensitive to a voltage-dependent blocking action of cesium (Fig. 6B; 3 mm). It is important to point out that no cesium-sensitive currents were detectable in these cells at RMP. However, during 1 Hz stimulation, these cells responded to application of cesium with a depolarization (3.45 ± 0.55 mV,n = 5), whereas exposure to cesium without concomitant 1 Hz stimuli (dashed lines, Fig. 6C1,left) failed to produce any effect. The effects of cesium on postsynaptic interneuronal excitatory potentials (at 1 Hz) are shown in Figure 6C2. The cesium-induced depolarization was sufficient to allow occasional firing of the interneurons.
Fig. 6.
A subpopulation of interneurons lacking Ih is affected by cesium during 1 Hz stimulation. Voltage-clamp analysis revealed that no h-type currents were detectable in this cell; under current clamp, no “sag” potentials were recorded. a, Current-clamp recording. As expected in interneurons lacking Ih, no sag of membrane potential was detectable upon hyperpolarization. The asterisks refer to orthodromically evoked EPSPs. Voltage-clamp analysis of a wide range of potentials from a holding potential of −35 mV. At cell resting potential (indicated by the dashed line), no effect of cesium was detectable. However, at hyperpolarized potentials, both the instantaneous and the steady-state currents were reduced. Note that no effects on outward current were detected. c, Perfusion with cesium during 1 Hz stimulation caused cell depolarization during time-matched exposures that in control conditions did not cause any effects. The cesium-induced depolarization reached a ceiling level and was reversed upon washout (c1). This depolarization was sufficient to elicit above-threshold responses in the cell (c2).
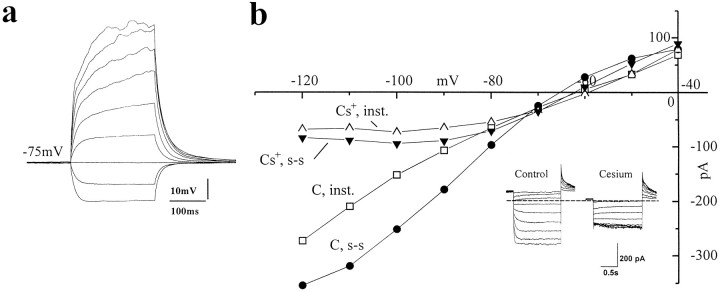
Cs+ blocks an inwardly rectifying current in CA1 glial cells
The main actions of cesium on astrocytes are believed to be specific for inwardly rectifying potassium and Ih-like currents (Sontheimer et al., 1992; Sontheimer and Waxman, 1993; Guatteo et al., 1996). We performed intracellular recordings from voltage-clamped CA1 stratum radiatum hippocampal slice glial cells (Fig. 7). Negative-going voltage steps evoked a large inward current, as reported previously for both cultured and in situ hippocampal and cortical astrocytes (Sontheimer and Waxman, 1993; Guatteo et al., 1996). Both the instantaneous and the steady-state whole-cell currents were drastically reduced in a voltage-dependent manner by brief bath applications of cesium; in addition, cesium caused a depolarization in these cells (3.1 ± 0.6 mV, n = 9). The inset in Figure 7B shows the actual current traces; similar results were obtained from all of the glial cells tested. Figure 7A shows a current-clamp recording performed in the same cell shown in Figure 7B to illustrate one of the criteria used for electrophysiological identification of hippocampal glial cells. Cells were held at their resting potential (−68.9 ± 4.5 mV, n = 9), and increasing depolarizing current steps were delivered in an attempt to elicit “active” responses. As shown in the figure, this procedure failed to evoke any action potential-like event when recording from stratum radiatum glia.
Fig. 7.
Effects of cesium on glial currents in hippocampal slices. Recordings from stratum radiatum glial cells were performed under both current and voltage clamp. a, Current clamp from an electrophysiologically identified glial cell. Note the absence of action potentials even with large depolarizing current injections (RMP = −75 mV). b, Voltage-clamp analysis revealed that a Cs+-sensitive inwardly rectifying current with a reversal potential of −72 mV was expressed in these cells (holding potential −35 mV).
Cs+ increases stimulus-induced [K+]out accumulation during LTD
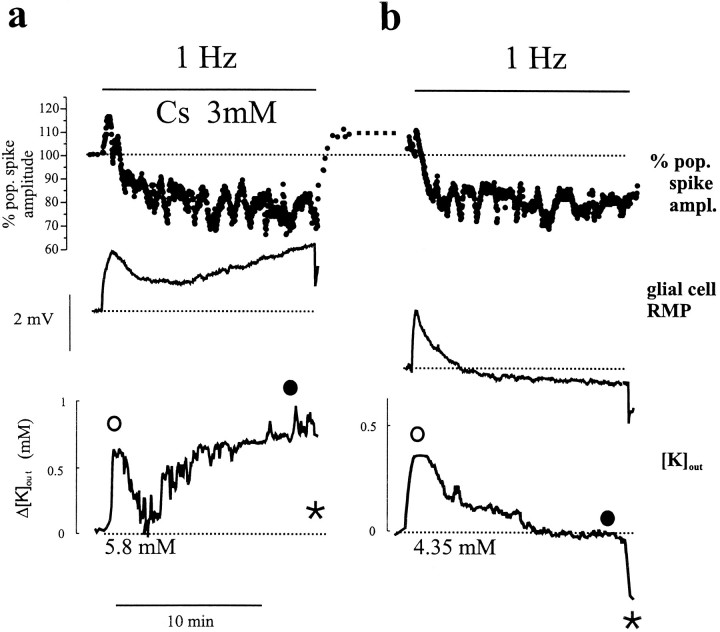
All of the effects of Cs+ independent from Ih blockade thus far described were consistent with a direct action on glia. Astrocytic membrane potential and [K+]out were monitored, therefore, during induction of LTD. The field potential was monitored as population spike amplitude in stratum pyramidale. As shown in Figure8B, the early events occurring during 1 Hz stimuli consisted, in control conditions, of a small and transient depolarization of glia followed by an undershoot below pre-LTD RMP. Cessation of 1 Hz stimulation caused an additional hyperpolarizing response. [K+]out changes closely followed the glial membrane potential. Cs+ had virtually no effect on the early glial and [K+]out responses (Fig. 8A). However, Cs+ prevented recovery of RMP and [K+]out; RMP remained depolarized, and extracellular [K+]outremained elevated during stimulation. The experiment was performed in the sequence shown (cesium before control) to avoid experiments on previously depressed pathways. Similar results were obtained in five different slices. Baseline [K+]out in control solution during 0.1 Hz stimulation was 4.35 mm. The maximum increase in [K+]out in control solution during 1 Hz stimulation (○ in Fig. 8) was 0.9 ± 0.4 mm. At the end of 1 Hz stimulation (•), [K+]out returned to pre-1 Hz values (4.35 mm). Two minutes after termination of 1 Hz stimulation, [K+]out transiently decreased to 4.1 ± 0.2 mm. In the presence of cesium, [K+]out increased over time; during pre-LTD, 0.1 Hz stimulation led to a new baseline of 5.8 ± 0.3 mm. In cesium during 1 Hz stimulation [K+]out increased further to 6.9 ± 0.2 mm and was 6.3 ± 0.4 mm at the end of the protocol. Two minutes after completion of the LTD protocol in Cs+, [K+]out transiently decreased to 5.8 ± 0.6 mm.
Fig. 8.
Recordings of population spike amplitude, glial resting membrane potential, and [K+]outduring the induction of LTD. In control solution, the [K+]out initial glial RMP was −72.5 mV and [K+]out baseline was 4.35 mm; cesium induced cell depolarization (by 2.7 mV, as expected from increased [K]out) and shifted baseline potassium values to 5.8 mm. a, Experiment performed in Cs+ (3 mm) added 10 min before 1 Hz stimulation. Note that the decrease of field potential amplitude during 1 Hz stimulation rapidly reversed after cessation of the stimulation. At the same time, [K+]out remained significantly above baseline and the glial cell was depolarized.b, Control solution. Field potential amplitude decreased after cessation of stimulation and remained constant thereafter. During 1 Hz stimulation, [K+]out and glial RMP both transiently increased and then returned to pre-LTD values. Upon cessation of the LTD induction protocol, a large glial hyperpolarization and an undershoot of [K+]out were observed. See text for comparison between cesium and control values.
DISCUSSION
The results obtained from our whole-cell, field-potential, and [K+]out recordings demonstrate that the actions of cesium on neuronal activity and synaptic plasticity in the hippocampus cannot be fully explained by cesium’s direct effects on CA1 pyramidal cell and interneuronal conductances. Although all of the short-term effects of cesium on neuronal (and glial) ion currents presented in this study are consistent with previous findings (Halliwell and Adams, 1982; McCormick and Pape, 1990; Maccaferri et al., 1993; Bayliss et al., 1994; Guatteo et al., 1996), prolonged applications of cesium or applications during 1 Hz stimulation unmasked a novel effect of cesium and allowed investigations of the effects of glial K+ uptake blockade on hippocampal synaptic plasticity and excitability. Our results demonstrate that when glial voltage-dependent K+ uptake is impaired, neuronal LTD cannot be maintained, possibly because of a concomitant synaptic potentiation resulting from K+-mediated depolarization of neighboring neurons. To our knowledge, this is the first report indicating a direct role for glial voltage-dependent K+channels in the regulation of synaptic plasticity. We have also shown that reduced glial K+ uptake might result in the appearance of interictal-like events and epileptiform afterdischarges. The effects of cesium on glia, interneurons, and pyramidal cells were investigated in our study and will be discussed separately. Because the effects of cesium on astrocytic potassium channels are central to the interpretation of our results, these will be discussed first.
Cs+ prevents potassium uptake by glia
Recordings from both hippocampal slices and cultured astrocytes have shown that cesium causes a blockade of inwardly rectifying potassium currents in voltage-clamped cells (Sontheimer and Waxman, 1993; Guatteo et al., 1996). We have extended this observation and demonstrated that cesium directly depolarizes glia and alters the stimulation-induced glial responses. This implies that cesium can interfere with normal potassium uptake into glia by two separate mechanisms: (1) by directly blocking inward rectifier K+channels, and (2) by causing depolarization, thus collapsing the gradient for K+ entry into the cell. Consistent with the action of cesium on the inwardly rectifying potassium current, we recently found that cesium decreases glial cell depolarizations elicited by orthodromic stimulation by antagonizing the influx of K+. We also found that application of cesium exaggerated the [K+]out transients induced by tetanic stimulation, resulting in higher increases and a slower recovery of [K+]out (D’Ambrosio et al., 1996).
During low-frequency, unstimulated, and asynchronous neuronal activity, potassium buildup is buffered independently from glial depolarizations (Casullo and Krnjevic, 1987). However, electrical stimulation at frequencies as low as 0.2 Hz are sufficient to cause glial depolarizations in the hippocampus. These depolarizing responses are concomitant with increased [K+]in in glial cells resulting from uptake from the extracellular space (Ballanyi et al., 1987) aimed at reestablishing physiological [K+]out. When these homeostatic mechanisms fail, abnormal accumulation of potassium occurs, resulting in neuronal synchronous burst firing (Lux and Neher, 1973; Lux et al., 1986;Dietzel et al., 1989). Unquestionably, mechanisms other than glial inward rectifiers are likely to be involved in potassium homeostasis (Ransom and Sontheimer, 1992, 1995). Nevertheless, even small changes in extracellular potassium can cause profound changes in neuronal function (Traynelis and Dingledine, 1988; Kawasaki et al., 1990;McBain, 1995). Our results demonstrate that blockade of the portion of glial uptake mediated by a voltage-dependent ion channel mechanism is sufficient to elevate hippocampal [K+]out.
Direct and indirect effects of cesium on neurons
Previous studies have shown that monovalent cations interfere with permeation through so-called “pacemaker” channels (such as the cardiac channel responsible for If; DiFrancesco, 1982). Since the discovery of similar currents in CNS neurons (Halliwell and Adams, 1982; Spain et al., 1987), cesium has been used to investigate the functional properties of these ion currents (Maccaferri et al., 1993). The direct effects of cesium on thalamic, hippocampal, and neocortical neurons are explained in terms of blockade of the Ih current. Short-lasting application of cesium results in cell hyperpolarization associated with increased RIN. Our study also provides evidence for Ih expression in CA1 interneurons. These interneurons were similarly hyperpolarized by brief applications of cesium. In contrast, in the interneuronal population lacking Ih, cesium failed to cause any effect on either RMP or RIN (Fig.6A,B,C1). We did not attempt to characterize further the expression of Ih in identified interneuronal populations, but our results are consistent with Ih being present in a subpopulation of cells (see below).
Although the short-term effects of cesium are consistent with its direct actions on Ih (Maccaferri et al., 1993), the reversal of the early hyperpolarization with no change in RIN observed in neurons after prolonged exposures cannot be explained by a direct action on neuronal ion channels; rather, it is consistent with an indirect effect mediated by blockade of glial K+ channels. However, alternative explanations of the late effects of cesium need to be discussed. A parsimonious explanation of the anomalous late depolarization is that during whole-cell recordings we were unable to quantify the RIN changes attributable to blockade of cesium-sensitive outward currents remotely located on the dendritic tree of pyramidal cells. This explanation seems unlikely, because the delay required for the depolarizing response to develop (tens of seconds) is not consistent with the time-constant of CA1 pyramidal neurons (milliseconds). It is also worth noting that the depolarizing response always followed hyperpolarizations and was never observed in isolation. Application of neuronal potassium channel blockers leads to enhanced neuronal activity or even to neuronal synchronization (Rutecki et al., 1987). None of these effects was observed after perfusion with cesiumalone, thus further ruling against a possible interaction of cesium with neuronal potassium currents. Furthermore, the depolarizing response to cesium was characterized by a voltage dependency inconsistent with a direct action on potassium channels because the response grew larger at more hyperpolarized potentials and did not reverse at EK (see Fig. 3). Finally, we have demonstrated that the depolarization induced by cesium depended on the configuration used to apply the solution bathing the cells. The delayed response to cesium was fully prevented by positioning the perfusion pipette close to the recording electrode, consistent with the hypothesis that prolonged applications of the blocker caused an accumulation of K+ in the extracellular space that could be prevented by increasing the rate of tissue perfusion.
Anomalous effects of Cs+ in cells lacking Ih
Although the majority of pyramidal cells and interneurons expressed Ih, we identified five interneurons that had no expression of cesium-sensitive ion currents evident at cell resting potential during either current- or voltage-clamp experiments (Fig. 6). No change in RIN or RMP was observed in these cells during a brief application of cesium. Similarly, stimulation of Schaffer collaterals at 1 Hz for 5 min did not cause any RMP change (Fig.6C1). However, when cesium was applied during 1 Hz stimulation, a depolarization occurred; this effect was not attributable to any detectable change in RIN. Furthermore, this depolarization was not attributable to prolonged 1 Hz stimulation alone because it was promptly reversed after cesium washout. Thus, this response observed in “cesium-insensitive” cells was similar to the late response of pyramidal cells and interneurons expressing Ih (depolarization with no change in RIN). Comparison of RMP changes with the results of ISM measurements demonstrated that these later effects of cesium were not attributable to a direct action on neurons but, rather, to an increase of extracellular potassium induced by 1 Hz stimuli in the presence of blocked glial voltage-dependent K+ channels.
Effects of cesium on synaptic plasticity
In a previous paper (Maccaferri et al., 1994), we described the effects of cesium pretreatment on LTD. In this study, we have extended the investigations of this phenomenon by applying cesium before, immediately after, or during LTD. Following these protocols, cesium abolished the maintenance of LTD induced by 1 Hz trains of orthodromic stimuli. In contrast, delayed applications of cesium several minutes after LTD induction were not as effective. This could not be explained by the direct hyperpolarizing action of cesium on pyramidal cells; nor could it be accounted for by the effects on neuronal excitability, because cesium decreased postsynaptic firing because of its effects on CA1 pyramidal cell RMP. The effects of cesium were invariably accompanied by bursting of CA1 pyramidal cells. Cesium or LTD-induction alone failed to induce hyperexcitability; the specific Ih channel blocker Zeneca ZD-7288 was also ineffective (Gasparini et al., 1996). LTD can be erased by a subsequent potentiation of synaptic transmission (Artola and Singer, 1993). Thus, the bursting behavior (and concomitant release of glutamate) observed after LTD induction in the presence of cesium might have caused an increased efficacy of synaptic potential generation, masking, or overlapping with the previously developed LTD. This interpretation of our results leads to the conclusion that under impaired glial function, and after even modest elevation in [K+]out, stimulation paradigms normally used to elicit LTD might result in long-term potentiation of synaptic activity (see also Sastry et al., 1988).
Reduced glial potassium buffering causes neuronal synchronization
Neuronal synchronization occurs after perfusion of hippocampal slices with high extracellular potassium. In our experiments, hyperexcitability and synchronization occurred only when Cs+ was applied concomitantly with stimulation paradigms aimed at eliciting intense synaptic activation, suggesting that under resting conditions K+ uptake by astrocyte voltage-dependent channels is minimal. Our findings and results from other laboratories clearly demonstrate that cesium-induced neuronal synchronization is not via a direct effect on neurons but, rather, results from interference with glial potassium uptake. These results can be summarized as follows. (1) Cesium causes synchronization in bicuculline-treated slices but not when normal synaptic inhibition is present (Hwa and Avoli, 1991). (2) Cesium induced depolarizations in neurons lacking cesium-sensitive currents only during 1 Hz stimulation, a protocol sufficient to increase [K+]out and thus stimulate glial uptake. (3) The depolarizing effects of cesium were not accompanied by changes in RIN. (4) Specific neuronal ion channel blockade (by Zeneca ZD-7288) did not cause reversal of LTD or bursting of CA1 cells (Gasparini et al., 1996). (5) Cesium-induced neuronal hyperexcitability and synchronization outlasted the actions of the drug on neuronal ion channels. The last finding is in agreement with a previously published observation (Aniksztejn and Ben Ari, 1991) demonstrating a form of cesium-induced short-term potentiation. Washout of cesium in our experiments was similarly characterized by a marked increase in EPSP frequency.
Recently, Hochman et al. (1995) showed that furosemide is capable of terminating seizures both in vitro and in vivo, possibly because of the drug’s blockade of the glial Na+/K+/2Cl− cotransporter. These findings are in apparent contrast with our hypothesis that blockade of potassium uptake into glia causes seizure-like activity. However, this discrepancy can be explained if one considers that the activation of cotransport-mediated potassium uptake results in glial swelling and subsequent shrinkage of the extracellular space, whereas potassium entry through voltage-dependent channels occurs in the absence of significant volume changes. It thus appears that, because of blockade of glial voltage-dependent potassium fluxes by Cs+, epileptiform activity develops because of increased [K+]out, possibly combined with an abnormal dependency on cotransport-dependent K+ uptake by astroglia. These mechanisms would result in a decrease of extracellular space size and an increased ephaptic coupling between neurons, both of which are known to be epileptogenic (Lux et al., 1986).
One of the possible mechanisms of action of cesium involves its known ability to interfere with the Na/K-ATPase pump (Sachs, 1977; Sohn and Vassalle, 1995). Blockade or reduction of pump activity might explain several of our findings, including abnormal accumulation of potassium, depolarization without changes in membrane resistance, and hyperexcitability. However, the actions of cesium on the Na/K pump cause transient pump activation (Sohn and Vassalle, 1995) and hyperpolarization. We have never observed Cs-mediated hyperpolarization not attributed to specific blockade of Ih (Figs. 3, 6). Finally, it has been shown that involvement of metabotropic glutamate receptors is involved in the generation of LTD (Bashir et al., 1993). It thus remains possible that cesium interferes with this subgroup of glutamate receptors. Our experimental design did not directly test this hypothesis, and further experiments are required to clarify this issue.
In conclusion, we have shown that application of cesium ions can cause profound and sometimes long-lasting changes in hippocampal CA1 physiology by acting on glial ion channels. Our results further support the hypothesis that glia contribute to the regulation of hippocampal plasticity and excitability. Future experiments performed by using glial-specific ion channel blockers will become useful tools toward understanding the functional significance of astroglia K+buffering in cortical structures. Finally, we have shown that suppression of neuronal h-currents causes a reduction of hippocampal excitability, further supporting an important role for these currents in hippocampal function.
Footnotes
This work was supported by National Institutes of Health Grants NIEHS ES 07033, NS 51614, and NS 21076 (D.J.), IF 32 NS10217-01, Research Foundation of the AANS (G.M.M.), and Consiglio Nazionale delle Ricerche CT9304376 (D.D.). We thank Gianmaria Maccaferri for participating in an early part of this work and B. Strowbridge, P. A. Schwartzkroin, and W. Crill for comments on this manuscript.
Correspondence should be addressed to Damir Janigro, Department of Neurological Surgery, 325 Ninth Avenue, P.O. Box 359914, Seattle, WA 98104.
REFERENCES
- 1.Ammann D. Ion selective microelectrodes. Springer; Berlin: 1986. [Google Scholar]
- 2.Aniksztejn L, Ben Ari Y. Novel form of long-term potentiation produced by a K+ channel blocker in the hippocampus. Nature. 1991;349:67–69. doi: 10.1038/349067a0. [DOI] [PubMed] [Google Scholar]
- 3.Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- 4.Ballanyi K, Grafe P, Bruggencate GT. Ion activities and potassium uptake mechanisms of glial cells of guinea-pig olfactory cortex slices. J Physiol (Lond) 1987;382:159–174. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballanyi K, Branchereau P, Champagnat J, Fortin G, Velluti J. Extracellular potassium, glial and neuronal potentials in the solitary complex of rat brainstem slices. Brain Res. 1993;607:99–107. doi: 10.1016/0006-8993(93)91493-c. [DOI] [PubMed] [Google Scholar]
- 6.Bashir ZI, Jane DE, Sunter DC, Watkins JC, Collingridge GL. Metabotropic glutamate receptors contribute to the induction of long-term depression in the CA1 region of the hippocampus. Eur J Pharmacol. 1993;239:265–266. doi: 10.1016/0014-2999(93)91009-c. [DOI] [PubMed] [Google Scholar]
- 7.Bayliss DA, Viana F, Bellingham MC, Berger AJ. Characteristics and postnatal development of a hyperpolarization-activated inward current in rat hypoglossal motoneurons in vitro. J Neurophysiol. 1994;71:119–128. doi: 10.1152/jn.1994.71.1.119. [DOI] [PubMed] [Google Scholar]
- 8.BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol. 1993;110:343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brines ML, Robbins RJ. Cell-type specific expression of Na+/K+-ATPase catalytic subunits in cultured neurons and glia: evidence for polarized distribution in neurons. Brain Res. 1993;631:1–11. doi: 10.1016/0006-8993(93)91179-v. [DOI] [PubMed] [Google Scholar]
- 10.Casullo J, Krnjevic K. Glial potentials in hippocampus. Can J Physiol Pharmacol. 1987;65:847–855. doi: 10.1139/y87-136. [DOI] [PubMed] [Google Scholar]
- 11.Christie BR, Kerr DS, Abraham WC. Flip side of synaptic plasticity: long-term depression mechanisms in the hippocampus. Hippocampus. 1994;4:127–135. doi: 10.1002/hipo.450040203. [DOI] [PubMed] [Google Scholar]
- 12.D’Ambrosio R, McKhann GM, Janigro D. Whole cell recording from hippocampal astrocytes during orthodromic stimulation. Soc Neurosci Abstr. 1996;22:128.16. [Google Scholar]
- 13.Dietzel I, Heinemann U, Lux HD. Relations between slow extracellular potential changes, glial potassium buffering, and electrolyte and cellular volume changes during neuronal hyperactivity in cat brain. Glia. 1989;2:25–44. doi: 10.1002/glia.440020104. [DOI] [PubMed] [Google Scholar]
- 14.DiFrancesco D. Block and activation of the pacemaker channel in calf Purkinje fibers: effects of potassium, cesium, and rubidium. J Physiol (Lond) 1982;329:485–507. doi: 10.1113/jphysiol.1982.sp014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenman G. Glass electrodes for hydrogen and other cations: principles and practice. Dekker; New York: 1967. [Google Scholar]
- 17.Gasparini S, D’Ambrosio R, DiFrancesco D, Janigro D. Cesium prevents LTD maintenance and causes epileptiform activity by an effect on both neuronal and glial cells. Soc Neurosci Abstr. 1996;22:596.9. [Google Scholar]
- 18.Guatteo E, Stanness KA, Janigro D. Hyperpolarization-activated currents in cultured rat cortical and spinal cord astrocytes. Glia. 1996;16:196–209. doi: 10.1002/(SICI)1098-1136(199603)16:3<196::AID-GLIA2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Hagland MM, Schwartzkroin PA. Role of Na-K pump potassium regulation and IPSPs in seizures and spreading depression in immature hippocampal slices. J Neurophysiol. 1990;63:225–239. doi: 10.1152/jn.1990.63.2.225. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- 21.Hochman DW, Baraban SC, Owens JW, Schwartzkroin PA. Dissociation of synchronization and excitability in furosemide blockade of epileptiform activity. Science. 1995;270:99–102. doi: 10.1126/science.270.5233.99. [DOI] [PubMed] [Google Scholar]
- 22.Hwa GG, Avoli M. Cesium potentiates epileptiform activities induced by bicuculline methiodide in rat neocortex maintained in vitro. Epilepsia. 1991;32:747–754. doi: 10.1111/j.1528-1157.1991.tb05529.x. [DOI] [PubMed] [Google Scholar]
- 23.Janigro D, Schwartzkroin PA. Effects of GABA on CA3 pyramidal cell dendrites in rabbit hippocampal slices. Brain Res. 1988a;453:265–274. doi: 10.1016/0006-8993(88)90166-7. [DOI] [PubMed] [Google Scholar]
- 24.Janigro D, Schwartzkroin PA. Effects of GABA and baclofen on pyramidal cells in the developing rabbit hippocampus: an in vitro study. Brain Res. 1988b;469:171–184. doi: 10.1016/0165-3806(88)90180-0. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki K, Traynelis SF, Dingledine R. Different responses of CA1 and CA3 regions to hypoxia in the rat hippocampal slice. J Neurophysiol. 1990;68:385–394. doi: 10.1152/jn.1990.63.3.385. [DOI] [PubMed] [Google Scholar]
- 26.Kriegstein AR, Suppes T, Prince DA. Cellular and synaptic physiology and epileptogenesis of developing rat neocortical neurons in vitro. Brain Res. 1987;431:161–171. doi: 10.1016/0165-3806(87)90206-9. [DOI] [PubMed] [Google Scholar]
- 27.Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci. 1988a;8:1411–1424. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. J Neurosci. 1988b;8:1400–1410. doi: 10.1523/JNEUROSCI.08-04-01400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lux HD, Neher E. The equilibration time course of [K] in rat cortex. Exp Brain Res. 1973;17:190–205. doi: 10.1007/BF00235028. [DOI] [PubMed] [Google Scholar]
- 31.Lux HD, Heinemann U, Dietzel I. Ionic changes and alterations in the size of extracellular space during epileptic activity. In: Delgado-Escueta AV, Ward AA, editors. Advances in neurology. Raven; New York: 1986. pp. 619–639. [PubMed] [Google Scholar]
- 32.Maccaferri G, Mangoni M, Lazzari A, DiFrancesco D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J Neurophysiol. 1993;69:2129–2136. doi: 10.1152/jn.1993.69.6.2129. [DOI] [PubMed] [Google Scholar]
- 33.Maccaferri G, Janigro D, Lazzari A, DiFrancesco D. Cesium prevents maintenance of long-term depression in rat hippocampal CA1 neurons. NeuroReport. 1994;5:1813–1816. doi: 10.1097/00001756-199409080-00032. [DOI] [PubMed] [Google Scholar]
- 34.McBain CJ. Hippocampal inhibitory neuron activity in the elevated potassium model of epilepsy. J Neurophysiol. 1995;72:2853–2863. doi: 10.1152/jn.1994.72.6.2853. [DOI] [PubMed] [Google Scholar]
- 35.McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol (Lond) 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 37.Nicolsky BP. Theory of glass electrodes. Zh Fis Khim. 1937;10:495. [Google Scholar]
- 38.Orkand RK. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphybia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- 39.Ransom BR, Sontheimer H. The neurophysiology of glial cells. J Clin Neurophysiol. 1992;9:224–251. doi: 10.1097/00004691-199204010-00005. [DOI] [PubMed] [Google Scholar]
- 40.Ransom CB, Sontheimer H. Biophysical and pharmacological characterization of inwardly rectifying potassium currents in rat spinal cord astrocytes. J Neurophysiol. 1995;73:333–346. doi: 10.1152/jn.1995.73.1.333. [DOI] [PubMed] [Google Scholar]
- 41.Rutecki PA, Lebeda FT, Johnstone D. 4-Aminopyridine produces epileptiform activity in the hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 1987;57:1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- 42.Sachs JR. Kinetic evaluation of the Na-K pump reaction mechanism. J Physiol (Lond) 1977;273:489–514. doi: 10.1113/jphysiol.1977.sp012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sastry BR, Goh JW, May PBY, Chirwa SS. The involvement of non-spiking cells in long-term potentiation of synaptic transmission in the hippocampus. Can J Physiol Pharmacol. 1988;66:841–844. doi: 10.1139/y88-135. [DOI] [PubMed] [Google Scholar]
- 44.Schwartzkroin PA. Hippocampal slices in experimental and human epilepsy. Adv in Neurology. 1986;44:991–1010. [PubMed] [Google Scholar]
- 45.Schwartzkroin PA, Prince DA. Changes in excitatory and inhibitory synaptic potentials leading to electrogenic activity. Brain Res. 1980;183:61–76. doi: 10.1016/0006-8993(80)90119-5. [DOI] [PubMed] [Google Scholar]
- 46.Schwartzkroin PA, Wyler AR. Mechanisms underlying epileptiform burst discharge. Ann Neurol. 1980;7:95–107. doi: 10.1002/ana.410070202. [DOI] [PubMed] [Google Scholar]
- 47.Sohn HG, Vassalle M. Cesium effects on dual pacemaker mechanisms in guinea pig sinoatrial node. J Mol Cell Cardiol. 1995;27:563–577. doi: 10.1016/s0022-2828(08)80051-x. [DOI] [PubMed] [Google Scholar]
- 48.Somjen GG. Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- 49.Sontheimer H, Waxman SG. Expression of voltage-activated ion channels by astrocytes and oligodendrocytes in the hippocampal slice. J Neurophysiol. 1993;70:1863–1873. doi: 10.1152/jn.1993.70.5.1863. [DOI] [PubMed] [Google Scholar]
- 50.Sontheimer H, Black JA, Ransom BR, Waxman SG. Ion channels in spinal cord astrocytes in vitro. I. Transient expression of high levels of Na+ and K+ channels. J Neurophysiol. 1992;68:985–1000. doi: 10.1152/jn.1992.68.4.985. [DOI] [PubMed] [Google Scholar]
- 51.Spain WJ, Schwindt PC, Crill WE. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987;57:1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- 52.Traynelis SF, Dingledine R. Potassium-induced spontaneous electrographic seizures in rat hippocampal slices. J Neurophysiol. 1988;59:259–276. doi: 10.1152/jn.1988.59.1.259. [DOI] [PubMed] [Google Scholar]
- 53.Walz W, Hertz L. Functional interactions between neurons and astrocytes. II. Potassium omeostasis at the cellular level. Prog Neurobiol. 1983;20:133–183. doi: 10.1016/0301-0082(83)90013-8. [DOI] [PubMed] [Google Scholar]