Abstract
Diagnosing dizzy patients remains a daunting challenge to the clinician in spite of modern imaging and increasingly sophisticated electrophysiological testing. Here we review the major bedside tests of the vestibulo-ocular reflex and how, when combined with a proper examination of the other eye movement systems, one can arrive at an accurate vestibular diagnosis.
Keywords: Vestibular, Examination, Labyrinth, Vestibulo-ocular, Nystagmus
1. Introduction
The evaluation of patients with dizziness and imbalance is always challenging and often frustrating, but with a careful, physiologically-based bedside examination one can arrive at the correct diagnosis, gratifying for both patient and physician. We can now probe the function of each of the semicircular canals and the utricle in isolation at the bedside. Only testing of the saccule requires the electrophysiology laboratory, but recording of vestibular evoked myogenic potentials (VEMPs) from the eye muscles and the neck muscles in response to sounds and vibration, allows us to test the function of both the saccule and the utricle. Here we will emphasize testing of eye movements, but, of course, the findings must be put into the context of a thorough history, general neurological examination, and when needed, neurootological laboratory testing, especially formal tests of hearing. A more detailed comprehensive description of bedside testing of the patient with a vestibular disorder as well as the evaluation and treatment of positional vertigo, and upon which some of this review is based, can be found in Kheradmand et al. (2013) and Nuti and Zee (2013).
2. The key physiological principles underlying the examination of the vestibulo-ocular reflex
Vestibulo-ocular reflexes assure clear vision when the head moves. The rotational vestibulo-ocular reflex (rVOR) produces a slow-phase eye movement that compensates for horizontal (yaw), vertical (pitch) or torsion (roll) head rotations. The normal rVOR is perfectly compensatory in direction and speed during yaw and pitch head rotations. In roll rotation, the eye movements are also well aligned with the head in direction but the gain (eye speed/head speed) is lower (Aw et al., 1996; Migliaccio et al., 2006; Schubert et al., 2012). The translational vestibuloocular reflex (tVOR) produces a slow phase that compensates for head translation; side-to-side (interaural), fore and aft (anterior-posterior), or up and down (vertical). The amplitude and direction of the tVOR depend on the point of regard. The amplitude must increase for near targets and the direction may have to change when the target is eccentric. For example, when moving fore and aft if one looks straight ahead the correct response of the tVOR is convergence and divergence respectively, while if one looks to the side the tVOR must have a disconjugate horizontal component.
Normally when the head is still, the left and right vestibular nerves and the neurons in the vestibular nucleus to which they project have equal resting discharge rates (vestibular tone). Movement of the head toward one side excites that labyrinth and inhibits the other. If the tone becomes relatively less on one side, either from a pathological lesion or a simulated lesion as with a caloric irrigation, a spontaneous nystagmus develops with slow phases directed toward, and quick phases directed away from the “lesioned” side.
The intensity of nystagmus often depends on the position of the eye in the orbit (Alexander’s law). With peripheral lesions the slow-phase velocity is higher when gaze is in the direction of the quick phase. With central lesions the opposite sometimes occurs.
With an imbalance in otolith and especially utricular pathways, the ocular tilt response (OTR) emerges, consisting of a lateral head tilt, vertical misalignment of the eyes (skew deviation with the eye being higher in the higher orbit) and ocular counterroll (torsion of both eyes with the top poles rotating toward the side of lower eye). There is also a consequent perceptual tilt of the visual world. The OTR reflects a tone imbalance analogous to the spontaneous nystagmus that occurs when there is a tone imbalance between the semicircular canals in each labyrinth.
A dynamic imbalance between the response from the two labyrinths during head rotation or translation produces a directional asymmetry in the compensatory slow phases. This is usually best appreciated with high acceleration, high velocity, and high frequency stimuli (head impulses in the case of the rVOR) because of Ewald’s second law; stimuli that excite the labyrinth produce a better response than those that inhibit it. With a unilateral loss of function in one labyrinth a greater response is elicited with rotation toward the intact than toward the lesioned side. Similarly, a dynamic otolith imbalance can be elicited with a brief, high-acceleration, side-to-side head translation along the interaural axis (head heave).
The central vestibular system improves the ability of the brain to faithfully transduce low-frequency head motion, for example, during prolonged, constant-velocity rotations. This phenomenon is called “velocity storage”. Velocity storage is important for understanding the significance and mechanism of post-head-shaking nystagmus.
3. The examination
3.1. Attitude of the head
A tilt of the head can be due to the ocular tilt reaction (OTR) that can emerge when there is imbalance in the otolith-ocular and otolith-spinal pathways. Peripheral vestibular damage or lesions within the vestibular nuclei in the medulla or caudal pons cause a head tilt toward the affected side, and lesions within the rostral pons and midbrain (commonly in the medial longitudinal fasciculus or MLF) usually cause a head tilt away from the affected side (Brandt and Dieterich, 1993). Patients with trochlear nerve palsy usually tilt their head away from the side of the paretic (higher) eye.
3.2. Dynamic visual acuity (DVA)
With a normal VOR, there is little difference in visual acuity when the head is still or moving. Patients with vestibular hypofunction, however, have a marked degradation of visual acuity and illusory movement of the environment (oscillopsia) while the head is moving. This is the rationale for comparing visual acuity with the head still and moving. DVA is assessed while the head is oscillated horizontally, vertically, or in the roll plane (from ear to shoulder) at a relatively high frequency of about two cycles per second. At this frequency visual tracking systems are too sluggish to contribute much to gaze stability, and therefore the function of the rVOR can be assessed acting alone, almost as if one were testing it in the dark. While oscillating the head, patients should not be allowed to stop or slow down too much near the turnaround points to prevent them from “sneaking” a look at the acuity chart. Normal individuals may lose one or two lines of acuity with head rotation, whereas patients with vestibular abnormalities often lose more than two. With unilateral vestibular hypofunction DVA is symmetric during pitch (vertical) and roll (torsion) but not yaw (horizontal) rotation; DVA is degraded more during yaw rotations toward the paretic than toward the healthy ear (Schubert et al., 2012). For roll movements of the head, the fovea (line of sight) is not displaced far from the visual target and so causes smaller decreases in visual acuity, even when labyrinthine function is completely lost. The specificity and sensitivity of DVA for detecting unilateral and especially bilateral loss is quite high and diagnosis can be made with a relatively few number of head rotations if the visual stimuli are adapted to the performance of the patient (Vital et al., 2010; Kim et al., 2011c).
3.3. Subjective visual vertical (SVV)
A tilt of the subjective visual vertical (SVV) is a sensitive sign of a disturbance in the otolith-ocular pathway (Dieterich and Brandt, 1993) and often occurs together with the OTR. Most patients with acute vestibular neuritis show a small and transient ipsilateral deviation of the SVV (Bohmer and Rickenmann, 1995). More central lesions can produce larger and more enduring tilts of the SVV. In the rostral medullary tegmentum and caudal pons the tilt of the SVV is usually ipsilateral to the lesion; in the rostral pons and caudal mesencephalic tegmentum the tilt of the SVV is usually contralateral to the lesion (Brandt and Dieterich, 1994).
The SVV can be measured at the bedside using the bucket method (Zwergal et al., 2009). The subject estimates verticality by aligning a dark straight line visible on the bottom of a bucket that is rotated to the right or left by the examiner. On the outside, there is a plumb line on the bottom of the bucket that originates from the center of a semicircle divided into degrees with the zero line adjusted to the dark line inside. In this way the deviation from true vertical can be measured by averaging a few trials.
3.4. Ophthalmoscopy
Ophthalmoscopy can detect subtle forms of nystagmus and other ocular oscillations such as saccadic microflutter (Ashe et al., 1991). The examiner should cover and uncover the other eye alternately to see if any drift of the disc is brought out or increased by removing fixation. Peripheral vestibular nystagmus is typically suppressed by fixation while central vestibular nystagmus is not. Recall that motion of the disc will be opposite to the direction of movement of the front of the eye because the disc is behind the axis around which the globe is rotating. For example, if the disc is moving upward it means the front of the globe is rotating downwards. The rVOR can also be evaluated during ophthalmoscopy. The head is oscillated horizontally or vertically (about two cycles per second), the subject fixes on a distant target, and then one eye is covered and the disc of the other is observed while the subject imagines still looking at the distant target. The observer notes the direction of motion of the optic disc relative to the direction of motion of the head. If the VOR is intact the optic disc does not move, as movement of the eye in the orbit is equal and opposite to the movement of the head. If the disc appears to move in the opposite direction to the head the reflex is hypoactive, and if it moves in the same direction the reflex is hyperactive. Finally, one must remember that if subjects habitually wear corrective spectacles their VOR gains will be adaptively adjusted to their habitual viewing condition, so during head rotation the VOR can appear hyperactive if the spectacles correct for farsightedness (hyperopia), or hypoactive if the spectacles correct for nearsightedness (myopia) (Cannon et al., 1985). Alternatively, one can rotate the head during ophthalmoscopy while subjects wear their corrective spectacles
3.5. Ocular alignment
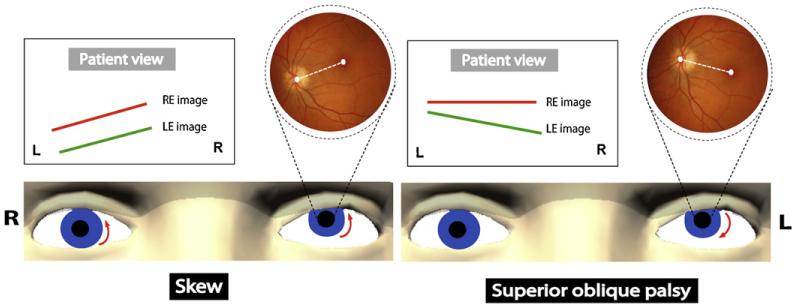
Examination of the alignment of the eyes can provide important information about otolith-ocular pathways. Cover testing and Maddox rod and red glass testing are the major tools and are used in the same way as when evaluating any patient with diplopia or ocular misalignment. When there is a vertical misalignment, the usual differential diagnosis is between trochlear nerve palsy and a skew deviation (Fig. 1). Skew deviations are usually from an imbalance in otolithocular pathways, though imbalance in the vertical semicircular canals may also contribute to the ocular misalignment (Brandt and Dieterich, 1993; Brodsky et al., 2006). The degree of misalignment with a skew deviation is relatively independent of where the eyes are pointed, i.e., the deviation is comitant. On the other hand, the hallmark of a superior oblique palsy is that the degree of misalignment changes with the direction of gaze, i.e., the deviation is noncomitant. The affected eye is higher and the vertical misalignment is greatest with the affected eye adducted and depressed, and also when the head is tilted toward the side of the higher eye (toward the side of the lesion). One should measure ocular alignment in both supine and upright positions as a skew deviation may decrease in the supine position while the misalignment with a superior oblique palsy does not (Wong, 2010). The relative direction of the torsion in the elevated eye – intorsion with skew and extorsion with a superior oblique palsy – is also helpful in diagnosis (Fig. 1). The direction of torsion can be determined by examining the fundus with the ophthalmoscope, by the bucket test for SVV examining each eye separately, or using visual field perimetry to detect the location of the blind spot relative to the fovea (Versino and Newman-Toker, 2010; Zwergal et al., 2009).
Fig. 1.
Skew deviation versus superior oblique palsy. Examiner view: a left hypertropia is shown with relative direction of the torsion in the elevated eye (dashed white line between the macula and optic disc): intorsion with skew deviation and extorsion with superior oblique palsy. Patient view with the double maddox rod test: relative position of the images from each eye is shown. In skew deviation, there is no or little torsional disparity. In superior oblique palsy, there is a torsional disparity with the lines pointing to the side of the paretic eye. RE: right eye; LE: left eye.
3.6. Examination of eye movements
A complete examination of the different ocular motor subsystems is central to the successful diagnosis of the patient with a vestibular disorder. It should include gaze holding, both in straight-ahead position (looking for nystagmus and other intrusions on fixation) and in eccentric positions (looking for gaze-evoked nystagmus), saccades (looking for delayed, slow or inaccurate movements), pursuit (looking for inadequate tracking requiring catch-up saccades) and convergence (primarily looking for its effect on any spontaneous nystagmus).
Spontaneous jerk nystagmus is the hallmark of a tone imbalance between the inputs from the semicircular canals of the two labyrinths. The slow phase is directed toward and the quick phase away from the hypoactive side. When nystagmus is peripheral in origin it characteristically increases or only becomes apparent when fixation is eliminated. This may also be detected with gentle eyelid closure and by observing movement of the corneal bulge, or even by palpating the globes. Note that saccadic oscillations such as opsoclonus may also be brought out or exacerbated by eye closure. One can also determine the static position of the eyes under closed lids by noting corrective movements when the patients open their eyes. In Wallenberg’s syndrome, for example, the eyes are often deviated toward the side of the lesion under closed lids so that when the eyes open there is a corrective saccade back to straight-ahead fixation.
The effect of removing fixation on nystagmus can be appreciated using Frenzel goggles, or during occlusive ophthalmoscopy when one eye is covered and the fundus of the other eye observed for the appearance of or increase in amplitude of any spontaneous drift. Since human beings have a relatively poor torsional fixation mechanism, the torsional component of a spontaneous nystagmus will be less suppressed by fixation than the horizontal or vertical components. Thus the true direction of a vestibular nystagmus can only be determined in the absence of fixation. Note that a mixed horizontaltorsional nystagmus with the slow phase toward the paretic side usually indicates a peripheral lesion. A pure vertical or pure torsional nystagmus usually has a central origin.
When a horizontal nystagmus only appears in one direction of eccentric gaze, the cause may be a mild unilateral peripheral vestibular lesion (based on Alexander’s law that states that vestibular nystagmus is more intense when looking in the direction of the quick phase). Spontaneous nystagmus may also occur in patients with lateral canal BPPV with the head upright. This is likely due to the natural inclination of lateral semicircular canals (SCC) with respect to the horizontal plane. The nystagmus typically stops after forward flexion of the head in the sitting position (about 30°) (De Stefano et al., 2011). The pathologic gaze-evoked nystagmus due to brainstem or cerebellar lesions can also be accompanied by signs of vestibular imbalance. The combination may manifest as lowfrequency, large-amplitude nystagmus on looking toward the lesioned side and high-frequency, small-amplitude nystagmus on looking away from the lesion. This is called Bruns’ nystagmus and is typically seen with relatively large cerebellopontine angle tumors (LIoyd et al., 2009). The largeamplitude nystagmus is due to abnormal gaze holding and the small-amplitude nystagmus due to vestibular imbalance.
A strong down-beating component brought out on lateral gaze or brought out by convergence implies central vestibular or cerebellar dysfunction. Down-beating or up-beating nystagmus that changes direction with convergence is usually a sign of a lesion in the medulla and commonly occurs with B1 deficiency in Wernicke’s disease. After prolonged eccentric gaze, when the eyes return to straight ahead there may be a transient rebound nystagmus with slow phases directed toward the previously held eccentric position. This is commonly a sign of cerebellar and specifically floccular/parafloccular (tonsil) dysfunction (Zee et al., 1981; Lin and Young, 1999). Periodic alternating nystagmus (PAN) changes its horizontal direction every few minutes and is a sign of abnormal function of the cerebellar nodulus (Waespe et al., 1985). PAN reflects instability in the velocity-storage mechanism within the vestibular nuclei because of a loss of inhibition by Purkinje cells from the nodulus.
3.7. Examination of the vestibulo-ocular reflex
3.7.1. Rotational vestibulo-ocular reflex (rVOR)
The rVOR compensates for rotation of the head. It should first be tested with relatively low-frequency (0.5 cycle per second) rotation of the head around the horizontal (yaw) and vertical (pitch) axes while the patient fixates on the nose of the examiner. Corrective saccades opposite to the rotation of the head indicate a hypoactive rVOR. Just as for dynamic visual acuity (DVA) smooth pursuit can make up for a defective rVOR when the head rotation is relatively slow and of low frequency, but when the loss of vestibular function is profound some catch-up saccades will be seen even during low-frequency oscillations as the patient attempts to fix upon a stationary target. An abnormally high amplitude of the rVOR with slow phases that are too fast (e.g., from a cerebellar lesion) requires corrective backup saccades in the direction of head rotation to maintain fixation. For rotation of the head in roll (ear to shoulder, around the nasal-occipital axis), one can evaluate the torsional VOR by noting the torsional quick phases, best seen using the movement of a blood vessel in the conjunctiva near the limbus. With a unilateral midbrain lesion that involves the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) the quick phases of torsional nystagmus are absent as the head is being tilted toward the side of the lesion (Helmchen et al., 1996).
A brief, high-acceleration head “impulse” is an effective bedside maneuver for detecting loss of function of a semicircular canal (Halmagyi and Curthoys, 1988). The patient is instructed to fix on a target, usually the nose of the examiner, while the head is quickly rotated by the examiner from one position to another. The rotation should not be large (less than 15°), but must be abrupt and of high acceleration. For the lateral semicircular canals the head impulse is applied horizontally, and for the vertical semicircular canals, the head is rotated vertically but only after turning the head about 30° to the right or left so that coplanar canals: right anterior, left posterior (RALP), or left anterior, right posterior (LARP) are stimulated (Migliaccio and Cremer, 2011). A corrective catchup saccade (horizontally for the lateral canals and vertically for the vertical canals) is the signature of an underactive VOR response.
In some patients with cerebellar disease the VOR response may be inappropriately directed “cross-coupled” for example, an upward slow-phase component followed by a downward corrective saccade with horizontal impulse testing (Walker and Zee, 2005). In patients with acute vestibular symptoms and a spontaneous nystagmus, a normal head impulse response suggests a central etiology whereas an abnormal head impulse is non-localizing as it can occur with central (usually an anterior inferior cerebellar artery distribution infarct which can affect both the vestibular nuclei and the labyrinth) as well as with isolated peripheral lesions (Newman-Toker et al., 2008; Braun et al., 2011). Two other signs that point to a central lesion in patients with an acute vestibular syndrome are a skew deviation and a bilateral gaze-evoked nystagmus (i.e., direction-changing) (Kattah et al., 2009).
Patients with chronic complete bilateral loss of labyrinthine function and occasionally patients with a long-standing unilateral loss may appear to have an intact impulse response because they trigger preprogrammed compensatory saccades so early during the movement. These movement then become “covert” saccades, embedded in the response during the head rotation and complete by the time the head stops moving, making them hard to discern (Weber et al., 2008). The short latency of the covert saccades cannot be attributed to visual feedback or patient anticipation and is likely triggered by any residual labyrinthine input or information from proprioceptors in the neck, acting as an “event detector”. (Tian et al., 2000; Peng et al., 2005). Some patients with unilateral vestibular loss may show not only large ipsilesional, but also smaller contralesional catch-up saccades. This finding does not necessarily indicate a bilateral vestibular deficit (Weber et al., 2008).
3.7.2. Translational vestibulo-ocular reflex (tVOR)
The head heave test, analogous to the head impulse test for the rVOR, evaluates the translational VOR (tVOR) and in turn the function of the utricle. An abrupt, high-acceleration lateral movement of the head (along the interaural axis) is imposed while the subject is instructed to look at a close target such as the nose of the examiner, especially since the tVOR must generate a larger response when targets are close. The lateral excursion of the head need be no more than 3–4 cm. A corrective catch-up saccade indicates the tVOR is hypoactive. Normal individuals usually show a hypoactive tVOR with a corrective saccade in either direction of head motion, so the response to horizontal translation must be asymmetrical to be useful for identifying an abnormality. While the “positive” head impulse sign is permanent after a complete loss of labyrinthine function (though the corrective saccades may become covert and hard to discern at the bedside), the head heave asymmetry as a sign of a hypoactive tVOR is usually rapidly compensated and so apparent only in the first days after a unilateral loss of function. A positive head heave sign, however, portends a delayed or less complete recovery following an acute vestibular neuritis (Mandala et al., 2008).
3.8. Valsalva-induced nystagmus
The Valsalva maneuver can induce nystagmus either by increasing intracranial pressure (straining against closed glottis as with lifting a heavy weight) or by increasing pressure in the middle ear (blowing out against pinched nostrils). The nystagmus may be induced in patients with cranio-cervical junction anomalies such as Arnold-Chiari malformation, with perilymph fistula, or superior canal dehiscence. Compression of the tragus can also provoke nystagmus by changing the middle-ear pressure (Hennebert’s sign). Increasing the pressure in the middle ear, however, is best done with a pneumatic otoscope. Jugular venous compression can also increases intracranial pressure and induce nystagmus similar to the Valsalva maneuver.
Tullio’s phenomenon (noise-induced nystagmus and oscillopsia) often occurs in patients who have Valsalva-induced nystagmus and is commonly associated with a superior canal dehiscence. Other possible pathologies include perilymph fistula, chronic middle ear disease, petrous meningioma, perilymphatic fistula, Ménière’s disease, enlarged vestibular aqueduct, mastoid venous thrombosis and congenital deafness (Kaski et al., 2012). Vestibular evoked myogenic potentials (VEMPs), low-frequency audiometry and high resolution CT scans of the temporal bone are often the keys to making the correct diagnosis in these complicated problems (Kaski et al., 2012).
3.9. Head-shaking induced nystagmus (HSN)
Head-shaking induced nystagmus (HSN) is usually a sign of a dynamic imbalance of vestibular function (Hain et al., 1987). The bedside HSN test is performed with the patient wearing Frenzel lenses. The head is rotated within a comfortable range at a frequency of about three cycles per second for about ten seconds and an induced nystagmus is looked for after the head has stopped moving. With a unilateral loss of vestibular function, a vigorous nystagmus with slow phases directed initially toward the affected side usually appears, sometimes followed by a reversal phase with slow phases directed toward the intact side (Kim et al., 2012a, b; Lee et al., 2012). In patients with unilateral labyrinthine loss, there is an asymmetry of peripheral inputs during high-velocity head rotations (Ewald’s second law), which leads to an unequal accumulation of activity in the central velocity-storage mechanism within the vestibular nuclei. Immediately after head shaking, the initial phase of HSN appears as a result of a decay of activity within the velocity-storage mechanism. A reversal phase may appear with the slow phases directed toward the intact ear (i.e., biphasic HSN). This reversal of the HSN reflects a short-term adaptation mechanism that balances out the initial phase (Hain et al., 1987). The biphasic conversion of head shaking is correlated with the severity of the initial canal paresis (Kim et al., 2012a; Lee et al., 2012). Also, monophasic HSN indicates less severe vestibular hypofunction than biphasic nystagmus (Lee et al., 2012). The mechanism underlying the different patterns of HSN may also relate to “recovery” nystagmus which refers to the appearance of a nystagmus with slow phases emanating from the lesioned ear. When there has been a prior adaptive rebalancing of vestibular tone after a unilateral lesion, and the tone from the paretic side is suddenly restored as peripheral function recovers, the new level of spontaneous activity on the paretic side becomes excessive relative to the central state of compensation. This leads to a new imbalance causing a spontaneous nystagmus with slow phases directed toward the intact year. The brain, however, catches up soon and rebalances vestibular tone to eliminate the “recovery” nystagmus. The direction of HSN can be particularly confusing in Ménière’s syndrome as it may be related to an excitatory phase, paretic phase, or recovery phase (Marques and Perez-Fernandez, 2012).
HSN can be also induced in the vertical planes. With unilateral peripheral lesions, vertical head shaking may cause a small-amplitude horizontal nystagmus with slow phases directed toward the intact ear (away from the affected side). This oppositely-directed response likely reflects an asymmetry in the normal contribution of excitation of the posterior SCC (which lies somewhat tilted relative to the up-down axis of the head) to the horizontal VOR during vertical head shaking (Hain et al., 1987). In patients with unilateral loss of labyrinthine function the presence of HSN is associated with a greater disability (Angeli et al., 2011). In the very acute phase, however, there may be no HSN, probably due to a temporary disengagement of the velocity-storage mechanism (Fetter et al., 1990).
HSN may appear with central lesions, usually in the medulla and cerebellum (Walker and Zee, 1999a; Kim et al., 2005). A cross-coupled “perverted” HSN such as a vertical nystagmus following horizontal head shaking is almost always a sign of a central and usually a cerebellar disturbance, though focal brain stem lesions with involvement of the crossed ventral tegmental tract (CVTT; an important pathway of the vertical VOR) may also produce this pattern (Kim et al., 2011a). With unilateral cerebellar lesions HSN usually beats ipsilaterally and there may also be a vertical downward beating component (Huh and Kim, 2011). In Wallenberg’s syndrome a unilateral loss of cerebellar inhibition over the velocity-storage mechanism within the vestibular nuclei may lead to head-shaking nystagmus with quick phases directed toward the side of the lesion (Choi and Kim, 2009). HSN from central causes usually is due to lesions involving the vestibulocerebellum, which is comprised of the flocculus/paraflocculus, nodulus and ventral uvula.
Finally, circular head shaking (tracing out a circular path with the chin) in normal individuals produces a torsional nystagmus that is probably similar to a post-rotatory nystagmus when a subject is rotated in the roll plane (around the naso-occipital axis). Patients with bilateral vestibular loss have a reduced or absent response so that circular head shaking, similar to a sustained horizontal chair rotation with the head tilted back and looking up, can be used to identify or confirm bilateral vestibular hypofunction (Haslwanter and Minor, 1999).
3.10. Vibration-induced nystagmus (VIN)
Vibration applied to the mastoid tip or vertex may bring out nystagmus in patients with unilateral loss of vestibular function and occasionally in other conditions such as superior canal dehiscence. The bedside VIN test is performed with the patient wearing Frenzel lenses. In general low-frequency vibration (e.g., 60 Hz) is more effective than higher frequency vibration (e.g., 120 Hz) in eliciting the nystagmus. In the case of a unilateral loss of function, vibration on either mastoid or over the vertex can elicit a nystagmus, usually with a slow phase toward the paretic ear. The direction of nystagmus usually is independent of the site of stimulation as the vibration impulses are transmitted throughout the skull nearly equally to both labyrinths. Because of this symmetry, normal individuals show no nystagmus or only a smallamplitude vibration induced nystagmus. In patients with a unilateral loss of labyrinthine function, the intensity of the VIN is correlated with the severity of the vestibular loss (Ohki et al., 2003). In these patients, stimulating with a vibrator (which normally excites both ears at once) is comparable to a hot water caloric irrigation to the intact ear (Ohki et al., 2003). The VIN shows considerable test-retest reliability and sensitivity to vestibular imbalance though the direction of the nystagmus may not always indicate the side of the lesion (Park et al., 2008, 2010; Koo et al., 2011; Dumas et al., 2011). For example, due to the “recovery” nystagmus mechanism, the VIN may occur with the slow phase away from the paretic side (see the section on head shaking-induced nystagmus). When vibration elicits a vertical nystagmus a central lesion should be suspected. A vibration-induced torsional or vertical nystagmus may also occur with a superior canal dehiscence (White et al., 2007).
3.11. Hyperventilation-induced nystagmus (HVN)
Hyperventilation may induce symptoms in patients with anxiety and phobic disorders but usually does not induce nystagmus (i.e., the hyperventilation syndrome). The HVN test is also performed with the patient wearing Frenzel lenses. Patients with demyelinating lesions of the vestibular nerve due to compression by a tumor (e.g., acoustic neuroma) or small blood vessels (microvascular compression) or with demyelination in central pathways (e.g., in multiple sclerosis) may develop nystagmus with hyperventilation. The alkalosis and decrease in extracellular calcium caused by 30 to 60 seconds of hyperventilation can improve conduction on demyelinated axons leading to a recovery nystagmus (slow phases directed toward the intact ear). Hyperventilation may also induce nystagmus in patients with vestibular neuritis in the acute stage (the slow phase can be ipsi or contralesional) as well as in the compensated stage (the slow phase is usually directed to the lesioned side) (Choi et al., 2007; Park et al., 2011; Califano et al., 2011). A contralesionally directed slow-phase pattern of HVN has been also reported after radiotherapy of vestibular schwannoma and is likely due to a similar demyelination mechanism (Bradley et al., 2011). In addition to a recovery mechanism HVN may also be related to a transient decompensation of a prior adaptive rebalancing of vestibular tone in the chronic stage. For example, hyperventilation may induce a nystagmus beating away from the lesion side in patients with larger vestibular schwannomas or in patients who have undergone surgical resection of a vestibular schwannoma. In these cases in which there is an ipsilesional slow phase pattern of HVN, the hyperventilation is thought to temporarily disrupt the adaptive mechanisms that compensates for the axonal disruption caused by tumor growth or surgical removal (Choi et al., 2007). The induced reduction in tissue oxygenation – due to cerebral vasoconstriction and serum alkalinization – have been suggested as possible mechanisms for the decompensation of the adaptive mechanisms during hyperventilation (Park et al., 2011).
Hyperventilation can also enhance spontaneous downbeat nystagmus in cerebellar patients which is likely mediated through metabolic effects on calcium channels of Purkinje cells (Walker and Zee, 1999b). Moreover, hyperventilation may induce nystagmus by changing intracranial pressure in patients with cranio-cervical junction anomalies or with abnormal connections between the subarachnoid space and the inner ear as occurs with a perilymph fistula. With superior canal dehiscence, intracranial hypotension induced by the hyperventilation provokes an ampullofugal endolymphatic flow in the superior canal, and leads to a nystagmus that aligns with the plane of the dehiscent superior canal (i.e., down beating and torsional) (Minor et al., 2003).
Some key features of the bedside examination and how the findings are incorporated into a correct anatomical diagnosis are summarized in Boxes 1 and 2 (Kim and Kim, 2012).
Box 1. Key maneuvers to elicit unilateral labyrinthine hypofunction.
Subjective visual vertical (SVV) with bucket test: Deviation toward the side of lesion
Eliminate fixation to look for spontaneous nystagmus: Slow phases directed toward the affected ear
Head impulse test: There is an impaired slow phase when rotating toward the affected ear requiring a corrective saccade (opposite to the head movement)
Horizontal head shaking: There is a horizontal nystagmus afterward (usually with a torsional component) with slow phases directed toward the affected ear
Vibration over skull (mastoids and vertex): There is a nystagmus with slow phases directed toward the affected ear
Box 2. Distinguishing superior and inferior division vestibular neuritis.
Superior division (lateral SCC, anterior SCC and utricle are affected)
Spontaneous nystagmus: horizontal (beating toward intact ear, from lateral canal) and vertical-torsional (small1 upward beating and a torsional component with the top pole beating toward intact ear, from anterior SCC)
Abnormal head impulse (rVOR from lateral and anterior SCC)
Abnormal head heave (tVOR from utricle)
Abnormal ocular VEMPs (from utricle)
Abnormal caloric (from lateral SCC)
Abnormal ocular counter-roll and SVV (tilt toward the side of the lesion, from utricle)
Inferior division (posterior SCC and saccule are affected)
Spontaneous nystagmus: vertical-torsional (down beating and a torsional component with the top pole beating toward intact ear, from posterior SCC)
Abnormal head impulse (rVOR from posterior SCC)
Abnormal cervical VEMPs (from saccule)
Occasional associated high frequency hearing loss (basal cochlea)
1Relatively small vertical component of spontaneous nystagmus with a superior division lesion since it arises from activity in the intact, unopposed posterior SCC (which has an oblique rather than a pure vertical orientation).
3.12. Positional testing
Positional testing is an essential part of the vestibular examination in all patients with the complaint of dizziness. The positional maneuvers are best performed with the patient wearing Frenzel lenses to remove fixation. The patient is first moved from the sitting position to the Hallpike position (the head is turned 45° to the left and then the patient is moved backward) to stimulate the left posterior SCC and one looks for a positional nystagmus. The patient is then brought back again to the sitting position. This maneuver is then repeated with the right ear down to stimulate the right posterior SCC. Then the head is placed in the straight back head-hanging position to look for a vertical, usually downward beating, nystagmus that commonly occurs with central disorders though there are probably some patients who have positional downbeating nystagmus due to anterior canal BPPV. Finally the patient is turned 90° to the left ear down and then 180° to the right ear down positions to stimulate the lateral canals and look for a lateral canal BPPV (i.e., supine head roll test). The diagnosis of a posterior canal BPPV (PC BPPV) is straightforward. When the head is placed with the offending labyrinth down, a mixed vertical torsional nystagmus appears, usually after a latency of a few seconds. The quick phases beat upward in the orbit with a torsional component such that the top pole beats toward the lower ear. With a positive maneuver, the direction of nystagmus may change for a brief period after the patient sits up from the Hallpike position. Many patients with PC BPPV may not show nystagmus at the time of their clinical evaluation but seemingly can still respond to treatment (Balatsouras and Korres, 2012). Both the Semont and the Epley maneuvers seem to be equally effective in treating PC BPPV (Mandalà et al., 2012).
Diagnosing a lateral canal BPPV (LC BPPV) can be challenging but the key features are outlined in Box 3. The most important diagnostic finding in LC BPPV is a horizontal and direction-changing positional nystagmus provoked by the supine head roll test. The latency of the nystagmus is commonly less than PC BPPV and, although it usually fades away, it is generally more intense and longer lasting than with PC BPPV. Rotation of the head towards one side causes an intense horizontal positional nystagmus beating toward the lower ear (geotropic) or beating toward the higher ear (apogeotropic). Rotation of the head to the other side reverses the direction of the nystagmus so that the nystagmus remains geotropic or apogeotropic. This inversion of the direction of the nystagmus, caused by two different head positions, 180° apart, is comparable to the inversion of direction of nystagmus that occurs when the patient with PC BPPV is brought from the Hallpike position to the sitting position. With LC BPPV, the pathological ear can usually be identified by comparing the intensity of the nystagmus in the right and left ear down positions. The key finding is that when in the position in which the nystagmus is most intense it will be beating toward the affected ear. This is the case for both geotropic and apogeotropic LC BPPV. Geotropic nystagmus is usually seen when the particles are free-floating in the lateral canal (canalolithiasis) relatively far from the cupula. Apogeotropic nystagmus can be due to the either canaloor cupulolithiasis of the lateral canal. The particle mass is attached to either side of the cupula of the lateral canal or there are free-floating particles in the long arm of the lateral canal close to the cupula. In both cases the cupula deviates away from the utricle, inhibiting activity from the lateral canal, and provoking apogeotropic nystagmus, beating away from the affected (lower) ear. The opposite occurs when the patient turns on the side of the normal ear; the mass causes the cupula to deviate toward the utricle, exciting activity from the lateral canal, which again leads to an apogeotropic nystagmus. LC BPPV may occur as a transient complication of treatment of PC BPPV. There are many maneuvers to treat LC BPPV (Kim et al., 2012b; Oh et al., 2009; Korres et al., 2011). Rarely an isolated direction changing horizontal nystagmus can be the sole sign of an acute focal cerebellar (nodulus) lesion (Kim et al., 2011b).
Box 3. Lateral canal BPPV.
Geotropic nystagmus: When supine with right or left ear down, nystagmus beats toward the ground (usually canalolithiasis)
Apogeotropic nystagmus: When supine with right or left ear down, nystagmus beats away from the ground (cupulolithiasis or canalolithiasis)
Side of pathology: When lying on the side in which the nystagmus is most intense the nystagmus will be beating toward the affected ear (i.e., geotropic with affected ear down and apogeotropic with affected ear up)
Bow and lean test: When head upright or tilted straight back (bow), there is a spontaneous horizontal nystagmus which beats to normal side in geotropic and beats toward affected side in apogeotropic nystagmus. When head pitched forward (lean) the spontaneous nystagmus reverses direction
Positional downbeating nystagmus may rarely be due to cupulolithiasis of the anterior canal though there is also commonly a torsional component. In anterior canal BPPV (AC BPPV), the Hallpike maneuver provokes a positional nystagmus with a downbeating component. The anterior canals are roughly coplanar to the posterior canals of the opposite side. Therefore, in AC BPPV a mixed vertical torsional nystagmus can appear when the head is placed in the Hallpike position with the normal labyrinth down. For example, when the right anterior canal is affected, the left Hallpike maneuver provokes downbeating quick phases with a torsional component such that the top pole beats toward the right ear. The nystagmus in AC BPPV can also be induced in the straight head-hanging position (about 30° below the earth-horizontal). The nystagmus is often of low intensity, relatively sustained, and it may not reverse when the patient is returned to the sitting position. Several maneuvers have been suggested to treat AC BPPV (Yacovino et al., 2009)
To epitomize, a careful, methodical and physiologically-based bedside examination usually provides the essential clues to the origin of the sometimes vague and hard to describe complaints of so many patient with dizziness and imbalance. Indeed, imaging and ancillary testing are far more productive when guided by the findings of a skillful clinical evaluation.
Acknowledgements
This work was supported by a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD);2T32DC000023.
Footnotes
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
REFERENCES
- Angeli SI, Velandia S, Snapp H. Head-shaking nystagmus predicts greater disability in unilateral peripheral vestibulopathy. Am J Otolaryngol. 2011;32:522–7. doi: 10.1016/j.amjoto.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Ashe J, Hain T, Zee D, Schatz N. Microsaccadic flutter. Brain. 1991;114:461–72. doi: 10.1093/brain/114.1.461. [DOI] [PubMed] [Google Scholar]
- Aw ST, Haslwanter T, Halmagyi GT, Curthoys IS, Yavor RA, Todd MJ. Three dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations I. Responses in normal subjects. J neurophysiol. 1996;76:4021–30. doi: 10.1152/jn.1996.76.6.4021. [DOI] [PubMed] [Google Scholar]
- Balatsouras DG, Korres SG. Subjective benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2012;146:98–103. doi: 10.1177/0194599811425158. [DOI] [PubMed] [Google Scholar]
- Bohmer A, Rickenmann J. The subjective visual vertical as a clinical parameter of vestibular function in peripheral vestibular disease. J Vestib Res. 1995;5:35–45. [PubMed] [Google Scholar]
- Bradley JP, Hullar TE, Neely JG, Goebel JA. Hyperventilation-induced nystagmus and vertigo after stereotactic radiotherapy for vestibular schwannoma. Otol Neurotol. 2011;32:1336–8. doi: 10.1097/MAO.0b013e31822e8666. [DOI] [PubMed] [Google Scholar]
- Brandt T, Dieterich M. Skew deviation with ocular torsion: a vestibular brainstem sign of topographic diagnostic value. Ann Neurol. 1993;33:528–34. doi: 10.1002/ana.410330518. [DOI] [PubMed] [Google Scholar]
- Brandt T, Dieterich M. Vestibular syndromes in the roll plane: topographic diagnosis from brainstem to cortex. Ann Neurol. 1994;36:337–47. doi: 10.1002/ana.410360304. [DOI] [PubMed] [Google Scholar]
- Braun EM, Tomazic PV, Ropposch T, Nemetz U, Lackner A, Walch C. misdiagnosis of acute peripheral vestibulopathy in central nervous ischemic infarction. Otol Neurotol. 2011;32:1518–21. doi: 10.1097/MAO.0b013e318238ff9a. [DOI] [PubMed] [Google Scholar]
- Brodsky MC, Donahue SP, Vaphiades M, Brandt T. Skew deviation revisited. Surv Ophthalmol. 2006;51:105–28. doi: 10.1016/j.survophthal.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Califano L, Melillo MG, Vassallo A, Mazzone S. Hyperventilation-induced nystagmus in a large series of vestibular patients. Acta Otorhinolaryngol Ital. 2011;31:17–26. [PMC free article] [PubMed] [Google Scholar]
- Cannon SC, Leigh RJ, Zee DS, Abel LA. The effect of the rotational magnification of corrective spectacles on the quantitative evaluation of the VOR. Acta Otolaryngol. 1985;100:81–8. doi: 10.3109/00016488509108591. [DOI] [PubMed] [Google Scholar]
- Choi KD, Kim JS. Head-shaking nystagmus in central vestibulopathies. Ann N Y Acad Sci. 2009;1164:338–43. doi: 10.1111/j.1749-6632.2008.03737.x. [DOI] [PubMed] [Google Scholar]
- Choi KD, Kim JS, Kim HJ, Koo JW, Kim JH, Kim CY, et al. Hyperventilation-induced nystagmus in peripheral vestibulopathy and cerebellopontine angle tumor. Neurology. 2007;69:1050–9. doi: 10.1212/01.wnl.0000271378.54381.6a. [DOI] [PubMed] [Google Scholar]
- De Stefano A, Kulamarva G, Citraro L, Neri G, Croce A. Spontaneous nystagmus in benign paroxysmal positional vertigo. Am J Otolaryngol. 2011;32:185–9. doi: 10.1016/j.amjoto.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. Ocular torsion and tilt of subjective visual vertical are sensitivebrainstem signs. Ann Neurol. 1993;33:292–9. doi: 10.1002/ana.410330311. [DOI] [PubMed] [Google Scholar]
- Dumas G, Karkas A, Perrin P, Chahine K, Schmerber S. High-frequency skull vibration-induced nystagmus test in partial vestibular lesions. Otol Neurotol. 2011;32:1291–301. doi: 10.1097/MAO.0b013e31822f0b6b. [DOI] [PubMed] [Google Scholar]
- Fetter M, Zee DS, Koenig E, Dichgans J. Head-shaking nystagmus during vestibular compensation in humans and rhesus monkeys. Acta Otolaryngol. 1990;110:175–81. doi: 10.3109/00016489009122534. [DOI] [PubMed] [Google Scholar]
- Hain T, Fetter M, Zee D. Head-shaking nystagmus in patients with unilateral peripheral vestibular lesions. Am J Otolaryngol. 1987;8:36–47. doi: 10.1016/s0196-0709(87)80017-0. [DOI] [PubMed] [Google Scholar]
- Halmagyi G, Curthoys I. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–9. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- Haslwanter T, Minor L. Nystagmus induced by circular head shaking in normal human subjects. Exp Brain Res. 1999;124:25–32. doi: 10.1007/s002210050596. [DOI] [PubMed] [Google Scholar]
- Helmchen C, Glasauer S, Bartl K, Buttner U. Contralesionally beating torsional nystagmus in a unilateral rostral midbrain lesion. Neurology. 1996;47:482–6. doi: 10.1212/wnl.47.2.482. [DOI] [PubMed] [Google Scholar]
- Huh YE, Kim JS. Patterns of spontaneous and head-shaking nystagmus in cerebellar infarction: imaging correlations. Brain. 2011;134:3662–71. doi: 10.1093/brain/awr269. [DOI] [PubMed] [Google Scholar]
- Kaski D, Davies R, Luxon L, Bronstein AM, Rudge P. The Tullio phenomenon: a neurologically neglected presentation. J Neurol. 2012;259:4–21. doi: 10.1007/s00415-011-6130-x. [DOI] [PubMed] [Google Scholar]
- Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504–10. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand A, Bronstein AM, Zee DS. Clinical bedside examination. In: Bronstein AM, editor. Vertigo and disorders of Balance. OUP; Oxford: 2013. [Google Scholar]
- Kim JS, Kim HJ. Inferior vestibular neuritis. J Neurol. 2012;259:1553–60. doi: 10.1007/s00415-011-6375-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kim JS, Ahn KW, Moon SY, Choi KD, Park SH, Koo JW. Isolated perverted head-shaking nystagmus in focal cerebellar infarction. Neurology. 2005;64:575–6. doi: 10.1212/01.WNL.0000150729.87682.79. [DOI] [PubMed] [Google Scholar]
- Kim HA, Lee H, Sohn SI, Kim JS, Baloh RW. Perverted head shaking nystagmus in focal pontine infarction. J Neurol Sci. 2011a;301:93–5. doi: 10.1016/j.jns.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Kim HA, Yi HA, Lee H. Apogeotropic central positional nystagmus as a sole sign of nodular infarction. Neurol Sci. 2011b doi: 10.1007/s10072-011-0884-x. doi: 10.1007/s10072-011-0884-x [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kim S, Oh YM, Koo JW, Kim JS. Bilateral vestibulopathy: clinical characteristics and diagnostic criteria. Otol Neurotol. 2011c;32:812–7. doi: 10.1097/MAO.0b013e31821a3b7d. [DOI] [PubMed] [Google Scholar]
- Kim MB, Huh SH, Ban JH. Diversity of head shaking nystagmus in peripheral vestibular disease. Otol Neurotol. 2012a;33:634–49. doi: 10.1097/MAO.0b013e31824950c7. [DOI] [PubMed] [Google Scholar]
- Kim SH, Jo SW, Chung WK, Byeon HK, Lee WS. A cupulolith repositioning maneuver in the treatment of horizontal canal cupulolithiasis. Auris Nasus Larynx. 2012b;39:163–8. doi: 10.1016/j.anl.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Koo JW, Kim JS, Hong SK. Vibration-induced nystagmus after acute peripheral vestibular loss: comparative study with other vestibule-ocular reflex tests in the yaw plane. Otol Neurotol. 2011;32:466–71. doi: 10.1097/MAO.0b013e31820d9685. [DOI] [PubMed] [Google Scholar]
- Korres S, Riga MG, Xenellis J, Korres GS, Danielides V. Treatment of the horizontal semicircular canal canalithiasis: pros and cons of the repositioning maneuvers in a clinical study and critical review of the literature. Otol Neurotol. 2011;32:1302–8. doi: 10.1097/MAO.0b013e31822f0bc5. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Shin JE, Park MS, Kim JM, Na BR, Kim CH, et al. Comprehensive analysis of head-shaking nystagmus in patients with vestibular neuritis. Audiol Neurootol. 2012;17:228–34. doi: 10.1159/000336958. [DOI] [PubMed] [Google Scholar]
- Lin CY, Young YH. Clinical significance of rebound nystagmus. Laryngoscope. 1999;109:1803–5. doi: 10.1097/00005537-199911000-00015. [DOI] [PubMed] [Google Scholar]
- LIoyd SK, Baguley DM, Butler K, Donnelly N, Moffat DA. Bruns’ Nystagmus in patients with vestibular schwannoma. Otol Neurotol. 2009;30:625–8. doi: 10.1097/MAO.0b013e3181a32bec. [DOI] [PubMed] [Google Scholar]
- Mandala M, Nuti D, Broman AT, Zee DS. Effectiveness of careful bedside examination in assessment, diagnosis, and prognosis of vestibular neuritis. Arch Otolaryngol Head Neck Surg. 2008;134:164–9. doi: 10.1001/archoto.2007.35. [DOI] [PubMed] [Google Scholar]
- Mandalà M, Santoro GP, Asprella Libonati G, Casani AP, Faralli M, Giannoni B, et al. Double-blind randomized trial on short-term efficacy of the Semont maneuver for the treatment of posterior canal benign paroxysmal positional vertigo. J Neurol. 2012;259:882–5. doi: 10.1007/s00415-011-6272-x. [DOI] [PubMed] [Google Scholar]
- Marques PS, Perez-Fernandez N. Bedside vestibular examination in patients with unilateral definite Ménière’s disease. Acta Otolaryngol. 2012;32:498–504. doi: 10.3109/00016489.2011.646357. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Cremer P. The 2D modified head impulse test: a 2D technique for measuring function in all six semi-circular canals. JVR. 2011;21:227–34. doi: 10.3233/VES-2011-0421. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Della Santina CC, Carey JP, Minor LB, Zee DS. The effect of binocular eye position and head rotation plane on the human torsional vestibuloocular reflex. Vision Res. 2006;46:2475–86. doi: 10.1016/j.visres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Minor lB, Carey JP, Cremer Pd, Lustig LR, Streubel SO, Ruckenstein MJ. Dehiscence of bone overlying the superior canal as a cause of apparent conductive hearing loss. Otol Neurotol. 2003;24:270–83. doi: 10.1097/00129492-200303000-00023. [DOI] [PubMed] [Google Scholar]
- Newman-Toker DE, Kattah JC, Alvernia JE, Wang DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology. 2008;70:2378–85. doi: 10.1212/01.wnl.0000314685.01433.0d. [DOI] [PubMed] [Google Scholar]
- Nuti D, Zee DS. Positional vertigo and BPPV. In: Bronstein AM, editor. Vertigo and disorders of balance. OUP; Oxford: 2013. [Google Scholar]
- Oh SY, Kim JS, Jeong SH, Oh YM, Choi KD, Kim BK, et al. Treatment of apogeotropic benign positional vertigo: comparison of therapeutic head-shaking and modified Semont maneuver. J Neurol. 2009;256:1330–6. doi: 10.1007/s00415-009-5122-6. [DOI] [PubMed] [Google Scholar]
- Ohki M, Murofushi T, Nakahara H, Sugasawa K. Vibration-induced nystagmus in patients with vestibular disorders. Otolaryngol Head Neck Surg. 2003;129:255–328. doi: 10.1016/S0194-5998(03)00529-1. [DOI] [PubMed] [Google Scholar]
- Park H, Hong SC, Shin J. Clinical significance of vibration-induced nystagmus and head-shaking nystagmus through follow-up examinations in patients with vestibular neuritis. Otol Neurotol. 2008;29:375–9. doi: 10.1097/MAO.0b013e318169281f. [DOI] [PubMed] [Google Scholar]
- Park H, Lee Y, Park M, Kim J, Shin J. Test-retest reliability of vibration-induced nystagmus in peripheral dizzy patients. J Vestib Res. 2010;20:427–31. doi: 10.3233/VES-2010-0389. [DOI] [PubMed] [Google Scholar]
- Park HJ, Shin JE, Lee YJ, Park MS, Kim JM, Na BR. Hyperventilation-induced nystagmus in patients with vestibular neuritis in the acute and follow-up stages. Audiol Neurootol. 2011;16:248–53. doi: 10.1159/000320841. [DOI] [PubMed] [Google Scholar]
- Peng GCY, Minor LB, Zee DS. Gaze position corrective eye movements in normal subjects and in patients with vestibular deficits. Ann NY Acad Sci. 2005;1039:337–48. doi: 10.1196/annals.1325.032. [DOI] [PubMed] [Google Scholar]
- Schubert M, Migliaccio AA, Ng TWC, Shaikh A, Zee DS. The under-compensatory roll aVOR does not affect dynamic visual acuity. JARO. 2012;13:517–25. doi: 10.1007/s10162-012-0330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Crane BT, Demer JL. Vestibular catch-up saccades in labyrinthine deficiency. Exp Brain Res. 2000;131:448–57. doi: 10.1007/s002219900320. [DOI] [PubMed] [Google Scholar]
- Versino M, Newman-Toker DE. Blind spot heterotopia by automatedtatic perimetry to assess static ocular torsion: centrocecal axis rotation in normals. J Neurol. 2010;257:291–3. doi: 10.1007/s00415-009-5341-x. [DOI] [PubMed] [Google Scholar]
- Vital D, Hegemann SC, Straumann D, Bergamin O, Bockisch CJ, Angehrn D, et al. A new dynamic visual acuity test to assess peripheral vestibular function. Arch Otolaryngol Head Neck Surg. 2010;136:686–91. doi: 10.1001/archoto.2010.99. [DOI] [PubMed] [Google Scholar]
- Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. 1985;228:199–202. doi: 10.1126/science.3871968. [DOI] [PubMed] [Google Scholar]
- Walker MF, Zee DS. Directional abnormalities of vestibular and optokinetic responses in cerebellar disease. Ann NY Acad Sci. 1999a;871:205–20. doi: 10.1111/j.1749-6632.1999.tb09186.x. [DOI] [PubMed] [Google Scholar]
- Walker MF, Zee DS. The effect of hyperventilation on downbeat nystagmus in cerebellar disorders. Neurology. 1999b;53:1576–9. doi: 10.1212/wnl.53.7.1576. [DOI] [PubMed] [Google Scholar]
- Walker MF, Zee DS. Cerebellar disease alters the axis of the high-acceleration vestibuloocular reflex. J Neurophysiol. 2005;94:3417–29. doi: 10.1152/jn.00375.2005. [DOI] [PubMed] [Google Scholar]
- Weber KP, Aw ST, Todd M, McGarvie L, Curthoys I, Halmagyi G. Head impulse test in unilateral vestibular loss. Neurology. 2008;70:454–63. doi: 10.1212/01.wnl.0000299117.48935.2e. [DOI] [PubMed] [Google Scholar]
- White JA, Hughes GB, Ruggieri PN. Vibration-induced nystagmus as an office procedure for the diagnosis of superior semicircular canal dehiscence. Otol Neurotol. 2007;28:911–6. [PubMed] [Google Scholar]
- Wong AMF. Understanding skew deviation and a new clinical test to differentiate it from trochlear nerve palsy. J AAPOS. 2010;14:61–7. doi: 10.1016/j.jaapos.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacovino DA, Hain TC, Gualtieri F. New therapeutic manoeuvre for anterior canal benign paroxysmal positional vertigo. J Neurol. 2009;256:1851–5. doi: 10.1007/s00415-009-5208-1. [DOI] [PubMed] [Google Scholar]
- Zee DS, Yamazaki A, Butler PH, Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981;46:878–99. doi: 10.1152/jn.1981.46.4.878. [DOI] [PubMed] [Google Scholar]
- Zwergal A, Rettinger N, Frenzel C, Dieterich M, Brandt T, Strupp M. A bucket of static vestibular function. Neurology. 2009;72:1689–92. doi: 10.1212/WNL.0b013e3181a55ecf. [DOI] [PubMed] [Google Scholar]