Abstract
Objective
Cardiovascular complications including cardiac arrest and arrhythmias remain a clinical challenge in the management of acute traumatic spinal cord injury (SCI). Still, there is a lack of knowledge regarding the characteristics of arrhythmias in patients with acute traumatic SCI. The aim of this prospective observational study was to investigate the occurrence of cardiac arrhythmias and cardiac arrests in patients with acute traumatic SCI.
Methods
As early as possible after SCI 24-hour Holter monitoring was performed. Additional Holter recordings were performed 1, 2, 3, and 4 weeks after SCI. Furthermore, 12-lead electrocardiograms (ECGs) were obtained shortly after SCI and at 4 weeks.
Results
Thirty patients were included. Bradycardia (heart rate (HR) <50 b.p.m.) was present in 17–35% of the patients with cervical (C1–C8) SCI (n = 24) within the first 14 days. In the following 14 days, the occurrence was 22–32%. Bradycardia in the thoracic (Th1–Th12) SCI group (n = 6) was present in 17–33% during the observation period. The differences between the two groups were not statistically significant. The mean minimum HR was significantly lower in the cervical group compared with the thoracic group both on 12-lead ECGs obtained shortly after SCI (P = 0.030) and at 4 weeks (P = 0.041).
Conclusion
Many patients with cervical SCI experience arrhythmias such as bradycardia, sinus node arrest, supraventricular tachycardia, and more rarely cardiac arrest the first month after SCI. Apart from sinus node arrests and limited bradycardia, no arrhythmias were seen in patients with thoracic SCI. Standard 12-lead ECGs will often miss the high prevalence these arrhythmias have.
Keywords: Spinal cord injury, Arrhythmias, Cardiac arrest, Holter monitor, Supraventricular tachycardia, Bradycardia
Introduction
It is well known that acute traumatic spinal cord injury (SCI) may cause cardiovascular challenges including changes of blood pressure and heart rate (HR) with arrhythmias and these issues remain a challenge to the clinician.1
The human heart receives a balanced innervation from sympathetic and parasympathetic fibers causing a HR of 60 b.p.m.) in healthy individuals at rest.2 The sympathetic preganglionic fibers run through the spinal cord and exit at levels Th1–Th4 to form synapses in the thoracic ganglia. From here, the sympathetic postganglionic fibers exit to innervate the heart where they are responsible for increasing HR and cardiac output. The parasympathetic fibers to the heart exit the central nervous system through the vagal nerve at brainstem level to form the cardiac plexus close to the aortic arch together with the sympathetic postganglionic fibers. Parasympathetic action results in a decrease of HR and a constriction of the coronary arterioles.2
Thus, according to the hypothesis, patients with SCI at the cervical and high thoracic level have impaired sympathetic innervation to the heart implying a greater risk of episodes of bradycardia, atrioventricular blocks (AV blocks), and cardiac arrest due to the unopposed parasympathetic activity.
Two studies regarding acute SCI do report episodes of bradycardia, AV block, and cardiac arrest.1,3 However, these studies have predominantly used 12-lead electrocardiograms (ECGs) to document arrhythmias corresponding to a short recording period of only 10 seconds.1,3 The short recording period makes the documentation potentially insufficient because the risk of not recording arrhythmias is greater compared with continuous monitoring using 24 hours recordings. The aim of this prospective observational study was to investigate the occurrence of cardiac arrhythmias systematically in patients with acute traumatic SCI including comparing this prevalence between patients with cervical and thoracic SCI as well as comparing the prevalence of arrhythmias between 12-lead ECGs and 24 hours recordings.
Methods
Patient selection
The Copenhagen University Hospital, Rigshospitalet, Denmark receives all patients with acute traumatic SCI in the Eastern part of Denmark, i.e. an up-take area corresponding to a population of 2.5 million.
The inclusion period started 1 January 2010. All patients with traumatic SCI admitted to Department of Neurosurgery (until 1 October 2010), the Spine Unit of Department of Orthopedic Surgery (from 1 October 2010), or the Neurological Intensive Care Unit (NICU) were screened for inclusion in the study. The inclusion criteria were: (1) traumatic SCI, (2) neurological level C1–Th12, (3) 18 years or older, and (4) signed informed consent. The exclusion criteria were: (1) ongoing pregnancy, (2) use of cardiac pacemaker prior to the traumatic SCI, and (3) concomitant brain injury, i.e. Glasgow Coma Score <144 because this may bias our study objective related to HR as well as compromise the cooperation related to the neurological classification of the patient. The protocol was approved by the Scientific Ethics Committee of the Capital Region, Denmark. Seven patients who met the inclusion criteria refused to participate, partly because they did not want unnecessary procedures and examinations.
For each patient, the SCI was characterized according to the International Standards for Neurological Classification of SCI by the neurological level and severity as defined by the American Spinal Injury Association (ASIA) Impairment Scale (AIS).5 Patients who during their admission were scored differently from time to time regarding neurological level and the AIS were classified with their first score in this study.
Due to the limited number of patients with lower thoracic SCI (Th5–Th12) and only two patients with upper thoracic (Th1–Th4) lesion patients were divided into two groups for further analysis; the cervical SCI group (C1–C8; n = 24) and the thoracic SCI group (Th1–Th12; n = 6).
Data acquisition
Holter (DelMar Reynolds, Lifecard CF, Lifecard: SE, Issaquah, WA) recordings were used to document HR and cardiac rhythm continuously for 24 hours. The first Holter monitor (Holter 1) was placed on the patients as quickly as possible after admission. Afterwards, Holter recordings were obtained consecutively as close as possible to 1 week after SCI (Holter 2), 2 weeks (Holter 3), 3 weeks (Holter 4), and 4 weeks after SCI (Holter 5). It was not possible to rely on continuous ECG monitoring because the patients might only have been in departments with constant ECG monitoring in part of the first month or not at all. In addition, some of the patients were monitored in other hospitals due to transfer within the first month.
The nurses, physiotherapists, other professionals and when possible the patients themselves were instructed on a premade list to document all relevant procedures performed during the Holter recordings, i.e. tracheal suctioning, movements like turning and transferring of patients, respiratory exercise, bladder- and bowel emptying, etc. This was to document if recorded arrhythmias were triggered by any of these procedures. Two experienced readers of ECG recordings analyzed the Holter recordings. In the Holter analysis system (Sentinel, Spacelabs Healthcare, version 8.1, Sentinel: SE, Snoqualmie, WA), sinus bradycardia was defined in two ways: as an episode of six beats or more with a HR <50 b.p.m. (SB50) and as an episode of six beats or more with a HR <60 b.p.m. (SB60). This was due to different definitions of bradycardia in the literature.1,3 Supraventricular tachycardia (SVT) was defined as an episode of six beats or more of narrow complexes with a HR >120 b.p.m. that is not ordinary sinus tachycardia. Ventricular tachycardia was defined as an episode of six beats or more of broad complexes with a HR >120 b.p.m. A sinus node arrest was defined as an arrest of heart rhythm ≥1 second. A QRS pause was an arrest of heart rhythm ≥3 seconds not preceded by a sinus beat. Primary cardiac arrest was an event necessitating cardiopulmonary resuscitation such as heart–lung compression or isoprenaline infusion used for extreme uncontrolled bradycardia.
Twelve-lead ECGs were obtained together with Holter 1 recording (shortly after SCI) and the Holter 5 recording (4 weeks after SCI) and were analyzed individually by an experienced reader trained in the Department of Cardiology in diagnostics of arrhythmias and ischemia. The corrected QT interval (QTc) was calculated according to Bazett's formula as the ratio of the QT interval to the square root of the RR interval, both measured in seconds.
Information about prior or ongoing heart disease was registered according to the International SCI Cardiovascular Function Basic Data Set.6 This information was cross-checked with the patient charts as well. Information about the patients' medication and use of antiarrhythmogenic and inotropic drugs was registered in the electronic patient chart system.
Data analysis
Statistical assessment of categorical variables, i.e. comparison of lesion level (cervical vs. thoracic) and recording method (12-lead vs. 24-hour recordings) was performed using Fisher's exact test. Continuous Gaussian distributed variables were compared with Student's t-test. Repeated measures analysis of variance (ANOVA) was performed to investigate if there was any change in cardiac arrhythmias over time.
P values <0.05 were considered statistically significant. IBM SPSS for Windows, version 20.0 (IBM Corp., Armonk, NY, USA) was used.
Results
Thirty-seven patients were eligible for the study but seven patients refused to participate. Accordingly, 30 patients were included. The patient characteristics are shown in Table 1. Patients with cervical and thoracic lesions were demographically similar except for age. The number of patients with prior cardiovascular history (CVH) in the two groups did not differ significantly. Using the International SCI Cardiovascular Function Basic Data Set,6 11 patients of the cervical group was identified with a history of cardiovascular disease (CVD). Validation with the patient charts revealed additional patients with CVH: one patient in the thoracic group and two in the cervical group. The seven patients that refused to participate included four cervical lesions: C2 (AIS D), two C3 (AIS C), and C4 (AIS D) and three thoracic lesions: Th6 (AIS A), Th9 (AIS A), and Th11 (AIS A). One patient died during the 4-week period of the study: an 81-year-old man with a C4 lesion (AIS A) died due to respiratory insufficiency.
Table 1 .
Patient characteristics
| Cervical spinal cord injuries | Thoracic spinal cord injuries | P value | |
|---|---|---|---|
| Number of patients | 24 | 6 | |
| Males | 18 (75%) | 4 (67%) | |
| Females | 6 | 2 | |
| Age (years) | |||
| Mean | 65 | 27 | <0.001 |
| Range | 36 to 87 | 22 to 59 | |
| Previous cardiovascular history | 13 (54%) | 1 (17%) | |
| Surgery performed | 24 (100%) | 6 (100%) | |
| Mechanism of injury* | |||
| Sports and leisure activities | 3 (13%) | 2 (33%) | |
| Transport activities | 5 (21%) | 3 (50%) | |
| Falls | 15 (63%) | 1 (17%) | |
| Other traumatic causes | 1 (4%) | 0 |
*Prioritized according to the International Spinal Cord Injury Core Data Set.23
The characteristics of the patients included in the study. Apart from age these were comparable between the cervical and the thoracic groups.
Results from Holter recordings
The numbers of days after SCI before the patients were equipped with Holter monitors varied mainly due to practical challenges including procuring informed consent, due to sedation, surgery, other investigations, transfer to other departments/hospitals, etc. The Holter recordings were performed as follows: Holter 1: median 4 days (range: 1–8 days) postinjury, Holter 2: median 7 days (range: 5–18 days), Holter 3: median 14 days (range: 11–28 days), Holter 4: median 21 days (range: 17–30 days), and Holter 5: median 29 days (range: 24–41 days) after the SCI. Three patients had delayed hospital admission (and thus delayed Holter recordings) corresponding to the wide range seen above. One patient did not develop palsy of her left arm and legs until 6 days after SCI. Two other patients had delayed admission due to late referral from other hospitals including a hospital in Greenland.
The results of the Holter recordings are shown in Table 2. SB50 was found in 17–35% of patients with cervical SCI and in 17–33% patients in the thoracic group. SB60 was found in 50–61% of patients shortly after SCI and 52–68% in 3–4 weeks after SCI (Holters 4 and 5) in the cervical patients. SB60 was present in 17–67% in the thoracic group without any pattern of prevalence. None of the results between the cervical and thoracic groups were statistically significant. Fig. 1 illustrates the mean minimum HR and Fig. 2 the maximum HR in the cervical and thoracic patients in the observation period. The mean minimum HR in the period shortly after SCI (corresponding to Holter 1) was significantly lower in the cervical group compared with the thoracic group even after adjustment for age (P = 0.030). Repeated measures ANOVA analyses showed no difference in minimum HR over time (between the five Holter recordings). The mean maximum HR in the period shortly after SCI (corresponding to Holter 1) was significantly higher in the thoracic group (P = 0.04) compared with the cervical group. The results were significant in weeks 2–4 (corresponding to Holters 3–5) as well (P = 0.03 and P = 0.008).
Table 2 .
Occurrence of arrhythmias in patients with acute traumatic SCI
| Holter 1 | Holter 2 | Holter 3 | Holter 4 | Holter 5 | |
|---|---|---|---|---|---|
| Sinus bradycardia (SB50) | |||||
| Cervical SCI | 8 (35%) | 4 (17%) | 6 (25%) | 5 (22%) | 7 (32%) |
| Thoracic SCI | 0 | 2 (33%) | 1 (17%) | 2 (22%) | 0 |
| Sinus bradycardia (SB60) | |||||
| Cervical SCI | 14 (61%) | 12 (50%) | 14 (58%) | 12 (52%) | 15 (68%) |
| Thoracic SCI | 1 (17%) | 4 (67%) | 2 (33%) | 4 (67%) | 2 (33%) |
| Mean minimum heart rate | |||||
| Cervical SCI (±SD) | 57 (12)## | 61 (13) | 61 (14) | 60 (14) | 57 (15) |
| Thoracic SCI (±SD) | 74 (12)## | 58 (12) | 64 (12) | 57 (9) | 63 (9) |
| Mean maximum heart rate | |||||
| Cervical SCI (±SD) | 98 (19)# | 99 (21) | 97 (20)## | 98 (20)## | 100 (19)### |
| Thoracic SCI (±SD) | 116 (7)# | 111 (11) | 115 (9)## | 117 (15)## | 125 (18)### |
| Supraventricular arrhythmias | |||||
| Cervical SCI | 8 (35%) | 9 (38%) | 11 (46%) | 8 (35%) | 5 (23%) |
| Thoracic SCI | 0 | 0 | 0 | 0 | 0 |
| Numbers of supraventricular arrhythmias | |||||
| Cervical SCI (range) | 325 (0–281) | 91 (0–71) | 76 (0–49) | 11 (0–3) | 26 (0–22) |
| Thoracic SCI | 0 | 0 | 0 | 0 | 0 |
| Sinus node arrests ≥1 seconds ≤3 seconds | |||||
| Cervical SCI | 7 (30%) | 2 (8%) | 2 (8%) | 1 (4%) | 4 (18%) |
| Thoracic SCI | 0 | 1(17%) | 1 (17%) | 1 (17%) | 2 (33%) |
| Two or more ventricular ectopic beats in run | |||||
| Cervical SCI | 7 (30%) | 6 (25%) | 3 (13%) | 5 (22%) | 2 (9%) |
| Thoracic SCI | 0 | 0 | 0 | 0 | 0 |
#P < 0.05, ##P ≤ 0.04, ###P ≤ 0.01.
SB50: bradycardia <50 b.p.m.; SB60: bradycardia <60 b.p.m.; Mean (±SD).
Cervical SCI: n = 24, thoracic SCI: n = 6.
The table shows the number of patients in the two groups recorded with different arrhythmias during the observation period. The first Holter monitor (Holter 1) was placed on the patients as quickly as possible after admission. Holter recordings were obtained consecutively as close as possible to 1 week after SCI (Holter 2), 2 weeks (Holter 3), 3 weeks (Holter 4), and 4 weeks after SCI (Holter 5).
Figure 1 .
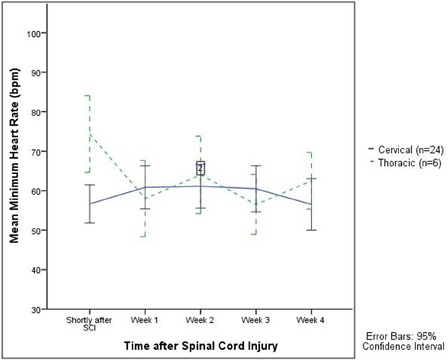
The mean minimum HR in the period shortly after SCI (corresponding to Holter 1) was significantly lower in the cervical group (P = 0.030) even after age adjustment.
Figure 2 .
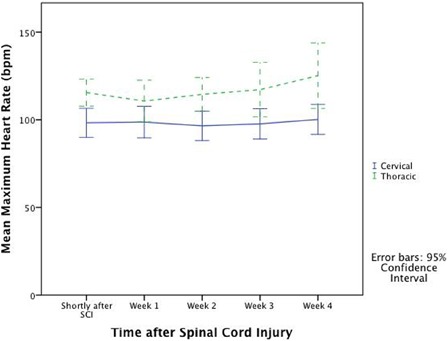
Mean maximum HR in cervical and thoracic patients after SCI.
Sinus node arrests ≥1 second occurred in both groups but none of the arrests were longer than 3 seconds. Two cervical patients had events of 2° AV block types 1 and 2 and one cervical patient had events of 3° AV block with ventricular escape rhythm. There were episodes of short lasting SVTs in the cervical patients; which were not seen in the thoracic group. The SVTs were most obvious in the first 2 weeks after SCI (Holters 1–3) and were gradually declining over the next 2 weeks (Holters 4 and 5). There was an insignificant difference between the number of SVTs in Holter 3 between the two groups (P = 0.06). There was no difference between the number of sinus node arrests, two ectopic beats in run or bradycardia (SB50 and SB60) between the recording periods. A few patients had different AIS scores during the observation period, but it was not possible to recognize any specific pattern suitable for statistical analysis regarding the prevalence of arrhythmias. Thus, we were not able to investigate whether different AIS scores affected our data.
Primary cardiac arrest occurred in three cervical SCI patients. These three patients (two aged 49 and one aged 60, respectively) had complete spinal cord lesions (AIS A) and are described in the following three cases.
Case 1: Forty-nine-year-old man had a Holter recorded arrest in the first week after SCI. Marked bradycardia and hypotension was followed by a few sinus node arrests of 3–8 seconds duration prior to a 20-second QRS pause accompanied by several ventricular escape beats.
Case 2: Twenty-eight days after SCI, this 60-year-old man with known CVD developed 3° AV block for 60 seconds with 5–6 ventricular escape beats with the longest QRS pause of 26 seconds. He then switched into sinus rhythm for a few beats accompanied by five sinus node arrests >3 seconds of which the longest was 46 seconds. This patient had no recorded bradycardia or hypotension prior to the arrest.
Case 3: Forty-nine-year-old man had a cardiac arrest shortly after admission in the emergency room. According to his admission chart, he was bradycardic and hypotensive prior to the arrest but detailed information about the episode is lacking because the episode was not Holter recorded. The exact reason to this cardiac arrest is unknown.
No fatal outcome due to cardiac arrests occurred, but all three patients (13% of cervical patients) received a cardiac pacemaker due to uncontrolled bradycardia. Furthermore, one patient received a pacemaker due to atrial fibrillation.
Results from 12-lead ECGs
One patient died before the last 12-lead ECG at 4 weeks could be obtained and 1 ECG was not obtained due to logistic reasons. The results of the analysis of all the other ECGs are shown in Table 3. SB50 was not observed on any ECGs. However in the cervical SCI group, SB60 was present in 21% of the initially recorded ECGs (corresponding to Holter 1) and in 9% of those recorded around 4 weeks after SCI (corresponding to Holter 5). The mean minimum HR was significantly lower in the cervical group compared with the thoracic group both on ECGs obtained shortly after SCI (P = 0.030) and at 4 weeks (P = 0.041). Repolarization changes were equally present in the cervical and the thoracic groups in the first and the last ECG recording ranging from negative T-waves to ST depressions and early repolarization. The mean QTc and PQ interval was also similar in the two groups. However, the mean QRS-complex duration in the ECGs obtained 4 weeks after SCI was significantly longer in the cervical group (P = 0.01) compared with those from the thoracic group. We found a prevalence of a longer QTc interval in the ECGs obtained a month after SCI as well. We did not find any supraventricular or ventricular arrhythmias on the ECGs. Comparing the ECGs independent of SCI level revealed a significantly longer PQ interval in the ECGs recorded 4 weeks after SCI.
Table 3 .
ECG abnormalities in patients with acute traumatic SCI
| Twelve-lead ECG as soon as possible after SCI (n = 30) |
Twelve-lead ECG 4 weeks after SCI (n = 28) |
|||||
|---|---|---|---|---|---|---|
| Cervical SCI (n = 24) | Thoracic SCI (n = 6) | All patients (n = 30) | Cervical SCI (n = 22) | Thoracic SCI (n = 6) | All patients (n = 28) | |
| Bradycardia <50 b.p.m. (SB50) | 0 | 0 | 0 | 0 | 0 | 0 |
| Bradycardia <60 b.p.m. (SB60) | 5 (21%) | 0 | 5 (17%) | 2 (9%) | 0 | 2 (7%) |
| Minimum HR (mean ± SD) | 72 ± 14# | 86 ± 14# | 75 ± 15 | 75 ± 15## | 90 ± 12## | 79 ± 15 |
| Repolarization changes | 4 (16%) | 2 (33%) | 6 (20%) | 2 (9%) | 3 (50%) | 5 (18%) |
| QTc interval, ms (mean ± SD) | 416 ± 23 | 403 ± 16 | 413 ± 22 | 423 ± 24 | 425 ± 41 | 423 ± 28 |
| QRS-interval, ms (mean ± SD) | 88 ± 11 | 85 ± 5 | 87 ± 10 | 90 ± 23### | 82 ± 4### | 87 ± 20 |
| PQ interval, ms (mean ± SD) | 170 ± 16 | 162 ± 16 | 168 ± 16### | 177 ± 14 | 170 ± 21 | 175 ± 16### |
| Supraventricular tachycardia | 0 | 0 | 0 | 0 | 0 | 0 |
| Ventricular tachycardia | 0 | 0 | 0 | 0 | 0 | 0 |
QTc, corrected QT interval.
#P < 0.05, ##P ≤ 0.04, ###P ≤ 0.01.
SB50: bradycardia <50 b.p.m.; SB60: bradycardia <60 b.p.m. SD, standard deviation.
Unless stated, values represent absolute numbers of patients (percentage of patients).
The table shows the ECG abnormalities on the ECGs obtained shortly after SCI and at 4 weeks.
Discussion
To our knowledge, this is the first prospective study which systematically uses 24 hours Holter recordings to document arrhythmias in the acute phase in patients with SCI. Our results demonstrate that acute SCI is accompanied by specific cardiovascular abnormalities including arrhythmias.
Bradycardia, AV block, and sinus node arrest
Bradycardia (SB50 and SB60) was a frequent complication in our study present in 35% (SB50) and 61% (SB60) of the patients with cervical SCI during the first days. The occurrence of SB60 increased within the end of first week and reached 58–68% in weeks 2–4 in the cervical patients. In the thoracic group, SB50 was present in 17–33% and SB60 in 17–67% of the patients but without any tendency to a pattern. Our observation was not expected as most studies report declining frequency of bradycardia during the first 2 weeks and a return to normal 2–6 weeks after SCI1,3 independently of the definition of bradycardia, i.e. less than 50 or 60 b.p.m. However, these studies relied on 12-lead ECGs only, which may give other results. In our study we did not observe any episodes of SB50 on the 12-lead ECGs in either the cervical or the thoracic group. In the cervical group, SB60 was found on 21% of the ECGs obtained shortly after SCI and 9% a month after SCI. The occurrence of SB50 as well as SB60 is clearly underestimated using ECGs compared with the Holter recordings in our study. Winslow et al.7 found no episodes of bradycardia in patients with thoracic (and lumbar) SCI. Some of the bradycardia episodes occurred during tracheal suctioning as previously described.3 However, using ECGs alone revealed that the mean minimum HR is significantly lower in cervical patients compared with thoracic patients throughout the observation period (P = 0.041 and P = 0.030).
Evidence of bradycardia (SB50) were present in a patient with upper thoracic SCI (Th2) corresponding to our theoretical knowledge of sympathetic cardiac innervation, but the thoracic group was too small to conclude whether the rather limited arrhythmias were qualitatively the same between upper and lower thoracic SCI.
However, we find an occurrence of bradycardia (SB60) on the Holter recordings quiet high even though we have used the definition recommended to be used after SCI and presented by ASIA and the International Spinal Cord Society.8 It is questionable whether this definition of bradycardia is appropriate when more than half of the study population met the criteria in our recordings. In addition, a healthy individual at rest may have a HR < 60 b.p.m. On the other hand, the definition might have been sufficient enough so far because studies have relied on 12-lead ECGs only as documentation. However, it could be advisable to change the bradycardia definition in the International Autonomic Standards for SCI from less than 60 b.p.m. to less than 50 b.p.m.9 or alternatively use two definitions depending on recording method. Still, a substantial proportion of the patients with cervical SCI will according to our investigation be recorded with bradycardia.
Our results demonstrated that thoracic patients had a significantly higher mean maximum HR almost during the whole observation period. This was not surprising because the major part of the thoracic patients had lower thoracic lesions (Th5–Th10) corresponding to no loss of sympathetic innervation and thus no unopposed parasympathetic activity. The thoracic patients may also be more physically mobile compared with the cervical patients causing a higher HR during the observation period. As expected, we identified several sinus node arrests as well as events of 2° AV block types 1 and 2 and 3° AV block, respectively. AV blocks were found on entry ECGs in two studies.1,3 Known CVD might have been predisposing to these events. For unknown reasons we did see sinus node arrests in one of the thoracic patients. This patient was not in the NICU (no suctioning) neither had any known CVD nor use of antiarrhythmogenic medicine. The notations made regarding performed procedures etc. during the Holter recordings did not reveal anything further. The reason to these events remains unclear.
Cardiac arrest
Of potentially great clinical importance were the three cardiac arrests requiring cardiopulmonary resuscitation. All three arrests occurred in patients with C1–C2 AIS A lesions. It is known that the level of cervical SCI is associated with severity of hemodynamic compromise and the need for more invasive interventions.10 One of the patients with no known CVH had bradycardia <30 b.p.m. and severe hypotension prior to cardiac arrest, but the other patients was neither hypotensive, hypoxic, nor bradycardic. Franga et al.11 recommend pacemaker consideration for patients with SCI with symptomatic bradyarrhythmic events when these still occur 2 weeks after injury. Our results show no significant difference between SB50 and SB60 over time (from Holter 1 to Holter 5). Thus it might not be necessary to wait with pacemaker implantation until 2 weeks after SCI. It was not possible to locate specific common warning features in order to identify those at highest risk of subsequent arrest besides a high cervical injury level as already described previously.12 Whether appropriate early intervention could protect against this risky event remains to be determined by further studies when more warning features have been identified.
Although the exact mechanism behind these events is not fully understood, they are likely to arise due to disruption of cardiac sympathetic innervation in especially cervical and upper thoracic SCIs. A complete lesion causes loss of supraspinal control and has been termed “decentralization” of the sympathetic nervous system.13 A term that encompasses both a loss of excitatory drive and a change in input–output relations of the autonomic brainstem reflexes. This causes loss of distal reflexes and vasomotor tone,14 which results in low resting arterial blood pressure caused by vasodilatation and loss of cardiac inotropy15 – elements that may cause neurogenic shock. Bradycardia results from unopposed parasympathetic vagal tone to the sinus atrial node, while the same stimulus reduces conduction in the AV node and the bundle of His resulting in episodes of AV blocks. Thus, some patients with cervical SCI might be predisposed for bradycardia and sinus node arrests during for instance tracheal stimulation (suctioning) due to vagal over activity.16 In persons with intact spinal cord tracheal stimulation results in tachycardia and hypertension – a response which is dependent on sympathetic efferent pathways under supraspinal control.14
Supraventricular tachycardia
We observed SVTs in the cervical SCI patients peaking in the first 2 weeks (Holters 1–3). This was not seen in patients with thoracic SCI. Lehmann et al.3 reported that 19% of patients with severe cervical SCI (Frankel A and B similar to AIS A and B) had episodes of SVTs. However, this result was predominantly caused by atrial fibrillation with 12-lead ECG as documentation. We found SVTs in patients with all severity grades (AIS A–D). SVTs can originate from the atria as a response to sympathetic stimulation of the sinus node.17 Thus it is a paradox to observe this trend in our study population. One explanation could be that they are triggered by pain or as a result of postoperative stress. However, the SVTs were not seen in thoracic patients as would be expected if triggered from any of the above as the thoracic patients underwent surgery and subsequent pain as well. The notes on the procedures performed during the Holter recordings did not reveal information on the possible provoking mechanisms. The SVTs might as well be triggered due to the use of vasopressor treatment in the acute phase. The NICU uses noradrenaline for treating hypotensive patients and isoprenaline for bradycardic patients. Noradrenaline is a catecholamine that increases blood pressure by increasing vascular tone through α-adrenergic receptor activation, which only has a limited effect on the heart. Isoprenaline is a β1- and β2-adrenoreceptor agonist which has positive inotropic and chronotropic effects on the heart that might produce tachycardia or arrhythmias. Our data revealed that one patient in the NICU received isoprenaline infusion within the same period recording a SVT. No patients suffered from blood loss or hemodynamic compromise. Twenty-seven episodes of SVT were Holter recorded on patients admitted to NICU. Only 10 of these episodes were recorded in a 24-hour period in which noradrenaline had been administered. Thus, use of ionotropics is only part of the truth, especially since noradranline only influences cardiac conduction poorly. Some patients had SVTs without being admitted to the NICU (no vasopressor treatment). Other centrally acting drugs, e.g. antidepressants, antiepileptics, and barbiturates crosses the blood–brain barrier and affects the central neurological tone and by that the heart as well which may cause arrhythmias. However, no patients received that type of medicine during the observation periods. It is possible the patients would have the SVTs independent of their SCI, i.e. related to their age or comorbidity. In the present study, the mean age in the cervical group was 65 while it was 27 in the thoracic group (three patients in the thoracic group was aged 22, 23, and 24, respectively). Thus, the age or comorbidity might be a source of bias. The patients with SVTs had a lot of supraventricular ectopic complexes (SVEC) as well. Approximately 10–15% of the middle-aged or elderly population may have excessive supraventricular ectopic activity without any known heart disease.18 Several theories about the mechanism and origin of SVEC and SVTs exist: it might be genetically determined or act as a forerunner of atrial fibrillation or be an early manifestation of hypertension.18 We were not able to find any correlation between SVT length or speed and neurological level or severity of the SCI.
Another reason to the occurrence of SVTs could be an indicator that the heart itself undergoes secondary changes (myocardial injury) in the weeks after SCI as suggested by Guha and Tator19 in an experimental study. Furthermore, an additional explanation regarding the observed SVTs might be that they were triggered by spontaneous sympathetic activity caused by loss of central control and the reflexes controlling the heart3 perhaps reinforced as a result of denervation hypersensitivity in the sympathetic synapse giving an excessive response to physiologically amounts of transmitter release. Yet another explanation could be that the SVTs are an early manifestation of atrial fibrillation in an early phase of autonomic dysreflexia as described in previous studies.20,21 This corresponds with the occurrence of the SVTs (2–3 weeks after SCI). The exact reason to the occurrence of SVTs is still unknown.
ECG analysis
Our analyses of the 12-lead ECGs did not reveal anything specific regarding ECG abnormalities shortly after and 1 month after acute SCI. The same trend is found by Prakash et al.,22 who found the prevalence of ECG abnormalities in chronic SCI similar to that in the able-bodied population. However, Lehmann et al.3 reported a higher incidence of non-specific ST-segment elevations among individuals with SCI. These individuals did also have an increased risk of developing bundle branch blocks and intraventricular conduction delays compared with the non-disabled population. As described, we did not find any SVTs on the ECGs even though we know these events do occur. The occurrence of bradycardia (SB50 and SB60) as well was less present on the ECGs compared with our 24-hour Holter recordings. Thus, our knowledge regarding arrhythmias in patients with acute SCI seems more divergent than we think because studies so far published have used mainly ECGs for documentation.
Limitations with this study
Our small number of patients is a limitation. For the same reason the statistical comparisons between the cervical and the thoracic SCI groups may be questioned. The thoracic group may be considered too small in order to document arrhythmias. Even though we have monitored continuously for 24 hours five times during the first month after acute SCI, we might have missed important events. During the Holter recordings, the staff might have forgotten to document important procedures carried out. Thus, there is a risk that the arrhythmias we consider occurred spontaneously might have been triggered by procedures not documented. Three patients with delayed hospital admission were included in the study which may have influenced the results. The large difference in age between the two groups is a limitation. However, it was not possible to include more patients in a specific group since this study prospectively included consecutive patients admitted with a traumatic SCI, so the inherent nature of the study makes this potential bias inevitable. New studies have to include a larger number of patients as well as they might use more sophisticated methods such as digital ECG recording, more intensified (longer duration) Holter recordings, etc. Further investigation could give information about how the heart adapts to a situation without sympathetic innervation and which patients are especially vulnerable to the cardiac instability resulting from acute traumatic cervical SCI.
Conclusion
Many patients with cervical SCI experience arrhythmias such as bradycardia, sinus node arrest, SVT, and more rarely cardiac arrest the first month after SCI. Apart from sinus node arrests and limited bradycardia, no arrhythmias were seen in patients with thoracic SCI. Standard 12-lead ECGs will often miss the high prevalence these arrhythmias have. However, 12-lead ECGs were enough to detect a significantly lower mean minimum HR in the cervical group compared with the thoracic group.
Acknowledgements
This project is financially supported by Trygfonden (j.nr.7531-09) and Toyotafonden (j.nr. 6358).
References
- 1.Piepmeier JM, Lehmann KB, Lane JG. Cardiovascular instability following acute cervical spinal cord trauma. Cent Nerv Syst Trauma 1985;2(3):153–60 [DOI] [PubMed] [Google Scholar]
- 2.Bonica JJ Autonomic innervation of the viscera in relation to nerve block. Anesthesiology 1968;29(4):793–813 [DOI] [PubMed] [Google Scholar]
- 3.Lehmann KG, Lane JG, Piepmeier JM, Batsford WP. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: incidence, time course and severity. J Am Coll Cardiol 1987;10(1):46–52 [DOI] [PubMed] [Google Scholar]
- 4.Sternbach GL The Glasgow coma scale. J Emerg Med 2000;19(1):67–71 [DOI] [PubMed] [Google Scholar]
- 5.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (Revised 2011). Spinal Cord 2011;34(6):535–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krassioukov A, Alexander MS, Karlsson A-K, Donovan W, Mathias CJ, Biering-Sørensen F. International spinal cord injury cardiovascular function basic data set. Spinal Cord 2010;48(8):586–90 [DOI] [PubMed] [Google Scholar]
- 7.Winslow EB, Lesch M, Talano JV, Meyer PR. Spinal cord injuries associated with cardiopulmonary complications. Spine 1986;11(8):809–12 [DOI] [PubMed] [Google Scholar]
- 8.Krassioukov AV, Karlsson A-K, Wecht JM, Wuermser L-A, Mathias CJ, Marino RJ. Assessment of autonomic dysfunction following spinal cord injury: rationale for additions to International Standards for Neurological Assessment. J Rehabil Res Dev 2007;44(1):103–12 [DOI] [PubMed] [Google Scholar]
- 9.Alexander MS, Biering-Sorensen F, Bodner D, Brackett NL, Cardenas D, Charlifue S, et al. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord 2008;47(1):36–43 [DOI] [PubMed] [Google Scholar]
- 10.Dixit S Bradycardia associated with high cervical spinal cord injury. Surg Neurol 1995;43(5):514. [DOI] [PubMed] [Google Scholar]
- 11.Franga DL, Hawkins ML, Medeiros RS, Adewumi D. Recurrent asystole resulting from high cervical spinal cord injuries. Am Surg 2006;72(6):525–9 [PubMed] [Google Scholar]
- 12.Bilello JF Cervical spinal cord injury and the need for cardiovascular intervention. Arch Surg 2003;138(10):1127–9 [DOI] [PubMed] [Google Scholar]
- 13.Claus-Walker J, Halstead LS. Metabolic and endocrine changes in spinal cord injury: II (section 1). Consequences of partial decentralization of the autonomic nervous system. Arch Phys Med Rehabil 1982;63(11):569–75 [PubMed] [Google Scholar]
- 14.Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil 2000;81(4):506–16 [DOI] [PubMed] [Google Scholar]
- 15.Mathias CJ, Frankel HL Autonomic disturbances in spinal cord lesions. In: Bannister R, Mathias CJ, (eds.) Autonomic failure. 3rd ed. Oxford: Oxford University Press; 1992:839–81.
- 16.Mathias CJ, Frankel HL. The cardiovascular system in tetraplegia and paraplegia. In: Frankel HL, (ed.) Spinal cord trauma, handbook of clinical neurology. Amsterdam: Elsevier Science Publishers; 1992. p. 435–56 [Google Scholar]
- 17.Rubart M, Zipes D. Genesis of cardiac arrhythmias: electrophysiological considerations. In: Lippy P, Bonow R, Mann D, Zipes DP, (eds.) Braunwald's heart disease Volume 1. 8th ed. Philadelphia: Saunders Elsevier; 2008, p. 746–50 [Google Scholar]
- 18.Binici Z, Intzilakis T, Nielsen OW, Køber L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation 2010;121(17):1904–11 [DOI] [PubMed] [Google Scholar]
- 19.Guha A, Tator CH. Acute cardiovascular effects of experimental spinal cord injury. J Trauma 1988;28(4):481–90 [DOI] [PubMed] [Google Scholar]
- 20.Pine ZM, Miller SD, Alonso JA. Atrial fibrillation associated with autonomic dysreflexia. Am J Phys Med Rehabil 1991;70(5):271–3 [DOI] [PubMed] [Google Scholar]
- 21.Forrest GP Atrial fibrillation associated with autonomic dysreflexia in patients with tetraplegia. Arch Phys Med Rehabil 1991;72(8):592–4 [PubMed] [Google Scholar]
- 22.Prakash M, Raxwal V, Froelicher VF, Kalisetti D, Vieira A, O'Mara G, et al. Electrocardiographic findings in patients with chronic spinal cord injury. Am J Phys Med Rehabil 2002;81(8):601–8 [DOI] [PubMed] [Google Scholar]
- 23.DeVivo M, Biering-Sørensen F, Charlifue S, Noonan V, Post M, Stripling T, et al. International spinal cord injury core data set. Spinal Cord 2006;44(9):535–40 [DOI] [PubMed] [Google Scholar]


