Abstract
Advances in mechanical circulatory support have been critical in bridging patients awaiting heart transplantation. In addition, improvement in device durability has enabled left ventricular assist device therapy to be applied as destination therapy in those not felt to be transplant candidate. Because of the increasing complexity of patients, there continues to be a need for alternative strategies for device implantation to bridge high-risk patients awaiting heart transplantation, wherein the risks of numerous previous sternotomies may be prohibitive. We present a unique technique for placement of the HeartWare ventricular assist device via left anterior thoracotomy to the descending aorta in a patient awaiting heart transplantation with a history of multiple previous sternotomies.
Keywords: HeartWare ventricular assist device, descending aorta, heart failure
A 49-year-old male with a history of ischemic cardiomyopathy presented to an outside hospital with unstable angina. He was emergently transferred to the Vanderbilt Heart Institute for management of end-stage heart failure. He was in New York Heart Association Functional Class IV at the time of presentation and was listed as a status 1B. He had already been on a course of home Milrinone with progressive failure of therapy.
The patient had three previous sternotomies before listing. His past surgical history was significant for coronary artery bypass grafting × 1 (2004), subsequent bioprosthetic mitral valve replacement (MVR) in 2009, and a redo mechanical MVR for structural valve deterioration 1 year before listing. Coronary angiography revealed disease not amenable to percutaneous intervention. Left ventricular ejection fraction was 25%, and right heart catheterization revealed significantly elevated filling pressures. Attempts were made at diuresis, but the patient became progressively oliguric and hypotensive. Given the patient’s progressive decline, it became clear that he would require mechanical support1. Due to his history of multiple previous sternotomies and high-risk comorbidities, an alternative implant approach was considered. Consent was obtained, and the patient was taken to the operating room.
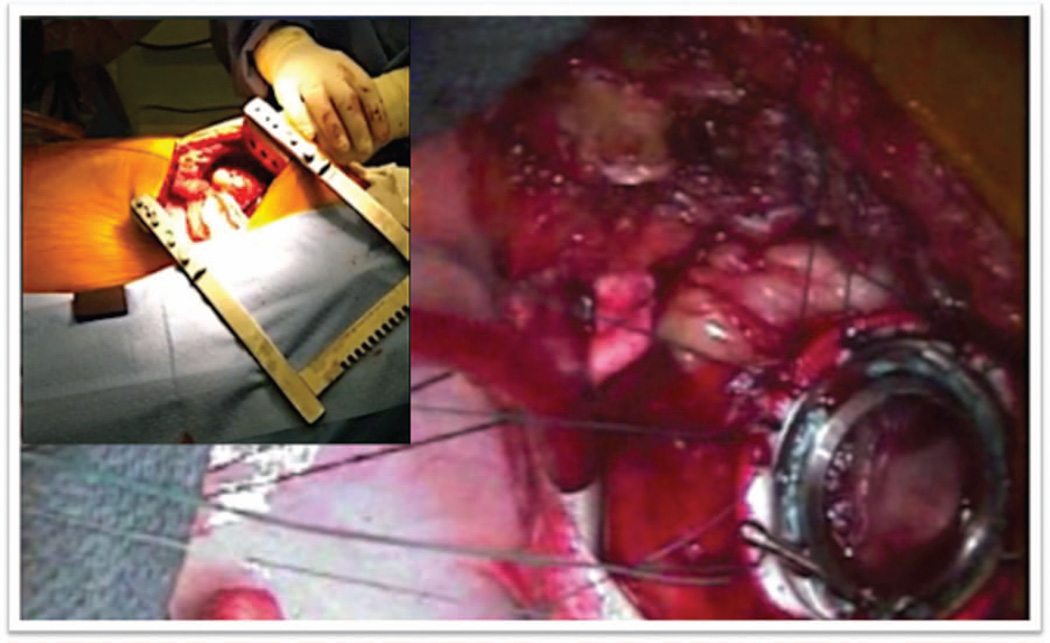
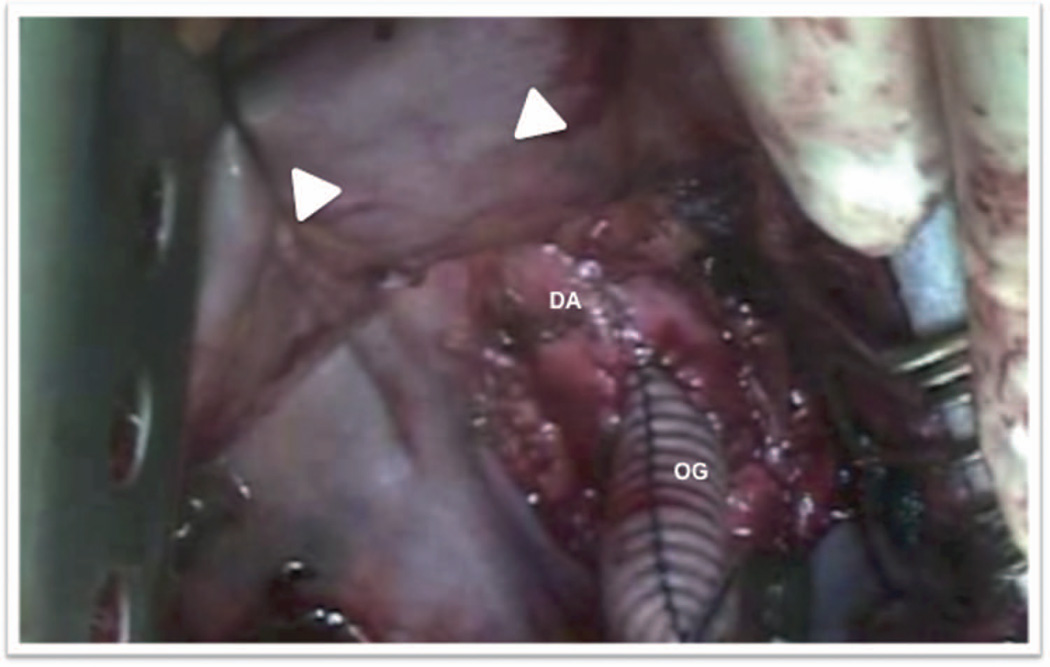
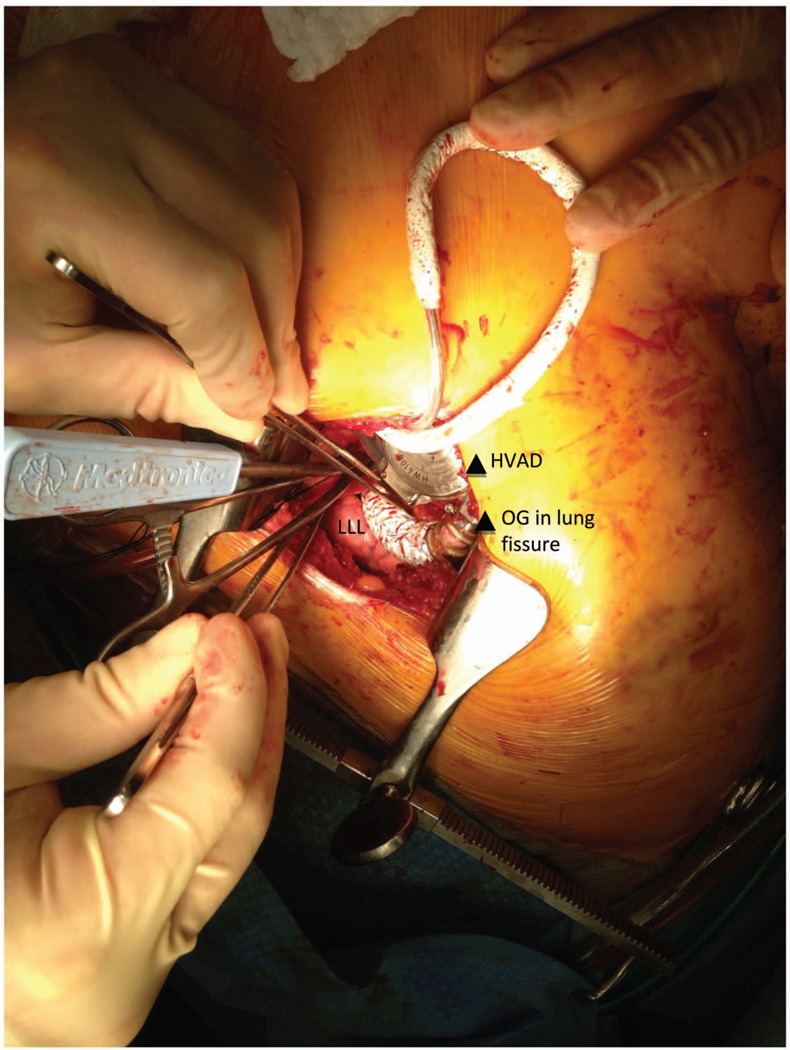
Strategies employed a left thoracotomy approach with outflow graft anastomosis to the descending aorta. A left anterior thoracotomy was performed through the fifth intercostal space to expose the left ventricular (LV) apex (Figure 1). The fifth rib was cut for better access. Lung adhesions were dissected, and the inferior pulmonary ligament was freed to optimize exposure. Ring placement was confirmed utilizing transesophageal echocardiogram (TEE), as a wire was advanced through the LV apex. Before heparin was administered, the strain relief and a portion of the driveline were wrapped in Gore-Tex to reduce adhesions during subsequent transplant. The driveline was brought out of the thoracic cavity and then tunneled through the subcutaneous tissue, terminating four finger-widths away from the costal margin along the mid-clavicular line on the left. The patient was heparinized, the femoral vessels were used, and cardiopulmonary bypass (CPB) was initiated. The sewing ring portion was confirmed with TEE, and the apical sewing ring portion of the HeartWare ventricular assist device (HVAD) inflow was secured in place utilizing Ethibond 2-0 sutures with felts as recommended (Figure 1).2 After coring the LV under TEE guidance, the HVAD was secured in place (Figure 2). The outflow graft was measured and deaired. A loop was created to bury the outflow graft in the left pulmonary fissure creating a straight but gentle angle to the descending aorta (Figure 3). An end-to-side anastomosis using a HeartPort-assisted technique was performed on the descending aorta using a partial cross-clamp in the vicinity of the junction of the diaphragm. The graft was de-aired with a de-airing needle, and carbon dioxide was utilized throughout the procedure. The patient was successfully weaned off CPB after initiating inotropes under TEE guidance. Right ventricular function was noted to be normal, and left ventricular assist device (LVAD) flows were satisfactory. No new valvular anomalies were observed. The intercostal space was approximated using a large polydioxanone stitch. The patient was dismissed approximately 3 weeks postoperatively. He was subsequently seen in VAD clinic and was successfully transplanted 3 months after LVAD implant. For device explant at the time of transplant, the posterior pericardium was opened during cardiectomy in order to access outflow graft anastomosis on the descending aorta. The outflow graft was ligated directly close to the aorta.
Figure 1.
Left thoracotomy exposure and HeartWare ventricular assist device sewing cuff placement. The left ventricular apex is exposed through a left thoracotomy, and the sewing cuff is inserted to avoid injury from sternal reentry.
Figure 2.
Outflow graft anastomosis to the descending aorta. End-to-side anastomosis to the descending aorta with retracted diaphragm (arrows). DA, descending aorta; OG, outflow graft.
Figure 3.
HeartWare ventricular assist device and outflow graft positioning. A loop was created to bury the outflow graft in the left pulmonary fissure creating a straight but gentle angle to the descending aorta. HVAD, HeartWare ventricular assist device; LLL, left lower lobe; OG, outflow graft.
Discussion
The utilization of alternative approaches, and potentially less invasive ones, has now become a recurrent and popular theme in cardiac surgery as the field continues to evolve. Although median sternotomy may still provide the best access routes for visualization during a cardiac surgical procedure, it is not without its potential dangers, especially in patients with several prior sternotomies.
Reoperative median sternotomy can have several risks that are associated with it, such as inadvertent injury to the heart and great vessels. Studies have shown that patients undergoing valve reoperation can have a 4% rate of complications directly related to the sternal reentry portion of the procedure. These observations are critical in patients bridged with a LVAD, as the number of previous sternotomies may represent a barrier for future heart transplantation.
Alternative approaches for LVAD placement are becoming increasingly popular. The Jarvik 2000 and the HeartMate II have undergone successful placement via left subcostal and minithoracotomy approaches.3 The HeartWare HVAD has also been implanted (with anastomosis to the ascending aorta) using minimally invasive techniques4 or bilateral anterior thoracotomies.5 The advantages of the miniaturized HVAD are clear, as its intrapericardial nature allows an easy pump placement. This patient had three previous sternotomies. Given the multiple risks entailed with sternal reentry in such a high-risk reoperative candidate, we opted to implant the LVAD using an alternative approach. We however believe it is critical to adjust the pump speed in order to leave some aortic valve opening (at least 1:3) to allow aortic root washout and to decrease concerns associated with clot formation and stroke; given the long-term outcomes with this approach remains uncertain. The pump speed needs to be adjusted to provide adequate support and a certain degree of cardiac ejection to minimize risks of thromboembolic complications. Regular transthoracic echocardiography (monthly) was used in order to adjust the degree of aortic valve opening and to monitor the possibility of clot formation. The differences in flow dynamics and theoretical increased risks of blood stasis using this approach to the descending aorta should guide the need to keep these patients as high priority on the transplant list.
A total of four patients have been implanted using this technique in our institution (Table 1). We did not observe any thromboembolic events or valve-related problems in this group of patients. Colleagues at the Mayo Clinic in Rochester (Minnesota) have also performed three such cases with the HeartWare HVAD successfully. As such techniques continue to evolve, surgeons will become comfortable with the learning curves involved. Such alternative techniques for HVAD placement could prove to be a very valuable tool to address increasingly complex patients referred for transplantation.
Table 1.
Description of Cases
| Patients | Age (years) | Number of Previous Sternotomies |
Description of Surgeries | Status at Last Follow-up |
|---|---|---|---|---|
| 1 | 49 | 3 | CABG + MV repair, MVR (bioprosthesis), MVR (mechanical) | Transplanted |
| 2 | 57 | 2 | CABG, CABG, CABG | Ongoing support |
| 3 | 60 | 3 | CABG, CABG, CABG | Ongoing support |
| 4 | 62 | 2 | CABG, CABG | Ongoing support |
CABG, coronary artery bypass grafting; MV, mitral valve; MVR, mitral valve replacement.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
References
- 1.Slaughter MS, Rogers JG, Milano CA, et al. HeartMate II Investigators: Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS. Implantation of the HeartWare left ventricular assist device. Semin Thorac Cardiovasc Surg. 2011;23:245–247. doi: 10.1053/j.semtcvs.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Hill JD, Avery GJ, Egrie G, Turley K, Reichenbach S. Less invasive Thoratec LVAD insertion: a surgical technique. Heart Surg Forum. 2000;3:218–223. [PubMed] [Google Scholar]
- 4.Schmitto JD, Molitoris U, Haverich A, Struber M. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg. 2012;143:511–513. doi: 10.1016/j.jtcvs.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 5.Popov AF, Hosseini MT, Zych B, Simon AR, Bahrami T. HeartWare left ventricular assist device implantation through bilateral anterior thoracotomy. Ann Thorac Surg. 2012;93:674–676. doi: 10.1016/j.athoracsur.2011.09.055. [DOI] [PubMed] [Google Scholar]