Abstract
The present investigation examined whether leptin stimulation of ventral tegmental area (VTA) or nucleus of the solitary tract (NTS) has a role in body weight homeostasis independent of the medial basal hypothalamus (MBH). To this end, recombinant adeno-associated viral techniques were employed to target leptin overexpression or overexpression of a dominant negative leptin mutant (Leptin Antagonist). Leptin Antagonist overexpression in MBH or VTA increased food intake and body weight to similar extents over 14 days in rats. Simultaneous overexpression of leptin in VTA with antagonist in MBH resulted in food intake and body weight gain that were less than with control treatment but greater than with leptin alone in VTA. Notably, leptin overexpression in VTA increased P-STAT3 in MBH along with VTA, and Leptin Antagonist overexpression in the VTA partially attenuated P-STAT3 levels in MBH. Interestingly, leptin antagonist overexpression elevated body weight gain, but leptin overexpression in the NTS failed to modulate either food intake or body weight despite increased P-STAT3. These data suggest that leptin function in the VTA participates in the chronic regulation of food consumption and body weight in response to stimulation or blockade of VTA leptin receptors. Moreover, one component of VTA-leptin action appears to be independent of the MBH, and another component appears to be related to leptin receptor-mediated P-STAT3 activation in the MBH. Finally, leptin receptors in the NTS are necessary for normal energy homeostasis, but appear to have mostly a permissive role. Direct leptin activation of NTS slightly increases UCP1, but has little effect on food consumption or body weight.
Keywords: Leptin, Gene Therapy, Signal transduction, neuroendocrinology
Introduction
The adipocyte-derived hormone, leptin, regulates appetite and energy expenditure through its action in the hypothalamus and other brain sites (Li, 2011). Our previous studies involving central leptin gene delivery (leptin overexpression) into the third ventricle produced leptin elevation in the cerebral spinal fluid (Scarpace et al., 2002b) and stimulation of leptin signaling in several brain regions (Matheny et al., 2011). Therefore, the observed physiological effects of leptin overexpression impinge upon a distributed neural network that includes an integration of leptin activity in many, if not all brain regions bearing functional leptin receptors. It is currently unclear if leptin function in an individual brain region is dissociable from that of other regions.
The arcuate nucleus (ARC) of the hypothalamus within the forebrain (Scarpace et al., 2012), the ventral tegmental area (VTA) within the midbrain (Bruijnzeel et al., 2011), and the nucleus of the solitary track (NTS) within the hindbrain (Grill and Hayes, 2012) are three regions responsive to direct leptin stimulation. Whereas leptin action in hypothalamic region is firmly established, physiological regulation of body weight by leptin in the VTA midbrain and NTS hindbrain are still under investigation. Although leptin receptors in these regions couple to predictable signaling pathways (Grill and Hayes, 2012; Scarpace et al., 2012), the role in long-term body weight homeostasis is unclear. Knockdown of leptin receptors in the midbrain demonstrated a role for leptin in reward-based feeding without discernible effect on body weight (Davis et al., 2011). In contrast, direct leptin injection into the VTA induces short-term decreases in food consumption and body weight (Bruijnzeel et al., 2011) and our previous report demonstrating that chronic leptin overexpression in the VTA ameliorates body weight gain and tempers food consumption to the same extent as leptin overexpression in the medial basal hypothalamus (MBH) support a role for leptin VTA action in long-term body weight homeostasis (Scarpace et al., 2012). In the latter study, these physiological responses to targeted leptin overexpression in the VTA or MBH were accompanied by elevated phosphorylation of signal transducer and activator of transcription 3 (P-STAT3) within the respective brain sites. Moreover, VTA leptin overexpression also stimulated P-STAT3 in the MBH, whereas leptin overexpression in the MBH did not evoke activation of P-STAT3 in the VTA. This unidirectional trans-stimulation was apparently not due to migration of either the vector or the gene product, suggesting the activation of hypothalamic P-STAT3 was not simply inadvertent (Scarpace et al., 2012). However, it remains uncertain if leptin activation of the VTA on body weight regulation is independent, co-dependent on the MBH, or has no role, and therefore, the observed body weight reductions are solely the result of inadvertent action of leptin in the MBH.
Leptin receptors in the NTS are required for the maintenance of body weight. Knockdown or ablation of leptin receptors in the NTS results in increased body weight gain (Hayes et al., 2010; Scott et al., 2011). Whether activation of leptin receptors by exogenous leptin has a direct effect on food intake or body weight is less certain. Central leptin infusion into the fourth cerebral ventricle, in the vicinity of the NTS, activates NTS leptin receptor signaling and is associated with diminished body weight and food consumption (Grill and Hayes, 2012). However, these studies cannot rule out the inadvertent activation of hypothalamic leptin receptors due to the central infusion. For example, both fourth ventricle and lateral ventricle leptin infusions induce physiological responses and activate hypothalamic leptin signaling to similar degrees (Ruiter et al., 2010). The responses to fourth ventricle leptin infusion may be the inadvertent activation of hypothalamic leptin receptors, and thus, it is unclear if direct leptin stimulation of the NTS participates in body weight regulation.
The purpose of the present investigation was to determine whether direct leptin stimulation of the VTA or NTS has a role in long-term body weight homeostasis independent of the MBH. To this end, recombinant adeno-associated viral techniques were employed to target leptin overexpression in the VTA or NTS coupled with the simultaneous overexpression of a dominant negative leptin mutant (Leptin Antagonist) in the MBH. Food intake and BW were examined over 2-3 weeks and leptin signaling was evaluated at death in F344×BN male rats.
Materials and Methods
Experimental animals
Three-month-old male F344 × Brown Norway (F344×BN) rats were obtained from Harlan Sprague-Dawley (Indianapolis, IN). Upon arrival, rats were examined and remained in quarantine for one week. Animals were cared for in accordance with the principles of the Guide to the Care and Use of Experimental Animals and protocols were approved by the University of Florida Institutional Animal Care and Use Committee. Rats were housed individually with a 12:12 h light-dark cycle (07:00 to 19:00 hr) and were fed a standard rodent chow (17% kcal from fat, no sucrose, 3.3 kcal/g, diet 7912, Harlan Teklad; Madison, WI).
Experimental design
This study consists of four experiments. In experiment one, rats were administered recombinant adeno-associated virus (rAAV) encoding either green fluorescent protein (rAAV-Control), or a modified version of the rat leptin gene that acts as dominant-negative antagonist (rAAV-Leptin Antg) by microinjection into the MBH or VTA for 14 days (N=13 controls, N=7 rAAV-Antg MBH, N=8 rAAVAntg VTA). One-half of the control rats received the GFP vector into the MBH (N=7) and the other half received the GFP vector into the VTA (N=6). Rats were allowed free access to food and water, ad libitum, and food consumption and body weight were recorded daily. Fresh food was provided weekly. Food consumption was determined by difference in weight of remaining food accounting for spillage. Physiological responses were not different between sub-controls and these rats were subsequently treated as one control group (N=13). Prior to death, at day 14, those treated with control vector were divided into two groups and administered by intracerebroventricular (i.c.v.) injection either artificial cerebral spinal fluid (ACSF, N=6) or leptin (40 ng, N=7), whereas those treated with rAAV-leptin antagonist were administered leptin (40 ng) to determine leptin signaling in MBH and VTA brain regions.
In experiment two, three groups of rats were given two injections of rAAV vectors (N=7-8/group). In the first group, the rAAV-GFP vector was delivered to VTA and to MBH (rAAV-Control); in the second group, a rAAV vector encoding rat leptin (rAAV-Leptin) was delivered to the VTA and rAAV-GFP to the MBH (rAAV-Leptin VTA), and in the third group, rAAV-leptin was delivered to the VTA and rAAV-leptin antagonist to the MBH (rAAV-Leptin VTA/Antg MBH).
In experiment three, three groups of rats were given two injections of rAAV vectors (N=7/group). In the first group, the rAAV-GFP vector was delivered to NTS and to MBH (rAAV-Control); in the second group, rAAV-leptin was delivered to the NTS and rAAV-GFP to the MBH (rAAV-Leptin NTS), and in the third group, rAAV-leptin was delivered to the NTS and rAAV-leptin antagonist to the MBH (Leptin NTS/Antg MBH).
In experiment four, three groups of rats were given a single injection of a rAAV-vector to the NTS, either rAAV-GFP, rAAV-leptin, or rAAV-leptin antagonist (N= 8/group).
Production of rAAV vectors
Rat leptin antagonist DNA was a modification of a previously described construct (Matheny et al., 2009). Wild type sequence, TTGGACCTT, corresponding to amino acids Leucine (L39), Aspartic acid (D40) and Phenylalanine (F41), was mutated to GCAGCCGCC, corresponding to Alanine, Alanine, Alanine. In the present study this construct was further modified by mutating GAC, corresponding to Aspartic acid (D23) to CTG, corresponding to Leucine. The sequence of the leptin antagonist construct was verified after PCR based mutagenisis.
Viral vectors (serotype 1 rAAV) encoding rat leptin or leptin antagonist cDNA were under the control of a chicken beta-actin promoter and were packaged by University of Florida vector core or Vectorbiolabs (Philadelphia, PA).
rAAV-vector administration
In experiment one, a single dose (5 ×1012 viral genomes/ml) of rAAV-GFP (1 μl) was unilaterally delivered by microinjection; in half of the control animals, the injections were directed into the MBH, right of midline, targeting the arcuate nucleus, and in the other half, the injections targeted the VTA. In parallel, the experimental animals received an equivalent dose of rAAV-leptin antagonist delivered by microinjection into the MBH, right of midline or into the VTA. In experiments 2 and 3, two sites were injected per rat, as described in the experimental design section. The coordinates for the injection targeting the MBH, right of midline were 2 mm posterior to Bregma, 0.4 mm lateral from the midsagittal suture and 9.8 mm ventral from the surface of the skull. The coordinates for the injection targeting the VTA were 5.3 mm posterior to Bregma, 0.8 mm lateral from the midsagittal suture and 8.5 mm ventral from the surface of the skull. The coordinates for the microinjection targeting the NTS were 12.7 mm posterior to Bregma, 1.6mm lateral from the midsagittal suture and 8.5 mm ventral from the surface of the skull. Stereotaxic coordinates for microinjection into the brain were determined using the Rat Brain Atlas (Paxinos and Watson, 2005). Coordinates were verified and refined by use of a water soluble blue dye and visualization in brain sections using low-power microscopy. Additional verification of the accuracy of the vector delivery was performed by microinjections of rAAV-GFP into the NTS, MBH, and VTA followed by fluorescence imaging of fixed brain sections. Fluorescent images of rAAV-GFP for MBH and VTA were published previously (Scarpace et al., 2012).
Acute leptin administration
A single dose of leptin (40 ng) was injected into the third cerebral ventricle as previously described (Scarpace et al., 2007). The coordinates for injection are 1.3 mm anterior to Bregma, 9.4mm ventral from the skull surface, at an angle of 20 degrees anterior to posterior. Rats were killed one hour later to assess leptin-mediated STAT3 signaling.
Tissue harvest and preparation
Rats were killed by thoracotomy under 5% Isoflurane anesthetic. Whole blood was taken by cardiac puncture and serum collected following centrifugation in serum separator tubes. Subsequently, 40 ml of cold saline were perfused through the circulatory system. The perirenal, retroperitoneal and epididymal white adipose depots along with interscapular brown adipose tissue (BAT) were excised and their individual weights recorded.
Additionally, 2 mm coronal sections containing the regions of the MBH, VTA and NTS were sliced from fresh brains using a micrometer controlled tissue slicer (Stoelting Co, Wood Dale, Il.) and a section or punch of the respective regions were taken as subsequently described. Brain tissue punches or sections (MBH, VTA or NTS) were removed under a low power microscope that provides identification of landmarks found in each 2 mm tissue slice. Aligning a straight edge razor blade with the optic tract (−1.5 mm posterior Bregma), a 2 mm caudal coronal section was cut. For the MBH, a 1mm × 1mm section was taken just lateral to the 3rd ventricle and the ventral most border of the brain slice. Similarly, aligning a straight edge razor blade with the caudal end of the hypothalamus (−5 mm posterior to Bregma), a coronal section was cut 2 mm posterior. For the 2 mm section containing the VTA, a 1mm diameter punch was centered ventral to the red nucleus, parvicellular (RPC) and medial to the border of the substantia nigra (SN). The 2 mm section containing the NTS was obtained by slicing the brain 1mm anterior to the cerebellum edge and 2 mm anterior to the initial slice. This section, containing the NTS, was further trimmed by discarding the coronal section 1.5 mm ventral to the 4th ventricle and by discarding the lateral 1 mm edge. All punches and sections were taken bilaterally. Brain sections and BAT were briefly sonicated in (40 ul and 300 ul, respectively) 10 mM Tris, pH 6.8, 2% SDS for Western analysis. Protein was determined by DC Bradford (Bio-Rad, Hercules, CA).
Serum leptin
Serum leptin levels were determined by radioimmunoassay (Millipore, Billerica, MA).
Western analysis
Protein homogenates (MBH, VTA, NTS, 20μg; BAT, 5 μg) was separated on a SDS-PAGE gel and electro-transferred to nitrocellulose membranes (Scarpace et al., 2001). Immunoreactivity was assessed with antibodies specific to UCP1 (Abcam, Cambridge, MA) or phospho-tyrosine 705 of STAT3 and reprobed with antibodies specific to STAT3 regardless of phosphorylation state (Cell Signaling, Danvers, MA). Immunoreactivity to P-STAT3 was compared with that for G3PDH (Abcam, Cambridge, MA) and expressed as a ratio. Immunoreactivity was detected with ECL prime (GE Healthcare, Piscataway NJ), scanned with a ChemiDoc XRS+ (BioRad, Hercules, CA) and quantified using ImageQuant software (Molecular Dynamics).
Imaging analysis of NTS
Separate rats received rAAV-GFP injection into NTS. Three weeks after virus injections, animals were killed and perfused with 100 ml of PBS, followed by 400 ml of ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. Brains were placed in 30% sucrose and stored at 4°C until sectioning. A block containing the NTS was cut using a straight edge razor blade. The block containing the NTS was frozen on the freezing stage and 50μm sections were cut. Sections were placed on Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA), air-dried, and a cover slip placed with Vectashield mounting medium (Vector, Burlingame, CA). Digital images of native GFP fluorescence and contrast-optimized unstained sections in brightfield were collected using a motorized Nikon microscope (Tokyo, Japan) equipped with a Nikon DS digital camera interfaced with a computer.
Statistical analysis
Data were analyzed by one-way ANOVA. A post-hoc test (Newman-Keuls) was applied to determine individual differences between means. A p-value of less that 0.05 was considered significant.
Results
Exp. 1: Leptin Antagonist Overexpression in MBH and VTA
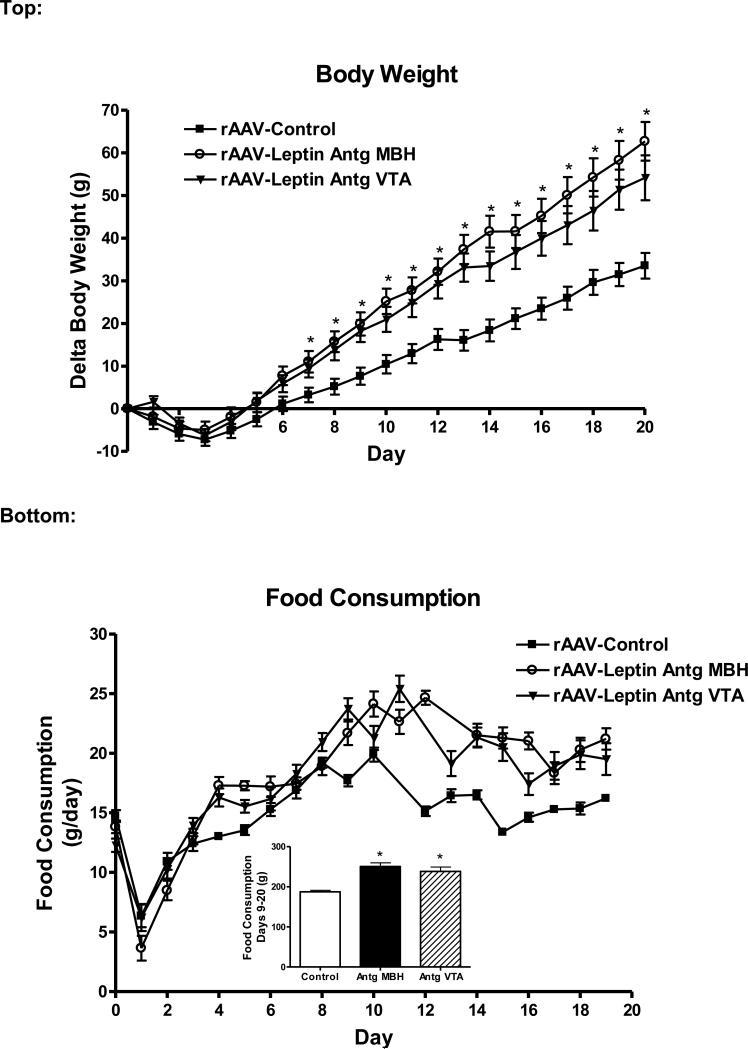
To identify if leptin receptor function in the VTA has a direct role in body weight and food intake regulation, Leptin Antagonist overexpression (rAAV-Leptin Antg) was targeted to the VTA. Prior to vector delivery, body weight was not different across groups (260 ± 6 g, Control; 251 ± 9 g, rAAVLeptin Antg into MBH; 257 ± 8 g, rAAV-Leptin Antg into VTA). Initially, there was a decrease in body weight due to the surgical delivery of the vector, after which delta body weight steadily increased through day 20 (Fig. 1, top). As expected, overexpression of the Leptin Antagonist in the MBH increased body weight gain compared with rats administered the control vector. Interestingly, weight gain was similar with Leptin Antagonist treatment in the VTA compared with treatment in the MBH (Fig. 1, top). Note, that one-half of the control rats received the GFP vector into the MBH and the other half received the GFP vector into the VTA. There were no significant differences in delta body weight (33.8 ± 3.2 g, GFP MBH; 33.1 ± 5.6 g, GFP VTA) between these two control sub-groups. For clarity, the rats administered control vector either into the MBH or into the VTA were combined into a single control group (rAAV-Control).
Figure 1.
Top: Change in body weight in rats following administration of control vector (closed squares) or rAAV-leptin antagonist to MBH (rAAV-Leptin Antg MBH, open circles) or VTA (triangles). The rAAV-leptin antagonist or control vectors were administered at day 0 in rats maintained on a standard diet. For clarity, the rats administered either control vector into the MBH or into the VTA were combined into a single control group (rAAV-Control). Delta body weight was significantly different with leptin antagonist treatment. (P<0.005, one-way ANOVA) beginning at day 7 (*P<0.01, MBH; *P<0.05, VTA by Newman-Keuls post-hoc analysis). Values represent the mean ± SE of 13 (control), 7 (rAAV-Antg MBH), or 8 (rAAV-Antg VTA) rats.
Bottom: Daily food consumption following administration of control vector (closed squares) or rAAV-leptin antagonist to MBH (open circles) or VTA (triangles). Inset: Cumulative food intake was significantly different with rAAV-leptin antagonist treatment (MBH or VTA) from day 9 through day 19. *P<0.0001, one-way ANOVA; P<0.001 by Newman-Keuls post-hoc analysis for difference from control.
Daily food consumption, for the most part paralleled the change in body weight. There was the initial expected surgery-related decrease in food intake followed by a rapid recovery (Fig 1, bottom). Beginning at day 9 in MBH and day 12 in the VTA and continuing through day 19, daily food intake was significantly greater with Leptin Antagonist compared with rAAV-control, and cumulative food consumption (days 9-19) was approximately 27-37% greater (Fig 1, bottom insert).
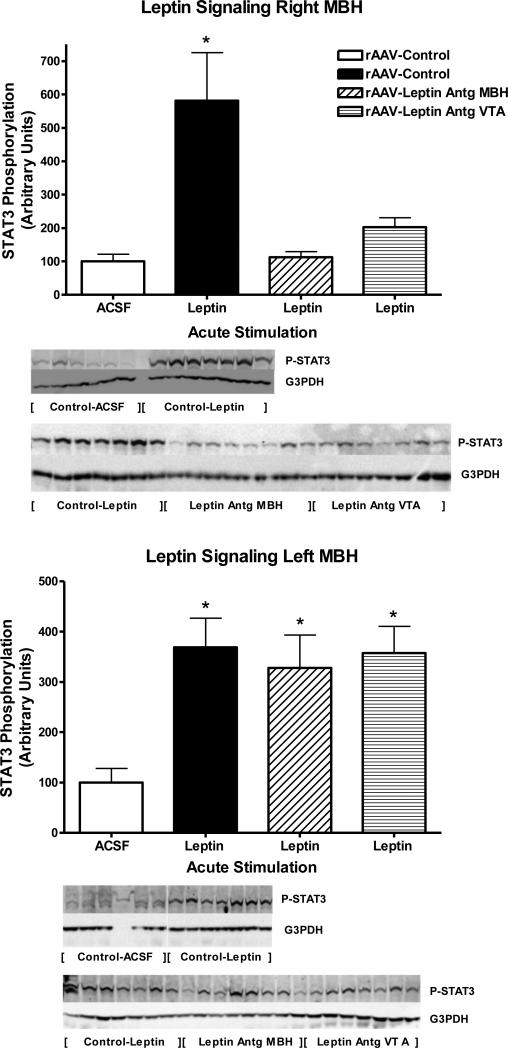
Exp. 1: Leptin signaling in right and left MBH following Leptin Antagonist overexpression
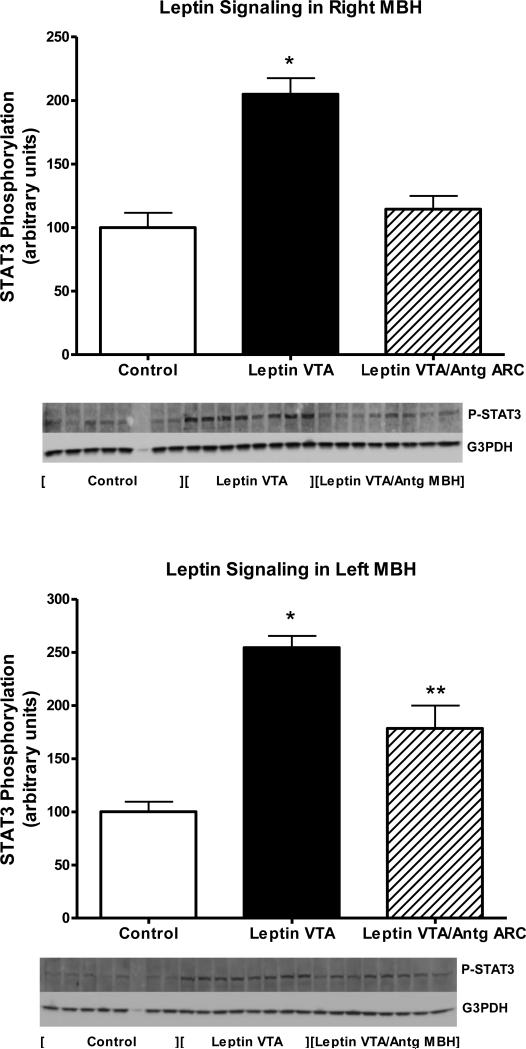
To determine the extent of leptin receptor blockade, leptin signaling following acute injection of 40ng of leptin into the third ventricle was examined in the right and left MBH at day 20 in the rats with Leptin Antagonist overexpression and corresponding controls. Note, the Leptin Antagonist was delivered using coordinates that targeted the MBH, right of midline, centered on the arcuate nucleus. This acute i.c.v. dose of 40ng leptin corresponded to a dose of leptin that results in half-maximal stimulation based on a previously determined full dose-response curve (Scarpace et al., 2001). As expected, the rAAV-control animals, half of whom were injected with ACSF and half with leptin, demonstrated a nearly six-fold increase in phosphorylated STAT3 (P-STAT3) in the right MBH with acute leptin administration as compared with ACSF administration (Fig 2 top, first two bars). However, in rats with Leptin Antagonist overexpression in the right MBH, acute leptin stimulation failed to elevate P-STAT3, suggesting the delivered antagonist fully blocked leptin signaling (Fig 2, top, third bar). Surprisingly, Leptin Antagonist overexpression in the VTA attenuated the acute leptin signaling in the right MBH. Although there was a two-fold elevation of P-STAT3 observed, it was not significantly different from control (Fig. 2, top, fourth bar).
Figure 2.
STAT3 phosphorylation following a single i.c.v. injection of ACSF (open bars) or 40 ng of leptin in rats administered control vector (solid bars), rAAV-leptin antagonist into the right MBH (hatched bars) or rAAV-leptin antagonist into the VTA (striped bars) 20 days earlier. STAT3 phosphorylation was assessed 1 hour later in the right MBH (Top) or left MBH (Bottom), and expressed as the ratio of STAT3 Phosphorylation to G3PDH.
Values represent the mean ± SE of 6-8 rats per group. Western analysis required two gels. Samples from Control-Leptin groups were present on both gels and used for normalization across gels. The value of ACSF injected control for each individual tissue is arbitrarily set to 100 with SE adjusted proportionally with remaining groups normalized to the level in ACSF injected control. Top: P< 0.001 for difference with treatment by one-way ANOVA. *P<0.01 for difference between AAV-Control with leptin injection and all other groups by Newman-Keuls post-hoc analysis. Bottom: *P<0.025 for difference with treatment by one-way ANOVA. *P<0.05 for difference between rAAV-Control with ACSF injection and all other groups by Newman-Keuls post-hoc analysis.
When the left MBH (contralateral to antagonist vector delivery) was examined, there was the predicted stimulation of P-STAT3 by 4-fold in rAAV-control animals following the acute leptin injection into the third ventricle. However, in contrast to the right MBH, no blockade of leptin signaling was observed in the left MBH by either antagonist delivery to the right MBH or antagonist delivery to VTA, thus confirming the limited spread of the overexpressed mutant (Fig 2, bottom). Because levels of total STAT3 decreased with Leptin Antagonist overexpression, changes in P-STAT3 were normalized to immunoreactivity of G3PDH. Protein levels of G3PDH were unchanged across treatments, and patterns of P-STAT3 signaling remained the same after the normalization (data not shown).
In previous studies, using super-maximal doses of leptin (1ug) injected into the 3rd ventricle, we identified a two-fold increase in P-STAT3 levels in the VTA compared with ACSF administration (Scarpace et al., 2012). In contrast, in the present study, with the sub-maximal dose of 40ng, we were unable to detect any elevation in P-STAT3 signaling following i.c.v. leptin administration (data not shown).
Exp. 1: Adiposity and BAT UCP1 levels
Adiposity levels were determined by the sum of the weights of perirenal, retroperitoneal and epididymal white adipose tissues at death in experiment one. Adiposity levels were increased by 83% and 40% with antagonist treatment in MBH and VTA, respectively (Table 1). Serum leptin levels, another marker of adiposity, paralleled the changes in adiposity with increases of 83% and 54%, respectively (Table 1).
Table 1.
Adiposity, serum leptin and BAT UPC1 levels following leptin antagonist overexpression in Exp. 1.
| rAAV-Control | rAAV-Antagonist into MBH | rAAV-Antagonist into VTA | |
|---|---|---|---|
| Adiposity, g | 7.31± 0.43a | 12.23± 0.81b | 10.19± 0.89c |
| Serum Leptin (ng/ml) | 5.8 ± 0.3a | 10.6 ± 0.9b | 8.9 ± 1.0c |
| UCP1/ μg Arbitrary units | 100 ± 10.8a | 46.8 ± 12.8c | 87.4 ± 17.6 |
Data represent the mean ± SE of 13 (control) or 7-8 (experimental groups) rats.
Adiposity represents the sum of perirenal, retroperitoneal and epididymal white adipose tissues at death.
The value of UCP1, expressed as UCP1/ μg BAT protein, in rAAV-Control for is arbitrarily set to 100 with SE adjusted proportionally with remaining groups normalized to the level in rAAV-Control.
P<0.0002 (Adiposity and Serum leptin) or P<0.05 (UCP1) for difference among groups at day 14 by one-way ANOVA.
P<0.001 for difference from control group by Newman-Keuls post-hoc analysis.
P<0.05 for difference from control group by Newman-Keuls post-hoc analysis.
Central overexpression of leptin is known to augment UCP1 in BAT (Scarpace et al., 2012). Consistent with this finding, overexpression of the Leptin Antagonist in the MBH decreased BAT UPC1 protein levels by 53%. However, overexpression of the Leptin Antagonist in the VTA did not change UCP1 relative to rAAV-control (Table 1).
Exp. 2: Overexpression of leptin in VTA in conjunction with antagonist overexpression in MBH
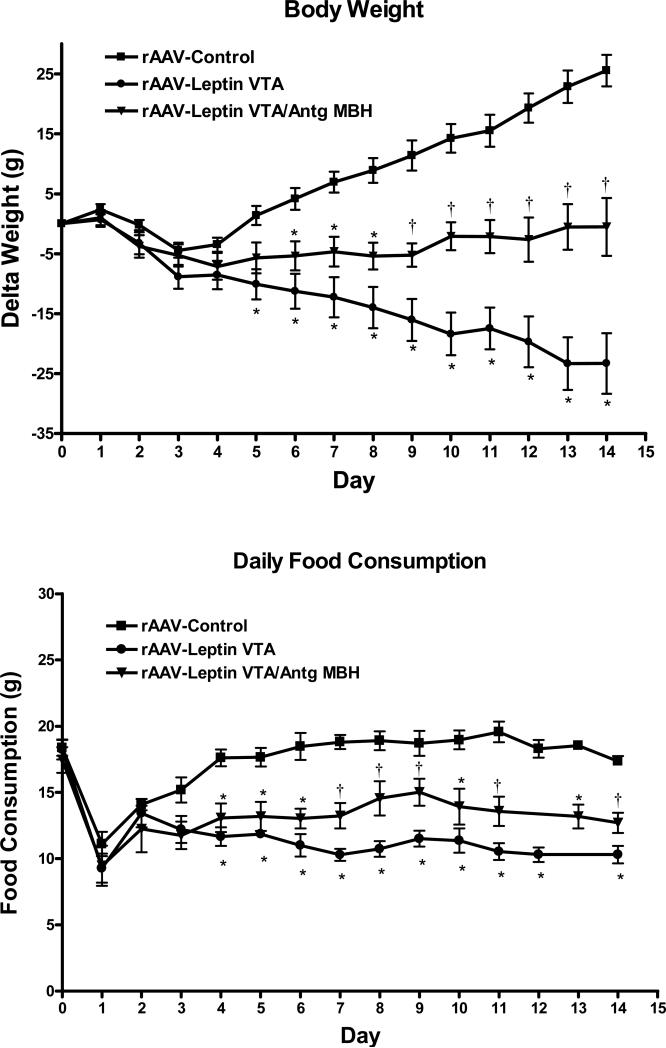
The unexpected finding that leptin receptor blockade of VTA both increases body weight and antagonizes leptin signaling in the MBH suggests the possibility that disturbances in MBH leptin receptors are responsible for antagonist-VTA-injected elevation in body weight. To help resolve this issue, we examined the simultaneous overexpression of leptin in the VTA along with the overexpression of Leptin Antagonist in the MBH. To this end, rAAV-GFP was delivered to VTA and MBH (rAAV-Control), rAAV-leptin to the VTA and rAAV-GFP to the MBH (rAAV-Leptin VTA), and rAAV-leptin to the VTA and rAAV-Leptin Antagonist to the MBH (rAAV-Leptin VTA/Antg MBH).
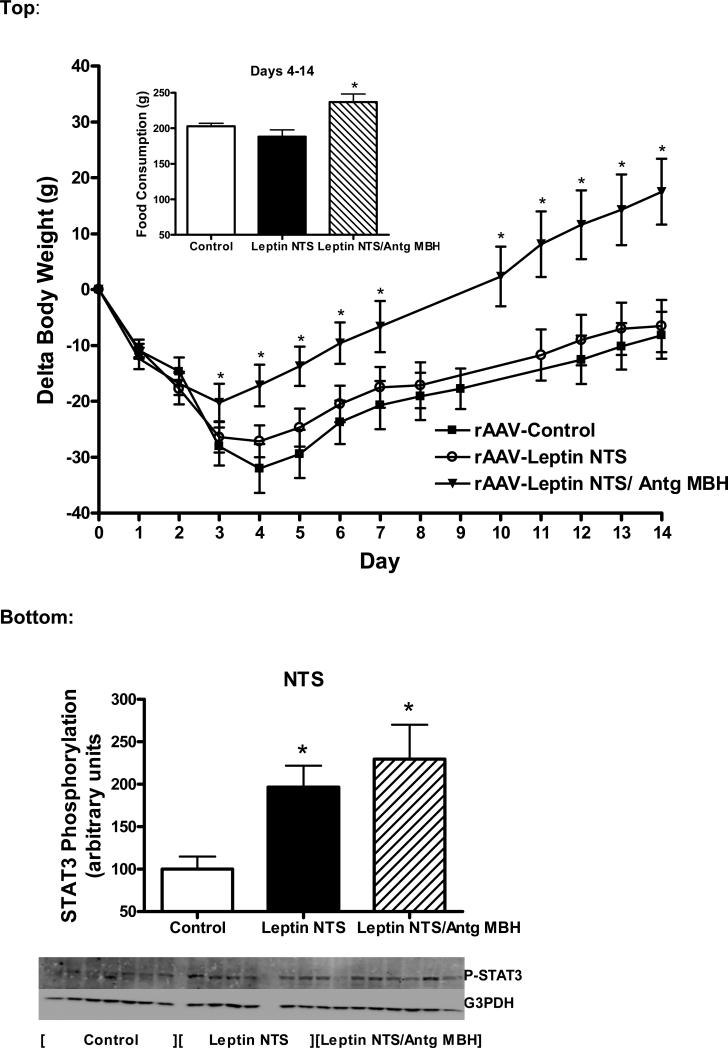
Leptin gene delivery to the VTA decreased body weight gain and food intake (Fig. 3, top), in a manner similar to our previous report and to same degree as leptin overexpression in the MBH (Scarpace et al., 2012). Simultaneous overexpression of the Leptin Antagonist in the MBH and leptin in the VTA resulted in body weight gain and decreased food intake that were less than rAAV-Control but greater than with leptin in the VTA (Fig. 3, top). Daily food consumption paralleled the body weight changes, with a decrease in food intake with rAAV-Leptin vector delivery to the VTA compared with control that was partially attenuated by the simultaneous treatment with the Leptin Antagonist in the MBH (Fig. 3, bottom).
Figure 3.
Change in body weight (Top) and daily food consumption (Bottom) following administration of control vector to MBH and VTA (squares), rAAV-leptin to VTA with rAAV-control to MBH (circles) or rAAV-leptin to VTA simultaneously with rAAV-leptin antagonist to MBH (rAAV-Leptin VTA/Antg MBH, triangles). The rAAV-vectors were administered at day 0. Values represent the mean ± SE of 7-8 rats per group.
Top: Delta body weight significantly diverged from controls starting at day 5 in the rAAV-Leptin VTA treated group (*P<0.01), at day 6 in rAAV-Leptin VTA /Antg MBH treated group (*P<0.01). The latter group was significantly different from both the controls and rAAV-leptin group starting at day 9 (†P<0.02) by one-way ANOVA and Newman-Keuls post-hoc analysis.
Bottom: Food consumption significantly diverged from controls starting at day 4 in the rAAV-Leptin VTA treated group (*P<0.001) and in rAAV-Leptin VTA /Antg MBH treated group (*P<0.01). The latter group was significantly different from both the control and the rAAV-leptin group starting at day 7 (†P<0.05) by one-way ANOVA and Newman-Keuls post-hoc analysis.
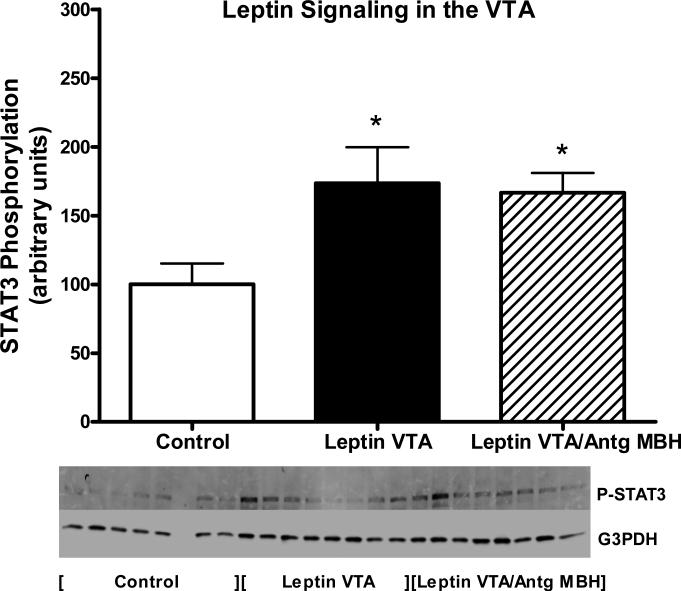
STAT3 phosphorylation was examined in the right and left MBH and the VTA at death on day 14. As opposed to Experiment 1, where leptin signaling was assessed following acute administration of leptin, in this experiment, no exogenous drug was administered, thus the levels of P-STAT3 reflected those stimulated by vector-mediated leptin overexpression in VTA relative to the basal non-stimulated P-STAT3 in control vector-treated animals or those with simultaneous antagonist delivery to the MBH. With respect to the VTA region, leptin overexpression in the VTA stimulated P-STAT3 by less than 2-fold over basal levels in control rats, and this stimulation was unaffected by simultaneous overexpression of antagonist in the right MBH (Fig 4). Examination of P-STAT3 in the right and left MBH revealed that leptin overexpression in the VTA resulted in elevated P-STAT3 levels in the right and left MBH (Fig 5, top & bottom). Further analysis revealed that the simultaneous overexpression of antagonist in the right MBH fully blocked receptor activation in the right MBH, but only partially blocked activation in the contralateral side (Fig 5, top & bottom).
Figure 4.
STAT3 phosphorylation in the VTA following treatment for 14 days with rAAV-Control vector (open bars), rAAV-leptin into the VTA (solid bars) or rAAV-Leptin VTA/Antg MBH (hatched bars). STAT3 phosphorylation represents that stimulated by leptin gene delivery. No further exogenous leptin was administered. Data is expressed as the ratio of STAT3 Phosphorylation to G3PDH.
Values represent the mean ± SE of 6-8 rats per group. The value of rAAV-Control for each individual tissue is arbitrarily set to 100 with SE adjusted proportionally with remaining groups normalized to the level in rAAV-Control. P< 0.05 for difference with vector treatment by one-way ANOVA; *P<0.05 for difference from rAAV-Control by Newman-Keuls post-hoc analysis.
Figure 5.
STAT3 phosphorylation in the right MBH (Top) or left MBH (Bottom) following treatment for 14 days with rAAV-Control vector (open bars), rAAV-Leptin into the VTA (solid bars) or rAAVLeptin VTA /Antg MBH (hatched bars). STAT3 phosphorylation represents that stimulated by leptin gene delivery. No further exogenous leptin was administered. Data is expressed as the ratio of STAT3 Phosphorylation to G3PDH.
Values represent the mean ± SE of 6-8 rats per group. The value of rAAV-Control for each individual tissue is arbitrarily set to 100 with SE adjusted proportionally with remaining groups normalized to the level in rAAV-Control. P< 0.0001 for difference with vector treatment in right MBH (top) and left MBH (bottom) by one-way ANOVA; *P<0.001 for difference between rAAV-Leptin and rAAV-Control by Newman-Keuls post-hoc analysis; **P<0.01 or for difference between leptin VTA /Antg MBH and either control or rAAV-Leptin to VTA by Newman-Keuls post-hoc analysis.
Exp. 2: Adiposity and BAT UCP1 levels
Adiposity levels were determined by serum leptin levels and the sum of the weights of perirenal, retroperitoneal and epididymal white adipose tissues at death. Both serum leptin and sum of white adipose tissues were markedly reduced with leptin treatment in the VTA (Table 2). Simultaneous antagonist treatment in the MBH tempered the reductions in adiposity and serum leptin, but not significantly (Table 2). UCP1 in BAT were elevated two-fold by leptin overexpression in VTA and this UCP1 stimulation was unaffected by the antagonist treatment in the MBH (Table 2).
Table 2.
Adiposity, serum leptin and BAT UPC1 levels following simultaneous VTA leptin overexpression and MBH leptin antagonist overexpression in Exp. 2.
| rAAV-Control | rAAV-Leptin into VTA | rAAV-Leptin into VTA rAAV antagonist into MBH | |
|---|---|---|---|
| Adiposity, g | 7.01 ± 0.45a | 1.40 ± 0.54b | 3.52 ± 0.76c |
| Serum Leptin, ng/ml | 4.1 ± 0.3a | 1.4 ± 0.2b | 2.0 ± 0.4b |
| UCP1/ μg Arbitrary units | 100 ± 13.7a | 207.4 ± 16.7c | 201.8 ± 27.0c |
Data represent the mean ± SE of 7-8 rats per group.
Adiposity represents the sum of perirenal, retroperitoneal and epididymal white adipose tissues at death.
P<0.0001 (Adiposity and Serum leptin) or P<0.002 (UCP1) for difference among groups by one-way ANOVA.
The value of UCP1, expressed as UCP1/ μg BAT protein, in rAAV-Control for is arbitrarily set to 100 with SE adjusted proportionally with remaining groups normalized to the level in rAAV-Control.
P<0.001 for difference from control group by Newman-Keuls post-hoc analysis.
P<0.01 for difference from control group by Newman-Keuls post-hoc analysis.
Exp. 3: Leptin overexpression in NTS with simultaneous antagonist overexpression in MBH
The NTS is another brain region that has leptin receptors (Grill and Hayes, 2012) and that purportedly participates in leptin mediated food and body weight regulation (Hayes et al., 2010; Scott et al., 2011). To examine whether the leptin receptors in the MBH have a role in the leptin response in the NTS, leptin overexpression in the NTS was compared with rAAV-control in the NTS. In addition, to distinguish whether the observed leptin interaction between VTA and MBH is specific, leptin was overexpressed in the NTS with the simultaneous overexpression of antagonist in the MBH. Unexpectedly, leptin gene delivery to the NTS failed to alter food consumption or body weight over 14 days compared with control vector treatment (Fig. 6, top). In the group with simultaneous overexpression of the antagonist and leptin in the MBH, there was the predicted increase in both body weight and food consumption due the presence of the antagonist in the MBH (Fig. 6, top).
Figure 6.
Top:Change in body weight and cumulative food consumption (inset) following administration of control vector into MBH and NTS (closed squares), rAAV-leptin to NTS with rAAV-control into MBH (open circles) or rAAV-leptin to NTS simultaneously with rAAV-leptin antagonist to MBH (rAAV-Leptin NTS /Antg MBH, triangles). The rAAV-vectors were administered at day 0. Delta body weight significantly diverged from controls starting at day 4 in rAAV-Leptin NTS /Antg MBH treated group (P<0.01) by one-way ANOVA and Newman-Keuls post-hoc analysis. *P< 0.005 for difference with vector treatment by one-way ANOVA; P<0.01 for difference between rAAV-leptin NTS/Antg MBH and rAAV-Control or P<0.05 for difference from Leptin NTS by Newman-Keuls post-hoc analysis. Values represent the mean ± SE of 6-7 rats per group.
Bottom: STAT3 phosphorylation in the NTS at death in the groups described in Fig. 6 top. STAT3 phosphorylation represents that stimulated by leptin gene delivery. No further exogenous leptin was administered. Data is expressed as the ratio of STAT3 Phosphorylation to G3PDH.
The value of rAAV-Control for each individual tissue is arbitrarily set to 100 with SE adjusted proportionally with remaining groups normalized to the level in rAAV-Control. Values represent the mean ± SE of 6-7 rats per group. P< 0.05 for difference with vector treatment by one-way ANOVA; *P<0.05 for difference from rAAV-Control by Newman-Keuls post-hoc analysis.
STAT3 phosphorylation was examined in the NTS and MBH at death on day 14. Similar to experiment 2, no exogenous leptin was administered, thus the levels of P-STAT3 reflected those stimulated by vector-mediated leptin overexpression in NTS relative to the basal non-stimulated PSTAT3 in control vector-treated animals. P-STAT3 levels were elevated in the NTS with leptin overexpression and this level of stimulation was unaffected by simultaneous antagonist treatment in the MBH (Fig. 6, bottom). More importantly, there were no changes in P-STAT3 levels in the MBH under any of the conditions investigated (data not shown).
Exp. 4: Leptin and Leptin Antagonist overexpression in NTS
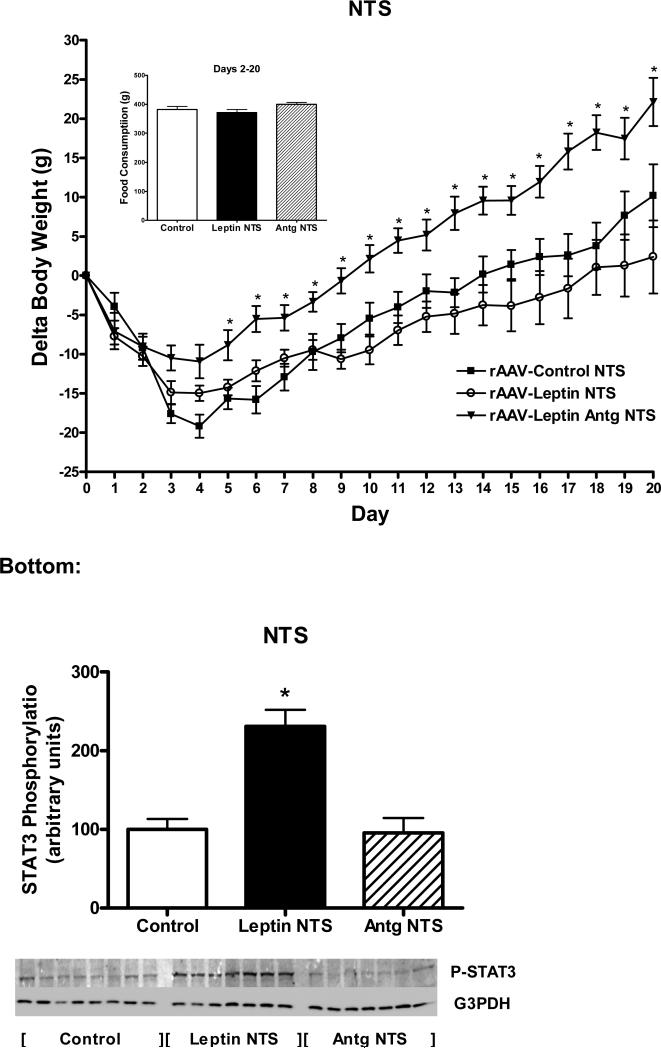
The unexpected lack of a response to leptin overexpression in the NTS coupled with previous observations that knockdown or ablation of leptin receptors in the NTS increases body weight gain (Hayes et al., 2010; Scott et al., 2011) prompted us to reexamine leptin overexpression in the NTS. However, in this experiment, leptin overexpression was followed for three weeks and was compared to leptin antagonist overexpression in the NTS (along with control). Prior to vector delivery, body weight was not different across groups (326 ± 9 g, Control; 321 ± 3 g, rAAV-Leptin; 312 ± 5 g, rAAVLeptin Antg). There was the usual initial decrease in body weight in all groups followed by significant increase in delta body weight in the leptin antagonist compared with the control group (Fig. 7, top). Similar to observations in Exp. 3, leptin overexpression in the NTS failed to significantly alter food consumption or body weight over 20 days compared with control vector treatment, although there was trend towards a decrease in body weight (Fig. 7, top). This data reinforced the lack of food intake response for leptin in the NTS. In contrast, antagonist overexpression, significantly increased body weight. Interestingly, this increase in body weight was less than half the elevated delta body weight observed with antagonist overexpression in the MBH (Fig. 1, top).
Figure 7.
Top: Change in body weight and cumulative food consumption (inset) following administration of control vector to NTS (closed squares), rAAV-leptin to NTS (open circles) or rAAV-leptin antagonist to NTS (rAAV-Leptin Antg, triangles). The rAAV-vectors were administered at day 0. Delta body weight in rAAV-Leptin Antg significantly diverged from controls starting at day 5 (P<0.02) by one-way ANOVA and Newman-Keuls post-hoc analysis. Values represent the mean ± SE of 7-8 rats per group.
Bottom: STAT3 phosphorylation in the NTS at death in the groups described in Fig. 7 top. STAT3 phosphorylation represents that stimulated by leptin gene delivery. No further exogenous leptin was administered. Data is expressed as the ratio of STAT3 Phosphorylation to G3PDH.
The value of rAAV-Control for each individual tissue is arbitrarily set to 100 with SE adjusted proportionally with remaining groups normalized to the level in rAAV-Control. Values represent the mean ± SE of 7-8 rats per group. P< 0.001 for difference with vector treatment by one-way ANOVA; *P<0.001 for difference from rAAV-Control or rAAV-Leptin Antg by Newman-Keuls post-hoc analysis.
STAT3 phosphorylation was examined in the NTS and MBH at death on day 20. Similar to experiment 3, no exogenous leptin was administered, thus the levels of P-STAT3 reflected those stimulated by vector-mediated leptin overexpression in NTS relative to the basal non-stimulated PSTAT3 in control vector-treated animals. P-STAT3 levels were elevated by nearly 2.5 fold in the NTS with leptin overexpression, whereas antagonist overexpression was not different than control (Fig. 6, bottom). There were no changes in P-STAT3 levels in the MBH under any of the conditions investigated (data not shown).
Exp. 4: Adiposity and BAT UCP1 levels
The sum of the weights of perirenal, retroperitoneal and epididymal white adipose tissues were not significantly different at death, however there was a trend towards a decrease with leptin overexpression and an increase with antagonist overexpression (Table 3). These trends were paralleled by a significant decrease in serum leptin and a significant increase in serum leptin with leptin and leptin antagonist overexpression, respectively (Table 3). UCP1 levels were also significantly increased with leptin gene delivery into the NTS (Table 3).
Table 3.
Adiposity, serum leptin and BAT UPC1 levels following leptin or leptin antagonist overexpression in NTS from Exp. 4.
| rAAV-Control | rAAV-Leptin into NTS | rAAV-Antagonist into NTS | |
|---|---|---|---|
| Adiposity, g | 5.43± 0.34 | 4.45± 0.67 | 6.07± 0.31 |
| Serum Leptin (ng/ml) | 2.80 ± 0.23a | 1.72 ± 0.41b | 3.46 ± .32c |
| UCP1/ μg Arbitrary units | 100 ± 8.2a | 134.2± 7.3b | 113.9± 7.5 |
Data represent the mean ± SE of 7 rats/group.
Adiposity represents the sum of perirenal, retroperitoneal and epididymal white adipose tissues at death.
The value of UCP1, expressed as UCP1/ μg BAT protein, in rAAV-Control for is arbitrarily set to 100 with SE adjusted proportionally with remaining groups normalized to the level in rAAV-Control.
P<0.005 (Serum leptin) or P<0.05 (UCP1) for difference among groups at day 20 by one-way ANOVA.
P<0.05 for difference from control group by Newman-Keuls post-hoc analysis.
P<0.01 for difference from rAAV-Leptin into NTS by Newman-Keuls post-hoc analysis.
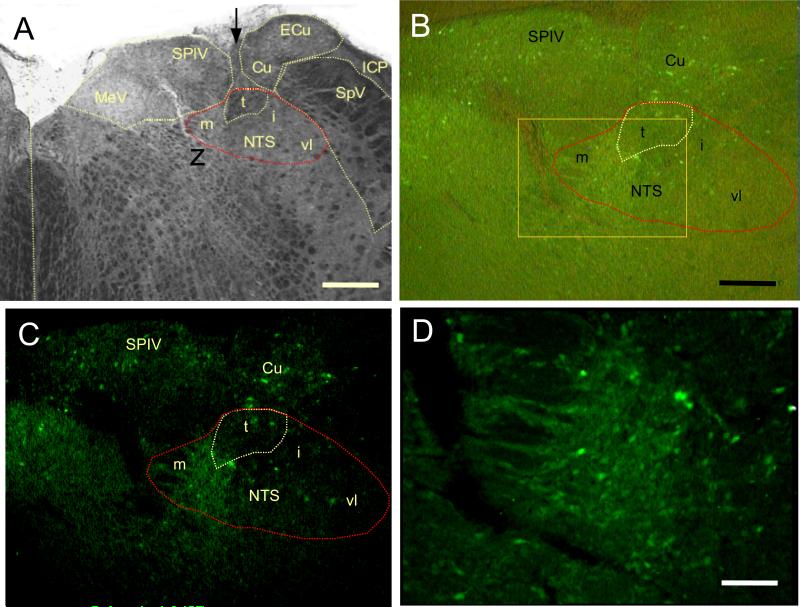
GFP Vector Image Analysis in NTS
To validate NTS injection parameters, the specificity and potential spread following delivery of the control vector encoding GFP was determined by examining native GFP fluorescence in selected coronal sections in conjunction with DAPI as a fluorescent nuclear counterstain (not shown) and unstained brightfield images (Figure 8). Expression was robust in NTS, with abundant numbers of GFP-fluorescent somata in medial, intermediate, and ventrolateral subdivistions. GFP-fluorescent cells were exclusively neuronal based on size and morphology, and were observed only in the ipsilateral dorsal medulla. Axons could occasionally be followed from NTS to the contralateral side. Some neurons in overlying structures (vestibular and cuneate nuclei) were observed, but few neurons were transduced ventral to the target.
Figure 8.
A: A trans-illuminated (brightfield) image of a coronal 50 um section through the unstained brainstem shows the position of nucleus tractus solitarius (NTS; t, tract; m, medial; i, intermediate; vl, ventrolateral compartments) in relation to prominent medulla structures as denoted in a standard atlas (Paxinos and Watson, 2005). Arrow marks vector injection needle track; vertical dotted line marks midline. Scale bar 500 um. MeV, medial vestibular nucleus; SPIV, superior vestibular nucleus; Cu, cuneate nucleus; ECu, external cuneate nucleus; SpV, spinal nucleus of cranial nerve V; ICP, inferior cerebellar peduncle.
B: Combining trans-illumination to visualize tissue organization (as in A) with epi-illumination to excite GFP fluorescence, vector expression was observed to be largely constrained to neurons in and near the NTS. Transduced neurons can be observed clearly in the entire medial-lateral and dorsalventral extent of NTS. Some neurons were transduced in vestibular and cuneate nuclei, but few were observed in areas ventral to NTS. Scale bar 250 um.
C: Image represents the same field described in B with epi-illumination alone. The field shows the abundance and restricted distribution of transduced neurons, and the absence of GFP fluorescence outside the immediate vector delivery site. B and C represent the same magnification.
D: At higher magnification the approximate area denoted by the box in B shows dozens of transduced NTS neurons expressing GFP closely packed in the medial zone. Epifluorescence is relatively stronger than in B and C due to the 10× objective focusing the excitation beam on to a smaller region than the 4X objective used for B and C. Trans-illumination intensity was reduced to optimize the image information from both illumination sources, so the brightness and contrast are different from B. Scale bar 100 um.
Discussion
This study employed recombinant adeno-associated viral techniques to target gene overexpression as a surrogate for pharmacological activation and blockade of leptin receptors specifically in localized brain regions. The expressed gene constructs include secretory sequences. As such, leptin or the dominant negative leptin mutant were expressed within cells comprising the MBH, VTA or NTS, and the peptide products secreted into the surrounding tissue. Thus, the secreted peptides were available to interact with leptin receptors on nearby cells in a manner, not unlike endogenous leptin reaching the brain via circulatory system, but in the present case the activation was limited to the targeted brain region. As detailed below, we demonstrated, using GFP fluorescence imaging analysis in rats receiving rAAV-GFP vector in either the VTA or MBH (Scarpace et al., 2012) or NTS (current study), that the GFP expression was found mainly in the targeted tissue and nearby adjacent areas, but not in non-injected brain regions.
The present study revealed several salient findings regarding the role of leptin action in the VTA and MBH. First, it established the usefulness of targeted overexpression of a leptin mutant as a tool for probing leptin action in specific brain regions. As described previously, overexpression of this mutant of rat leptin yields a protein that acts as neutral leptin receptor antagonist (Matheny et al., 2009, Zhang et al., 2007). Brain-wide overexpression increased food consumption, body weight and adiposity and blocked leptin signaling within the hypothalamus (Matheny et al., 2009). In the present study, targeted overexpression of the antagonist to the MBH, right of midline, fully blocked leptin signaling in response to exogenous leptin stimulation. Moreover, the viral vector-mediated overexpression was functionally focused in the delivered region, as evidence by differential blockade in the right and left MBH. Second, targeted leptin receptor blockade in the VTA increased body weight gain and food consumption to the same degree as the MBH, suggesting that leptin receptor function in both brain regions equally participate in body weight homeostasis. Third, the simultaneous overexpression of leptin in the VTA in conjunction with Leptin Antagonist overexpression in the MBH resulted in food intake and body weight gains that were less than with rAAV-control (GFP) but greater than with leptin in the VTA (Fig. 3). This, coupled with the observation that leptin overexpression in the VTA activates leptin receptors in the MBH, suggests that the physiological action of leptin in the VTA has, at least, one component related to P-STAT3 activation in the MBH.
Previous evidence linked leptin receptor activity in the VTA to modulation of HF and sucrose consumption with no discernible effect on body weight (Hommel et al., 2006, Davis et al., 2011). Direct leptin injection into the VTA induced short-term decreases in food consumption and body weight (Bruijnzeel et al., 2011). We reported that chronic leptin overexpression in the VTA ameliorates body weight gain and tempers high-fat food consumption to the same extent as leptin overexpression in the MBH (Scarpace et al., 2012). Collectively, these data support a role for leptin VTA action in body weight homeostasis. However, in the latter study, the physiological responses to targeted leptin overexpression in the VTA were associated with elevated P-STAT3 in the MBH, whereas leptin overexpression in the MBH did not evoke activation of P-STAT3 in the VTA (Scarpace et al., 2012). The present study expanded on these findings by simultaneously overexpressing a Leptin Antagonist in the right MBH along with the overexpression of leptin in the VTA. The present data revealed that the VTA-leptin-mediated stimulation of P-STAT3 in the right MBH was completely blocked, suggesting this elevated P-STAT3 was the result of leptin receptor activation. Thus, the action of leptin in the VTA may be fully or partially mediated by leptin receptors in the MBH. However, because co-expression of the antagonist in the MBH only partly blocks the physiological responses to VTA leptin, suggesting a VTA response independent of the MBH. This interpretation is complicated by the observation that antagonist overexpression in the right MBH does not fully block the VTA-leptin mediated activation of P-STAT3 in the left MBH. Thus, the action of the unblocked component of leptin receptors in the left MBH may account for some of the physiological responses to VTA-leptin overexpression.
The mechanism by which leptin gene delivery to the VTA activates the MBH could be either specific or inadvertent. The latter includes migration of either the vector or the leptin transgene product. We previously employed GFP as a surrogate marker for viral transduction and transgene expression and performed GFP fluorescence imaging analysis in rats receiving rAAV-GFP vector in either the VTA or MBH. GFP expression was found in the VTA and adjacent SN area post the VTA delivery, while GFP was detected mainly in the lateral and medial arcuate hypothalamic nuclei as well as in the VMH post MBH delivery. Therefore, GFP fluorescence was focused within the injected site and spread into a few indicated neighboring structures but with no appearance in the non-injected MBH or VTA brain region (Scarpace et al., 2012). Moreover, increased leptin expression and elevated leptin protein was detected only within the rAAV-leptin injected brain region and was notably absent in the non-injected brain-region (Scarpace et al., 2012). It is possible that a small amount of leptin, below our level of detection, diffuses through tissue and is transported either through the brain's ventricular system or the subarachnoid space (Ruiter, et al., 2010). Whereas this is plausible, it is not apparent. Rats demonstrate decreasing body weight and food intake in a dose response manner to either i.c.v. infusion of leptin (Wilsey et al., 2004) or rAAV-leptin delivery (data not shown). Considering that targeted overexpression of leptin to either the MBH or the VTA result in similar decreases in body weight gain and food consumption (Scarpace et al., 2012), it seems unlikely that levels of leptin below detection acting on MBH leptin receptors could results in the same physiological responses as direct leptin overexpression in the MBH. It seems more likely that a neural connection, such as along the medial forebrain bundle from the VTA to the hypothalamus (Simmons, et al., 1998) underlies the mechanism.
If VTA-leptin stimulation mediates a specific communication between the VTA and MBH, then leptin overexpression in other brain regions should not activate MBH leptin receptors. It was recently reported that microinjection of leptin into the hindbrain triggers STAT3 phosphorylation both in the hindbrain and hypothalamic neurons (Ruiter, et al 2010). The authors suggested that hindbrain leptin activates neurons in the hypothalamus via neuronal projections, or that leptin reaches leptin-sensitive hypothalamic neurons via the CSF in the subarachnoid space. In the present report, targeted overexpression of leptin in the NTS elevated P-STAT3 levels in the NTS suggesting activation of leptin receptors, however, MBH leptin receptors were not activated. Thus, there appears to be no leptin receptor neurons ascending to the MBH that modulate STAT3 phosphorylation. This implies that the observed leptin-mediated neural communication between the VTA and MBH is specific, or at least, not universal among all regions expressing leptin receptors.
Interestingly, the targeted overexpression of leptin to the NTS elevated P-STAT3, but there was no significant effect on body weight or food consumption in two separate experiments. Previous reports have established that knockdown of NTS leptin receptors (Hayes, et. al., 2010) or ablation of leptin receptors in the glucagon-like 1 peptide expressing neurons in the NTS (Scott, et. al., 2011) result in hyperphagia and elevated weight gain. The present findings partially support the earlier data, in that overexpression of the Leptin Antagonist exacerbates body weight gain, although food intake was not different. In contrast, our demonstration that direct leptin activation of the NTS has little role in body weight homeostasis, at first glance, appears to be incongruent with observations following leptin receptor knockdown, ablation, or blockade. However, from a pharmacological perspective, these apparent disparate findings suggest that the leptin receptors in the NTS are saturated by endogenous leptin in the basal state, and thus, unresponsive to further stimulation by leptin overexpression, but still sensitive to receptor blockade or reductions. This interpretation seems unlikely for two reasons. First, antagonist overexpression did not decrease P-STAT3, and second, overexpression of leptin in the NTS increases P-STAT3 by approximately two-fold, which is similar to the increase in P-STAT3 levels in MBH when leptin is overexpressed in the MBH (Scarpace et al, 2012), and leptin receptors in the MBH are fully responsive to exogenous leptin. Alternatively, the NTS leptin receptor could be permissive, supporting the actions of other peptides or facilitating leptin action in other brain sites. It is established that NTS leptin receptors are necessary for the actions of cholecystokinin, and that the NTS is a site of integration for leptin and gastrointestinal signals of satiation (Hayes, et. al., 2010).
Despite the lack of a significant effect of NTS leptin overexpression on food intake or body weight, serum leptin was diminished and UCP1 levels were elevated. This suggests that direct activation of NTS leptin receptors stimulates energy expenditure through activation of BAT. Thus, it is possible that prolonged stimulation of NTS leptin receptors would result in significant differences in body weight compared with control. Collectively, these data suggest that NTS leptin receptors are necessary for normal energy homeostasis, but their role is mostly permissive, with direct activation of NTS leptin receptors yielding a small elevation in energy expenditure, but little effect on body weight.
GFP fluorescence in rats receiving rAAV-GFP vector into the NTS indicated that expression was centered in NTS, with abundant fluorescent in neurons in all three subdivisions. The limited expression in vestibular and cuneate nuclei would not be predicted to exert direct action on feeding circuitry. Moreover, any leptin secretion from these nuclei would empty into the 4th ventricle, where the natural CSF flow would take it caudally and laterally rather than toward the MBH. This is consistent with the observations that NTS leptin expression did not stimulate P-STAT3 in the MBH, and leptin receptor blockade in MBH, did not reduce leptin mediated P-STAT3 in the NTS.
Leptin is known to both suppress appetite and augment energy expenditure (Li, 2011). One reliable marker of the latter effect of leptin is an increase in non-shivering thermogenesis through induction in UCP1 protein in BAT (Scarpace, et al., 1997). Although energy expenditure was not directly assessed in this study, BAT UCP1 protein levels were examined. We previously reported that leptin overexpression in the VTA elevated UCP1 in BAT (Scarpace, et al., 2012). In the present study, UCP1 in BAT was augmented with leptin overexpression in the VTA with or without simultaneous overexpression of the antagonist in the MBH. Moreover, by visual inspection, the BAT in both groups was moderately red compared with control, suggestive of activated UCP1. These data suggest that the VTA has an independent action from that of the MBH in the induction and activation of UCP1. Interestingly, Leptin Antagonist treatment in the MBH diminishes UCP1 levels in BAT, but not antagonist treatment in the VTA. A reasonable explanation is that the MBH sets the dominant sympathetic tone for BAT UCP1 induction and this induction is sensitive to any reduction in that MBH-mediated tone by antagonist treatment, whereas the VTA has little role in maintaining the dominant tone, but appears to have an independent stimulatory role.
The most perplexing finding in this report is that Leptin Antagonist treatment in the VTA attenuates STAT3 phosphorylation in the ipsilateral side of the MBH leptin treated rats. In contrast, in these same animals, P-STAT3 levels in the contralateral side are mostly unaffected as is leptin-MBH induced BAT UCP1. Presumably, the unknown mechanism by which leptin application in the VTA activates leptin receptors in the MBH would be subject to leptin receptor blockade, suggesting that the VTA plays a permissive role in MBH signaling.
In summary, leptin function in the VTA participates in the chronic regulation of food consumption and body weight in response to stimulation or blockade of VTA leptin receptors. Moreover, the simultaneous overexpression of the leptin in the VTA with antagonist in the MBH resulted in food intake and body weight gains that were less than with control but greater than with leptin in the VTA. These data along with the elevated UCP1 protein suggest a component of VTA-leptin action is independent of the MBH, although we cannot rule out participation by the MBH. Furthermore, another component of the physiological action of leptin in the VTA appears to be related to leptin receptor-mediated P-STAT3 activation in the MBH, whose mechanism remains uncertain. Finally, leptin receptors in the NTS are necessary for normal energy homeostasis, and demonstrate a mild increase in UCP1 protein, but appear to have mostly a permissive role while direct leptin activation of NTS leptin receptors has little effect on food consumption or body weight.
These data implicate an integrative response involving brain regions accounts for the observed physiological effects to the central stimulation of leptin with an emphasis on an interplay between the VTA the MBH and a mostly permissive role for leptin receptors in the NTS. Leptin action in the VTA has one body weight homeostatic component co-dependent on MBH leptin receptors and another component apparently independent of the MBH.
Acknowledgments
Funding
Supported by the NIH DK091710 (PJS) and the Medical Research Service of the Department of Veterans Affairs.
Footnotes
Declaration of interest
There are no conflicts of interest.
References
- Bruijnzeel AW, Corrie LW, Rogers JA, Yamada H. Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behav Brain Res. 2011;219:254–264. doi: 10.1016/j.bbr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, Figlewicz DP, Benoit SC. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry. 2011;69:668–674. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16(3):296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Li MD. Leptin and beyond: an odyssey to the central control of body weight. Yale J Biol Med. 2011;84:1–7. [PMC free article] [PubMed] [Google Scholar]
- Matheny M, Shapiro A, Tumer N, Scarpace PJ. Region-Specific Diet-induced and Leptin-Induced Cellular Leptin Resistance Includes the Ventral Tegmental Area in Rats. Neuropharmacology. 2011;60:480–487. doi: 10.1016/j.neuropharm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny M, Zhang Y, Shapiro A, Tümer N, Scarpace PJ. Central overexpression of leptin antagonist reduces wheel running and underscores importance of endogenous leptin receptor activity in energy homeostasis. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1254–61. doi: 10.1152/ajpregu.90449.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- Ruiter M, Duffy P, Simasko S, Ritter RC. Increased hypothalamic signal transducer and activator of transcription 3 phosphorylation after hindbrain leptin injection. Endocrinology. 2010;151:1509–19. doi: 10.1210/en.2009-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Pollock BH, Tümer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997;273:E226–230. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience. 2001;104:1111–1117. doi: 10.1016/s0306-4522(01)00142-7. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Tümer N, Zhang Y. Leptin Overexpression in VTA Trans-activates the Hypothalamus whereas Prolonged Leptin Action in either Region Cross-Desensitizes. Neuropharmacology. 2012;65:90–100. doi: 10.1016/j.neuropharm.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Zhang Y, Cheng KY, Tumer N. Leptin antagonist reveals an uncoupling between leptin receptor signal transducer and activator of transcription 3 signaling and metabolic responses with central leptin resistance. J Pharmacol Exp Ther. 2007;320:706–712. doi: 10.1124/jpet.106.112813. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Zhang Y, Tumer N, Frase CD, Shek EW, Hong B, Prima V, Zolotukhin S. Central leptin gene delivery evokes persistent leptin signal transduction in young and aged-obese rats but physiological responses become attenuated over time in aged-obese rats. Neuropharmacology. 2002b;42:548–561. doi: 10.1016/s0028-3908(02)00003-5. [DOI] [PubMed] [Google Scholar]
- Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest. 2011;121:2413–2421. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JM, Ackermann RF, Gallistel CR. Medial forebrain bundle lesions fail to structurally and functionally disconnect the ventral tegmental area from many ipsilateral forebrain nuclei: implications for the neural substrate of brain stimulation reward. J Neurosci. 1998;18:8515–8533. doi: 10.1523/JNEUROSCI.18-20-08515.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsey JT, Scarpace PJ. Caloric restriction reverses the deficits in leptin receptor protein and leptin signaling capacity associated with diet-induced obesity: role of leptin in the regulation of hypothalamic ObRb expression. J Endocrinology. 2004;181:297–306. doi: 10.1677/joe.0.1810297. [DOI] [PubMed] [Google Scholar]
- Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol. 2005;184:291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]
- Zhang J, Matheny MK, Tumer N, Mitchell MK, Scarpace PJ. Leptin antagonist reveals that the normalization of caloric intake and the thermic effect of food after high-fat feeding are leptin dependent. Am J Physiol Regul Integr Comp Physiol. 2007;292:R868–874. doi: 10.1152/ajpregu.00213.2006. [DOI] [PubMed] [Google Scholar]