Abstract
Insights into the important contribution of inflammation and immune functions in the development and progression of atherosclerosis have greatly improved our understanding of this disease. Although the role of T cells has been extensively studied for decades, only recently has the role of B cells gained more attention. Recent studies have identified differential effects of different B-cell subsets and helped to clarify the still poorly understood mechanisms by which these act. B1 cells have been shown to prevent lesion formation, whereas B2 cells have been suggested to promote it. Natural IgM antibodies, mainly derived from B1 cells, have been shown to mediate atheroprotective effects, but the functional role of other immunoglobulin classes, particularly IgG, still remains elusive. In this review, we will focus on recent insights on the role of B cells and various immunoglobulin classes and how these may mediate their effects in atherosclerotic lesion formation. Moreover, we will highlight potential therapeutic approaches focusing on B-cell depletion that could be used to translate experimental evidence to human disease.
Keywords: antibodies; atherosclerosis; B-lymphocytes; complement system proteins; immunity, humoral; immunoglobulin M
Atherosclerosis is a chronic inflammatory disease of large- and medium-sized arteries. It is characterized by the deposition and trapping of low-density lipoproteins (LDLs) in the artery wall, which then undergo a variety of changes, both enzymatic and nonenzymatic, that lead to a cascade of inflammatory responses followed by the recruitment of mostly macrophages and T cells.1 The accumulation of LDL in the subendothelial space makes it susceptible to oxidation by various processes, and the resultant formation of oxidized LDL (OxLDL) has been suggested to be the key event in driving the subsequent inflammation that characterizes atherosclerosis.2 Infiltrating macrophages take up OxLDL via scavenger receptors leading to the formation of lipid-laden foam cells, which are the hallmark cells of atherosclerotic lesions. With plaque progression, many foam cells undergo apoptosis resulting in the formation of an acellular necrotic core that is full of lipid gruel and cellular debris. Once the fibrous cap covering the necrotic core erodes or ruptures, allowing contact between the exposed material and the circulating coagulation system, thrombus formation is triggered, resulting in clinical events such as stroke and myocardial infarction,3,4 which are leading causes of death globally.5
Inflammation is the response of the immune system to the presence of exogenous and endogenous antigens, and it is now widely accepted that immune mechanisms are involved in all phases of lesion development. However, knowledge of the antigens involved in initiating and sustaining the inflammatory response is only beginning to be understood. Although immune responses to infectious pathogens have been implicated in atherogenesis, evidence to support a primary role has been lacking, although such responses could have an important contributory role.6 Among endogenous antigens, most studies have focused on heat shock protein 60 (Hsp60) and epitopes of OxLDL.7–9 Hsp60, which shares high homology with mycobacterial Hsp65, has been shown to be expressed in endothelial cells in response to several proatherogenic triggers such as a high-cholesterol diet. This in turn results in the recall of a cross-reactive autoimmune response against endothelial Hsp60, but which originally developed against microbial Hsp65 following an infection. The resultant humoral and cellular response induces endothelial damage. Furthermore, oxidative modification of LDL results in the generation of a variety of oxidation-specific epitopes (OSEs), such as the formation of adducts between apolipoprotein B and reactive oxidized lipid moieties, including phosphocholine-containing oxidized phospholipids (OxPLs), and their degradation products such as malondialdehyde and 4-hydroxynonenal. In addition, it is possible that immunogenic apolipoprotein B–derived peptides are also generated. Based on this, several model antigens are used to study these immune responses, including malondialde-hyde-modified LDL (MDA-LDL) and copper-oxidized LDL (CuOx-LDL).2,7,10 Although the exact contribution of these and potentially other antigens is not fully understood, it is now clear that both innate and adaptive immune responses are intimately involved in atherogenesis.
Innate immunity uses a variety of preformed, germline pattern recognition receptors to effect immune responses to pathogens and endogenous antigens. Because these pattern recognition receptors are limited in number, they recognize pathogen-associated molecular patterns on infectious agents and by analogy, danger-associated molecular patterns on endogenous antigens, which form common recognition motifs. These endogenous responses parallel antimicrobial responses and are necessary to sense tissue damage that needs to be resolved by the recruitment of phagocytes through sterile inflammation. In the case of atherogenesis, as noted above, heat shock proteins, cholesterol crystals, and OSEs seem to be important danger-associated molecular patterns that are recognized by innate pattern recognition receptors.11 For example, OSEs, which are lipid peroxidation-derived modifications of proteins and lipids, represent conserved ligands for several arcs of innate immunity, including macrophage scavenger receptors, soluble innate proteins, and natural antibodies (NAbs). Notably, the same OSEs have been found on the surface of OxLDL and apoptotic cells, which both accumulate inside athero-sclerotic lesions and provide a continuous trigger for sterile inflammatory responses.11
Adaptive immunity also plays an important role in lesion development. Antibodies with specificity for plaque antigens (eg, OxLDL) and activated T cells are present in human lesions.12,13 Important experimental evidence regarding the involvement of adaptive immunity came from studies with atherosclerosis-prone LDL receptor (Ldlr−/−) or apolipoprotein E–deficient (Apoe−/−) mice that lack both functional T and B cells as a result of recombination activating gene deficiency or a severe combined immunodeficiency background.14–16 These mice were found to exhibit significantly less lesion development indicating an important role for adaptive immunity. However, lesions still develop in these mice, and in the face of marked hypercholesterolemia, the immune-deficient mice develop similar lesions compared with those with intact adaptive immunity. Thus, although not a prerequisite for lesion development, adaptive immunity has an important modulatory role that can both enhance and retard lesion progression. There is now extensive evidence that Th1 responses and interferon-γ (IFN-γ) in particular are critical drivers of lesion formation. These effects are primarily mediated by CD4+ T cells, but CD8+ T cells seem to play a critical role as well.9 Compelling evidence also demonstrates that the activity of proatherogenic T-cell subsets is tightly regulated by regulatory T cells, which act via multiple mechanisms and may directly alter plaque characteristics by the secretion of the fibrogenic cytokine transforming growth factor-β.8 The roles of other T-cell subsets such as Th17 and Th2 are less consistent, and their effects may be best understood by the cytokines they secrete and how these function in relationship to IFN-γ–secreting Th1 cells. In contrast to the pronounced presence of T cells within lesions, only few B cells can be detected.17 B cells participate in lymphocyte infiltrates of the adventitia surrounding affected arteries, which can be organized in tertiary lymphoid organs that have been suggested to regulate the inflammatory response of lesions.18 Emerging data reviewed by Perry et al19 and Kyaw et al20 have recently identified an important role for B cells in atherogenesis. The potential mechanisms by which B cells may affect atherosclerosis include the production of immunoglobulins and the subsequent formation of immune complexes with their cognate antigens. Antibodies with specificity for plaque antigens, such as OxLDL, have been detected in atherosclerotic lesions, and plasma antibody titers to OSEs and other antigens such as Hsp60 have been shown to correlate with several clinical manifestations of atherosclerosis.12 Depending on the class of immunoglobulin, this can lead to the engagement of different Fc receptors resulting in cellular activation or inhibition. Moreover, antibodies may also provide an important function by neutralizing antigens and promoting their clearance. In addition, B cells mediate functions independent of antibody production, such as antigen presentation and the release of cytokines, including interleukin-10 (IL-10). In this review, we will discuss how different B-cell subsets may affect atherogenesis and particularly focus on the role of humoral immunity in mediating some of these effects.
B Cells Play a Role in Atherosclerosis
A recent network-driven integrative analyses of data from genome-wide association studies and whole blood gene expression profiles from Framingham Heart Study participants identified B-cell immune responses as causative in coronary heart disease (CHD).21 Based on differential gene expression in whole blood extracts between healthy controls and patients with CHD, the authors identified modules enriched in genes involved in B-cell function, which were integrated with tissue-specific Bayesian networks and protein–protein interaction networks and led to the identification of B-cell–related genes as key drivers in CHD.
Evidence for a functional role of B cells in experimental atherosclerosis first came from studies by Caligiuri et al,22 who demonstrated that splenectomized Apoe−/− mice develop aggravated atherosclerosis compared with sham-treated mice. This effect could be reversed by adoptive transfer of splenocytes. Interestingly, transfer of educated splenic B cells from older Apoe−/− donor mice not only rescued the proatherogenic effect, but also reduced lesion size below the size of sham-treated mice.22 (It should be noted that transfer of T cells into splenectomized mice also rescued mice from enhanced atherosclerosis.) In fact, adoptive transfer of splenic B cells, but not T cells, into intact Apoe−/− mice also mediated moderate protection.22 In line with this, Major et al23 demonstrated that cholesterol-fed Ldlr−/− mice that were reconstituted with B-cell–deficient bone marrow from μMT mice had increased plaque formation compared with mice reconstituted with wild-type bone marrow. Although these studies suggest an overall protective role of B cells, recent data have challenged this: 2 groups independently reported that anti-CD20–mediated B-cell depletion in atherosclerotic ApoE−/− and Ldlr−/− mice results in a significant reduction of lesion size.24,25 Thus, the general concept of B-cell involvement in atherosclerosis and the role of individual players still need to be elucidated.
The apparent discrepancy between the studies described above may be rooted in part in the differential roles of unique B-cell subsets (Table 1). Extensive studies of the peripheral blood, peritoneal cavity, spleen, and other lymphoid tissues have identified B1 and B2 cells as the 2 main B-cell subsets in mice based on their developmental origin. B2 cells, which represent the vast majority of B cells, are bone marrow derived and include follicular as well as marginal zone B cells. B1 cells, which can be further divided into B1a and B1b based on their surface marker expression, seem to be a developmentally and functionally distinct subset that is localized primarily in the peritoneal and pleural cavities.26,27 Although B1a cells seem to be atheroprotective, the role of B2 cells in atherosclerosis is still debatable although the majority of studies suggest a proatherogenic role (Table 2 and see below). In addition, a distinct B1-cell subset termed innate response activator (IRA) that responds to lipopolysaccharide stimulation by secretion of granulocyte-macrophage colony-stimulating factor (GMCSF) has recently been identified.33 It has been shown to play a vital role in protection against sepsis (Table 1). IRA B cells seem to be phenotypically distinct from other B1 cells because they also express the immature B-cell marker CD93. Moreover, in contrast to B1 cells, IRA B-cell survival is dependent on B-cell–activating factor receptor (BAFFR) signaling because these cells are depleted in BAFFR-deficient mice. Hilgendorf et al32 showed that cholesterol-fed Ldlr−/− mice deficient in GM-CSF expressing B cells lack IRA B cells and develop reduced atherosclerosis. The authors propose that IRA B cells promote atherosclerosis by stimulating the expansion of mature dendritic cells that results in the generation of IFN-γ–secreting Th1 cells, accompanied by a switch from IgG1 to IgG2c antibodies against OxLDL. Moreover, these cells are increased in the spleen of patients with symptomatic compared with nonsymptomatic cardiovascular disease (CVD).32 Finally, regulatory B cells with a distinct surface marker expression profile have been identified and are being discussed as modulators of autoimmunity, for example, by the secretion of IL-10 or direct interaction with pathogenic T cells.34 The role of regulatory B cells in atherogenesis remains to be identified.
Table 1. Summary of Murine B-Cell Subsets and Their Surface Markers, Localization, and Frequencies in the Spleen.
| Murine B Cells | Cell Surface Markers | Localization | Frequency (of Splenic B Cells) |
|---|---|---|---|
| B1 | |||
| B1a | CD19+ B220low IgMhigh CD5+ CD43+ CD23− | Spleen, peritoneum | ≈2% |
| B1b | CD19+ B220low IgMhigh CD5− CD43+ CD23− | Spleen, peritoneum | <1% |
| IRA | |||
| Innate response activator | IgMhigh CD43+ CD23low CD93+ | Spleen | <0.5%* |
| B2 | |||
| Marginal zone | CD19+ B220+ IgMhigh CD21high CD43− CD23− | Spleen | ≈10% |
| Follicular | CD19+ B220+ IgMlow CD21+ CD43− CD23+ | Spleen, circulation | ≈80% |
| Bregs | |||
| B regulatory | CD19+ B220+ IgMhigh CD1dhigh CD43− | Spleen | ≈1% |
Bregs indicates regulatory B cells; and IRA, innate response activator.
The frequency of IRA B cells increases to ≈4% in response to lipopolysaccharide injections.
Table 2. Summary of Experimental Studies With Respect to the Role of Different B-Cell Subsets in Atherosclerosis.
| Atherosclerosis Studies in Mice That Involve Different B-Cell Subsets | Effect on Atherosclerosis | B-Cell Subsets (Potentially) Affected |
|||||
|---|---|---|---|---|---|---|---|
| B1a | B1b | IRA | MZ | FO | Bregs | ||
| Adoptive CD19+ B-cell transfer (20×106) into splenectomized or shamoperated Apoe−/− mice22 | ↓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Reconstitution of Ldlr−/− mice with B-cell–deficient (μMT) bone marrow23 | ↑ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| CD20 antibody treatment of Apoe−/− and Ldlr−/− mice24,25 | ↓ | (✓) | ✓ | ? | ✓ | ✓ | ✓ |
| Adoptive transfer of splenic CD43− B cells into lymphocyte-deficient Rag2−/−γ-chain−/−Apoe−/− (5×106 or 5×107) or B-cell–deficient μMT/Apoe−/− (5×106) mice25 | ↑ | … | … | … | ✓ | ✓ | ✓ |
| Adoptive transfer of splenic CD43− B cells (3×107 or 6×107) into B-cell–deficient μMT/Apoe−/− mice28 | ↓ | … | … | … | ✓ | ✓ | ✓ |
| Adoptive transfer of wild-type but not sIgM−/− B1a cells (105 every 3 wk) into splenectomized atherosclerotic Apoe−/− mice29 | ↓ | ✓ | … | … | … | … | … |
| BAFFR-deficient Apoe−/− or Ldlr−/− mice24,30 | ↓ | … | … | (✓) | ✓ | ✓ | ? |
| Anti-BAFFR treatment of Apoe−/− mice31 | ↓ | … | … | (✓) | ✓ | ✓ | ? |
| Reconstitution of Ldlr−/− mice with 50% GM-CSF and 50% B-cell–deficient (μMT) bone marrow to achieve GM-CSF deficiency in B cells only32 | ↓ | … | … | ✓ | … | … | … |
Apoe−/− indicates apolipoprotein E deficient; BAFFR, B-cell–activating factor receptor; Bregs, regulatory B cells; FO, follicular; IRA, innate response activator; Ldlr−/−, low-density lipoprotein receptor; and MZ, marginal zone.
In humans, different B-cell subsets have been described as well, but data regarding these are mostly derived from peripheral blood analysis. There is only limited knowledge regarding B cells that lack circulating capacities and substantial disagreement on the identity of such subsets.
B2 and B1 Cells: The Villain and the Good Guy?
A series of studies has pointed toward a differential role of B1 and B2 cells in atherogenesis (Table 2). For example, anti-CD20 treatment in vivo preferentially induces rapid death of B2 cells within a few hours on administration. Indeed, in a study by Ait-Oufella et al,24 B2 cells were effectively depleted on CD20 antibody treatment, whereas B1a cells remained unchanged. These data were also confirmed by Kyaw et al,25 who in addition showed that adoptive transfer of splenic B2 cells, but not B1 cells, into lymphocyte-deficient Rag2−/−γ-chain−/−Apoe−/− or B-cell–deficient μMT/Apoe−/− mice aggravated atherosclerosis.24,35 Moreover, disruption of the B-cell–activating factor (BAFF) signaling in atherosclerotic mouse models has further supported a proatherogenic function of B2 cells specifically. BAFF is expressed by monocytes, dendritic cells, and T cells and binds to 3 receptors predominately found on B cells, the BAFFR, transmembrane activator and calcium modulator and cyclophilin ligand interactor, and B-cell maturation antigen.36 The interaction between BAFF and BAFFR is of crucial importance for the survival of B2 cells because mice deficient in either BAFF or BAFFR lack mature B2 cells but have near-normal B1 cell numbers.37,38 In line with this, BAFFR-deficient Apoe−/− mice as well as BAFFR-deficient Ldlr−/− bone marrow chimeric mice display a nearly complete lack of mature B2 cells with fully preserved B1a cell numbers.30,39 In both cases, atherosclerotic lesion formation was significantly reduced. In addition, in a recent study, Kyaw et al31 showed that inhibition of the BAFFR using a blocking anti-BAFFR antibody in atherosclerotic Apoe−/− mice resulted in a selective depletion of B2 cells and decreased plaque formation. These data support a proatherogenic role for B cells and B2 cells in particular. The exact mechanisms by which B2 cells promote atherosclerosis remain elusive. Nevertheless, several possibilities have been suggested. For example, a link between anti-CD20–mediated B2-cell depletion and IL-17 upregulation has been proposed to mediate the atheroprotective effect of this treatment, because anti–IL-17 treatment abrogated the effect of anti-CD20.24 In turn, increased Th17 cells were suggested to counterbalance the effects of proatherogenic IFN-γ secreting Th1 cells. On the contrary, the robust reduction of T-cell–dependent serum IgG antibodies as result of B-cell depletion (Figure 1) may have resulted in the removal of potentially pathogenic antibodies directed against plaque antigens. Furthermore, B-cell–mediated antigen presentation to pathogenic T cells would also be impaired after B-cell depletion. Interestingly, IRA cells, which secrete GM-CSF, were also shown to be depleted in BAFFR-deficient mice, and thus their depletion may also be responsible for the decreased atherogenesis in these mice.33
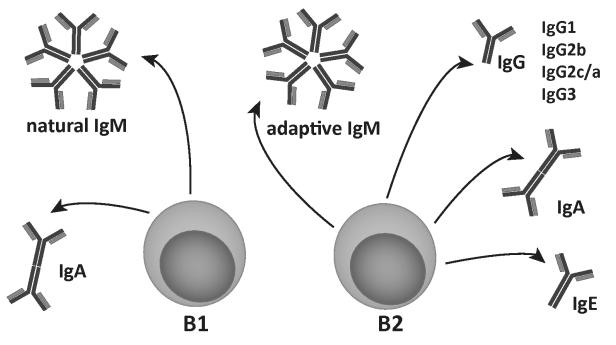
Figure 1. B1 and B2 cells have different immunoglobulin production profiles in mice.
B cells are divided in 2 main subfamilies, the B1 and B2 cells. B1 cells produce germline-encoded natural IgM and IgA antibodies. B2 cells respond to T-cell help on antigen stimulation and produce adaptive IgM, followed by IgG (IgG1, IgG2a/c, IgG3), IgA, or IgE antibodies via a process termed class switching.
In contrast to the studies discussed above, McNamara and colleagues found that adoptive transfer of splenic B2 cells from ApoE−/− mice into cholesterol-fed μMT/Apoe−/− mice resulted in robust protection from atherosclerosis. In addition, they found that the effect was absent when B cells from Apoe−/− mice, which were also deficient in the transcription factor inhibitor of differentiation-3 (Id3), were transferred.28 Of note, transferred Id3−/− B cells failed to home to aortic sites, which was accompanied by decreased chemokine receptor (CCR)6 expression on Id3-deficient B cells. A possible explanation for these contradictory data could be the different numbers and different genetic background (wild type versus Apoe−/−) of transferred B2 cells, which may affect their fate and function in the recipient. Moreover, differences in the purity of transferred cells may also have contributed to these conflicting results. One may even speculate that the CD43− B-cell preparations also contained potentially atheroprotective regulatory B cells and that an increased frequency of such cells could have contributed to the beneficial outcome in the study by McNamara and colleagues, for example, via IL-10 secretion.
Thus, at present, there is conflicting evidence concerning the role of B2 cells in atherogenesis. To clarify this seemingly complicated issue, it will be critical to dissect the functions of individual B2-cell subsets (marginal zone versus follicular) and to separate intrinsic biological properties of the B2 cells from effects mediated by the antibodies they secrete.
B1a cells on the contrary, which represent the much smaller subset of B cells, clearly protect from atherosclerosis in mice. It is currently not known whether B1b cells have the same atheroprotective potential as B1a cells. An intact spleen has been shown to be required for B1a cell maintenance, because splenectomized or genetically asplenic mice have significantly lower B1 cell numbers in the circulation and perito-neal cavities.40 Moreover, it has been shown that the splenic microenvironment enhances the antibody production capacity of B1 cells.41 Thus, splenectomy may result in the loss of this potentially atheroprotective B-cell subset. Indeed, Kyaw et al29 showed that the peritoneal B1a cells of splenectomized Apoe−/− mice are reduced by 50%. Importantly, transfer of peritoneal B1a cells but not splenic B2 cells into splenectomized Apoe−/− mice resulted in an amelioration of the splenectomy-induced accelerated atherosclerosis.29 Interestingly, in a long-term follow-up study on the cause of death of 740 World War II American servicemen who underwent splenectomy consequent to trauma, the risk of death attributable to ischemic heart disease was found to be 2-fold higher, suggesting a similar net protective effect of splenic cells in humans as well.42 A human B1 cell equivalent has been recently described by Griffin et al43 applying the criteria of spontaneous IgM secretion, efficient T-cell stimulation, and tonic intracellular signaling. Based on these criteria, they identified a small population of B1 cells present in umbilical cord and adult peripheral blood that express the unique phenotype of CD20+CD27+CD43+ CD70−.43 A recent study, however, has challenged this fact, as Covens et al44 analyzed characteristics of this newly described B-cell population and found them to have a gene expression signature more similar to preplasmablasts than to murine B1 cells. Interestingly, this population of B cells was particularly enriched in umbilical cord blood and found to decline with age. It is tempting to speculate that such a decline correlates with a potential loss of protective capacities resulting in increased cardiovascular risk. Because the secretion of natural IgM antibodies is one of the major functions of B1 cells, it has been hypothesized that the atheroprotective effect of B1 cells depends on the secretion of IgM antibodies. Splenectomy of Apoe−/− mice is associated with decreased IgM levels, and patients subjected to splenectomy after trauma have been reported to have significantly lower serum IgM titers.45 Of note, unlike transfer of wild-type B1a cells, B1a cells isolated from sIgM−/− donor mice, which express but do not secrete IgM, failed to attenuate the accelerated atherosclerosis in splenectomized mice, demonstrating that the IgM conferred the atheroprotection.29 This is particularly interesting because we and others have previously characterized atheroprotective properties of specific B1 cell–derived natural IgM antibodies.46–50
IgM: The House Keeper
Several studies in human subjects have shown that plasma levels of IgM antibodies to OSEs are inversely correlated with CVD. For example, levels of IgM antibodies to CuOx-LDL and MDA-LDL are inversely correlated with the carotid intima–media thickness or the risk of developing a >50% diameter stenosis in the coronary arteries.51–53 Similarly, IgM titers to the OSE phosphocholine have been reported to be inversely correlated with the incidence of stroke54 and heart attack55 as well as with the CVD risk in patients with lupus.56,57 Although these data are primarily correlative in nature, there is now increasing experimental evidence supporting a mechanistic role for IgM antibodies in protection against CVD, as reviewed below (Figure 2).
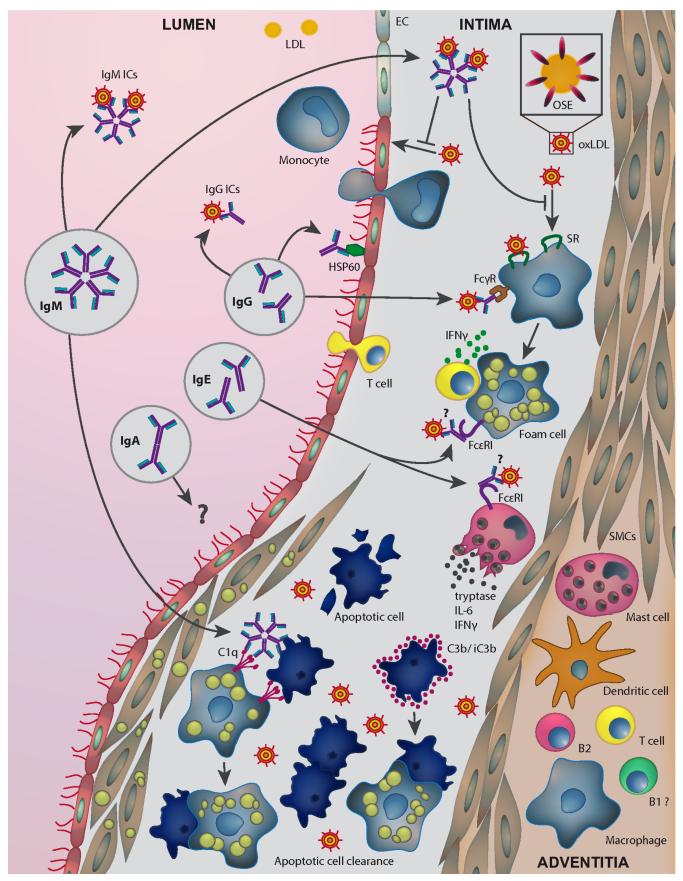
Figure 2. Role of immunoglobulins in lesion development.
Different immunoglobulins are present in atherosclerotic plaques, which are directed against relevant antigens such heat shock proteins (HSPs) and oxidized low-density lipoproteins (OxLDLs). IgM antibodies may mediate atheroprotection by neutralizing the proinflammatory properties of OxLDL, inhibiting the uptake of OxLDL by macrophages, and by promoting apoptotic cell clearance. Their protective capacity may be largely dependent on their ability to recognize oxidation-specific epitopes (OSEs) present on both OxLDL and apoptotic cells. OxLDL-specific IgG antibodies could activate macrophages via Fcγ receptor engagement, thereby promoting atherogenesis, but may also exhibit protective neutralizing capacities. IgM and IgG immune complexes with LDL carrying OSEs are also found in the circulation and may promote clearance of proatherogenic LDL particles. HSP60/65-specific IgG recognize stressed endothelial cells and induce damage via antibody-dependent cellular cytotoxicity. IgE antibodies activate mast cells and macrophages via the engagement FcεRI resulting in plaque destabilization. Specificities of IgE for atherosclerosis antigens, for example, OxLDL, remain to be shown. The role of IgA antibodies in atherosclerosis is still elusive. Opsonization of apoptotic cells with C3b and iC3b enhances their uptake by macrophages. EC indicates endothelial cells; IC, immune complexes; IFN-γ interferon-γ; IL-6, interleukin-6; SMCs, smooth muscle cells; and SR, scavenger receptors.
IgM antibodies comprise both natural IgM antibodies and adaptive IgM antibodies. NAbs are primarily derived from B1 cells that spontaneously secrete T-cell–independent antibodies, whereas adaptive IgMs are secreted by B2 cells in a T-cell–dependent manner (Figure 1). In uninfected mice, 80% to 90% of the total IgM pool is derived from B1 cells.58 Natural IgM antibodies are pre-existing germline-encoded products that do not require exogenous antigen stimulation for their generation.59 Because of their specificity for microbial antigens, they play an important role in the first-line defense against infections with bacteria, viruses, and fungi. For example, sIgM−/− mice are particularly susceptible to bacterial peritonitis in a cecal ligation and puncture model.60 Moreover, IgM antibodies are also necessary to mount an efficient protective response against influenza virus as well as to promote the recognition and clearance of fungi, such as Pneumocystis murina.60–63 NAbs also have specificity for certain self antigens, and in addition to their antimicrobial properties, possess—so-called—housekeeping functions by regulating the safe disposal of apoptotic cells and (neo)self antigens, for example, altered self proteins. NAbs facilitate the removal of cellular debris,64 because their repertoire includes specificities for highly conserved structures on apoptotic cells and other (neo)self antigens, such as OSEs that are found on apoptotic cells and on OxLDL.65 We have shown that OSEs are a major target of natural IgM antibodies in mice and humans.66 These studies originated in the characterization of a set of monoclonal IgM antibodies with specificity for epitopes of OxLDL that were cloned from the spleens of hypercholesterolemic Apoe−/− mice, which develop high anti-OxLDL autoantibody titers.67 One clone that was studied in more detail is the anti-OxLDL IgM E06, which was shown to have fine specificity for the phosphocholine head group of OxPLs, but not the phosphocholine of native phospholipids.68 Of relevance, E06 could bind to OxLDL and prevent its binding to CD36 and SR-B1 of macrophages and thus inhibit OxLDL uptake and prevent foam cell formation in vitro.69 These and other data demonstrated that the phosphocholine of OxPL is a ligand for CD36 and mediated the uptake of OxLDL by macrophages.11
Subsequently, sequence analysis of the CDR3 region of E06 revealed a germline sequence, which was 100% identical in both the VL and VH chains to that of the prototypic B1 cell–derived NAb T15, which is an IgA. T15 has specificity for phosphocholine, which is also a prominent constituent of the capsular polysaccharide of Streptococcus pneumonia (but is not part of a phospholipid) and has been shown to provide optimal protection to mice from pneumococcal infections.50,70,71 We first demonstrated an atheroprotective function of T15/ E06 IgM in Ldlr−/− mice that were immunized with heat-killed pneumococcal extracts and fed an atherogenic diet for 16 weeks. This immunization resulted in high anti-OxLDL IgM titers attributable to a near monoclonal expansion of T15id+ IgM and concomitantly decreased lesion formation.47 Plasma from these mice were able to block the uptake of OxLDL by macrophages effectively. Subsequently, Faria-Neto et al49 confirmed the atheroprotective function of T15/E06 by showing that passive infusion of T15/E06 IgM antibodies reduced vein graft atherosclerosis in Apoe−/− mice. Moreover, several atheroprotective interventions are associated with an increase in T15/E06 IgM levels. For example, we could show that the atheroprotective immunization with homologous MDA-LDL resulted not only in the generation of high titers of antibodies against MDA-LDL, but also in the expansion of T15/E06 IgM. We found that this effect was dependent on the induction of IL-5, because MDA-LDL immunization of Il5−/− mice did not show an expansion of T15/E06 IgM. Moreover, IL-5–deficient Ldlr−/− bone marrow chimeras had significantly lower T15/E06 levels and developed accelerated atherosclerosis. The potential atheroprotective function of IL-5 and its role in modulating anti-OxLDL IgM levels was further confirmed in a human population, in which low serum IL-5 levels were associated with increased carotid intima–media thickness and low anti-OxLDL IgM levels.72,73 Of note, in a genome-wide association study analysis of 15 596 patients with coronary artery disease and 34 992 controls, a single-nucleotide polymorphism (SNP) variant in the 3′ untranslated region of the IL-5 gene was associated with increased risk of coronary artery disease.74
The mechanisms that underlie the protective properties of T15/E06 IgM are not entirely clear. As noted above, experiments performed in vitro have shown that T15/E06 prevents uptake of OxLDL by binding to the phosphocholine of OxPL, thereby inhibiting foam cell formation.46,47 An additional mechanism by which T15/E06 IgM may limit plaque burden is by limiting the accumulation of apoptotic cells in developing lesions through the recognition of phosphocholine of OxPL formed on apoptotic cell surfaces.65 Impaired efferocytosis has been linked to enhanced atherogenesis, and T15/E06 has the capacity to promote apoptotic cell clearance by macrophages in a C1q-dependent manner.64,75,76 Finally, a key protective function is found in the ability of T15/E06 IgM to neutralize proinflammatory gene expression induced by OxPL present in OxLDL and the membranes of apoptotic cells.11,48 For example, T15/ E06 has been shown to inhibit IL-8 and adhesion molecule expression in endothelial cells stimulated with apoptotic cells or blebs and decreased monocyte adherence.48,77 Moreover, T15/ E06 was able to abrogate the recognition of POVPC (1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine) (an OxPL) by the macrophage scavenger receptor CD36,69 which is also critically involved in the proinflammatory response of macrophages to OxPL by cooperating with Toll-like receptor 4 and 6.78 Indeed, T15/E06 IgM has been found to prevent OxPAPC (oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine)–induced IL-6 secretion by macrophages79 and to block the ability of OxPL to decrease macrophage phagocytosis.80 Many more natural IgMs with specificity for other OSEs exist, which represent a prominent fraction (20%–30%) of all natural IgMs in mice and humans.66 For example, IgM with specificity for malondialdehyde epitopes, such as the natural IgM NA17, also bind apoptotic cells and enhance the in vivo clearance of injected apoptotic cells by peritoneal macrophages.66 Malondialdehyde represents another important danger signal that is present in atherosclerotic lesions and promotes inflammatory cytokine expression in vivo.66 Because of the prominent presence of malondialdehyde adducts in lesions, malondialdehyde-specific IgM may have a particularly important role in atheroprotection. Indeed, we have shown that the atheroprotective immunization of animal models of atherosclerosis with autologous MDA-LDL also leads to the induction of high titered IgM antibodies against MDA-LDL, which may in part be responsible for the protective effect of immunization.72
Because ~30% of natural IgM antibodies bind different OSEs, it can be expected that low IgM levels in general or total IgM deficiency would be associated with an increased propensity for lesion formation. Lewis et al81 demonstrated the atheroprotective role of IgM antibodies using sIgM−/− mice, which cannot secrete IgM but possess surface-bound IgM antibodies and have the capacity to class switch and secrete all other immunoglobulin classes. When sIgM−/− mice were crossed onto Ldlr−/− mice, they develop dramatically accelerated atherosclerosis both on a low cholesterol diet and on an atherogenic diet. Considering the above described role for IgM in apoptotic cell clearance, the authors evaluated the apoptotic cell accumulation in the aortic root lesions of these mice. Although there was a trend toward increased numbers of apoptotic cells in the lesions of sIgM−/−Ldlr−/− mice fed a low cholesterol diet, differences were not significant when stringent statistical tests were applied. Moreover, no differences in apoptotic cell accumulation were found in sIgM−/−Ldlr−/− mice fed a high-cholesterol diet, despite differences in lesion size.81 Similarly, adoptive transfer of B1a cells into splenectomized Apoe−/− mice, which rescued the aggravated atherosclerosis in these mice and increased lesional IgM deposition, did not significantly alter the apoptotic cell content compared with lesions from mice in which B1a cells from sIgM−/− mice were transferred.29 These data suggest that promoting the clearance of apoptotic cells within the lesions alone does not fully explain the atheroprotective mechanisms of IgM, and additional mechanisms may be operative.
In this regard, immunoregulatory properties of IgM may be of particular importance. Natural IgMs possess a biased repertoire toward (neo)self antigens, and this interaction may be a critical factor for immune homeostasis. Mice deficient in sIgM display strong differences in their splenic B-cell populations with increased B1 and marginal zone cells and decreased follicular B cells, likely as a result of impaired B-cell receptor signaling. Moreover, IgG2a and IgG3 as well as IgA plasma levels are increased in sIgM−/− mice, which also show impaired IgG antibody responses at least to suboptimal doses of a T-cell–dependent antigen.82–85 Paradoxically, Lewis et al81 reported decreased IgG2a/c titers against CuOx-LDL in atherosclerotic sIgM−/−Ldlr−/− mice, which may reflect increased immune complex formation with OxLDL in the absence of circulating IgM. Interestingly, infusion of polyclonal IgM into Apoe−/− mice during the last 4 weeks of a 29-week atherogenic diet has been reported to delay lesion formation, whereas infusion of a monoclonal T15id+ IgM preparation failed to do so in this setting.86 Atheroprotection in mice receiving polyclonal IgM was associated with a decreased frequency of CD4+ cells in the spleen. These data suggest that immunoregulatory functions of IgM, which may require the full repertoire of IgM specificities, provide an additional atheroprotective mechanism. In this regard, it is worthwhile to note that the repertoire (antibody specificities) of IgM antibodies differs between different B-cell subsets. For example, B1a cell–derived IgMs are more restricted to a germline-encoded repertoire, whereas the specificities of B1b cell–derived IgM can be more adaptive to antigen challenge.27 Therefore, insights into the contribution of the polyclonal repertoire of natural IgM as well as the role of IgM with defined specificities, such as phosphocholine and malondialdehyde, will be critical for the understanding of the atheroprotective mechanism of IgM.
IgG: Old Friend or Foe?
IgG is the main immunoglobulin subtype in the circulation of humans. The IgG consists of 4 different subclasses both in humans (IgG1, IgG2, IgG3, and IgG4) and in mice (IgG1, IgG2a/c, IgG2b, and IgG3) that exhibit different affinity to Fcγ receptors as well as a different capacity to activate complement.87,88 IgG antibodies are present in atherosclerotic lesions, and some of them have been shown to have specificity for OxLDL.12 In addition, IgG antibodies with specificity for OxLDL and other plaque antigens have been documented in human plasma, and thus several epidemiological studies have measured their titers in association with CVD risk. Although many studies documented a positive correlation of anti-Ox-LDL IgG with manifestations of CVD, others have not.9 In most cases, however, these correlations lose significance when multivariate analyses are performed with other risk factors of CVD. Another explanation for the inconsistency of these studies may also be found in the lack of reproducible antigens used to make such measurements. We have recently identified and characterized peptide mimotopes for malondialdehyde epitopes that can serve as a standardized antigen to assess antibodies specific to malondialdehyde epitopes.89 Studies are currently ongoing to evaluate their use in large clinical studies. Hsp65-specific IgG titers have also been evaluated in relationship to CVD manifestations and found to be an independent risk factor for carotid intima–media thickness in one study,90 but not in another.91
Despite all these caveats, IgG antibodies have often been suggested to be proatherogenic. However, there is little experimental evidence for this or even any functional role of IgG antibodies in atherosclerosis. Apart from the recent mouse studies, in which B-cell depletion was associated with decreased atherosclerosis and a profound reduction of both total and OxLDL-specific IgG antibodies and to a lesser extent IgM antibodies,24,25,30,31,39 their role has been mostly implicated from immunization studies with either Hsp65 or models of OxLDL. For example, immunization of normocholesterolemic rabbits with Hsp65 induced arteritis even in the absence of elevated serum cholesterol levels and accelerated atherosclerosis on a cholesterol-enriched diet.92 These effects were reproduced in studies using Ldlr−/− mice fed a regular chow diet, which developed high anti-Hsp65 IgG antibodies and increased atherosclerotic lesions.93 A proatherogenic effect of anti-Hsp65 IgG was also demonstrated by George et al,94 who showed that intraperitoneal injections of chow-fed Ldlr−/− mice with IgG preparations from Hsp65-immunized mice promoted fatty streak formation. These data suggest that at least in part Hsp65-specific IgGs have proatherogenic properties, for example, by damaging Hsp60-expressing endothelial cells95 (Figure 2).
In contrast, immunization of Ldlr−/− Watanabe heritable hyperlipidemic rabbits with homologous MDA-LDL, which resulted in the induction of high IgG antibody titers against MDA-LDL, was shown to protect from atherosclerotic lesion formation compared with control PBS and keyhole limpet hemocyanin-immunized rabbits.96 Similar data were obtained after immunization of hypercholesterolemic rabbits with homologous CuOx-LDL.97 Moreover, immunization of either Apoe−/− or hypercholesterolemic Ldlr−/− mice with MDA-LDL resulted in the induction of robust IgG titers against MDALDL and decreased atherosclerosis.72,98–100 In such immunization experiments, many complex immunoregulatory changes may be induced including both cellular and humoral effects, and the mechanisms by which immunization with OSEs protect from lesion formation remain to be defined. Clearly, more complex regulatory mechanisms may be involved beyond the simple induction of neutralizing antibodies. In support of this, immunization of Ldlr−/− mice with native LDL also resulted in atheroprotection in the absence of any measurable IgG titers to LDL antigens.100 Nevertheless, there is considerable data to support a direct protective effect of OSE-specific antibodies. For example, passive transfer of a recombinant human IgG1 to MDA-LDL into Apoe−/− mice inhibited lesion formation.101 Passive infusion of Ldlr−/− mice with the human recombinant Fab antibody IK17 against MDA-LDL102 or adenoviral-mediated expression in vivo of an scFv version of IK17 both inhibited lesion formation, and in both of these cases, there was an enhanced capacity of plasma antibodies to inhibit OxLDL binding to macrophages.102 Thus, considerable data demonstrate direct atheroprotective properties for malondialdehyde-specific antibodies. Importantly, to our knowledge; there is no information to date of adverse effects of OxLDL-specific IgG antibodies in vivo.
However, there are only limited insights into the mechanisms by which these anti-OxLDL IgGs act in vivo (Figure 2). Based on in vitro analysis of plasma from mice with high IgG titers to MDA-LDL, it seems that such IgGs have the capacity to block the binding of OxLDL to macrophages in vitro, but whether this occurs in vivo is unknown. On the contrary, Schiopu et al101 reported that the recombinant human MDALDL–specific IgG1 that inhibited atherosclerosis actually increased uptake of OxLDL by macrophages in vitro. Of importance, the contributing role of Fc effector functions of these anti-OxLDL IgG antibodies is still elusive. Fcγ receptors, which bind to the Fc portion of antibodies, are critical mediators of functions of IgG, and several studies in mouse models of atherosclerosis have addressed the global role of Fcγ receptors. Two categories of Fcγ receptors exist: the activating receptors that include the FcγRI, FcγRIIA, FcγRIII, and on the contrary, the inhibitory receptor FcγRIV. Apoe−/− mice lacking the Fc γ-chain (γ−/−, which lack all activating receptors), thus only express the inhibitory receptor FcγRIIB, develop significantly less atherosclerosis when fed either regular chow or a high-cholesterol diet. Decreased lesion formation was associated with reduced numbers of lesional macrophages and T cells.103 Consistent with a proatherogenic role of activating Fcγ receptors, another study also found strongly reduced lesion size in CD16 (FcγRIII)-deficient Ldlr−/− mice at both the aortic root and innominate artery, despite increased plasma total cholesterol. Interestingly, Cd16−/−Ldlr−/− mice exhibited elevated IgG1 and IgG2c plasma titers against MDA-LDL and CuOx-LDL compared with Ldlr−/− control mice, whereas total plasma IgG levels were not different.104 Although these data point to a proatherogenic role of activating Fcγ receptors, it is not clear whether these effects correspond directly to the engagement of Fc receptors by disease-specific IgGs, such as those to OSEs, or to a more global property of activating Fcγ receptors. It has been shown that human OxLDL-IgG immune complexes activate proinflammatory mitogen-activated protein kinase signaling in THP-1 human monocytes in an FcγRI (the human equivalent of murine FcγRI)-dependent manner.105 In addition, indirect mechanisms may also have contributed to the atheroprotective effects in FcγR-deficient mice. For example, does the increase of OxLDL-specific IgGs mediate protection or is the relative activity of the inhibitory FcγRIIB increased in these mice? The latter point is of great interest because expression of FcγRIIB has been shown to protect mice from several autoantibody-mediated disease.106 Indeed, Apoe−/− mice deficient in the inhibitory FcRγIIB receptor develop enhanced atherosclerosis, indicating a protective role for this immunomodulatory Fc receptor. Notably, these mice also had elevated IgG1 and IgG2c plasma titers against MDALDL and CuOx-LDL.107
In summary, it is clear that we are still far from a complete understanding of the biological roles and functions of OxLDL-specific IgGs in atherosclerosis. In addition to binding to OxLDL, these OSE antibodies also bind to apoptotic cells, although their impact on clearance or the proinflammatory properties of these cells is unknown. It still needs to be investigated whether their effects are mediated by FcγR and, if so, which cells expressing FcγR are involved. Furthermore, the roles of the different IgG isotypes, which have different affinities for Fcγ receptors, as well as different complement activating properties, need to be determined. These considerations add yet another layer of complexity to understanding their effector functions and net impact on atherogenesis.
IgE: The Underestimated Player
IgE antibodies have been extensively studied in allergy and asthma, where they are considered critical mediators of these pathologies. Little is known about their role in atherosclerosis, although a few epidemiological studies exist that suggest a contribution for this usually tightly controlled immunoglobulin. For example, Kovanen et al108 found that high IgE levels are a prognostic factor for myocardial infarction and cardiac death in dyslipidemic men in the Helsinki Heart Study. This association was later confirmed by Wang et al,109 who found that patients with CHD have elevated IgE levels compared with subjects without CHD. Moreover, in this study, IgE levels were positively correlated with the CVD severity, with patients with acute myocardial infarction having the highest serum IgE levels compared with patients with unstable angina pectoris and stable angina pectoris. The association with IgE was independent of sex, age, body mass index, hypertension, diabetes mellitus, or serum lipid profiles. This study and several others, including the one by Kovanen et al,108 have also shown that IgE levels were directly associated with smoking. Therefore, IgE may be involved in the increased risk of atherosclerosis associated with smoking. Thus, epidemiological data suggest a proatherogenic role for IgE antibodies.
Although the role of IgE antibodies in experimental atherosclerosis has not been directly investigated, indirect evidence comes from studies in atherosclerosis-prone mice deficient in Fcε receptors. IgE antibodies mainly bind to the high-affinity IgE receptor (FcεRI) and to the low-affinity IgE receptor (FcεRII/CD23). Moreover, the IgE-binding protein galectin-3 has been shown to cross-link receptor-bound IgE and FcεRI.110 A recent study demonstrated that Apoe−/− mice deficient in FcεRI develop significantly decreased atherosclerosis with reduced macrophage and apoptotic cell content. As a potential proatherogenic mechanism, the authors demonstrated that FcεRI on macrophages cooperates with Toll-like receptor 4 and on IgE binding led to cell activation, inflammatory cytokine secretion, and apoptosis (Figure 2). However, these effects were achieved with IgE concentrations that were >200-fold higher than the concentrations measured in athero-sclerotic mice. Therefore, these effects may not entirely reflect an in vivo situation.109
Other FcεRI-mediated effects may also be operative because IgE antibodies are a major stimulus for mast cells, which have been implicated in atherosclerosis and in the destabilization of lesions in particular.111 Studies in atherosclerotic Ldlr−/− mice have shown that mast cell deficiency results in reduced lesion formation and that this may be promoted by mast cell–derived IL-6 and IFN-γ.112 Another study by Bot et al113 also demonstrated a proatherogenic effect of mast cell activation in Apoe−/− mice and a role for mast cell degranulation in particular. In line with this, in vitro degranulation of mast cells by IgE treatment has been shown to promote OxLDL uptake by macrophages in coculture experiments.114 Thus, mast cells may be a critical mediator of the proatherogenic effects of IgE binding to FcεRI (Figure 2). Interestingly, galectin-3 was found to be upregulated in atherosclerotic lesions of rabbits and humans. Apoe−/− mice deficient in galectin-3 or treated with a galectin-3 inhibitor were shown to develop significantly less atherosclerosis.115,116 Although galectin-3 has many other potential functions, its proatherogenic effect may in part be mediated by its ability to bind and cross-link IgE antibodies. Critical insights into a direct role of IgE in atherosclerosis are still missing. Moreover, it will be important to demonstrate whether such effects are dependent on certain antibody specificities for relevant antigens, for example, OxLDL, or whether they are antigen independent.117,118
IgA: Waiting to Go on Stage
IgA immunoglobulins have been selected to provide the first line of defense in mucosal areas (mucosal IgA). On the contrary, IgA antibodies are also found in the circulation. In humans, there are 2 classes of IgA, IgA1 and IgA2, whereas in mice, only 1 class exists. In addition, the human secreted IgA comprised monomeric IgA1 and IgA2, whereas in mice, the circulating IgA antibodies form dimers and oligomers.119 There is little information about the role of IgA antibodies in CVD. Two studies by Muscari et al120,121 found elevated IgA levels in patients with advanced vascular disease and myocardial infarction, respectively. Moreover, Kovanen et al108 reported IgA levels to be correlated with myocardial infarction and cardiac death in dyslipidemic men after adjustment for CVD risk factors. However, there are no mechanistic studies available with respect to IgA antibodies in atherosclerosis. Recent insights on the effects of the gut microbiome on CVD,122 however, could suggest a potentially important mechanism by which IgA antibodies could be modulated to impact atherogenesis.
Complement in Atherosclerosis
Besides its antimicrobial properties, complement is known to participate in the maintenance of immune homeostasis by sensing endogenous danger signals such as cellular debris and apoptotic cells. The complement cascade is composed of 3 pathways, the classical, the alternative, and the lectin pathway, which converge at the level of the C3 convertase resulting, if unopposed, in the generation of proinflammatory C5a and C5b leading to the formation of the lytic terminal complement complex. The classical pathway, which involves C1 and C4 upstream of the C3 convertase, is also strongly initiated by IgM and IgG antibodies bound to their cognate antigens (eg, microbes, apoptotic cells). Complement components have been described to be deposited in atherosclerotic lesions, and there are growing numbers of animal studies addressing the role of certain complement components. For a detailed review, see Speidl et al.123 However, because of the fact that many complement components also play a key role in homeostasis, including the removal of apoptotic cells, the interpretation of these studies as indicators of immunoglobulin action is difficult. Ldlr−/− mice deficient in C3, the central component of all 3 pathways, have been shown to develop lesions of similar size in the abdominal and thoracic aorta. However, increased lipid and macrophage deposition and decreased collagen and smooth muscle cell content were found in the aortic root lesions of C3-deficient mice, suggesting an overall protective role for C3.124 In addition, C3-deficient mice crossed on an Apoe−/−Ldlr−/− background have been shown to develop 84% increased lesions in the aorta.125 C3b and its degradation product iC3b have been shown to facilitate apoptotic cell uptake by macrophages in vitro. Similarly, the member of the classical pathway C1q, which also binds to apoptotic cells directly or via IgM,123 has been found to be atheroprotective, because Ldlr−/− mice deficient in C1q develop significantly larger lesions compared with control mice.81,126 In support of a proatherogenic role for complement activation, it has been shown that pharmacological inhibition of the C5a receptor CD88 in Apoe−/− mice results in decreased plaque size in the aortic root.127 This finding is supported by epidemiological data showing a positive association of C5a levels with increased CVD risk independent of nonspecific inflammatory markers such as C-reactive protein or serum amyloid A.128 Moreover, epidemiological studies have shown that C4 levels are associated with severe atherosclerosis.120
Thus, studies on the role of complement components do not allow any conclusion on immunoglobulin effector functions in atherosclerosis. In fact, homeostatic functions of complement may be of great relevance. This should also include regulators of complement activation, such as C4bp and complement factor H (CFH), which are present in atherosclerotic lesions and may also modulate immunoglobulin function.129–131 CFH provides cofactor activity for factor I–mediated degradation of C3b into iC3b fragments, and deposition of iC3b on the surface of apoptotic cells has been shown to promote anti-inflammatory clearance mechanisms.132 Our recent discovery that CFH binds malondialdehyde epitopes on cellular debris and inhibits their proinflammatory effects shines light on a potential function in atherosclerosis. In fact, we showed that the malondialde-hyde-binding sites in CFH are localized to domains that are also hot spots of disease-associated mutations. For example, a common SNP rs1061170, which is highly associated with an increased risk for age-related macular degeneration, significantly impairs the binding of CFH to malondialde-hyde. Although meta-analyses of clinical studies involving ≈48 000 individuals do not support a role for this specific SNP in CVD,133 it is tempting to speculate that the combination of several rare SNPs in the malondialdehyde-binding sites results in functional alterations of CFH binding to malondialdehyde. Such SNPs might not be detected in genetic association studies because of their low frequencies. In this regard, it will be interesting to evaluate the association of CFH binding to malondialdehyde with CVD in large clinical cohorts.
Translational Opportunities by Targeting B Cells and Their Effect in Antibody Production
Clearly, the involvement of immune mechanisms in atherosclerosis offers several novel therapeutic opportunities for targeting immune responses in atherosclerosis. The broad spectrum of such approaches is summarized in several reviews.8,9,134 However, the advent and clinical application of several B-cell targeting compounds in autoimmune disease offer a hitherto unrecognized opportunity for novel therapeutic strategies in atherosclerosis (Table 3). This is of particular interest because diseases for which these compounds are mainly being used or developed, including rheumatoid arthritis and systemic lupus erythematosus (SLE), are also associated with a significantly increased risk of CVD. There are several B-cell–depleting compounds that are currently approved or in clinical trials. Foremost, the CD20-depleting antibody (Rituximab) results in B-cell elimination by cross-linking the CD20 receptor, which is expressed on all B cells.35 It has been approved by the US Food and Drug Administration as a treatment for rheumatoid arthritis. Moreover, in 2011, the US Food and Drug Administration approved a neutralizing BAFF antibody (Belimumab) as treatment for SLE. The effects of these 2 treatments on CVD are not known; but as discussed, several animal studies demonstrated an athero-protective effect of B-cell depletion by CD2024 or inhibition of BAFFR signaling with an anti-BAFFR antibody.31 In this regard, additional compounds that inhibit the BAFF–BAFFR signaling exist, such as the soluble form of BAFFR fused to an immunoglobulin backbone (BAFFR-Ig).136 Similar to this, a decoy form of the transmembrane activator and calcium modulator and cyclophilin ligand interactor receptor (TACIIg) is currently in clinical phase III trial for SLE. TACI-Ig binds both BAFF and another ligand termed a proliferation inducing ligand36 leading to the depletion of mature B2 as well as plasma cells with profound immunoglobulin reduction.137,138 A careful accounting of the impact of these interventions on CVD should be done, which may reveal whether any of these B-cell depleting strategies modulate CVD risk or at least decrease the increased CVD risk associated with rheumatoid arthritis or SLE.
Table 3. Summary of B-Cell Depleting Compounds Already Approved or in Development for Treatment SLE and RA and Their Effects in Experimental Atherosclerosis (if Applicable).
| Compound | Function | Effect on B Cells | Effect on Murine Atherosclerosis | Clinical Application | Reference |
|---|---|---|---|---|---|
| α-CD20 | Cross-linking of CD20 receptor | Fcγ receptor–mediated B-cell depletion | Decreased atherosclerosis | RA | 35 |
| α-BAFF | Neutralization of circulating BAFF | B2 cell apoptosis | Unknown | SLE (Belimumab) | 135 |
| BAFFR-Fc | Neutralization of both circulating and membrane-bound BAFF | B2 cell apoptosis | Unknown | Unknown | 136 |
| α-BAFFR | BAFF receptor blockage | B2 cell apoptosis | Decreased atherosclerosis | Unknown | 31 |
| TACI-Fc | Neutralization of circulating and membrane-bound BAFF and APRIL | B2 cell and plasma cell apoptosis | Unknown | Phase 3 clinical trial for SLE (Atacicept) | 137,138 |
APRIL indicates a proliferation inducing ligand; BAFFR, B-cell–activating factor receptor; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; and TACI, transmembrane activator and calcium modulator and cyclophilin ligand interactor.
Concluding Remarks
Several studies in mouse models of atherosclerosis have established an important modulatory role for B cells in experimental atherosclerosis, and their effect is dependent on specific B-cell subsets. The contribution and effector functions of immunoglobulins and the different immunoglobulin classes in these effects still requires further investigation, but natural IgM antibodies, particularly those directed to OSEs, clearly mediate atheroprotection. There is still much to learn about the importance of B-cell immunity in human disease, but some genetic evidence exists which for the most part corroborates findings from mouse studies. Insights from patients with SLE or rheumatoid arthritis with increased CVD risk that are being treated with novel B-cell–depleting drugs may help establish this link in humans and could help identify novel therapeutic strategies for atherosclerosis.
Acknowledgments
We are grateful to Vesna Krajina for help with the illustrations.
Sources of Funding C.J. Binder is supported by the Austrian Academy of Sciences and a grant of the Austrian Science Fund (SFB F30). J.L. Witztum is supported by National Institutes of Health grant HL088093.
Nonstandard Abbreviations and Acronyms
- Apoe−/−
apolipoprotein E deficient
- BAFF
B-cell–activating factor
- BAFFR
B-cell–activating factor receptor
- CFH
complement factor H
- CHD
coronary heart disease
- CuOx-LDL
copper-oxidized LDL
- CVD
cardiovascular disease
- Hsp60
heat shock protein 60
- IFN-γ
interferon-γ
- IL-10
interleukin-10
- IRA
innate response activator
- LDL
low-density lipoprotein
- Ldlr−/−
low-density lipoprotein receptor–deficient
- MDA-LDL
malondialdehyde-modified LDL
- NAbs
natural antibodies
- OSE
oxidation-specific epitope
- OxLDL
oxidized LDL
- OxPL
oxidized phospholipid
- SLE
systemic lupus erythematosus
- SNP
single-nucleotide polymorphism
Footnotes
Disclosures C.J. Binder has a patent on the use of complement factor H for oxidative stress disease conditions, which is held by Center for Molecular Medicine. J.L. Witztum has patents and patents disclosure for the commercial use of antibodies to oxidation-specific epitopes, which are held by University of California San Diego. The other authors report no conflicts.
References
- 1.Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 3.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 7.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 8.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–358. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 9.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weismann D, Binder CJ. The innate immune response to products of phospholipid peroxidation. Biochim Biophys Acta. 2012;1818:2465–2475. doi: 10.1016/j.bbamem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ylä-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dansky HM, Charlton SA, Harper MM, Smith JD. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1997;94:4642–4646. doi: 10.1073/pnas.94.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugherty A, Puré E, Delfel-Butteiger D, Chen S, Leferovich J, Roselaar SE, Rader DJ. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E-/- mice. J Clin Invest. 1997;100:1575–1580. doi: 10.1172/JCI119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Hansson GK. Detection of B cells and proinflammatory cytokines in atherosclerotic plaques of hypercholesterolaemic apolipoprotein E knockout mice. Scand J Immunol. 1999;50:25–30. doi: 10.1046/j.1365-3083.1999.00559.x. [DOI] [PubMed] [Google Scholar]
- 18.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry HM, Bender TP, McNamara CA. B cell subsets in atherosclerosis. Front Immunol. 2012;3:373. doi: 10.3389/fimmu.2012.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyaw T, Tipping P, Bobik A, Toh BH. Protective role of natural IgM-producing B1a cells in atherosclerosis. Trends Cardiovasc Med. 2012;22:48–53. doi: 10.1016/j.tcm.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Huan T, Zhang B, Wang Z, et al. Coronary ARteryDIsease Genome wide Replication and Meta-analysis (CARDIoGRAM) Consortium. International Consortium for Blood Pressure GWAS (ICBP) A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33:1427–1434. doi: 10.1161/ATVBAHA.112.300112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 24.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, Sirvent J, Van Snick J, Tedgui A, Tedder TF, Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P, Bobik A, Toh BH. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185:4410–4419. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 26.Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27:428–433. doi: 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 28.Doran AC, Lipinski MJ, Oldham SN, et al. B-cell aortic homing and atheroprotection depend on Id3. Circ Res. 2012;110:e1–e12. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A, Toh BH. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109:830–840. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 30.Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, MacKay F, Tipping P, Bobik A, Toh BH. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS One. 2012;7:e29371. doi: 10.1371/journal.pone.0029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyaw T, Cui P, Tay C, Kanellakis P, Hosseini H, Liu E, Rolink AG, Tipping P, Bobik A, Toh BH. BAFF receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE(-/-) mice. PLoS One. 2013;8:e60430. doi: 10.1371/journal.pone.0060430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilgendorf I, Theurl I, Gerhardt LM, et al. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation. 2014;129:1677–1687. doi: 10.1161/CIRCULATIONAHA.113.006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauch PJ, Chudnovskiy A, Robbins CS, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol. 2013;10:122–132. doi: 10.1038/cmi.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 37.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 39.Sage AP, Tsiantoulas D, Baker L, Harrison J, Masters L, Murphy D, Loinard C, Binder CJ, Mallat Z. BAFF receptor deficiency reduces the development of atherosclerosis in mice–brief report. Arterioscler Thromb Vasc Biol. 2012;32:1573–1576. doi: 10.1161/ATVBAHA.111.244731. [DOI] [PubMed] [Google Scholar]
- 40.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holodick NE, Tumang JR, Rothstein TL. Immunoglobulin secretion by B1 cells: differential intensity and IRF4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. Eur J Immunol. 2010;40:3007–3016. doi: 10.1002/eji.201040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinette CD, Fraumeni JF., Jr. Splenectomy and subsequent mortality in veterans of the 1939-45 war. Lancet. 1977;2:127–129. doi: 10.1016/s0140-6736(77)90132-5. [DOI] [PubMed] [Google Scholar]
- 43.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70−. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Covens K, Verbinnen B, Geukens N, Meyts I, Schuit F, Van Lommel L, Jacquemin M, Bossuyt X. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype. Blood. 2013;121:5176–5183. doi: 10.1182/blood-2012-12-471953. [DOI] [PubMed] [Google Scholar]
- 45.Drew PA, Kiroff GK, Ferrante A, Cohen RC. Alterations in immunoglobulin synthesis by peripheral blood mononuclear cells from splenectomized patients with and without splenic regrowth. J Immunol. 1984;132:191–196. [PubMed] [Google Scholar]
- 46.Hörkkö S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 48.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faria-Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, Cercek B, Shah PK. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006;189:83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 50.Shaw PX, Hörkkö S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karvonen J, Päivänsalo M, Kesäniemi YA, Hörkkö S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 52.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, Mayr A, Witztum JL, Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–2229. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 54.Fiskesund R, Stegmayr B, Hallmans G, Vikström M, Weinehall L, de Faire U, Frostegård J. Low levels of antibodies against phosphorylcholine predict development of stroke in a population-based study from northern Sweden. Stroke. 2010;41:607–612. doi: 10.1161/STROKEAHA.109.558742. [DOI] [PubMed] [Google Scholar]
- 55.Grönlund H, Hallmans G, Jansson JH, Boman K, Wikström M, de Faire U, Frostegård J. Low levels of IgM antibodies against phosphorylcho-line predict development of acute myocardial infarction in a population-based cohort from northern Sweden. Eur J Cardiovasc Prev Rehabil. 2009;16:382–386. doi: 10.1097/HJR.0b013e32832a05df. [DOI] [PubMed] [Google Scholar]
- 56.Anania C, Gustafsson T, Hua X, Su J, Vikström M, de Faire U, Heimbürger M, Jogestrand T, Frostegård J. Increased prevalence of vulnerable athero-sclerotic plaques and low levels of natural IgM antibodies against phosphorylcholine in patients with systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R214. doi: 10.1186/ar3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grönwall C, Akhter E, Oh C, Burlingame RW, Petri M, Silverman GJ. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol. 2012;142:390–398. doi: 10.1016/j.clim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 60.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, Plevy S, Bengtén E, Kolls JK. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med. 2010;207:2907–2919. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 65.Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci U S A. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Bäckhed F, Miller YI, Hörkkö S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palinski W, Hörkkö S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J Biol Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 69.Boullier A, Gillotte KL, Hörkkö S, Green SR, Friedman P, Dennis EA, Witztum JL, Steinberg D, Quehenberger O. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J Biol Chem. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 70.Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- 71.Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sämpi M, Ukkola O, Päivänsalo M, Kesäniemi YA, Binder CJ, Hörkkö S. Plasma interleukin-5 levels are related to antibodies binding to oxidized low-density lipoprotein and to decreased subclinical atherosclerosis. J Am Coll Cardiol. 2008;52:1370–1378. doi: 10.1016/j.jacc.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 74.The IBC 50K CAD Consortium Large-scale gene-centric analysis identifies novel variants for coronary artery disease. PLoS Genet. 2011;7:e1002260. doi: 10.1371/journal.pgen.1002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y, Khanna S, Goodyear CS, Park YB, Raz E, Thiel S, Grönwall C, Vas J, Boyle DL, Corr M, Kono DH, Silverman GJ. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183:1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, Binder BR, Leitinger N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 78.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thimmulappa RK, Gang X, Kim JH, Sussan TE, Witztum JL, Biswal S. Oxidized phospholipids impair pulmonary antibacterial defenses: evidence in mice exposed to cigarette smoke. Biochem Biophys Res Commun. 2012;426:253–259. doi: 10.1016/j.bbrc.2012.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 83.Ehrenstein MR, O’Keefe TL, Davies SL, Neuberger MS. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci U S A. 1998;95:10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baker N, Ehrenstein MR. Cutting edge: selection of B lymphocyte subsets is regulated by natural IgM. J Immunol. 2002;169:6686–6690. doi: 10.4049/jimmunol.169.12.6686. [DOI] [PubMed] [Google Scholar]
- 85.Notley CA, Baker N, Ehrenstein MR. Secreted IgM enhances B cell receptor signaling and promotes splenic but impairs peritoneal B cell survival. J Immunol. 2010;184:3386–3393. doi: 10.4049/jimmunol.0902640. [DOI] [PubMed] [Google Scholar]
- 86.Cesena FH, Dimayuga PC, Yano J, Zhao X, Kirzner J, Zhou J, Chan LF, Lio WM, Cercek B, Shah PK, Chyu KY. Immune-modulation by polyclonal IgM treatment reduces atherosclerosis in hypercholesterolemic apoE-/- mice. Atherosclerosis. 2012;220:59–65. doi: 10.1016/j.atherosclerosis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 88.Frank MM, Miletic VD, Jiang H. Immunoglobulin in the control of complement action. Immunol Res. 2000;22:137–146. doi: 10.1385/IR:22:2-3:137. [DOI] [PubMed] [Google Scholar]
- 89.Amir S, Hartvigsen K, Gonen A, Leibundgut G, Que X, Jensen-Jarolim E, Wagner O, Tsimikas S, Witztum JL, Binder CJ. Peptide mimotopes of malondialdehyde epitopes for clinical applications in cardiovascular disease. J Lipid Res. 2012;53:1316–1326. doi: 10.1194/jlr.M025445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu Q, Willeit J, Marosi M, Kleindienst R, Oberhollenzer F, Kiechl S, Stulnig T, Luef G, Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–259. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- 91.Knoflach M, Kiechl S, Kind M, Said M, Sief R, Gisinger M, van der Zee R, Gaston H, Jarosch E, Willeit J, Wick G. Cardiovascular risk factors and atherosclerosis in young males: ARMY study (Atherosclerosis Risk-Factors in Male Youngsters) Circulation. 2003;108:1064–1069. doi: 10.1161/01.CIR.0000085996.95532.FF. [DOI] [PubMed] [Google Scholar]
- 92.Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992;12:789–799. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- 93.Afek A, George J, Gilburd B, Rauova L, Goldberg I, Kopolovic J, Harats D, Shoenfeld Y. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J Autoimmun. 2000;14:115–121. doi: 10.1006/jaut.1999.0351. [DOI] [PubMed] [Google Scholar]
- 94.George J, Afek A, Gilburd B, Shoenfeld Y, Harats D. Cellular and humoral immune responses to heat shock protein 65 are both involved in promoting fatty-streak formation in LDL-receptor deficient mice. J Am Coll Cardiol. 2001;38:900–905. doi: 10.1016/s0735-1097(01)01440-1. [DOI] [PubMed] [Google Scholar]
- 95.Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–2577. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci U S A. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ameli S, Hultgårdh-Nilsson A, Regnström J, Calara F, Yano J, Cercek B, Shah PK, Nilsson J. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol. 1996;16:1074–1079. doi: 10.1161/01.atv.16.8.1074. [DOI] [PubMed] [Google Scholar]
- 98.George J, Afek A, Gilburd B, Levkovitz H, Shaish A, Goldberg I, Kopolovic Y, Wick G, Shoenfeld Y, Harats D. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138:147–152. doi: 10.1016/s0021-9150(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 99.Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- 100.Freigang S, Hörkkö S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol. 1998;18:1972–1982. doi: 10.1161/01.atv.18.12.1972. [DOI] [PubMed] [Google Scholar]
- 101.Schiopu A, Bengtsson J, Söderberg I, Janciauskiene S, Lindgren S, Ares MP, Shah PK, Carlsson R, Nilsson J, Fredrikson GN. Recombinant human antibodies against aldehyde-modified apolipo-protein B-100 peptide sequences inhibit atherosclerosis. Circulation. 2004;110:2047–2052. doi: 10.1161/01.CIR.0000143162.56057.B5. [DOI] [PubMed] [Google Scholar]
- 102.Tsimikas S, Miyanohara A, Hartvigsen K, et al. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J Am Coll Cardiol. 2011;58:1715–1727. doi: 10.1016/j.jacc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hernández-Vargas P, Ortiz-Muñoz G, López-Franco O, Suzuki Y, Gallego-Delgado J, Sanjuán G, Lázaro A, López-Parra V, Ortega L, Egido J, Gómez-Guerrero C. Fcgamma receptor deficiency confers protection against atherosclerosis in apolipoprotein E knockout mice. Circ Res. 2006;99:1188–1196. doi: 10.1161/01.RES.0000250556.07796.6c. [DOI] [PubMed] [Google Scholar]
- 104.Kelly JA, Griffin ME, Fava RA, Wood SG, Bessette KA, Miller ER, Huber SA, Binder CJ, Witztum JL, Morganelli PM. Inhibition of arterial lesion progression in CD16-deficient mice: evidence for altered immunity and the role of IL-10. Cardiovasc Res. 2010;85:224–231. doi: 10.1093/cvr/cvp300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang Y, Jaffa A, Koskinen S, Takei A, Lopes-Virella MF. Oxidized LDL-containing immune complexes induce Fc gamma receptor I-mediated mitogen-activated protein kinase activation in THP-1 macrophages. Arterioscler Thromb Vasc Biol. 1999;19:1600–1607. doi: 10.1161/01.atv.19.7.1600. [DOI] [PubMed] [Google Scholar]
- 106.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mendez-Fernandez YV, Stevenson BG, Diehl CJ, Braun NA, Wade NS, Covarrubias R, van Leuven S, Witztum JL, Major AS. The inhibitory FcγRIIb modulates the inflammatory response and influences atherosclerosis in male apoE(-/-) mice. Atherosclerosis. 2011;214:73–80. doi: 10.1016/j.atherosclerosis.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kovanen PT, Mänttäri M, Palosuo T, Manninen V, Aho K. Prediction of myocardial infarction in dyslipidemic men by elevated levels of immunoglobulin classes A, E, and G, but not M. Arch Intern Med. 1998;158:1434–1439. doi: 10.1001/archinte.158.13.1434. [DOI] [PubMed] [Google Scholar]
- 109.Wang J, Cheng X, Xiang MX, et al. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe-/- mice. J Clin Invest. 2011;121:3564–3577. doi: 10.1172/JCI46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 111.Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92:1084–1088. doi: 10.1161/01.cir.92.5.1084. [DOI] [PubMed] [Google Scholar]
- 112.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 113.Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, Biessen EA. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 114.Ma H, Kovanen PT. IgE-dependent generation of foam cells: an immune mechanism involving degranulation of sensitized mast cells with resultant uptake of LDL by macrophages. Arterioscler Thromb Vasc Biol. 1995;15:811–819. doi: 10.1161/01.atv.15.6.811. [DOI] [PubMed] [Google Scholar]
- 115.Nachtigal M, Ghaffar A, Mayer EP. Galectin-3 gene inactivation reduces atherosclerotic lesions and adventitial inflammation in ApoE-deficient mice. Am J Pathol. 2008;172:247–255. doi: 10.2353/ajpath.2008.070348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.MacKinnon AC, Liu X, Hadoke PW, Miller MR, Newby DE, Sethi T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology. 2013;23:654–663. doi: 10.1093/glycob/cwt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 118.Pandey V, Mihara S, Fensome-Green A, Bolsover S, Cockcroft S. Monomeric IgE stimulates NFAT translocation into the nucleus, a rise in cytosol Ca2+, degranulation, and membrane ruffling in the cultured rat basophilic leukemia-2H3 mast cell line. J Immunol. 2004;172:4048–4058. doi: 10.4049/jimmunol.172.7.4048. [DOI] [PubMed] [Google Scholar]
- 119.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muscari A, Bozzoli C, Gerratana C, Zaca’ F, Rovinetti C, Zauli D, La Placa M, Puddu P. Association of serum IgA and C4 with severe atherosclerosis. Atherosclerosis. 1988;74:179–186. doi: 10.1016/0021-9150(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 121.Muscari A, Bozzoli C, Puddu GM, Rovinetti C, Vallar G, Renzi C, Scarani P, Molinaro N, Puddu P. Increased serum IgA levels in subjects with previous myocardial infarction or other major ischemic events. Cardiology. 1993;83:383–389. doi: 10.1159/000175995. [DOI] [PubMed] [Google Scholar]
- 122.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? J Thromb Haemost. 2011;9:428–440. doi: 10.1111/j.1538-7836.2010.04172.x. [DOI] [PubMed] [Google Scholar]
- 124.Buono C, Come CE, Witztum JL, Maguire GF, Connelly PW, Carroll M, Lichtman AH. Influence of C3 deficiency on atherosclerosis. Circulation. 2002;105:3025–3031. doi: 10.1161/01.cir.0000019584.04929.83. [DOI] [PubMed] [Google Scholar]
- 125.Persson L, Borén J, Robertson AK, Wallenius V, Hansson GK, Pekna M. Lack of complement factor C3, but not factor B, increases hyperlipidemia and atherosclerosis in apolipoprotein E-/- low-density lipoprotein receptor-/- mice. Arterioscler Thromb Vasc Biol. 2004;24:1062–1067. doi: 10.1161/01.ATV.0000127302.24266.40. [DOI] [PubMed] [Google Scholar]
- 126.Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ, Haskard DO. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2007;170:416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manthey HD, Thomas AC, Shiels IA, Zernecke A, Woodruff TM, Rolfe B, Taylor SM. Complement C5a inhibition reduces atherosclerosis in ApoE-/- mice. FASEB J. 2011;25:2447–2455. doi: 10.1096/fj.10-174284. [DOI] [PubMed] [Google Scholar]
- 128.Speidl WS, Exner M, Amighi J, Kastl SP, Zorn G, Maurer G, Wagner O, Huber K, Minar E, Wojta J, Schillinger M. Complement component C5a predicts future cardiovascular events in patients with advanced atherosclerosis. Eur Heart J. 2005;26:2294–2299. doi: 10.1093/eurheartj/ehi339. [DOI] [PubMed] [Google Scholar]
- 129.Oksjoki R, Kovanen PT, Mäyränpää MI, Laine P, Blom AM, Meri S, Pentikäinen MO. Complement regulation in human atherosclerotic coronary lesions. Immunohistochemical evidence that C4b-binding protein negatively regulates the classical complement pathway, and that C5b-9 is formed via the alternative complement pathway. Atherosclerosis. 2007;192:40–48. doi: 10.1016/j.atherosclerosis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 130.Oksjoki R, Jarva H, Kovanen PT, Laine P, Meri S, Pentikäinen MO. Association between complement factor H and proteoglycans in early human coronary atherosclerotic lesions: implications for local regulation of complement activation. Arterioscler Thromb Vasc Biol. 2003;23:630–636. doi: 10.1161/01.ATV.0000057808.91263.A4. [DOI] [PubMed] [Google Scholar]
- 131.Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, Cano M, Brandstätter H, Tsimikas S, Skerka C, Superti-Furga G, Handa JT, Zipfel PF, Witztum JL, Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Amarilyo G, Verbovetski I, Atallah M, Grau A, Wiser G, Gil O, Ben-Neriah Y, Mevorach D. iC3b-opsonized apoptotic cells mediate a distinct anti-inflammatory response and transcriptional NF-kappaB-dependent blockade. Eur J Immunol. 2010;40:699–709. doi: 10.1002/eji.200838951. [DOI] [PubMed] [Google Scholar]
- 133.Sofat R, Casas JP, Kumari M, Talmud PJ, Ireland H, Kivimaki M, Marmot M, Hughes AD, Thom S, Ebrahim S, Whittaker JC, Smeeth L, Lawlor DA, Humphries SE, Hingorani AD. Genetic variation in complement factor H and risk of coronary heart disease: eight new studies and a meta-analysis of around 48,000 individuals. Atherosclerosis. 2010;213:184–190. doi: 10.1016/j.atherosclerosis.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 134.Amir S, Binder CJ. Experimental immunotherapeutic approaches for atherosclerosis. Clin Immunol. 2010;134:66–79. doi: 10.1016/j.clim.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ, 3rd, Goenka R, Miller JP, Cho YH, Long V, Ward C, Migone TS, Shlomchik MJ, Cancro MP. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A. 2008;105:15517–15522. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Matsushita T, Fujimoto M, Hasegawa M, Matsushita Y, Komura K, Ogawa F, Watanabe R, Takehara K, Sato S. BAFF antagonist attenuates the development of skin fibrosis in tight-skin mice. J Invest Dermatol. 2007;127:2772–2780. doi: 10.1038/sj.jid.5700919. [DOI] [PubMed] [Google Scholar]
- 137.Gross JA, Dillon SR, Mudri S, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 138.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]