Abstract
Objective
To evaluate the generation of rheumatoid arthritis (RA)–related autoantibodies in the lung.
Methods
Simultaneous collection of serum and induced sputum was performed in 21 healthy controls, 49 at-risk subjects without inflammatory arthritis but at risk of RA due to family history or seropositivity for anti–citrullinated protein antibodies, and 14 subjects with early RA. Samples were tested for anti–cyclic citrullinated peptide 2 (anti-CCP2), anti-CCP3, anti-CCP3.1, rheumatoid factor isotypes IgM, IgG, and IgA, and total IgM, IgG, and IgA.
Results
One or more autoantibodies were present in sputum of 39% of at-risk seronegative subjects, 65% of at-risk seropositive subjects, and 86% of subjects with early RA. In at-risk seronegative subjects, the rate of anti-CCP3.1 positivity and the median number of autoantibodies were elevated in sputum versus serum. In subjects with early RA, the rate of positivity for several individual autoantibodies and the median number of autoantibodies were higher in serum than in sputum. Results in at-risk seropositive subjects were intermediate between these groups. In at-risk subjects with autoantibody positivity in sputum, the ratios of autoantibody to total Ig were higher in sputum than in serum, suggesting that these autoantibodies are generated or sequestered in the lung.
Conclusion
RA-related autoantibodies are detectable in sputum in subjects at risk of RA and in subjects with early RA. In a subset of at-risk subjects, the presence of sputum autoantibodies in the absence of seropositivity, and the increased autoantibody-to–total Ig ratios in sputum, suggest that the lung may be a site of autoantibody generation in the early development of RA. These findings suggest an important role of the lung in the pathogenesis of RA.
The finding of serum elevations of rheumatoid factor (RF) and anti–citrullinated protein antibodies (ACPAs) prior to the symptomatic onset of inflammatory arthritis in rheumatoid arthritis (RA) suggests that RA-related autoimmunity may be initiated outside of the joints (1–5). Although the anatomic site of initiation of RA-related autoantibody production is unknown, emerging data, including the identification of elevations of IgA autoantibodies in subjects prior to the onset of symptomatic RA, suggest that initiation may occur at a mucosal site (6–9). Furthermore, the association of inhaled factors such as tobacco smoke and occupational dust with increased risk of RA (10,11), and reported findings of inflammatory lung abnormalities associated with serum RA-related autoantibody positivity in the absence of (and in some cases preceding) articular symptoms in RA (12–14), suggest that the lung may be a mucosal (and potentially an initiating) site of generation of RA-related autoimmunity.
Evaluating the lung to determine whether it is a site of generation of RA-related autoimmunity presents many challenges; however, prior studies demonstrating the generation of autoantibodies within the lung through comparative analyses of sputum and serum suggest that such an approach may be used to identify the lung as a site of generation of RA-related autoantibodies in the early development of RA (15–18). Therefore, to test the hypothesis that RA-related autoantibodies are generated in the lung, we evaluated ACPAs and RF isotypes in simultaneously collected sputum and serum from healthy subjects, subjects at elevated risk of developing RA due to family history of RA or seropositivity for ACPAs, and subjects classified as having RA according to established criteria.
SUBJECTS AND METHODS
Study subjects
Subjects were recruited from the Studies of the Etiology of Rheumatoid Arthritis (SERA) project, a prospective study established to investigate the natural history of RA (19). Enrollment was between February 2011 and September 2012 and included subjects with early (<12 months since diagnosis) seropositive RA (early RA) fulfilling the 1987 revised criteria of the American College of Rheumatology (ACR) (20), subjects at elevated risk of future RA (at-risk subjects) who were currently without inflammatory arthritis, and seronegative controls (healthy controls) without a known history of health care provider–diagnosed lung or autoimmune disease who were recruited through local advertising. The at-risk subjects included those with a first-degree relative who fulfilled the ACR 1987 revised criteria for RA and those identified through community health fair screening as being seropositive for ACPAs on at least one occasion (21). At-risk subjects were further categorized as seropositive (≥1 serum RA-related autoantibody) or seronegative as determined by serum testing at their lung study visit. All at-risk and healthy control subjects completed a standardized questionnaire and underwent a 68-joint examination performed by a rheumatologist or trained study nurse to confirm the absence of clinical evidence of inflammatory arthritis at their lung study visit.
Ethical considerations
All study procedures were approved by institutional review boards at participating institutions.
Sputum collection and processing
Sputum was obtained using an induced sputum protocol of inhalation of nebulized 5% saline over 15 minutes, with expectorated sputum collected at defined intervals and when subjects sensed the need for coughing/sputum release, and with oral rinse and drying prior to sputum expectoration to minimize salivary contamination (16,17). To additionally ensure that adequate bronchial samples were analyzed, only sputum samples with a squamous epithelial cell count of <10 cells per high-power field were used for these experiments (excluding 8% of subjects) (16).
Once collected, sputum samples were processed by diluting them with 3 ml of phosphate buffered saline (PBS) per 1 gram of sputum, followed by mechanical disruption of the sample through an 18-gauge needle (4 cycles per gram or a minimum of 12 cycles) (22). Next, to separate the cellular material, processed sputum samples were centrifuged at 1,200 revolutions per minute for 10 minutes, and that supernatant was further centrifuged at 3,500 rpm for 20 minutes, with this final supernatant used for testing described below.
Of note, the mucolytic agent dithiothreitol (DTT) has been used in some studies for processing sputum as this agent can separate cellular and mucus contents of sputum. However, we did not use DTT in this study as it disrupts disulfide bonds that may result in alteration of autoantibody detection, and prior studies have also shown that the addition of DTT does not improve antibody measurements in sputum compared to mechanical processing without DTT (18). Furthermore, we initially tested the raw, unprocessed sputum samples for RA-related autoantibodies using the enzyme-linked immunosorbent assay (ELISA)–based autoantibody tests described below. In testing these unprocessed sputum samples, there was a mean variability between ELISA wells of >20% for individual samples—a finding possibly related to heterogeneous distribution of autoantibodies between wells due to adherence to mucus. However, with the mechanical disruption processing method described above, the variability of autoantibody levels between wells was <5%, suggesting that with the process, the sputum samples were appropriately homogenized.
Autoantibody testing
All serum samples were tested for the following: RF isotypes IgM, IgA, and IgG by ELISA (Quanta Lite kits; Inova Diagnostics), anti–cyclic citrullinated peptide 2 (anti-CCP2) (IgG ELISA, Diastat; Axis-Shield), and anti-CCP3.1 (IgA/IgG ELISA; Inova Diagnostics). Serum positivity for each RF isotype was established based on levels positive in <5% of 491 blood donor controls; standard kit cutoffs were used for serum anti-CCP positivity (anti-CCP2 >5 units; anti-CCP3.1 ≥20 units). All serum samples were diluted 1:100 per manufacturer specifications; additional dilutions were performed (1:200, 1:400, etc.) as necessary to determine the final autoantibody level if initial dilution was not sufficient to identify the upper autoantibody level.
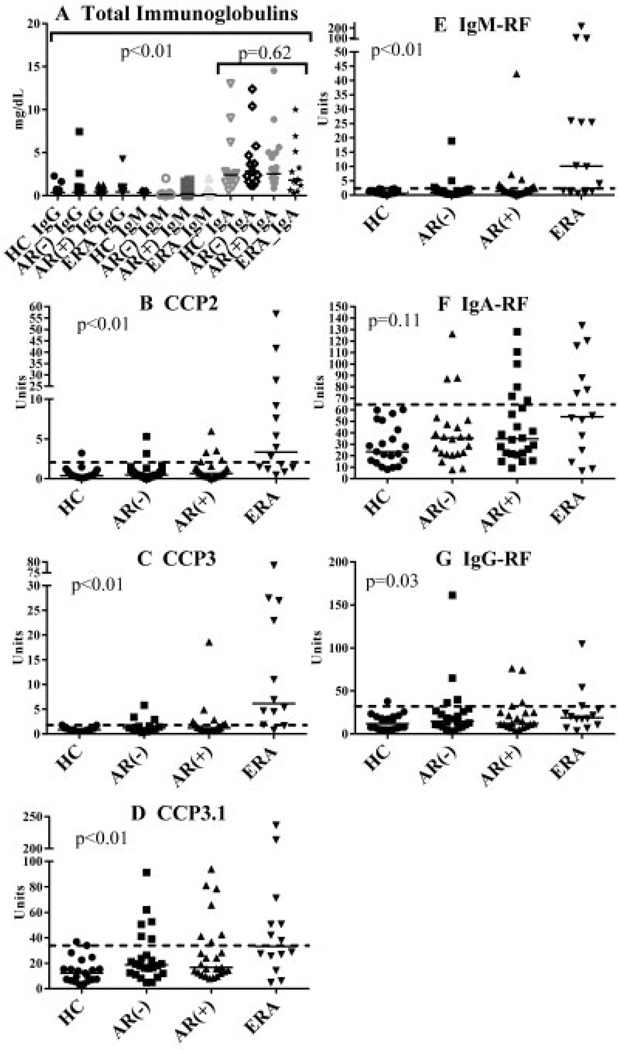
All sputum samples were collected simultaneously with serum samples and tested using the autoantibody assays described above, substituting 100 µl of PBS-processed sputum for serum, without further dilution beyond the weight-based dilution described above. All samples were tested with the laboratory technician blinded with regard to the group or serum autoantibody status of the subject. Sputum autoantibody levels were established by comparing concentrations to a standard curve. A “final” level for each autoantibody was established using an average of levels from 2 ELISA wells. Because there are no established levels for RA-related autoantibody positivity in sputum, we established a positive level for each autoantibody by applying a 2 SD increase to mean levels in processed sputum from healthy controls (see Figure 1 for cutoff levels).
Figure 1.
Immunoglobulin and autoantibody levels in sputum. Shown are plots depicting autoantibody levels in sputum from healthy controls (HC), at-risk seronegative subjects (AR−), at-risk seropositive subjects (AR+), and subjects with early rheumatoid arthritis (ERA). Each data point represents a single subject; solid horizontal lines show the median level for each group. Dashed lines indicate cutoff values generated by adding 2 SD to the mean autoantibody level in healthy controls. In A, P < 0.01 represents comparison of total IgA levels to other Ig levels across groups; P = 0.62 represents comparison of IgA levels across groups. In B–G, P values represent comparisons of levels of autoantibodies across the 4 groups. CCP2 = anti–cyclic citrullinated peptide 2; RF = rheumatoid factor.
In a subset of subjects with an adequate volume of sputum available (n = 56), an anti-CCP3 IgG ELISA (Inova Diagnostics) was also performed on sputum and matched serum using the methods described above (standard anti-CCP3 kit cutoff for serum ≥20 units).
Total Ig testing
Concentrations of total IgG, IgM, and IgA in sputum were determined using a Siemens BN II nephelometry cerebrospinal fluid (CSF) assessment system with polystyrene beads coated with antibodies to human IgG, IgM, and IgA, respectively, and according to the manufacturer’s specifications, with results reported in mg/dl. Of note, the CSF system was used to quantify relatively low levels of Ig compared to serum, with sensitivity for detection of Ig of up to 0.01 mg/dl. Concentrations of total IgG, IgM, and IgA in serum were determined using a Beckman-Coulter Synchron nephelometry system with anti-human IgG, IgM, and IgA antibodies, respectively, and according to the manufacturer’s specifications, with results reported in mg/dl.
Ratios comparing autoantibody levels to total Ig levels
For each sample, the ratio of each autoantibody to its corresponding total Ig (e.g., anti-CCP2 compared to total IgG) was calculated by using the autoantibody level (in arbitrary units) compared to the concentration of total Ig. Of note, for anti-CCP3.1, the autoantibody level was compared to the concentration of IgA and IgG because the anti-CCP3.1 assay detects both of these isotypes.
For sputum, these ratios were established with no correction for dilution because sputum autoantibodies and total Ig levels were determined on similarly diluted samples. To identify autoantibody levels in serum, all serum samples were diluted 1:100, although some samples required dilution up to 1:800 to identify the upper autoantibody value. As such, in the calculation of autoantibody-to–total Ig ratio in serum, autoantibody levels were multiplied by the dilution required to establish that level.
Anti–tetanus toxoid IgG testing
Concentrations of anti–tetanus toxoid IgG antibody were determined in sputum and serum using an ELISA (Cortez Diagnostics) that has a sensitivity of 0.0093 IU/ml, with protective levels of this antibody considered present at a level of 0.01 IU/ml (23). Testing was performed according to the manufacturer’s specifications, with the exception that the sputum samples were not further diluted after initial processing with PBS as described above.
Shared epitope (SE) testing
DNA from all subjects was analyzed for the presence of alleles containing the SE, using methods described elsewhere (19). The subtypes considered SE positive included DR4 alleles (DRB1*0401, *0404, *0405, *0408, *0409, *0410, *0413, *0416, *0419, and *0421) and DR1 alleles (DR1*0101, *0102, *0104, *0105, *0107, *0108, and *0111).
Statistical analysis
Subject characteristics were compared across groups using Kruskal-Wallis testing for continuous variables and one-way analysis of variance for dichotomous variables. Autoantibody levels were compared across groups using Kruskal-Wallis testing. The proportions of subjects with autoantibody positivity in sputum and serum were compared using nonparametric matched pair analyses. Ratios for autoantibody to total Ig levels in sputum and serum were compared using Wilcoxon matched pairs signed rank tests. All analyses were performed using SPSS software, version 20 (IBM) and GraphPad Prism software, version 6.
RESULTS
Subject demographics
Subjects’ characteristics are presented in Table 1. The healthy controls were younger than the other groups and fewer had ≥1 allele containing the SE; however, there were no significant differences in age, sex, smoking status, and history of lung disease across the at-risk subjects and subjects with early RA. Of note, aside from mild, self-limited postprocedural coughing in 20% of subjects, there were no complications from collection of induced sputum.
Table 1.
Characteristics of the study subjects
| Healthy controls (n =21)* |
At-risk seronegative subjects (n =23)† |
At-risk seropositive subjects (n =26)† |
Subjects with early RA (n =14)‡ |
P§ | |
|---|---|---|---|---|---|
| Age, median (range) years | 33 (26–58) | 58 (23–79) | 54 (28–70) | 50 (29–75) | <0.01 |
| Female, no. (%) | 17 (81) | 15 (65) | 16 (62) | 9 (64) | 0.73 |
| Ever smoker, no. (%) | 6 (29) | 8 (35) | 13 (50) | 5 (36) | 0.48 |
| Current smoker, no./total no. | 1/6 | 1/8 | 1/13 | 2/5 | – |
| History of lung disease, no. (%)¶ | 0 (0) | 6 (26) | 8 (31) | 6 (43) | 0.11 |
| Shared epitope (≥1 allele), no. (%) | 5 (24) | 15 (65) | 15 (58) | 9 (64) | 0.03 |
Without self-reported history of autoimmune disease or lung disease; seronegative for all rheumatoid arthritis (RA)–related autoantibodies tested.
Recruited from the Studies of the Etiology of Rheumatoid Arthritis project. Subjects were first-degree relatives of probands with RA or were seropositive for RA-related autoantibodies identified through health fair screening; all were without current inflammatory arthritis. Subjects were designated seronegative or seropositive based on serum testing for RA-related autoantibodies at the time of sputum collection.
RA of <12 months’ duration since diagnosis.
Comparison across groups using nonparametric testing.
Self-reported history of health care provider–diagnosed lung disease (asthma, emphysema, chronic bronchitis, or bronchiectasis).
Sputum Ig levels
There were no significant differences in total IgG, IgM, or IgA levels in sputum across groups (P > 0.05 for all comparisons), although for all groups, sputum IgA levels were significantly higher compared to levels of IgG and IgM (Figure 1A).
Sputum autoantibody levels
There was a trend toward higher median levels of all autoantibodies in sputum in at-risk seropositive subjects, at-risk seronegative subjects, and subjects with early RA when compared to healthy controls (Figure 1), with levels of anti-CCP2, anti-CCP3, anti-CCP3.1, IgM-RF, and IgG-RF being statistically significantly elevated across groups (Figures 1B–E and G).
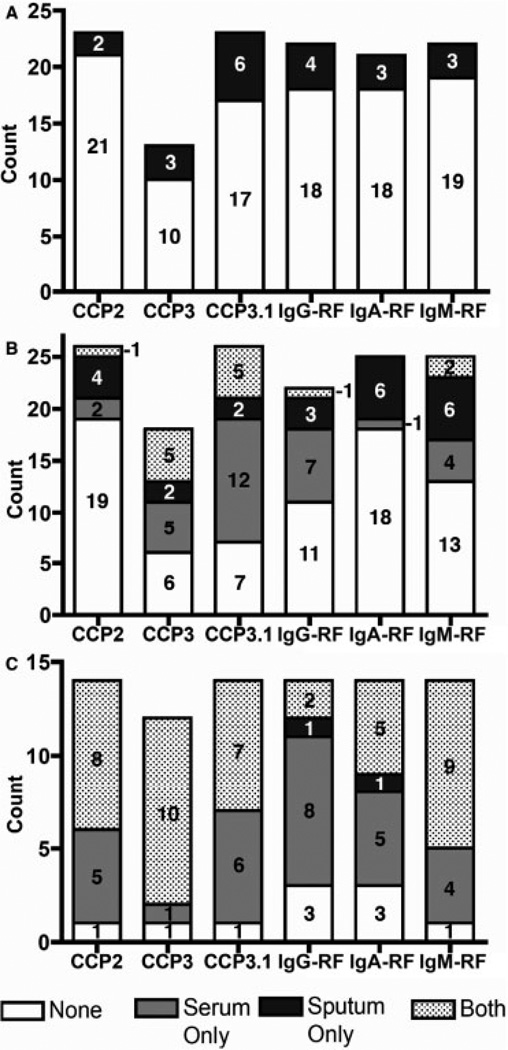
Sputum autoantibody positivity
For each autoantibody, the rates of positivity in serum and sputum are presented in Table 2 and Figure 2. Among the at-risk seronegative subjects, 9 of 23 (39%) were positive for at least 1 autoantibody in their sputum, although in paired analyses with sputum and serum, only the proportion of anti-CCP3.1 positivity in their sputum was statistically significantly elevated (6 of 23 [26%] in sputum versus 0 of 23 [0%] in serum; P = 0.03). Furthermore, in the at-risk seronegative subjects, the number of autoantibodies was significantly higher in sputum than in serum (median [range] 0 [0–5] versus 0 [0]; P < 0.01).
Table 2.
Comparison of serum and sputum autoantibody positivity*
| At-risk seronegative subjects | At-risk seropositive subjects | Subjects with early RA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum (n = 23) |
Sputum (n = variable)† |
P‡ | Serum (n = 26) |
Sputum (n = variable)† |
P‡ | Serum (n =14) |
Sputum (n = variable)† |
P‡ | |
| Anti-CCP2 | 0 (0) | 2/23 (9) | 0.50 | 3 (12) | 5/26 (19) | 0.69 | 13 (93) | 8/14 (57) | 0.06 |
| Anti-CCP3 | 0/13 (0) | 3/13 (23) | 0.25 | 10/18 (56) | 7/18 (39) | 0.45 | 13 (93) | 10/12 (83) | 1.00 |
| Anti-CCP3.1 | 0 (0) | 6/23 (26) | 0.03 | 17 (65) | 7/26 (27) | 0.01 | 13 (93) | 7/14 (50) | 0.03 |
| IgA-RF | 0 (0) | 3/21 (14) | 0.25 | 2 (8) | 6/25 (24) | 0.13 | 9 (64) | 6/14 (43) | 0.38 |
| IgG-RF | 0 (0) | 4/22 (18) | 0.13 | 11 (42) | 4/22 (18) | 0.34 | 10 (71) | 3/14 (21) | <0.01 |
| IgM-RF | 0 (0) | 3/22 (14) | 0.25 | 7 (27) | 8/25 (32) | 1.00 | 13 (93) | 9/14 (64) | 1.00 |
| ≥1 antibody present | 0 (0) | 9 (39) | – | 26 (100) | 17 (65) | – | 14 (100) | 12/14 (86) | – |
| No antibodies present | 23 (100) | 14 (61) | – | 0 (0) | 9 (35) | – | 0 (0) | 2 (14) | – |
| Antibodies present, median (range) | 0 (0) | 0 (0–5) | <0.01 | 2 (1–3) | 1 (0–6) | 0.13 | 6 (1–6) | 3.5 (0–6) | <0.01 |
Except where indicated otherwise, values are the number (%) positive or number/total number (%) positive of subjects tested. RA = rheumatoid arthritis; RF = rheumatoid factor.
All serum samples were tested for all autoantibodies except anti–cyclic citrullinated peptide 3 (anti-CCP3), which was only tested in subjects with matched anti-CCP3 sputum testing; however, the numbers of subjects with sputum testing for autoantibodies varied due to the availability of sufficient samples from testing. As such, the numbers of subjects tested for each autoantibody in sputum are included. Statistical analyses were performed only using those subjects who were tested for the particular autoantibodies.
For serum versus sputum positivity for autoantibodies.
Figure 2.
Positivity for autoantibodies in sputum and serum. Shown are counts of subjects testing positive for autoantibodies in serum, sputum, both, or neither in the following groups: at-risk seronegative subjects (i.e., seronegative for all anti-CCP2, anti-CCP3, anti-CCP3.1, IgG-RF, IgA-RF, and IgM-RF at the time of sputum evaluation) (A), at-risk seropositive subjects (i.e., seropositive for at least 1 RA-related autoantibody at the time of sputum evaluation) (B), and subjects with early RA (C). See Figure 1 for definitions.
Among the at-risk seropositive subjects, a higher proportion were positive for autoantibodies in their serum compared to sputum, although, when comparing each autoantibody in sputum versus serum within an individual, some individuals were positive for specific autoantibodies only in their sputum (Figure 2B). Additionally, there was no significant difference between the numbers of autoantibodies in sputum and serum in this group (median [range] 1 [0–6] in sputum versus 2 [1–3] in serum; P = 0.13).
Subjects with early RA had the highest prevalence of autoantibody positivity in serum and sputum. Additionally, the proportions of subjects with sputum positivity for anti-CCP2, anti-CCP3, and IgM-RF were significantly higher in subjects with early RA than in at-risk subjects (P < 0.05). Furthermore, a higher proportion of subjects with early RA were positive for autoantibodies in their serum than in their sputum; however, 2 subjects were positive for specific autoantibodies only in their sputum (Figure 2C). In these subjects with early RA, there was also a higher number of autoantibodies in the serum than in the sputum (median [range] 6 [1–6] versus 3.5 [0–6]; P < 0.01).
There was no significant association between age, sex, and smoking status with or without SE positivity and sputum autoantibody positivity or levels in at-risk subjects or subjects with early RA (data not shown).
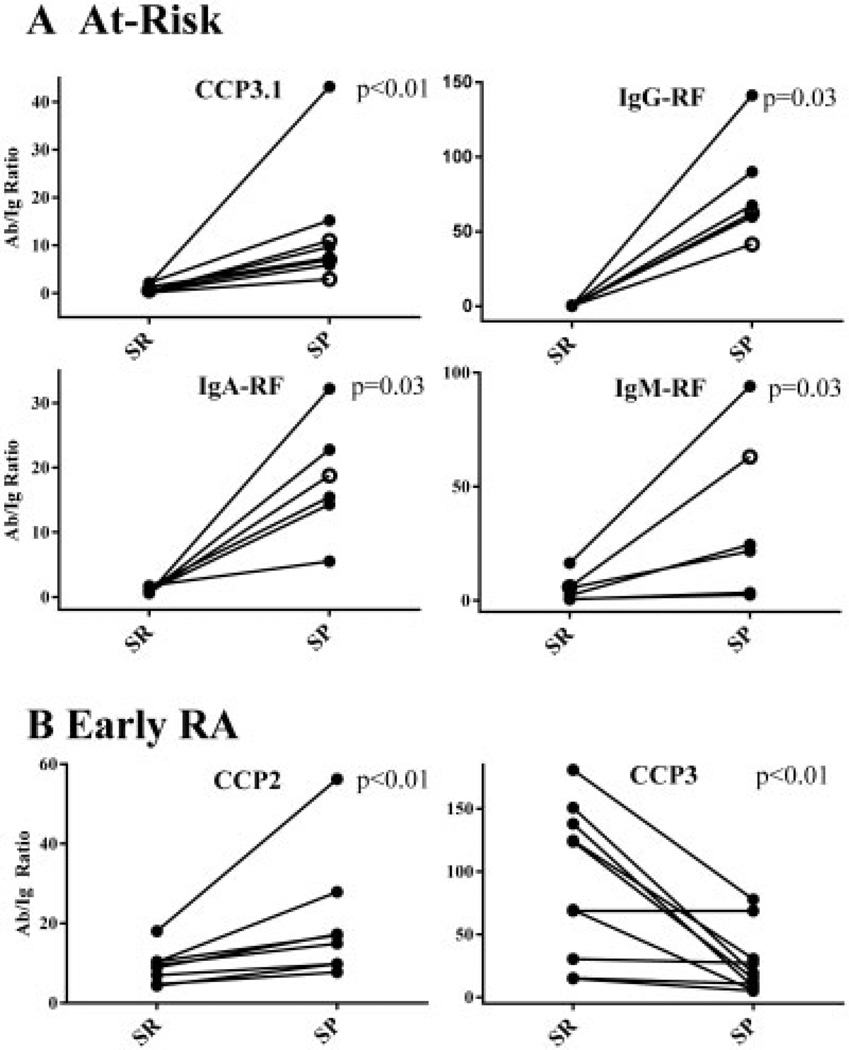
Ratio comparisons to evaluate lung generation of autoantibodies
Prior studies have compared the ratio of autoantibody to total Ig levels within a sample (e.g., synovial fluid or sputum) to the ratio of autoantibody to total Ig in the serum, with the conclusion being that if the ratio is higher in a given sample compared to that in the blood, the autoantibody is generated or sequestered in that sample (24–26). We therefore examined the ratio of autoantibody level to the concentration of its corresponding total Ig (e.g., ratio of anti-CCP2 level to total IgG) in the subset of 43 at-risk subjects and subjects with early RA for whom there was an adequate volume of sputum and serum for testing for all autoantibodies and for total IgG, IgM, and IgA (13 at-risk seronegative subjects, 18 at-risk seropositive subjects, and 12 subjects with early RA).
In at-risk seronegative and seropositive subjects who were positive for autoantibodies in sputum, the ratios of autoantibody to corresponding total Ig were higher in sputum than in serum for all autoantibodies, with the majority being statistically significantly higher in sputum: P = 0.06 for anti-CCP2, P = 0.13 for anti-CCP3, P < 0.01 for anti-CCP3.1, P = 0.03 for IgG-RF, P = 0.03 for IgA-RF, and P = 0.03 for IgM-RF (significant results are shown in Figure 3A). In subjects with early RA who were positive for autoantibodies in sputum, the ratios of autoantibody to corresponding total Ig were significantly higher in sputum than in serum for anti-CCP2 (P < 0.01) and significantly lower in sputum than in serum for anti-CCP3 (P < 0.01) (Figure 3B). The autoantibody-to–total Ig ratio was higher in serum than in sputum for the other autoantibodies, although these results were not statistically significant: P = 0.06 for anti-CCP3.1, P = 0.63 for IgA-RF, and P = 0.30 for IgM-RF (IgG-RF was not evaluated since only 3 subjects with early RA were positive for this autoantibody in sputum).
Figure 3.
Ratios of autoantibody (Ab) to total Ig levels in serum (SR) and sputum (SP). A, At-risk subjects (solid circles denote seropositive subjects; open circles denote seronegative subjects). In at-risk subjects, autoantibody-to–total Ig ratios were significantly higher in sputum than in serum for anti-CCP3.1, IgG-RF, IgA-RF, and IgM-RF. B, Subjects with early RA. In subjects with early RA, autoantibody-to–total Ig ratios were significantly higher in sputum than in serum for anti-CCP2, but higher in serum than in sputum for anti-CCP3. See Figure 1 for other definitions.
Anti–tetanus toxoid antibody in sputum compared to serum
An additional method to determine whether antibodies present in sputum are translocated from serum is to evaluate an antibody, such as anti–tetanus toxoid IgG, that is not normally present in mucosal samples (27). Therefore, in a subset of 29 at-risk subjects (13 seronegative; 16 seropositive) and 10 subjects with early RA for whom we had sufficient samples, we tested levels of anti–tetanus toxoid IgG antibody in matched sputum and serum samples.
All subjects had detectable anti–tetanus toxoid IgG antibody in their serum. However, only 6 of 39 subjects (15%) had detectable levels in their sputum (median [range] 4.0 units [0.5–7.0] in serum, 0.0 units [0–1.7] in sputum; P < 0.01). Of these 6 subjects, 3 were at-risk subjects who had sputum positivity for ≥1 RA-related autoantibody, and in these subjects the ratio of anti–tetanus toxoid IgG antibody to total IgG was lower in sputum than in serum. Conversely, in these 3 subjects the ratio of RA-related autoantibody to total Ig was higher in sputum than in serum (data not shown). The other 3 subjects with anti–tetanus toxoid IgG detectable in their sputum were negative for autoantibodies in their sputum, and none of the other at-risk subjects and subjects with early RA whose sputum was positive for RA-related autoantibodies had detectable anti–tetanus toxoid IgG antibody in their sputum.
Anti-CCP3 and anti-CCP3.1 comparisons
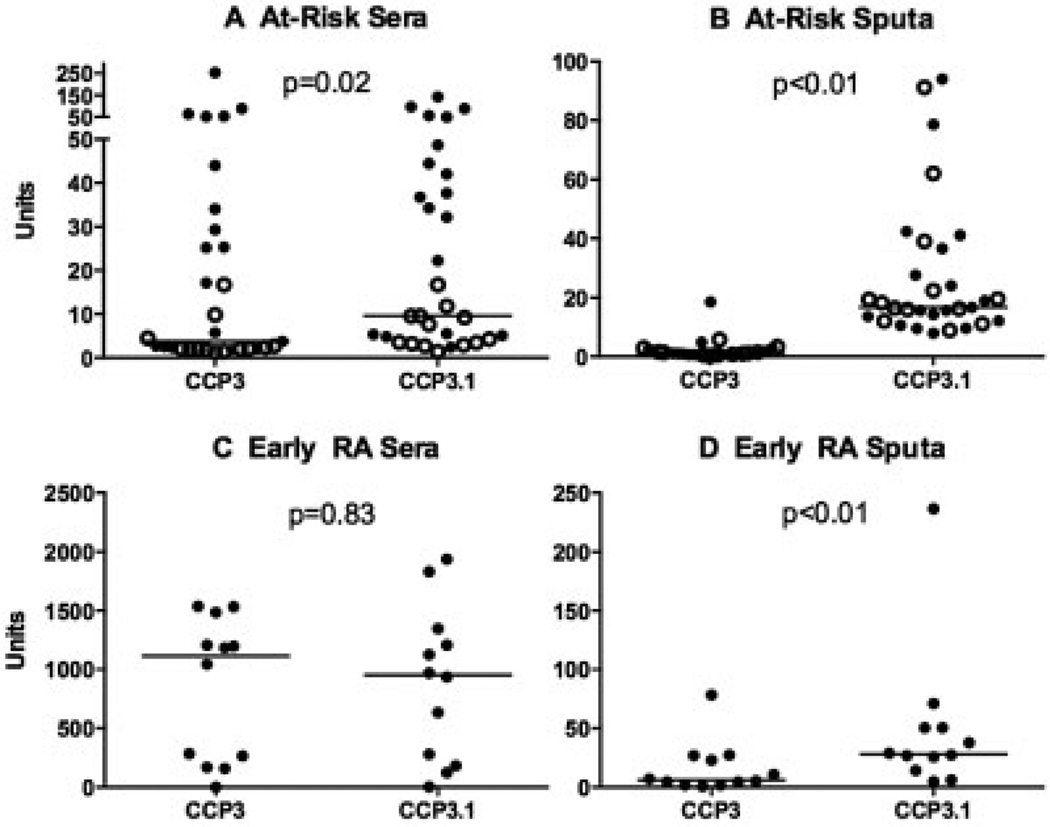
The anti-CCP3 and anti-CCP3.1 kits have similar antigen plates and cutoff levels for positivity in serum; however, the anti-CCP3 kit detects only IgG autoantibodies, while the anti-CCP3.1 kit detects both IgG and IgA autoantibodies. Therefore, in order to explore a potential role of IgA-related autoantibodies in autoimmune responses in the lung, we performed sputum and serum testing using the anti-CCP3 and anti-CCP3.1 kits in a subset of 59 subjects for whom we had sufficient samples.
In at-risk seronegative and seropositive subjects, both the serum and the sputum median levels of anti-CCP3.1 were statistically significantly higher than those of anti-CCP3 (Figures 4A and B). Furthermore, in subjects with early RA, the median sputum level of anti-CCP3.1 was significantly higher than that of anti-CCP3; however, in serum, the levels of these 2 autoantibodies were similar (Figures 4C and D).
Figure 4.
Levels of anti-CCP3 and anti-CCP3.1 autoantibodies. Shown are plots of anti-CCP3 and anti-CCP3.1 autoantibody levels in the serum (A and C) and sputum (B and D) of subjects at risk of RA (solid circles denote seropositive subjects; open circles denote seronegative subjects) (A and B) and subjects with early RA (C and D). Each data point represents a single subject; horizontal lines show the median level for each group. See Figure 1 for definitions
DISCUSSION
We demonstrate herein that elevations of RA-related autoantibodies are detectable in the sputum of subjects who have established RA, as well as in subjects who are at elevated risk of future RA. Additionally, the identification of autoantibody positivity in sputum but not in serum in a subset of subjects who are at risk of future RA, as well as higher ratios of certain autoantibodies in sputum than in serum in at-risk subjects, suggests that the lung may be a site of generation of RA-related autoantibodies.
Multiple studies have demonstrated that RA-related autoantibodies can be present in mucosal samples including saliva, tears, and gingival crevicular samples in established RA as well as other diseases, with several studies suggesting that these autoantibodies are generated at a mucosal site (28–32). Furthermore, in established RA and other diseases, bronchoalveolar lavage and sputum studies have identified elevated RF (33–35) and increases in immune complexes that appear to be generated within the lung (36).
There are several potential mechanisms for the generation of RA-related autoimmunity in the lung. In particular, within the airways, inducible bronchus-associated lymphatic tissue can develop in response to infections or other stimuli (37). Once present, inducible bronchus-associated lymphatic tissue produces immune factors, including antibodies (IgA, IgG, and IgM isotypes), that can be detected at the mucosal surface of the lung (37,38). Importantly, Rangel-Moreno and colleagues have linked inducible bronchus-associated lymphatic tissue to RA by demonstrating that many patients with RA-related lung disease have inducible bronchus-associated lymphatic tissue (37). Additionally, they demonstrated that plasma cells within RA-associated inducible bronchus-associated lymphatic tissue generate both RF and ACPAs. Therefore, it is possible that our findings of elevated RA-related autoantibodies in induced sputum, which preferentially samples the mucosal milieu of the bronchial tree, reflects underlying generation of RA-related autoimmunity within the airways.
We did not systematically evaluate all of the subjects for evidence of airway inflammation that may be associated with generation of autoantibodies. However, in prior work we have identified airway abnormalities consistent with inflammation by high-resolution computed tomographic lung imaging, both in subjects at risk of future RA but without clinically apparent inflammatory arthritis and in subjects with early RA (12). As such, it is possible that the subjects studied herein have such lung inflammation that is associated with the presence of RA-related autoantibodies in their sputum. This will need to be explored in future studies.
There are several caveats to the conclusion that RA-related autoantibodies that are detected in the sputum are being generated in the lung. It is possible that these autoantibodies are present in sputum due to translocation from the circulation. However, we believe this is unlikely in all cases for several reasons: first, several at-risk subjects and subjects with early RA had sputum autoantibody positivity in the absence of serum positivity; second, in at-risk subjects with sputum autoantibody positivity, the ratios of autoantibody to total Ig were higher in the sputum than in the serum; finally, anti–tetanus toxoid antibody was not elevated in the sputum in the majority of subjects with RA-related autoantibody positivity in sputum.
It is also possible that sputum concentrations across groups were unequal, perhaps as a result of collection or processing effects. This could lead to higher levels of autoantibodies in at-risk subjects and subjects with early RA compared to controls, and therefore false detection of positivity. However, the method of sputum collection and processing that we used was standardized across all groups, and should have yielded homogeneous sputum samples with equivalent concentrations throughout each sputum sample (16,17,22). In addition, the similarity in total Ig levels between groups (Figure 1) suggests that the concentration of sputum was similar across groups.
However, in contrast to our finding of elevated RA-related autoantibodies in sputum in at-risk seronegative subjects, we found that several at-risk seropositive subjects and the majority of subjects with early RA had greater positivity for RA-related autoantibodies in serum versus sputum (Table 2 and Figure 2), and several of these subjects also had higher ratios of autoantibodies to total Ig in serum compared to sputum (Figure 3). There are several potential explanations for these findings. It is unlikely that the lung is the site of generation of autoantibodies in all subjects during the natural history of RA. Other mucosal sites including the periodontal region and gut are also candidate sites for generation of autoantibodies (7–9,32,39). In addition, the joints are known to be a source of autoantibody generation in subjects with established RA, and that may be an important factor when assessing the lung generation of autoantibodies in subjects with early RA who have already developed synovitis (24,25). Furthermore, changes in autoantibody production within the lung may occur over time, resulting in greater positivity for autoantibodies in serum versus sputum. Such changes may include the development of autoantibody isotypes and subclasses that preferentially move to the circulation rather than to sputum, as well as the potential transition of autoantibody production from a mucosal source such as inducible bronchus-associated lymphatic tissue (that produces autoantibodies that can be readily detected in sputum) to production in regional lymph nodes leading to greater levels of systemic rather than mucosal autoantibodies (40).
Notably, we found that the differences in autoantibody positivity in sputum compared to serum changed across groups, with the proportions of subjects positive in sputum versus serum highest in at-risk seronegative subjects, intermediate in at-risk seropositive subjects, and lowest in subjects with early RA. The at-risk seropositive subjects had been seropositive for RA-related autoantibodies ≥1 year prior to their participation in this lung study, and we assume that the subjects with early RA similarly had a prolonged period of autoimmunity at the time of their sputum evaluation. As such, if these subjects had initial generation of autoimmunity in their lungs, then over time the types of changes in location of autoantibody generation described above may have led to greater autoantibody positivity in serum versus sputum.
Also of note, there were higher rates of positivity for anti-CCP3 and anti-CCP3.1 than for anti-CCP2 in the sputum (Table 2 and Figure 2). It is known that a variety of citrullinated proteins are present in the lung in patients with or without established RA (41), and therefore it may be that the differences seen between anti-CCP2 and anti-CCP3/anti-CCP3.1 reactivity are that the anti-CCP3/anti-CCP3.1 kits contain antigens more likely to be targeted by autoimmune reactions in the lung. We cannot evaluate reactivity to specific citrullinated proteins in these anti-CCP kits due to the proprietary nature of these assays. As such, autoantibody reactivity to specific citrullinated proteins will need to be explored using assays that can detect autoantibodies to specific citrullinated or other antigens. Additionally, the larger differences between levels of anti-CCP3 and anti- CCP3.1 in sputum compared to relatively small differences between these levels in serum (Figure 4) suggest that IgA responses are an important aspect of RA-related autoimmunity in the lung. Also, the higher levels and prevalence of anti-CCP3.1 positivity compared to the other anti-CCPs in the at-risk seronegative subjects (Table 2 and Figure 4) suggest that IgA autoimmunity, and perhaps natural IgA reactivity to citrullinated or other autoantigens, may be an important aspect of early RA autoimmunity (42,43). In the future, specific IgA isotypes for each of these anti-CCPs as well as specific citrullinated antigens, and larger control groups, need to be assessed to determine the importance of IgA reactivity in relation to RA-related autoimmunity in the lung.
Going forward, these findings need to be replicated in larger studies that should include longitudinal comparisons of simultaneously collected biospecimens from multiple sites (e.g., the eyes, oral cavity, lung, gut, genitourinary tract, blood, and joints) to understand the evolution of autoantibodies at different mucosal sites in the natural history of RA. Studies are also needed to evaluate the potential mechanisms of development of RA-related autoantibody responses in the lung, including isotype and subclass development and reactivity with specific antigens.
Many of these future investigations could use sputum samples, which are relatively inexpensive and safe to obtain. However, due to the paucity of T and B cells in sputum and the potential of sputum assessments to miss factors that may be present in underlying mucosal tissue, other methods such as bronchoscopy with lavage or lung biopsy may be necessary to explore fully the role of the lung in early RA pathogenesis. Future studies should also investigate the potential impact of production of autoantibodies in the lung on lung injury, such as interstitial lung disease, as well as genetic and environmental factors (such as the SE, tobacco smoke, and organisms [e.g., bacteria, viruses, and fungi]) that may drive the generation of autoimmunity in the lung. Informative animal models will also be important to understand how RA-related autoimmunity may be generated at mucosal surfaces.
Importantly, given our finding of RA-related autoantibodies in the sputum of at-risk seronegative subjects, it is likely that these subjects who are at risk of future RA but who have not yet developed circulating autoimmunity, or who have circulating autoimmunity in the absence of clinically apparent inflammatory arthritis, are ideal for investigating the earliest aspects of the initiation of RA. Such subjects could be identified through projects such as SERA or other natural history studies of RA development such as the Canadian North American Native project that has demonstrated elevations of circulating cytokines and chemokines in first-degree relatives of probands with RA in the absence of serum positivity for ACPAs or RF (44), and Dutch ACPA-positive subjects without inflammatory arthritis who have been followed up for the clinically apparent development of RA (45).
In conclusion, elevations of autoantibodies in sputum of subjects with established RA, and subjects at risk of developing disease, suggest that sputum testing may be a safe and informative method to investigate the lung’s role in the pathogenesis of RA, and especially as a site of initiation of RA-related autoimmunity.
Acknowledgments
Supported by the Rheumatology Research Foundation (Within Our Reach Program grant and Disease Targeted Research Initiative Program grant), the NIH (grants U01-AI-101981, R01-AR-051395, U19-AI-050864, and Colorado Clinical and Translational Sciences Institute grants UL1-TR-000154 and TL1-TR-000155), and the Walter S. and Lucienne Driskill Foundation.
Drs. Holers and Deane have submitted a patent application for the use of biomarkers to predict actionable outcomes in rheumatoid arthritis. Dr. Deane has received grants from Abbott Laboratories and AbbVie.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Deane had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Willis, Demoruelle, Derber, Weisman, Norris, Holers, Deane.
Acquisition of data. Willis, Demoruelle, Derber, Chartier-Logan, Parish, Pedraza, Weisman, Holers, Deane.
Analysis and interpretation of data. Willis, Demoruelle, Derber, Weisman, Holers, Deane.
REFERENCES
- 1.Del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum. 1988;31:1239–1244. doi: 10.1002/art.1780311004. [DOI] [PubMed] [Google Scholar]
- 2.Aho K, Palosuo T, Raunio V, Tuomi T. The timing of rheumatoid factor seroconversions [letter] Arthritis Rheum. 1987;30:719–720. doi: 10.1002/art.1780300623. [DOI] [PubMed] [Google Scholar]
- 3.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 4.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 5.Majka DS, Deane KD, Parrish LA, Lazar AA, Baron AE, Walker CW, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67:801–807. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokkonen H, Mullazehi M, Berglin E, Hallmans G, Wadell G, Ronnelid J, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther. 2011;13:R13. doi: 10.1186/ar3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, et al. Peptidylarginine deiminase from porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikuls TR, Thiele GM, Deane KD, Payne JB, O’Dell JR, Yu F, et al. Porphyromonas gingivalis and disease-related autoantibodies in individuals at increased risk of rheumatoid arthritis. Arthritis Rheum. 2012;64:3522–3530. doi: 10.1002/art.34595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64:3083–3094. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. for the Epidemiological Investigation of Rheumatoid Arthritis Study Group. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 11.Oliver JE, Silman AJ. Risk factors for the development of rheumatoid arthritis. Scand J Rheumatol. 2006;35:169–174. doi: 10.1080/03009740600718080. [DOI] [PubMed] [Google Scholar]
- 12.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Airways abnormalities and rheumatoid arthritis–related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64:1756–1761. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer A, Solomon JJ, du Bois RM, Deane KD, Olson AL, Fernandez-Perez ER, et al. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir Med. 2012;106:1040–1047. doi: 10.1016/j.rmed.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gizinski AM, Mascolo M, Loucks JL, Kervitsky A, Meehan RT, Brown KK, et al. Rheumatoid arthritis (RA)-specific autoantibodies in patients with interstitial lung disease and absence of clinically apparent articular RA. Clin Rheumatol. 2009;28:611–613. doi: 10.1007/s10067-009-1128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kips JC, Inman MD, Jayaram L, Bel EH, Parameswaran K, Pizzichini MM, et al. The use of induced sputum in clinical trials. Eur Respir J Suppl. 2002;37:47s–50s. doi: 10.1183/09031936.02.00004702. [DOI] [PubMed] [Google Scholar]
- 16.Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52:498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavord ID, Sterk PJ, Hargreave FE, Kips JC, Inman MD, Louis R, et al. Clinical applications of assessment of airway inflammation using induced sputum. Eur Respir J Suppl. 2002;37:40s–43s. doi: 10.1183/09031936.02.00004002. [DOI] [PubMed] [Google Scholar]
- 18.Baumann U, Gocke K, Gewecke B, Freihorst J, von Specht BU. Assessment of pulmonary antibodies with induced sputum and bronchoalveolar lavage induced by nasal vaccination against Pseudomonas aeruginosa: a clinical phase I/II study. Respir Res. 2007;8:57. doi: 10.1186/1465-9921-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolfenbach JR, Deane KD, Derber LA, O’Donnell C, Weisman MH, Buckner JH, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009;61:1735–1742. doi: 10.1002/art.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Deane KD, Striebich CC, Goldstein BL, Derber LA, Parish MC, Feser ML, et al. Identification of undiagnosed inflammatory arthritis in a community health fair screen. Arthritis Rheum. 2009;61:1642–1649. doi: 10.1002/art.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hector A, Jonas F, Kappler M, Feilcke M, Hartl D, Griese M. Novel method to process cystic fibrosis sputum for determination of oxidative state. Respiration. 2010;80:393–400. doi: 10.1159/000271607. [DOI] [PubMed] [Google Scholar]
- 23.Ramsay ME, Corbel MJ, Redhead K, Ashworth LA, Begg NT. Persistence of antibody after accelerated immunisation with diphtheria/ tetanus/pertussis vaccine. BMJ. 1991;302:1489–1491. doi: 10.1136/bmj.302.6791.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masson-Bessiere C, Sebbag M, Durieux JJ, Nogueira L, Vincent C, Girbal-Neuhauser E, et al. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by local plasma cells and constitute a higher proportion of IgG than in synovial fluid and serum. Clin Exp Immunol. 2000;119:544–552. doi: 10.1046/j.1365-2249.2000.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snir O, Widhe M, Hermansson M, von Spee C, Lindberg J, Hensen S, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010;62:44–52. doi: 10.1002/art.25036. [DOI] [PubMed] [Google Scholar]
- 26.Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6:e1. doi: 10.1371/journal.pmed.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvaggio JE, Waldman RH, Fruchtman MH, Wigley FM, Johnson JE., III Systemic and secretory antibody response of atopic and non-atopic individuals in intranasally administered tetanus toxoid. Clin Allergy. 1973;3:43–49. doi: 10.1111/j.1365-2222.1973.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 28.Svard A, Kastbom A, Sommarin Y, Skogh T. Salivary IgA antibodies to cyclic citrullinated peptides (CCP) in rheumatoid arthritis. Immunobiology. 2013;218:232–237. doi: 10.1016/j.imbio.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Dunne JV, Carson DA, Spiegelberg HL, Alspaugh MA, Vaughan JH. IgA rheumatoid factor in the sera and saliva of patients with rheumatoid arthritis and Sjögren’s syndrome. Ann Rheum Dis. 1979;38:161–165. doi: 10.1136/ard.38.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elkon KB, Gharavi AE, Patel BM, Hughes GR, Frankel A. IgA and IgM rheumatoid factors in serum, saliva and other secretions: relationship to immunoglobulin ratios in systemic sicca syndrome and rheumatoid arthritis. Clin Exp Immunol. 1983;52:75–84. [PMC free article] [PubMed] [Google Scholar]
- 31.Otten HG, Daha MR, van der Maarl MG, Hoogendoorn LI, Beem EM, de Rooy HH, et al. IgA rheumatoid factor in mucosal fluids and serum of patients with rheumatoid arthritis: immunological aspects and clinical significance. Clin Exp Immunol. 1992;90:256–259. doi: 10.1111/j.1365-2249.1992.tb07938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey GP, Fitzsimmons TR, Dhamarpatni AA, Marchant C, Haynes DR, Bartold PM. Expression of peptidylarginine deiminase-2 and -4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. J Periodontal Res. 2013;48:252–261. doi: 10.1111/jre.12002. [DOI] [PubMed] [Google Scholar]
- 33.Sakaida H. IgG rheumatoid factor in rheumatoid arthritis with interstitial lung disease. Ryumachi. 1995;35:671–677. In Japanese. [PubMed] [Google Scholar]
- 34.Martinez-Cordero E, Negrete-Garcia MC, Mendoza A. Rheumatoid factor activity in serum and bronchoalveolar lavage fluid from patients with acute hypersensitivity pneumonitis. J Investig Allergol Clin Immunol. 1992;2:254–257. [PubMed] [Google Scholar]
- 35.Schiotz PO, Egeskjold EM, Hoiby N, Permin H. Autoantibodies in serum and sputum from patients with cystic fibrosis. Acta Pathol Microbiol Scand C. 1979;87:319–324. [PubMed] [Google Scholar]
- 36.Jansen HM, Schutte AJ, Elema JD, Giessen MV, Reig RP, Leeuwen MA, et al. Local immune complexes and inflammatory response in patients with chronic interstitial pulmonary disorders associated with collagen vascular diseases. Clin Exp Immunol. 1984;56:311–320. [PMC free article] [PubMed] [Google Scholar]
- 37.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116:3183–3194. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foo SY, Phipps S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol. 2010;3:537–544. doi: 10.1038/mi.2010.52. [DOI] [PubMed] [Google Scholar]
- 39.Wegner N, Lundberg K, Kinloch A, Fisher B, Malmstrom V, Feldmann M, et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233:34–54. doi: 10.1111/j.0105-2896.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 40.Twigg HL., III Pulmonary host defenses. J Thorac Imaging. 1998;13:221–233. doi: 10.1097/00005382-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67:1488–1492. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 42.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandtzaeg P. Function of mucosa-associated lymphoid tissue in antibody formation [published erratum appears in Immunol Invest 2010;39:780] Immunol Invest. 2010;39:303–355. doi: 10.3109/08820131003680369. [DOI] [PubMed] [Google Scholar]
- 44.El-Gabalawy HS, Robinson DB, Smolik I, Hart D, Elias B, Wong K, et al. Familial clustering of the serum cytokine profile in the relatives of rheumatoid arthritis patients. Arthritis Rheum. 2012;64:1720–1729. doi: 10.1002/art.34449. [DOI] [PubMed] [Google Scholar]
- 45.Van de Stadt LA, Witte BI, Bos WH, van Schaardenburg D. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-202127. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]