Abstract
Proteoglycan core proteins are linked to four different classes of linear sugar chains referred to as glycosaminoglycans. Heparan sulfate constitutes one of these classes of glycosaminoglycans, and has been shown to be important in developmental processes as well as disease. We designed a low-density gene expression array to identify expression levels of heparan sulfate biosynthetic enzymes and proteoglycan core proteins in the aorta of late stage embryos (E18.5) and adult mice (12 weeks). Significant changes were found in mRNA expression of proteoglycan core proteins syndecan, glypican, decorin, perlecan, and versican from development to adulthood (n=8, p<0.05). Immunohistochemistry revealed a striking localization of both decorin and perlecan staining to the subendothelium in adult vessels, which differed from consistent staining of the endothelium, smooth muscle, and adventitia in development. Significant differences were also identified in the expression of the heparan sulfate modifying enzymes, glururonyl C5 epimerase, 2-O and 6-O sulfotransferases, and N-deacetylase/N sulfotransferases 1–3 (n =8, p<0.05). In conclusion, proteoglycan core proteins and heparan sulfate biosynthetic enzymes in the aorta undergo significant changes in their expression from development to adulthood. These findings may have important biological significance in the specific cell-defined roles of proteoglycan and heparan sulfate related targets in vascular development, maintenance, and response to various perturbations.
Keywords: Heparan sulfate, Proteoglycan, Gene expression, O-sulfotransferase, N-sulfotransferase
Introduction
Proteoglycan core proteins and the linear sugar chains (glycosaminoglycans) to which they are attached are important for embryonic development, maintenance of cellular function, and response to various stresses [1–3]. Four distinct families of sugar chains bind to proteoglycans: heparan sulfate, chrondroitin sulfate/dermatan sulfate, keratan sulfate, and hyaluronan. The importance of the specific sulfation pattern of the heparan sulfate chains, (regulated by heparan sulfate biosynthetic enzymes) in recruiting and binding growth factors, lipoproteins, and chemokines has just begun to be understood and received particular attention in the vasculature, liver, forebrain and lung [4, 5].
Heparan sulfates are co-receptors for various morphogens and growth factors including those essential for vascular development and maintenance including vascular endothelial growth factor, vascular endothelial growth factor [6], fibroblast growth factor (FGF) [7, 8], and transforming growth factor beta [9]. However, the relative expression pattern of the modification enzymes that regulate heparan sulfate modification and proteoglycan core proteins in the vessel have not been described. In this study, we identified significant differences in both heparan sulfate modifying enzymes as well as proteoglycan core proteins in the developing vs. the adult aorta.
Materials and Methods
Extraction of RNA
Aorta from wild type E18.5 embryos (n=8) and 12-week-old adult male mice (n=8) were harvested and snap frozen in liquid nitrogen. RNA was harvested from isolated vascular smooth muscle cells (VSMCs) and aorta by the TRizol method (Invitrogen) as previously described [10]. cDNA was synthesized by using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems).
Gene Expression
mRNA expression was assessed by designing a customized low density array using a TaqMan Array 384-well fluidic card platform. The gene array included heparan sulfate biosynthetic and degrading enzymes and proteoglycan core proteins (see Table 1 for Taqman Primer IDs). The experiment was replicated, samples were run in duplicate, and expression of each gene was normalized to the housekeeping gene hypoxanthine phosphoribosyl transferase1 (HPRT1). HPRT1 values were not significantly different between embryonic and adult vessels. Analysis was performed on an ABI7900 HT as previously described [10]. Gene expression was analyzed using the delta delta Ct method [11].
Table 1.
Gene abbreviation, Taqman primer ID on the low density array, normalized delta Ct values for RNA extracted from aortas of late stage embryo and adult C57BL6 mice and significance
| Gene | Primer ID | Expression Embryo (n=8) |
Adult (n=8) |
P value |
|---|---|---|---|---|
| Gpc1 | Mm00497305_m1 | 1.33±0.11 | 1.02±0.09 | 0.04 |
| Gpc3 | Mm00516722_m1 | 23.36±1.99 | 1.44±0.21 | 0.001 |
| Gpc4 | Mm00515035_m1 | 4.11 ±0.46 | 1.17±0.10 | 0.001 |
| Sdc1 | Mm00448918_m1 | 3.84±0.40 | 0.53±0.08 | 0.001 |
| Sdc2 | Mm00484718_m1 | 5.73±0.68 | 3.68±0.60 | 0.04 |
| Sdc3 | Mm01179831_m1 | 4.62±0.31 | 1.56±0.25 | 0.001 |
| Sdc4 | Mm00488527_m1 | 6.84±0.77 | 10.91 ±2.21 | ns |
| Hspg2 | Mm01181165_m1 | 24.94±2.39 | 17.28±1.97 | 0.03 |
| Dcn | Mm00514535_m1 | 30.34±2.70 | 17.34±3.71 | 0.01 |
| Acan | Mm00545807_m1 | 3.88±0.58 | 0.28±0.10 | 0.001 |
| Vcan | Mm01283063_m1 | 6.93±0.65 | 0.89±0.16 | 0.001 |
| Bcan | Mm00476090_m1 | ND | ND | ND |
| Lum | Mm00500510_m1 | ND | ND | ND |
| Ext1 | Mm00468769_m1 | 2.19±0.18 | 2.68±0.39 | ns |
| Ext2 | Mm00468775_m1 | 0.71 ±0.07 | 0.61±0.04 | ns |
| Ndst1 | Mm00447005_m1 | 2.18±0.11 | 1.77±0.16 | 0.05 |
| Ndst2 | Mm00447818_m1 | 0.99±0.08 | 0.52±0.02 | 0.001 |
| Ndst3 | Mm00453178_m1 | 0.78±0.14 | 0.12±0.04 | 0.001 |
| Ndst4 | Mm00480767_m1 | ND | ND | ND |
| GLCE | Mm00473667_m1 | 0.73±0.06 | 0.46±0.04 | 0.002 |
| HS2ST | Mm00478684_m1 | 1.46±0.13 | 0.47±0.04 | 0.001 |
| HS3ST1 | Mm01964038_s1 | 0.20±0.01 | 0.18±0.05 | ns |
| HS3ST2 | Mm00616933_m1 | ND | ND | ND |
| HS3ST3A1 | Mm00780907_s1 | 0.03±0.01 | 0.03±0.01 | ns |
| HS3ST3B1 | Mm00479621_m1 | 0.08±0.01 | 0.11 ±0.03 | ns |
| HS3ST6 | Mm01299930_m1 | ND | ND | ND |
| HS6ST1 | Mm01229698_s1 | 0.94±0.11 | 0.71±0.02 | ns |
| HS6ST2 | Mm00479296_m1 | 0.16±0.02 | 0.02±0.01 | 0.001 |
| HS6ST3 | Mm00479297_m1 | ND | ND | ND |
| Sulf1 | Mm00552283_m1 | 4.58±0.43 | 4.00±1.07 | ns |
| Sulf2 | Mm00511193_m1 | ND | ND | ND |
| HPSE | Mm00461768_m1 | 0.22±0.03 | 0.22±0.04 | ns |
Acan aggrecanl; Dcn decorin; Gpc1 glypican1; Gpc3 glypican3; Gpc4 glypican 4; Hspg2 heparan sulfate proteoglycan 2 (perlecan); Sdc1 syndecan 1; Sdc2 syndecan 2; Sdc3 syndecan 3; Sdc4 syndecan 4; Vcan versican; Lum lumican; Bcan brevican; Ext1 exostoses 1; Ext2 exostoses 2; H2ST1 heparan sulfate 2-O-sulfotransferase 1; HS3ST1 heparan sulfate 3-O-sulfotransferase; HS3ST3A1 heparan sulfate 3-O-sulfotransferase 3A1; HS3ST3B1 heparan sulfate, 3-O-sulfotransferase 3B1; HS3ST6 heparan sulfate 3-O-sulfotransferase 6; HS6ST1 heparan sulfate 6-O-sulfotransferase 1; HS6ST2 heparan sulfate 6-O-sulfotransferase-2; HS6ST3 heparan sulfate 6-O-sulfotransferase 3; GLCE glucuronyl C5-epimerase; Ndst1 N-deacetylase/N-sulfotransferase 1; Ndst2 N-deacetylase/N-sulfotransferase 2; Ndst3 N-deacetylase/N-sulfotransferase 3; Ndst4 N-deacetylase/N-sulfotransferase 4; Sulf1 sulfatase 1; Sulf2 sulfatase 2; HPSE heparanase; ND not determined; ns not significant
Immunohistochemistry
Immunolocalization of perlecan (Hspg2) and decorin (Dcn) was performed by utilizing the vectastain IgG kits according to the manufacturer’s instructions (Vector Laboratories). Five micrometer paraffin-embedded sections were stained with anti-perlecan antibody (Santa Cruz Biotechnology) or anti-decorin antibody (R&D Systems). Sections were observed and imaged under ×100 magnification using a Zeiss upright microscope.
Statistical Analysis
Data is represented as mean±SE. ANOVA was performed to analyze the statistical significance in gene expression between embryonic and adult aorta and p<0.05 was considered significant.
Results
Proteoglycan Core Proteins
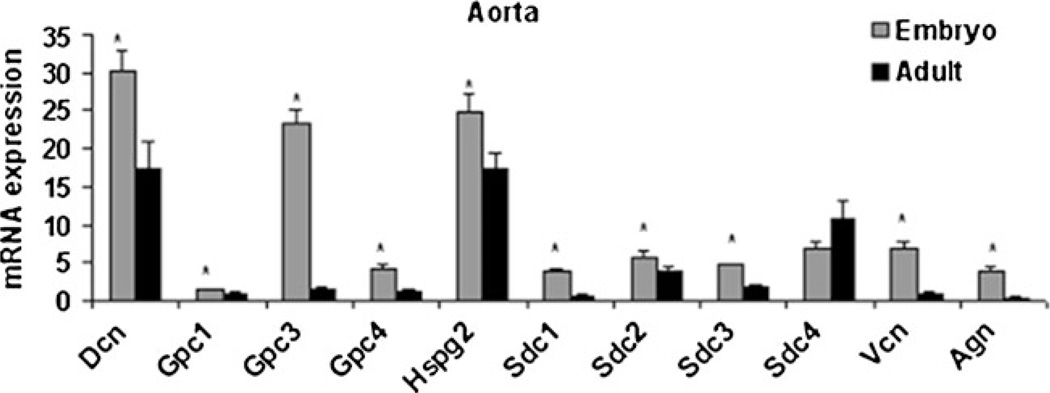
Glypican (Gpc) and syndecan (Sdc) are the two major classes of proteoglycans that bind heparan sulfate. Gpc1, 3, and 4 mRNA expression were significantly higher in embryonic aorta compared to adult aorta (Fig. 1, Table 1). The transcript abundance of Gpc3 was the highest among all its isoforms tested. Sdc1, 2, and 3 mRNA expression was also significantly higher in the embryonic aorta compared to the adult aorta (Fig. 1, Table 1). Sdc4 expression did not change (Fig. 1, Table 1).
Fig. 1.
mRNA expression of proteoglycan core proteins in aorta from late-stage embryos and adult male mice (n=8/ group, p<0.05)
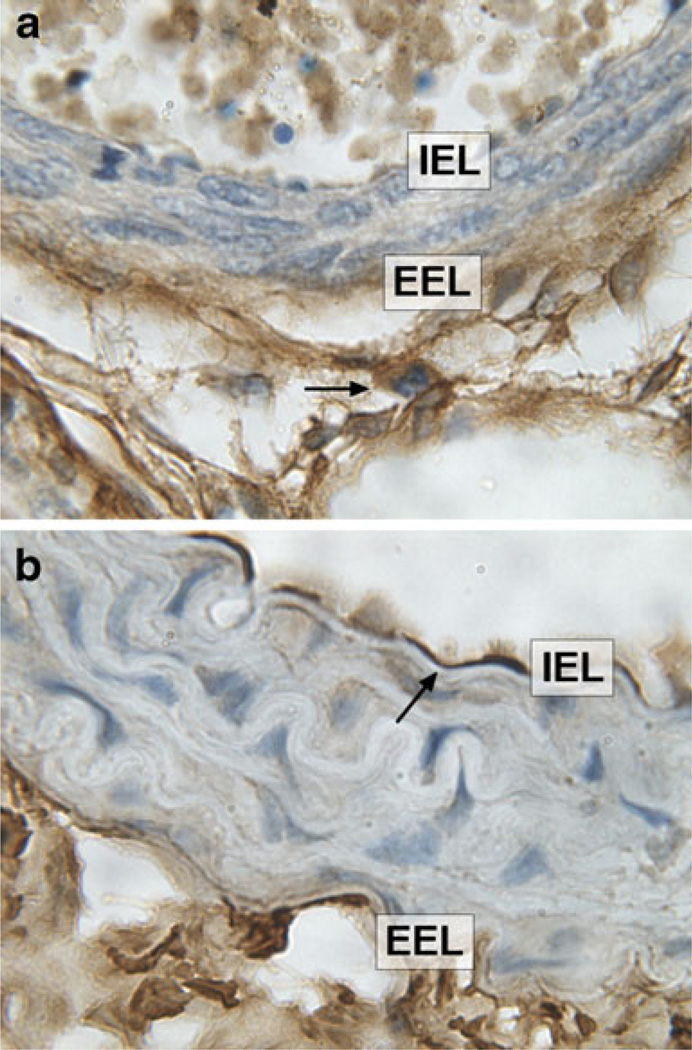
Chondroitin sulfate proteoglycans analyzed in this study include Dcn, lumican (Lum), versican (Vcan), aggrecan (Acan), and brevican (Bcan). Dcn is a proteoglycan that bears a single chondroitin/dermatan sulfate chain. Dcn was highly expressed in aorta at E18.5, which decreased significantly in adult mice (Fig. 1). Immunostaining at E18.5 revealed the Dcn staining pattern was strong in the medial and adventitial regions of the vessel as shown in Fig. 2a. In contrast, immunostaining revealed localized expression of Dcn in the subendothelial region of the adult aorta (Fig. 2b). Lum binds to keratan sulfate, and was not detected at any of the time points (Table 1). Both Acan and Vcan expression were significantly higher in the embryonic aorta as compared to adult aorta (Fig. 1, Table 1). Bcan, a proteoglycan mostly found in the CNS was not detected in any cohort (Table 1).
Fig. 2.
a Representative photomicrographs of E18.5 vessels showing Dcn staining in the medial and adventitial region (arrowhead) in aorta. b In contrast, Dcn staining was found predominantly in the subendothelial region (arrowhead) in adult aorta. Magnification ×100. IEL internal elastic lamina, EEL external elastic lamina
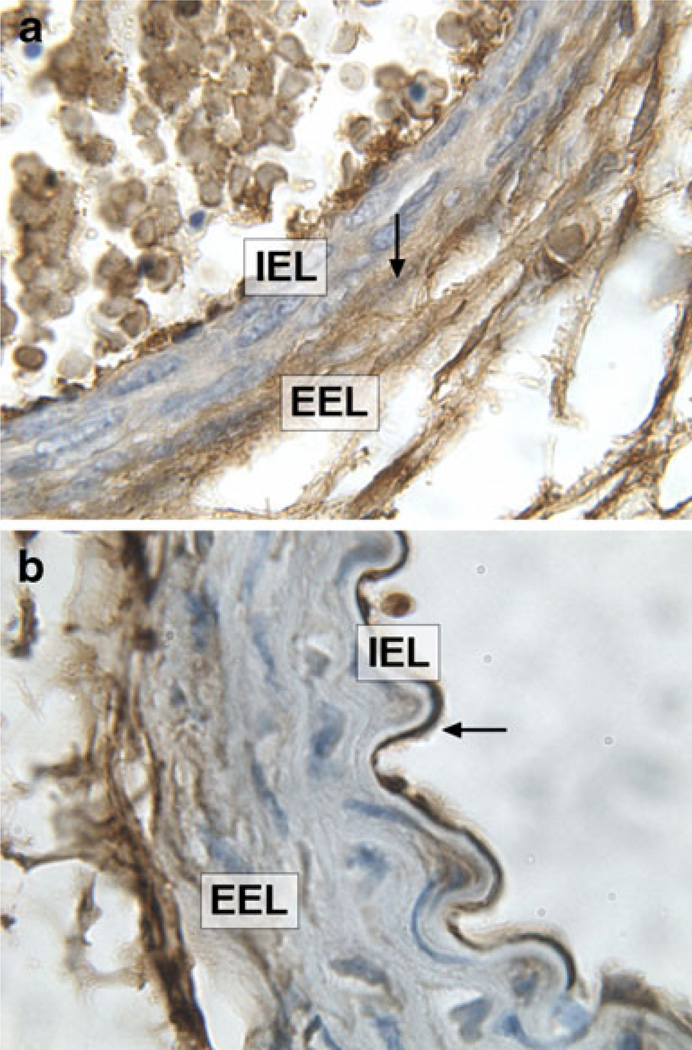
Hspg2, a major proteoglycan of the arterial basement membrane that also binds heparan sulfate, was significantly higher in embryonic aorta compared to adult aorta (Fig. 1, Table 1). Immunostaining of vessels at E18.5 for Hspg2 showed a strong immunostaining throughout the medial wall (Fig. 3a). In comparison, staining the adult vessel showed strongest Hspg2 expression localized to the subendothelial region in adult aorta (Fig. 3b).
Fig. 3.
a Representative photomicrographs showing Hspg2 staining in the medial (arrowhead) and adventitial region in aorta from E18.5 embryos b and predominantly in the subendothelial region (arrowhead) in aorta from adult male mice. Magnification ×100. IEL internal elastic lamina, EEL external elastic lamina
Heparan Sulfate Biosynthetic Enzymes
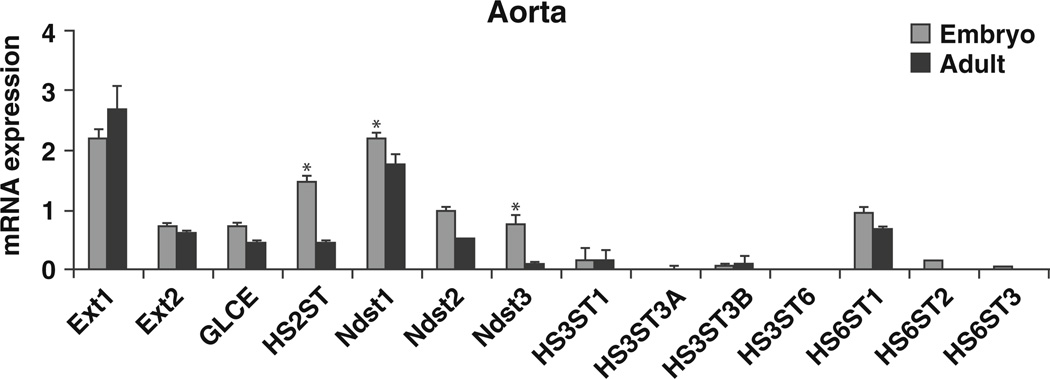
Heparan sulfate biosynthesis occurs in the Golgi complex and involves the participation of several enzymes. Exostosin 1 (Ext1) and exostosin 2 (Ext2) are the co-polymerases that catalyze the elongation of the disaccharides. Ext1 was highly expressed in both the embryonic and adult stages compared to Ext2. No differences in expression of Ext1 and 2 were seen between embryonic and adult aorta (Fig. 4, Table 1).
Fig. 4.
mRNA expression of heparan sulfate biosynthetic enzymes in aorta from embryos and adult male mice (n=8/ group, p<0.05)
N-sulfation is the initial step carried out by a family of N-deacetylase/N-sulfotransferases (Ndst1–4). In this study, Ndst1 was the predominant form expressed with a significant decrease in expression from embryonic to adult stage. The expression of Ndst2 and 3 also decreased significantly from embryonic to adult (Fig. 4, Table 1). Ndst4 transcript was undetected (Table 1).
Glucuronyl C5-epimerase (GLCE) catalyzes the epimerization of glucuronic acid to idouronic acid [12]. Expression of GLCE was significantly decreased in adult aorta compared to embryonic vessels (Fig. 4, Table 1). Heparan sulfate 2-O-sulfotransferase (HS2ST), a 2-O sulfotransferase, adds sulfate ion to iduronic acids following C5 epimerization of glucuronic acid to idouronic acid [12] and its transcript decreased significantly in adult aorta as compared to the developing aorta (Fig. 4, Table 1).
Out of the six isoforms of 3-O sulfotransferses, HS3ST1, was the most abundant transcript in the aorta. In contrast, HS3ST2 was undetected in aorta. All the other isoforms were expressed at low levels (Table 1). All three isoforms of heparan sulfate 6-O sulfotransferase (HS6ST) are expressed during embryogenesis with HS6ST1 showing the highest expression. HS6ST3 was undetected (Fig. 4, Table 1).
Ligands to heparan sulfate interactions are also affected by sulfatases and heparanases. There are two sulfatases, sulf1 and 2, that specifically remove sulfate ion present at 6-O position [13]. Transcript abundance of Sulf1 did not change from embryonic to adulthood (Table 1). Sulf2 was undetected. Heparanase (HPSE) is a major mammalian enzyme that degrades heparan sulfate chains [14]. It plays key roles in angiogenesis and cancer metastasis [15]. In the present study, HPSE expression did not differ between embryonic and adult aorta (Table 1).
Discussion
The major findings of this study were that the gene expression and distribution of proteoglycan core proteins changes significantly in the aorta from development to adulthood. With the exception of Sdc4, the transcript abundance of Gpc1–4, Sdc1–3, Acan, Dcn, Hspg2, and Vcan decreased significantly in the adult aorta compared to the late-stage embryo. Immunostaining showed that Dcn and Hspg2 expression in the adult vessels exhibited a gradient-like expression pattern with the strongest expression in the subendothelial space, just beneath the endothelial lining. In contrast, the developing vessels showed ubiquitous expression throughout the medial wall. We focused on these proteins given the high level of expression in the adult as well as the embryo. Wight et al. [16] showed that vascular smooth muscle cells secrete Dcn, which alters the properties of a fibrin matrix. The extent to which this may influence embryonic vascular elasticity vs. adult elasticity is not known. Hspg2 has been shown to inhibit VSMC proliferation [17]. The staining patterns reveal these proteins in a new light and highlight potential differences in a role for each of these core proteins in the vessel during development vs. adulthood.
In the current study, we show that Gpc3 is the most abundantly expressed of the Gpc family. Gpc3 mRNA expression is significantly higher in aortas during the embryonic stage compared to adulthood. Data from isolated murine VSMC from embryonic and adult aorta showed similar findings (not shown). In humans, loss of function mutations in Gpc3 causes an X-linked disorder known as Simpson–Golabi–Behmel syndrome, a rare congenital disease with severe abnormalities including congenital heart defects Gpc3 knockout mice exhibit excessive coronary arteries in comparison to the number of veins leading to coronary fistulas [18]. More work will be needed to define the cell-specific staining and functional role of Gpc3 in the vasculature.
We also identified a significant decrease in mRNA expression of the transmembrane proteoglycan family members Sdc1, 2, and 3 from embryo to adulthood. This suggests that Sdc1, 2, and 3 may play an important role in vascular development. Sdc1 null mice have been reported to be viable and fertile as well as exhibit normal hair, skin, and ocular surface epithelia [19]. The vasculature in the developing Sdc1 knockout mouse has not been assessed. Sdc1 knockout mouse do exhibit increased intimal lesion and increased VSMC proliferation in response to vascular injury [20]. Sdc1 knockout mouse also exhibit increased endothelial–leukocyte interaction and corneal angiogenesis [21]. Recent work has shown that Sdc1 is critical for migration of stem cells and that this may occur through its interaction with integrin assembly and function [22]. Ablation of Sdc4 leads to degeneration of fetal vessels in the placenta [23]. We did not detect significant differences in expression of Sdc4 in the embryo and adult aortas. However, there was a trend towards an increase in Sdc4 expression in adult aorta. The vascular structure and vascular reactivity of large and small vessels from Sdc4−/− mouse have not been elucidated. Taken together, these functions suggest that the family of Sdcs are important for vascular development and may play a role in VSMC, endothelial and leukocytes. This work provides new evidence showing expression of Sdc1, 2, and 3 is stronger in embryonic vessels than adult. How this translates into biological function and significance is not yet known.
Hspg2 is the major component of the arterial basement membrane and we found that it is significantly expressed in embryonic aorta. In addition, Hspg2 protein was predominantly localized to the subendothelial region of adult aorta, as previously reported [24, 25]. This is significant given the fact that subendothelial proteoglycans including Hspg2 play a major role in the retention of atherogenic lipoproteins [4, 26] and Hspg2 expression has been shown to be upregulated in response to vascular injury [27]. This contrasts from Hspg2 expression in the embryo, which is strong in the medial layer as well as the adventitial layer. These findings suggest that different circulating or local factors may regulate Hspg2 expression in both the embryo and adult. Hspg2 deficiency in mice leads to impaired corneal angiogenesis, delayed wound healing and retarded tumor growth [25]. Tran-Lundmark et al. [26] showed that loss of heparan sulfate chains specifically binding to perlecan in a genetic mouse model resulted in increased atherosclerosis. Thus perlecan and the heparan sulfate chains to which it binds are important in vascular development and disease.
We identified a significant decrease in the chondroitin sulfate family members including Acan and Vcan in adult aorta. Vcan is considered the major chondroitin sulfate of the vessel wall. Vcan is involved in the development and progression of atherosclerosis and restenosis [28] as well as in early cardiogenesis [29, 30]. The role of Vcan and Acan in vessel development has not been explored.
Heparin is perhaps best known in cardiovascular medicine for its role as an anticoagulant in which it binds antithrombin [31]. However, the anticoagulant properties of heparin can be chemically manipulated to either enhance or remove the anticoagulant capacity and uncouple the anticoagulant properties from the anti-inflammatory action of HSPGs [31]. The majority of our understanding of the role of heparan sulfate in vascular injury has come from work demonstrating that heparin inhibits vascular smooth muscle cell proliferation and neointimal formation in injured arteries and that this effect was independent of its anticoagulant capabilities [32–38]. Heparin administration in these studies resulted in a significant loss of bFGF content in the denuded vessel and an increase in the half-life of bFGF in the plasma [35]. Heparin also significantly inhibited proliferation of medial VSMC after denudation [35]. A new generation of heparan sulfate mimetic, PI-88 also inhibited intimal thickening after balloon injury [39]. PI-88 (phosphomannopentaose sulfate) is a 2-kD synthetic polysulfated oligosaccharide that inhibits the activity of the extracellular matrix-degrading enzyme heparanase [39]. In recent years, attention has been focused on the role of heparan sulfate modification in vascular remodeling. Our lab has demonstrated that deletion of the modifying enzyme, Ndst1, in the vessel wall leads to smaller vessels in development and in adulthood and reduced intimal hyperplasia in response to injury [10]. Taken together, these findings suggest that proteoglycan core proteins, heparan sulfate, and chondroitin sulfate play a role in vascular development, as well as remodeling.
In the present study, significant decreases were seen in the expression of Ndst1, 2 and 3 from embryo to adulthood. Of these isoforms, Ndst1 is the most abundant in the embryo and adulthood. Previous work in our lab has identified that knockout of Ndst1 using a smooth muscle specific promoter resulted in smaller vessels as well as defects in vascular remodeling. We documented that a loss of Ndst1 resulted in specific changes in the sulfation pattern of the glycosaminoglycans as measured by a well-characterized and sensitive HPLC technique [10, 40]. In addition, loss of Ndst1 in endothelial cells has been reported to decrease neutrophil trafficking [41]. Finally, MacArthur et al. [5] demonstrated that Ndst1 expression in hepatocytes was critical for the clearance of cholesterol and triglyceride-rich particles.
In this study, we identified a significant decrease in the heparan sulfate-modifying enzyme HS2ST in the adult aorta compared to E18.5. Similar findings were seen in the heparan sulfate-modifying enzyme, GLCE. Mice deficient in HS2ST phenocopy GLCE deficiency and die neonatally due to renal agenesis [42, 43]. Heart and vasculature development however, were reported as unperturbed. HS2ST is also critical for limb bud development [44] and triglyceride clearance [45]. A closer look at the role of HS2ST in vascular smooth muscle cell phenotypes and the role of HS2ST in vascular contractility may be warranted.
Although this study compared over 30 transcripts of major proteoglycan core proteins and modifying enzymes with multiple replicates, a limitation was that this we did not probe all proteoglycans and enzymes. Specifically, neuropillin-1 and betaglycan were not included. The role of these proteoglycans in vascular development has been studied. Neuropilin-1-deficient mice have vascular defects at E8.5 that become progressively more severe [46, 47]. Betaglycan-null mice lack coronary vessels [48, 49]. In the present study, with the exception of Sdc4, the transcript abundance of Gpc1–4, Sdc1–3, Acan, Dcn, Hspg2, and Vcan decreased significantly in the adult aorta compared to the late-stage embryo. This may be related to the composition of the cell types in the aorta. The staining patterns of both Dcn and Hspg2 suggest that the increased expression in the aorta is not due to a robust change in expression in the smooth muscle cells or the single layer of endothelium but rather from the cell types that make up the adventitia and/or any cells that were present in the lumen. Further studies are needed to fully understand the biological relevance of these changes in cardiovascular development and disease.
Acknowledgments
This work was funded by an R01 to JLH (NIH-R01HL081715).
Abbreviations
- Acan
aggrecan
- Dcn
decorin
- Gpc1
glypican 1
- Gpc3
glypican 3
- Gpc4
glypican 4
- Hspg2
heparan sulfate proteglycan 2 (perlecan)
- Sdc1
syndecan 1
- Sdc2
syndecan 2
- Sdc3
syndecan 3
- Sdc4
syndecan 4
- Vcan
versican
- Bcan
Brevican
- Lum
Lumican
- Ext1
exostosin 1
- Ext2
exostosin 2
- HS2ST
heparan sulfate 2-O-sulfotransferase
- HS3ST1
heparan sulfate 3-O-sulfotransferase 1
- HS3ST2
heparan sulfate 3-O-sulfotransferase 2
- HS3ST3A1
heparan sulfate 3-O-sulfotransferase 3A1
- HS3ST3B1
heparan sulfate 3-O-sulfotransferase 3B1
- HS3ST6
heparan sulfate 3-O-sulfotransferase 6
- HS6ST1
heparan sulfate 6-O-sulfotransferase 1
- HS6ST2
heparan sulfate 6-O-sulfotransferase-2
- HS6ST3
heparan sulfate 6-O-sulfotransferase 3
- GLCE
glucuronyl C5-epimerase
- Ndst1
N-deacetylase/N-sulfotransferase 1
- Ndst2
N-deacetylase/N-sulfotransferase 2
- Ndst3
N-deacetylase/N-sulfotransferase 3
- Ndst4
N-deacetylase/N-sulfotransferase 4
- Sulf1
sulfatase 1
- Sulf2
sulfatase 2
- HPSE
heparanase
- VSMC
vascular smooth muscle cell
- FGF
fibroblast growth factor
- HPRT1
hypoxanthine phosphoribosyl transferase1
Contributor Information
Neeta Adhikari, Lillehei Heart Institute, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
Marjorie Carlson, Lillehei Heart Institute, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
Ben Lerman, Lillehei Heart Institute, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
Jennifer L. Hall, Lillehei Heart Institute, Department of Medicine, University of Minnesota, Minneapolis, MN, USA Developmental Biology Center, University of Minnesota, Minneapolis, MN, USA; University of Minnesota, 312 Church Street, 4-106 NHH, 55455 Minneapolis, MN, USA jlhall@umn.edu.
References
- 1.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. The FASEB Journal. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 2.Forsberg E, Kjellen L. Heparan sulfate: lessons from knockout mice. Journal of Clinical Investigation. 2001;108:175–180. doi: 10.1172/JCI13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grobe K, Ledin J, Ringvall M, Holmborn K, Forsberg E, Esko JD, et al. Heparan sulfate and development: differential roles of the n-acetylglucosamine n-deacetylase/ n-sulfotransferase isozymes. Biochimica et Biophysica Acta. 2002;1573:209–215. doi: 10.1016/s0304-4165(02)00386-0. [DOI] [PubMed] [Google Scholar]
- 4.Adhikari N, Basi D, Rusch M, Mullegama S, Larson JD, Youseff J, et al. Genetic loss of n-sulfation of heparan sulfate alters post golgi trafficking of mcp-1 and remodeling of the vessel wall in response to injury. Circulation Supplement II. 2006;114:II_216. [Google Scholar]
- 5.MacArthur JM, Bishop JR, Stanford KI, Wang L, Bensadoun A, Witztum JL, et al. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of ldl receptor family members. Journal of Clinical Investigation. 2007;117:153–164. doi: 10.1172/JCI29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 7.Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends in Molecular Medicine. 2002;8:483–489. doi: 10.1016/s1471-4914(02)02394-8. [DOI] [PubMed] [Google Scholar]
- 8.Auguste P, Javerzat S, Bikfalvi A. Regulation of vascular development by fibroblast growth factors. Cell and Tissue Research. 2003;314:157–166. doi: 10.1007/s00441-003-0750-0. [DOI] [PubMed] [Google Scholar]
- 9.ten Dijke P, Arthur HM. Extracellular control of tgfbeta signalling in vascular development and disease. Nature Reviews. Molecular Cell Biology. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 10.Adhikari N, Basi DL, Townsend D, Rusch M, Mariash A, Mullegama S, et al. Heparan sulfate ndst1 regulates vascular smooth muscle cell proliferation, vessel size and vascular remodeling. Journal of Molecular and Cellular Cardiology. 2010;49:287–293. doi: 10.1016/j.yjmcc.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fermin DR, Barac A, Lee S, Polster SP, Hannenhalli S, Bergemann TL, et al. Sex and age dimorphism of myocardial gene expression in nonischemic human heart failure. Circulation: Cardiovascular Genetics. 2008;1:117–125. doi: 10.1161/CIRCGENETICS.108.802652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annual Review of Biochemistry. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 13.Holst CR, Bou-Reslan H, Gore BB, Wong K, Grant D, Chalasani S, et al. Secreted sulfatases sulf1 and sulf2 have overlapping yet essential roles in mouse neonatal survival. PLoS ONE. 2007;2:e575. doi: 10.1371/journal.pone.0000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bame KJ. Heparanases: endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology. 2001;11:91R–98R. doi: 10.1093/glycob/11.6.91r. [DOI] [PubMed] [Google Scholar]
- 15.Nasser N. Heparanase involvement in physiology and disease. Cellular and Molecular Life Sciences. 2008;65:1706–1715. doi: 10.1007/s00018-008-7584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson PY, Potter-Perigo S, Gooden MD, Vernon RB, Wight TN. Decorin synthesized by arterial smooth muscle cells is retained in fibrin gels and modulates fibrin contraction. Journal of Cellular Biochemistry. 2007;101:281–294. doi: 10.1002/jcb.21182. [DOI] [PubMed] [Google Scholar]
- 17.Segev A, Nili N, Strauss BH. The role of perlecan in arterial injury and angiogenesis. Cardiovascular Research. 2004;63:603–610. doi: 10.1016/j.cardiores.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Ng A, Wong M, Viviano B, Erlich JM, Alba G, Pflederer C, et al. Loss of glypican-3 function causes growth factor-dependent defects in cardiac and coronary vascular development. Developmental Biology. 2009;335:208–215. doi: 10.1016/j.ydbio.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, et al. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. Journal of Cell Science. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- 20.Fukai N, Kenagy RD, Chen L, Gao L, Daum G, Clowes AW. Syndecan-1: an inhibitor of arterial smooth muscle cell growth and intimal hyperplasia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1356–1362. doi: 10.1161/ATVBAHA.109.190132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotte M, Bernfield M, Joussen AM. Increased leukocyte-endothelial interactions in syndecan-1-deficient mice involve heparan sulfate-dependent and -independent steps. Current Eye Research. 2005;30:417–422. doi: 10.1080/02713680590956289. [DOI] [PubMed] [Google Scholar]
- 22.Stepp MA, Daley WP, Bernstein AM, Pal-Ghosh S, Tadvalkar G, Shashurin A, et al. Syndecan-1 regulates cell migration and fibronectin fibril assembly. Experimental Cell Research. 2010;316:2322–2339. doi: 10.1016/j.yexcr.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Nakamura E, Ito M, et al. Syndecan-4 deficiency impairs the fetal vessels in the placental labyrinth. Developmental Dynamics. 2000;219:539–544. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1081>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.Vikramadithyan RK, Kako Y, Chen G, Hu Y, Arikawa-Hirasawa E, Yamada Y, et al. Atherosclerosis in perlecan heterozygous mice. Journal of Lipid Research. 2004;45:1806–1812. doi: 10.1194/jlr.M400019-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM, et al. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Research. 2004;64:4699–4702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]
- 26.Tran-Lundmark K, Tran P-K, Paulsson-Berne G, Friden V, Soininen R, Tryggvason K, et al. Heparan sulfate in perlecan promotes mouse atherosclerosis: roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circulation Research. 2008;103:43–52. doi: 10.1161/CIRCRESAHA.108.172833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinsella MG, Tran PK, Weiser-Evans MC, Reidy M, Majack RA, Wight TN. Changes in perlecan expression during vascular injury: role in the inhibition of smooth muscle cell proliferation in the late lesion. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:608–614. doi: 10.1161/01.ATV.0000063109.94810.EE. [DOI] [PubMed] [Google Scholar]
- 28.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Current Opinion in Cell Biology. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 29.Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Developmental Biology. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- 30.Henderson DJ, Copp AJ. Versican expression is associated with chamber specification, septation, and valvulo-genesis in the developing mouse heart. Circulation Research. 1998;83:523–532. doi: 10.1161/01.res.83.5.523. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? The Journal of Clinical Investigation. 1997;99:2062–2070. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clowes AW, Clowes MM, Gown AM, Wight TN. Localization of proteoheparan sulfate in rat aorta. Histochemistry. 1984;80:379–384. doi: 10.1007/BF00495421. [DOI] [PubMed] [Google Scholar]
- 33.Clowes AW, Karnowsky MJ. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977;265:625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- 34.Guyton JR, Rosenberg RD, Clowes AW, Karnovsky MJ. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circulation Research. 1980;46:625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- 35.Lindner V, Reidy MA. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovich MA, Edelman ER. Tissue concentration of heparin, not administered dose, correlates with the biological response of injured arteries in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(20):11111–11116. doi: 10.1073/pnas.96.20.11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers C, Karnovsky MJ, Edelman ER. Inhibition of experimental neointimal hyperplasia and thrombosis depends on the type of vascular injury and the site of drug administration. Circulation. 1993;88(3):1215–1221. doi: 10.1161/01.cir.88.3.1215. [DOI] [PubMed] [Google Scholar]
- 38.Welt FG, Woods TC, Edelman ER. Oral heparin prevents neointimal hyperplasia after arterial injury: inhibitory potential depends on type of vascular injury. Circulation. 2001;104(25):3121–3124. doi: 10.1161/hc5001.100837. [DOI] [PubMed] [Google Scholar]
- 39.Francis DJ, Parish CR, McGarry M, Santiago FS, Lowe HC, Brown KJ, et al. Blockade of vascular smooth muscle cell proliferation and intimal thickening after balloon injury by the sulfated oligosaccharide pi–88: phosphomannopentaose sulfate directly binds fgf-2, blocks cellular signaling, and inhibits proliferation. Circulation Research. 2003;92(8):e70–e792. doi: 10.1161/01.RES.0000071345.76095.07. [DOI] [PubMed] [Google Scholar]
- 40.Toyoda H, Kinoshita-Toyoda A, Fox B, Selleck SB. Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu. Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. The Journal of Biological Chemistry. 2000;275:21856–21861. doi: 10.1074/jbc.M003540200. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs l-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nature Immunology. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 42.Bullock SL, Fletcher JM, Beddington RSP, Wilson VA. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes & Development. 1998;12:1894–1906. doi: 10.1101/gad.12.12.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li JPGF, Hagner-McWhirter Å, Forsberg E, Åbrink M, Kisilevsky R, Zhang X, et al. Targeted disruption of a murine glucuronyl c5-epimerase gene results in heparan sulfate lacking L-iduronic acid and in neonatal lethality. Journal of Biological Chemistry. 2003;278(31):28363–28366. doi: 10.1074/jbc.C300219200. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi T, Habuchi H, Tamura K, Ide H, Kimata K. Essential role of heparan sulfate 2-o-sulfotransferase in chick limb bud patterning and development. The Journal of Biological Chemistry. 2007;282:19589–19597. doi: 10.1074/jbc.M610707200. [DOI] [PubMed] [Google Scholar]
- 45.Stanford KI, Wang L, Castagnola J, Song D, Bishop JR, Brown JR, et al. Heparan sulfate 2-o-sulfotransferase is required for triglyceride-rich lipoprotein clearance. The Journal of Biological Chemistry. 2010;285:286–294. doi: 10.1074/jbc.M109.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, et al. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135:2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 48.Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, et al. Heart and liver defects and reduced transforming growth factor {beta}2 sensitivity in transforming growth factor {beta} type III receptor-deficient embryos. Molecular and Cellular Biology. 2003;23:4371–4385. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Compton LA, Potash DA, Brown CB, Barnett JV. Coronary vessel development is dependent on the type III transforming growth factor beta receptor. Circulation Research. 2007;101:784–791. doi: 10.1161/CIRCRESAHA.107.152082. [DOI] [PubMed] [Google Scholar]