Significance
Deep-sea ecosystem processes play a key role in global functioning of the planet. These functions are largely dependent upon deep-sea biodiversity. Industrial fisheries, after the depletion of fish stocks and destruction of the marine habitats on continental shelves, are now rapidly moving deeper into the ocean interior. We show here that bottom trawling along continental slopes has a major impact on deep-sea sedimentary ecosystems, causing their degradation and infaunal depauperation. Deep-sea fisheries, indeed, cause the collapse of benthic biodiversity and ecosystem functions, with potential consequences on the biogeochemical cycles. These findings support the claim of immediate actions for a sustainable management of fisheries in deep-sea environments.
Abstract
Bottom trawling has many impacts on marine ecosystems, including seafood stock impoverishment, benthos mortality, and sediment resuspension. Historical records of this fishing practice date back to the mid-1300s. Trawling became a widespread practice in the late 19th century, and it is now progressively expanding to greater depths, with the concerns about its sustainability that emerged during the first half of the 20th century now increasing. We show here that compared with untrawled areas, chronically trawled sediments along the continental slope of the north-western Mediterranean Sea are characterized by significant decreases in organic matter content (up to 52%), slower organic carbon turnover (ca. 37%), and reduced meiofauna abundance (80%), biodiversity (50%), and nematode species richness (25%). We estimate that the organic carbon removed daily by trawling in the region under scrutiny represents as much as 60–100% of the input flux. We anticipate that such an impact is causing the degradation of deep-sea sedimentary habitats and an infaunal depauperation. With deep-sea trawling currently conducted along most continental margins, we conclude that trawling represents a major threat to the deep seafloor ecosystem at the global scale.
Trawling represents one of the most common fishing practices along the coastal oceans of the world. However, it can have a plethora of impacts on the sea bottom, including stock impoverishment, alterations to the sea-bottom morphology, sediment resuspension, and increased bottom-water turbidity, epibenthos mortality, altered nutrient cycles, and alteration of the benthic biodiversity (1).
Historical records of this fishing practice date back to the mid-1300s, and it became widely practiced with the industrialization of fisheries in the late 19th century (2–4). Because shallow coastal water resources have steeply declined in the last 50 y (5, 6), fisheries are expanding offshore and trawling is being carried out at progressively increasing depths (7, 8).
In contrast to what was believed up to a few decades ago, deep-sea habitats (>200 m in depth) are rich in biodiversity, and they host many endemic and commercially important species (9, 10). Compared with shallow-water areas, the impact of trawling on deep-sea benthic ecosystems is deemed more severe and long-lasting, because of their lower resilience and higher vulnerability (10). However, our knowledge of the impact of trawling on deep-sea ecosystems has remained limited and has mainly focused on hard-bottom systems, such as seamounts and cold-water coral reefs (11, 12).
Sedimentary environments (i.e., the soft sea bottom) represent the greatest area of the deep-sea floor and host a vast fauna biodiversity (10). In these environments, the metazoan fauna (i.e., multicellular organisms) include almost all of the 35 modern animal Phyla. The smaller components of this fauna, the meiofauna, are characterized by relatively short life cycles, high turnover rates, and a lack of larval dispersion. For all oceanic seafloors, nematodes account for >90% of meiofauna abundance in the deep sea (13) and are characterized by very high species richness and recognizable feeding types and life strategies (14, 15). In this sense, nematodes have been recently used as a model to demonstrate that any loss in deep-sea fauna biodiversity is associated with an exponential decrease in ecosystem functioning (16).
Recent investigations carried out in the north-western Mediterranean Sea have revealed that the continuous stirring, mixing, and resuspension of surface sediments by intensive and chronic trawling activities has caused changes to the present-day sediment dynamics and has permanently smoothed the seafloor morphology of the continental slope over large spatial scales (17–19). In this region, deep-sea trawled grounds are subjected to levels of sediment disturbance whose effects are larger than the changes in sediment properties associated with seasonal variability (20). Smoothed trawling grounds are also exposed to a reduced habitat heterogeneity. Because high habitat heterogeneity is crucial to preserve high biodiversity levels (21, 22), trawling activities might represent a major threat to the integrity of deep-sea ecosystems (12, 18).
Results
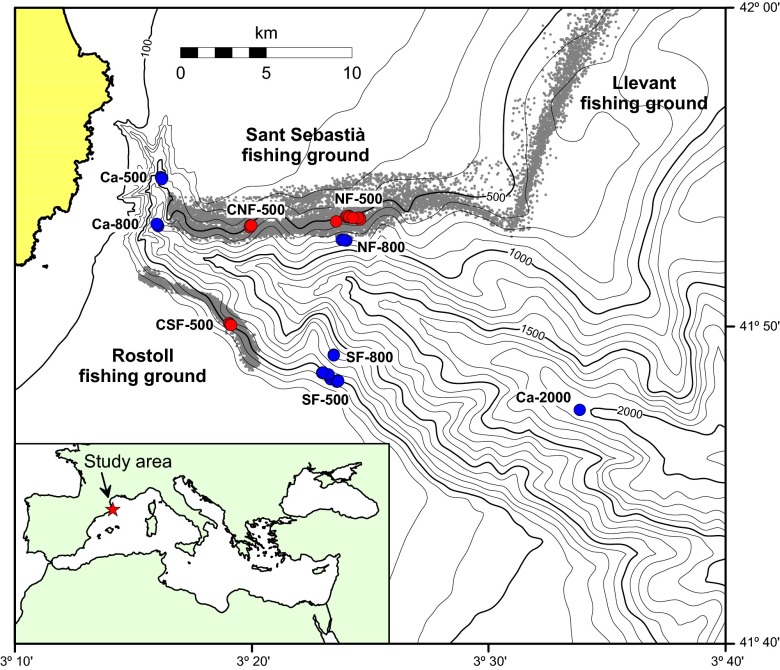
To determine the chronic impact of trawling on deep-sea sedimentary environments, we investigated several properties of the benthic ecosystems within La Fonera submarine canyon (north-western Mediterranean Sea; Fig. 1). In this region, trawling activities are conducted in the waters from 200 to 800 m in depth, although they are more intensive between 400 and 750 m (18, 19, 23) (Methods). Trawling activities in this region take place along the entire northern flank of the canyon, whereas the southern flank is trawled only partially, because of the rougher terrain conditions in the easternmost part (20) (Fig. 1). This allowed us to use the sediments from the untrawled southern canyon flank as a reference (compared with similar trawled depths), as well as from other untrawled canyon regions, to determine the impact of chronic trawling on the quantity, biochemical composition, and rate of decomposition of sedimentary organic matter; on the meiofauna abundance, biomass, and richness of taxa; and on the biodiversity of the nematodes (Table S1).
Fig. 1.
Bathymetric map of La Fonera Canyon. The sampling stations in the trawled and untrawled areas are shown as red and blue dots, respectively. The vessel monitoring system positions of large (>400 horsepower) trawlers for the years 2007 to 2010 are shown by the small gray dots. (Inset) Location of the study area in the north-western Mediterranean Sea.
Alteration of the Deep-Sea Sediment Column.
We analyzed several physical/chemical properties of the sediment column at ∼500-m water depth, at both the trawled and untrawled sites (Fig. S1). At the untrawled sites, the sediment column has a topmost unit of unconsolidated material with dry bulk densities as low as 0.6 g⋅cm−3 (Fig. S1A). There is progressive compaction with depth, with dry bulk density reaching values of 0.7–1.0 g⋅cm−3 toward the bottom of these sediment cores. Such vertical pattern in dry bulk density along the sediment column from the untrawled ground indicates that these sediments continuously receive and accumulate soft material from the water column. In contrast, the surface sediments from the trawled sites are much denser and reach consistently higher values near the surface: this indicates the absence of recently deposited unconsolidated sediment attributable to repeated trawling-induced resuspension. This large difference in the compaction of sediments between the two grounds, which reflects differences in the sedimentation patterns, is also consistent with the higher surface 210Pb concentrations and deeper excess 210Pb horizons in the reference (untrawled) sites than in the trawled sites (Fig. S1B). Moreover, the total organic carbon content in the trawled sediments (0.34–0.89% dry weight) were lower (by 28–36%) than in the untrawled sediments (0.68–1.02%), consistently along the upper 10 cm of the sediment column (Fig. S1C).
Quantity and Composition of Sedimentary Organic Matter.
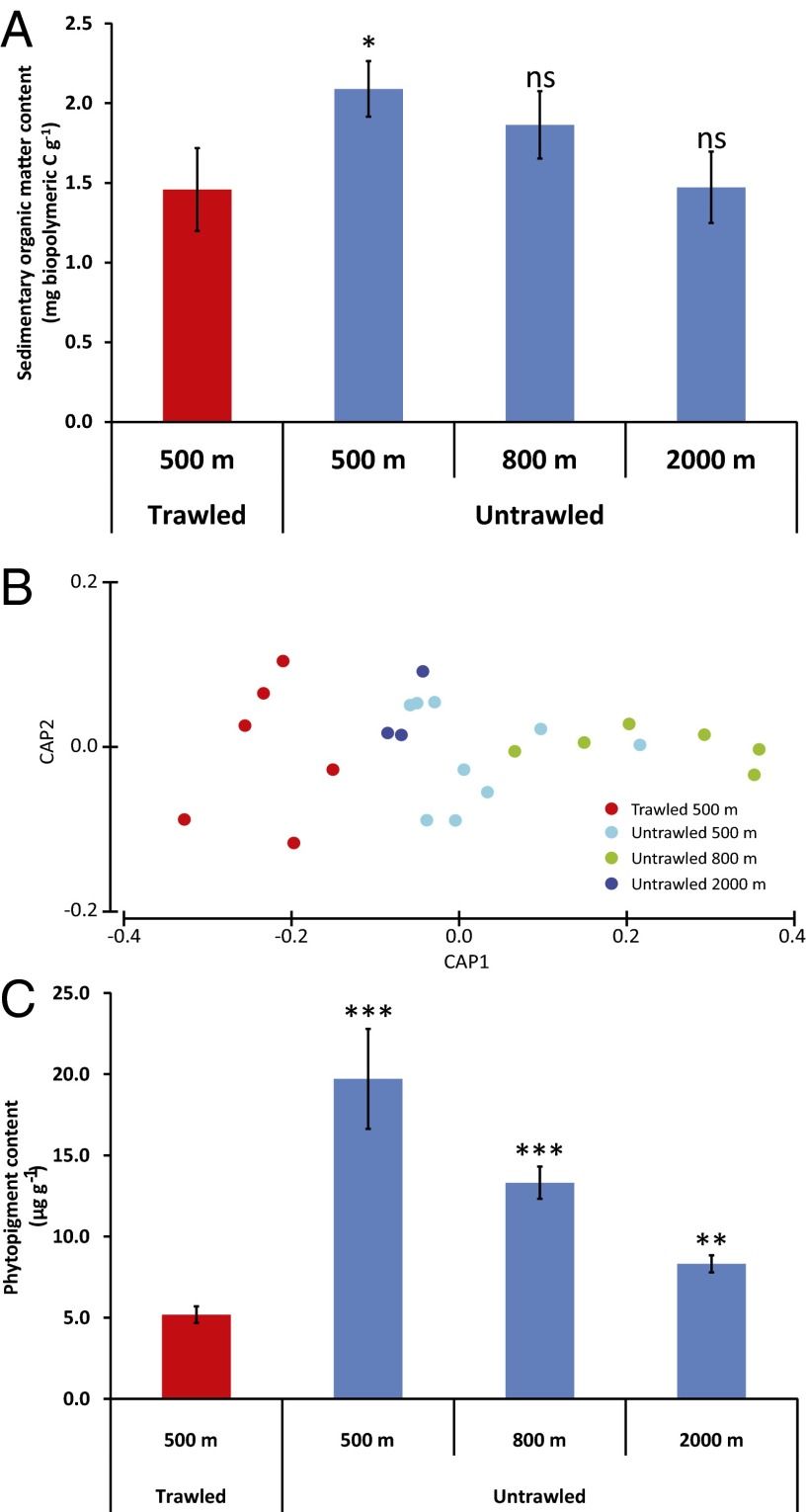
Compared with untrawled areas at the same depth and in deeper areas (800 m), the trawled sediments at 500 m in depth along the flanks of La Fonera Canyon are characterized by a significant 5–52% decrease in labile organic matter content (mean decrease in biopolymeric C content: 30% vs. 500 m, 22% vs. 800 m; Fig. 2A). Major alterations to the biochemical composition of the trawled sediments at 500 m in depth are also observed (Table S2 and Fig. 2B). The same applies to the primary organic matter deposited on the surface of the sea floor (i.e., the input of senescent and dead algae from the photic zone, measured here as the phytopigment concentrations in the sediments), which is, on average, lower by 74% vs. untrawled sediments at 500 m, 61% vs. 800 m, and 38% vs. 2,000 m (Table S2 and Fig. 2C). The concentrations of phytopigment in the trawled sediments at 500 m in depth in La Fonera Canyon are also lower than those from many other continental margins investigated over large spatial and temporal scales (Table S3).
Fig. 2.
Sedimentary organic matter in trawled and untrawled sediments at different depths of La Fonera Canyon. (A) Sediment content of biopolymeric C. (B) Variations in the biochemical composition of the sedimentary organic matter (biplot after canonical analysis of the principal coordinates). (C) Total phytopigment concentrations in the top 1 cm of sediment. Error bars indicate SEs among stations at similar depth and level of impact or SDs among replicates for the data at 2,000 m in depth. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant vs. untrawled samples from 500, 800, and 2,000 m.
Impacts on Ecosystem Functions and Biodiversity.
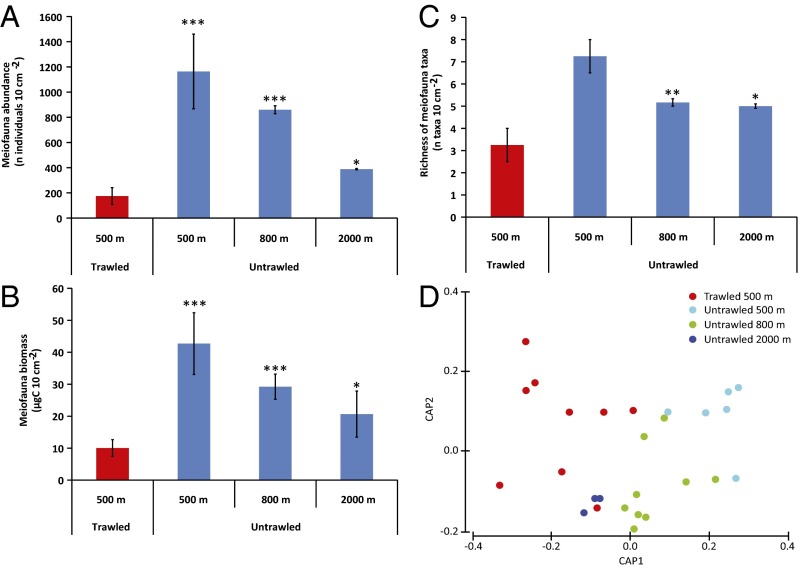
The trawling-induced continuous stirring of the upper part of deep-sea sediments in the fishing grounds leads to a decrease in the organic C turnover rates. The organic C turnover in the trawled sediments at 500 m in depth (mean, 0.078 d−1) is 37% slower, on average, than in the untrawled sediments at the same depth (mean, 0.124 d−1; P < 0.05), and even slower than in the untrawled sediments at ca. 2,000 m (by 59%; mean, 0.191 d−1; P < 0.001) (Table S2 and Fig. S2). Trawling of the deep-sea soft bottom also affects the meiofauna abundance, biomass, and biodiversity in the surface sediments. Here, compared with the untrawled areas, the meiofauna abundance in trawled areas was significantly reduced by up to >80% [mean decrease: 85% vs. 500 m (P < 0.001); 80% vs. 800 m (P < 0.001); 55% vs. 2,000 m (P < 0.05)], the meiofauna biomass by >70% [mean decrease: 76% vs. 500 m (P < 0.001); 66% vs. 800 m (P < 0.001); 51% vs. 2,000 m (P < 0.05)], and the meiofaunal taxa richness by >50% [mean decrease: 55% vs. 500 m (P < 0.002); 37% vs. 800 m (P < 0.01); 35% vs. 2,000 m (P < 0.05)] (Table S4 and Fig. 3 A–C). Values of meiofaunal abundance in the trawled grounds of La Fonera Canyon are among the lowest observed so far along the continental slope of the Mediterranean Sea (Fig. S3 A and B), and, although being obtained in the mesotrophic north-western Mediterranean Sea, they are similar only to those encountered in the ultraoligotrophic eastern Mediterranean Sea (Fig. S3C). Moreover, the structures of the meiofauna assemblages in trawled sediments at 500 m in depth do not differ significantly from those in bathyal sediments along the canyon axis at 2,000 m in depth (Table S5 and Fig. 3D).
Fig. 3.
Meiofauna in trawled and untrawled sediments at different depths of La Fonera Canyon. Abundance (A), biomass (B), and biodiversity (as richness of taxa) (C) and variations in the composition of the meiofauna communities (biplot after canonical analysis of the principal coordinates) (D). Error bars indicate SEs among stations at similar depth and level of impact or SDs among replicates for the data at 2,000 m in depth. *P < 0.05; **P < 0.01; ***P < 0.001 vs. untrawled samples from 500, 800, and 2,000 m.
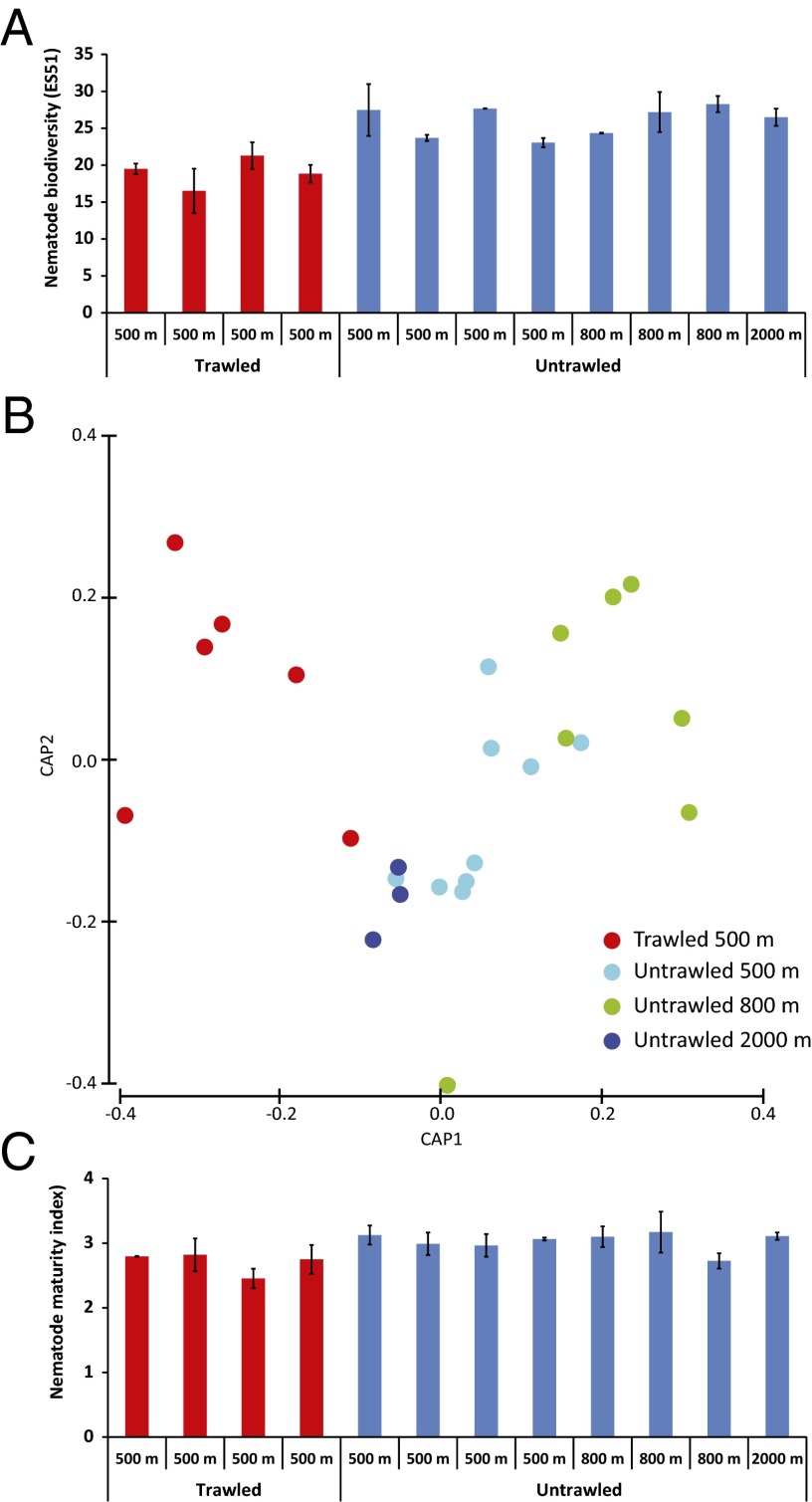
The enhanced trawling-induced sediment erosion is also associated with reductions in the fauna biodiversity and alterations to the biological traits of the benthic assemblages (e.g., the nematode life strategies). Compared with the untrawled areas, it is apparent that these trawling activities have significantly reduced the nematode biodiversity in the trawled sediments by ca. 25% [statistic F (Pseudo-F) =12.72; P < 0.01], in terms of the ES51 (mean decrease: 25% vs. 500 m; 28% vs. 800 m; 28% vs. 2,000 m; Fig. 4A). There are also significant differences in the species compositions of the nematode assemblages between the trawled and untrawled sediments at 500 m in depth and between trawled sediments at 500 and 2,000 m in depth (Table S5 and Fig. 4B). In addition, the nematode assemblages in the trawled sediments contain a significantly larger proportion of opportunistic species (Pseudo-F = 5.099; P < 0.01) than in the untrawled sediments, as indicated by the lower values of nematode maturity index shown in Fig. 4C (mean decrease: 11% vs. 500 m; 10% vs. 800 m; 13% vs. 2,000 m). Trawled sediments appear to be colonized by meiofauna of higher sizes (individual biomass ca. 60% higher, on average) than those in the untrawled sediments at the same depth and beyond but of similar size of those encountered in the bathyal adjacent plain at 2,000 m in depth (Fig. S4).
Fig. 4.
Nematode biodiversity in trawled and untrawled sediments at different depths of La Fonera Canyon. The expected number of nematode species for a theoretical sample of 51 nematode specimens (ES51) (A), variations in the composition of the nematode assemblages (canonical analysis of the principal coordinates) (B), and maturity index (C). Error bars indicate SEs among stations at similar depth and level of impact or SDs among replicates for the data at 2,000 m in depth. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant vs. untrawled samples from 500, 800, and 2,000 m.
Discussion
Our study reveals that compared with untrawled areas, trawled sediments in the deep-sea regions are characterized by a lower organic C turnover and are significantly depleted in organic matter content, meiofauna abundance and biodiversity, and nematode species richness and individual biomass. The continental slope of the region under investigation is subjected to trawling activities all over the year, on weekdays at working hours (18). Signals of a persistent trawling-induced sediment disturbance are indeed clearly visualized in the vertical structure of the sediment column over the fishing grounds (Fig. S1). Thus, although we limited our analysis to only one time over the year, we can reliably consider the differences observed between trawled and untrawled sites as being representative of the consolidated impact of chronic and intensive trawling more than of seasonal variability of ecosystem properties across the investigated region. In this regard, a recent study also provided evidence that changes in the quantity and composition of sedimentary organic matter caused by bottom trawling in the region are larger than those associated with the natural seasonality of the investigated variables (20). We also pinpoint that the seasonal and interannual changes in the phytopigment contents and meiofaunal abundance and community composition along the continental margins of the north-western Mediterranean Sea close to the study area are very weak (Table S6).
We show that these trawled sediments are generally characterized by a decrease in the fraction of organic matter of algal origin (i.e., total phytopigments), which is the fraction of sedimentary organic matter that is most digestible to heterotrophic consumption, and, as such, represents the most important food source for deep-sea benthic fauna (24–26).
The effects of deep-sea trawling extend not only to food availability for the benthos but also to the key ecosystem function of C cycling. We thus report here that the potential organic C turnover in trawled sediments is significantly lower than that estimated for untrawled sediments. This is consistent with what has been observed in coastal habitats, where natural (e.g., chronic bioturbation and episodic storms) and anthropogenic factors (e.g., trawling and dredging) that mechanically disturb the sediment can result in severe changes in the rates of organic matter degradation (27–29). Therefore, in this deep-sea ecosystem, the persistent trawling-induced resuspension of large amounts of high-quality nutritional resources, coupled with a slowdown of the organic C cycling, indicates that bottom trawling can exacerbate the natural food limitation of the deep-sea sediments.
Previous studies showed that the sediments that are resuspended by trawling along the northern canyon flank tend to flow down-slope incorporated in bottom-arrested gravity currents caused by the excess of density provided by the suspended sediment load, being channelized through the canyon’s tributary valleys. When these flows reach the main canyon axis, they are redirected seaward and further down-canyon, without affecting the southern canyons’ flank, and accumulate the sediment load in the lower canyon reaches at depths of ≥1,800 m (17–19, 30). Combined with the concurrent impoverishment of the benthic trophic resources in the upper slope sediments, this mechanism contributes to explain why also the composition of the meiofaunal assemblages in trawled sediments at 500 m in depth tends to resemble those recorded at much deeper depths along the canyon axis (i.e., at 2,000 m) (Table S5 and Fig. 3D).
The effects of intensive and chronic trawling-induced disturbance of sediments along the continental slope studied here resemble the severe impairment of meiofaunal and nematode abundance and diversity caused by chronic and intensive (highly frequent) bottom trawling in coastal habitats (31, 32) and appear to be as severe as the effects of trawling on hard-bottom substrata (12, 33). Moreover, our data pinpoint signs of severe impacts due to deep-sea trawling that can be also appreciated in terms of the life traits and body size of the nematode assemblages. Indeed, we report that the nematode assemblages in trawled sediments are characterized by life strategies that are more opportunistic than those observed in untrawled grounds, as previously reported also from shallow-water studies (34). The appearance and dominance of organisms with opportunistic life strategies (i.e., early colonizers) is a typical bioecological response that is reported for communities that are exposed to frequent disturbance events (35, 36). This observation, coupled with the significantly higher mean individual biomass of nematodes in trawled grounds (Fig. S4), suggests that the disturbance exerted by bottom trawling removed preferentially small individuals, more susceptible to be resuspended, while leaving on site larger and heavier organisms.
Cumulatively, the impacts of trawling on the sediment structure, the benthic biodiversity, and the most basic of all of the nutritional resources in these deep-sea sedimentary ecosystems resemble the catastrophic effects caused by man-accelerated soil erosion on land (37, 38) and the general environmental deterioration of abandoned agriculture fields exposed to high levels of human impact (39). Thus, ultimately, intensive and chronic bottom trawling is deemed to transform large portions of the deep continental slope into faunal deserts and highly degraded seascapes.
Deep-sea trawling is currently carried out along large sectors of the oceans, and it appears to have severe consequences on deep-sea sediment dynamics at a global scale. Using the same approach adopted for estimating the volume of sediment displaced by trawling in La Fonera Canyon (18), we have estimated that the continuous sediment resuspension induced by deep-sea trawling can daily subtract from 60% to 100% of the organic C flux from the trawled area (Methods). Despite the fact that the sediment composition and texture of surface sediments and the magnitude of the C flux can change considerably among continental margins, our results suggest that this fishing practice may have devastating consequences on the biodiversity and ecosystem functioning of the deep-sea sedimentary environments across the world oceans.
Methods
Study Area.
La Fonera (Palamós) submarine canyon is located in the Catalan margin (north-western Mediterranean), and it is about 40 km long with a maximum depth of ca. 2,200 m (30). The head of the canyon is forked and N-to-S oriented, with a deeply incised V shape and a major axis oriented WNW-to-ESE (Fig. 1). The steep canyon walls are indented by numerous gullies. The most important river on the coast is the Ter River, which is located about 15 km northwest of the canyon head, with an average annual flow of 10.3 m3⋅s−1. The hydrography of the area is characterized by a vertical density front (salinity-driven) (30) that is permanently positioned at the convergence between the shelf and the continental slope and separates the (superficial) waters of continental origin from the denser open-sea waters. The general circulation is governed by a baroclinic current along the continental slope toward the southwest, in geostrophic balance with the density front (40). The direction of the prevailing currents in the area means that a substantial part of the along-margin sediment transport bypasses the canyon head and enters the canyon directly from the northern canyon flank (41). Data from near-bottom current meter deployments within La Fonera Canyon showed a closed circulation inside the canyon confinement with low velocities (<20 cm/s) oriented along the local bathymetry and showing frequent current reversals at inertial frequencies (40, 42). However, those measurements and further monitoring studies revealed that the near-bottom water turbidity and the sediment dynamics in this submarine canyon is heavily dominated, both in its magnitude and temporal patterns, by trawling-induced sediment resuspension at the fishing ground (17–19, 40–42).
The local trawling fleet in this submarine canyon targets the deep-sea shrimp Aristeus antennatus (Risso, 1816) and operates on a daily basis (with interruption only during the weekend) and year round, down to ca. 800 m in depth (18). Otter trawl gears are used, comprising a towed net with sweeplines (bridles), iron otterboards, and warps. The mouth of the net is kept open by means of floats and weights and is spread horizontally by forces exerted on the otterboards, which are oriented obliquely to the trawl’s forward motion. The two otterboards are heavy (from hundreds of kilograms to tons) and can imprint furrows in the sediment with the resulting sediment resuspension. The sweeplines and the footrope also cause sediment resuspension (41). In La Fonera submarine canyon, there are two main areas that are routinely visited: the Sant Sebastià fishing ground along the northern flank of the canyon and the Rostoll fishing ground along the southern flank of the canyon (Fig. 1). Studies conducted over the last decade have shown that the continuous passage of the trawling equipment along the steep canyon flanks has triggered frequent sediment gravity flow (17). This has transported resuspended sediments downslope toward the main canyon axis (41, 42), which has affected the sediment accumulation rates in the lower canyon, at a depth of >1,200 m (30). The fishing grounds currently visited by trawlers had the same roughness as the one of the eastern southern flank of the canyon and have been smoothed over time and after decades of intensive bottom trawling (18).
Sampling Strategy.
Our analysis is primarily based on the refutation of the null hypothesis by which the content, composition, and degradation rates of organic matter, abundance, biomass, and structure of the meiofauna community, species richness, and life traits of nematode assemblages do not vary between trawled and untrawled sediments at 500 m and at greater depths in La Fonera submarine canyon. After identifying areas subjected to intensive trawling activities and reference areas (i.e., untrawled areas), sediment samples were retrieved from a total of 13 stations spread across La Fonera submarine canyon at depths ranging from 500 to 2,000 m (Table S1). The criteria we have followed to identify trawled and untrawled sites are based on the distribution of vessel monitoring system positions of large (>400 horsepower) trawlers operating in the study area over the course of four years (2007 to 2010) (Fig. 1) (18). Surface sediment samples were collected using a KC Denmark A/S interface multicorer during an oceanographic cruise from May 10–14, 2011, on board the R/V García del Cid. At each station, three Plexiglas cores were retrieved from three independent casts of the multicorer (total of nine corers per station) and immediately stored at −20 °C for subsequent analyses of organic matter and meiofauna in the laboratory (within 1 mo). An additional core was used for analysis of extracellular enzymatic activities in the topmost 2 cm of the sediment column at four stations, of which one was in the trawled area and one at each of the representative untrawled sampling depths along the major canyon axis (500, 800, 2,000 m) (Table S1). Separate casts of the multicorer were carried out to retrieve sediment cores at stations located at 500 m in both trawled and untrawled grounds along the canyon flanks and used for the analysis of 210Pb concentrations, sediment dry bulk density, and total organic C through the entire cores.
Organic Matter Composition and Turnover.
The trophic effects caused by bottom trawling were assessed through the analysis of the labile (phytopigment) and semilabile (biopolymeric C) fractions of sedimentary organic matter (43). Chlorophyll-a and phaeopigment were extracted (12 h at 4 °C, in the dark) from triplicate superficial (0–1 cm) sediment samples (about 1 g), using 3 mL to 5 mL 90% (vol/vol) acetone as the extractant. These extracts were analyzed fluorometrically, to estimate chlorophyll-a, and after acidification with 200 µL of 0.1 N HCl, to estimate the phaeopigments (44). Different methods for assessing chlorophyll-a concentrations in marine sediments can provide different underestimates and overestimates (45). For this reason, we summed the chlorophyll-a and phaeopigment concentrations (i.e., total phytopigments) (43). Protein, carbohydrate, and lipid analyses were carried out on the top 1 cm of three independent sediment cores using photometric protocols (44). Protein, carbohydrate, and lipid contents were converted to C equivalents using the conversion factors 0.49, 0.40, and 0.75 mg of C per milligram, respectively, and their sum is referred to as the biopolymeric C (44). The sedimentary contents of the phytopigments and biopolymeric C pools reflect the overall trophic conditions of the deep-sea sediments (43). For determination of the extracellular enzymatic activities, 2.5 mL of sediment subsamples was incubated at the in situ temperature (ca. 14 °C) in the dark for 2 h with 2.5 mL of filtered, sterile seawater containing 200 µM l-leucine-4-methylcumarinyl-7-amide and 75 µM 4-methylumbelliferyl β-d-glucopyranoside separately for aminopeptidase and β-glucosidase activities, respectively. After these incubations, the samples were centrifuged, and the supernatants were analyzed fluorometrically (44). The protease and glucosidase activities (micromoles of substrate per gram per hour) were converted into C degradation rates (micrograms of C per gram per hour), using 72 µg of C per mole of substrate as the conversion factor (46). The turnovers (per day) of the whole protein and carbohydrate pools (here referred as biopolymeric C) were calculated as the ratios of the hourly C degradation rates (once multiplied by 24) and the whole protein and carbohydrate C contents in the sediment.
Fauna Biodiversity.
The sediment samples were sieved through 1,000-µm and then 20-µm meshes, to retain the smallest organisms. The fraction that remained on the 20-µm sieve was resuspended and centrifuged three times with Ludox HS 40 (density, 1.18 g⋅cm−3) (15). All metazoans were counted and classified per taxon after staining with Rose Bengal (0.5 g⋅L−1), under a stereomicroscope using Delfuss cuvettes. For determination of the meiofauna biomass, the individual biomasses of all of the animals belonging to the different taxa were calculated. The nematode biomass was calculated from the biovolume (n = 100 per replicate) using Andrassy’s formula [V = L × W2 × 0.063 × 10−5; body length (L) in microns, width (W) in microns]. For all of the other taxa, the biovolume was measured for all of the specimens encountered. The body volumes were derived from measurements of body length (L) (in millimeters) and width (W) (in millimeters) using the formula V = L × W2 × C; where C is the approximate conversion factor for each meiofauna taxon (47). To obtain the biomass (micrograms dry weight), the body volumes were multiplied by an average density (1.13 g⋅cm−3), which assumes that the dry weight:wet weight ratio is 20–25%, and the C content was considered as 40% of the dry weight. For the analysis of nematode species diversity, 100 specimens (or all of the retrieved nematodes, if the number of extracted individuals was <100) were randomly picked from three independent replicates at each sampling station. The nematodes were mounted on slides (following the formalin–ethanol–glycerol technique, to prevent dehydration) (48) and identified to the species level. For unknown species, these were indicated as sp1, sp2, and sp3. Nematode species richness was estimated as the total number of species identified at each habitat. Because the species richness is strongly influenced by the number of individuals identified, to standardize the values of nematode diversity, the species abundance data were converted into rarefaction diversity indices (49, 50). Because the species richness is also strongly affected by the sample size, the expected number of species for a theoretical sample of 51 nematode specimens, the ES51, was calculated (51). Previous studies have shown that this approach enables the provision of robust data on deep-sea species richness, and the expected species number is the most commonly used density-independent index for the comparison of areas with nonstandardized sample sizes (52). The maturity index was calculated according to the weighted mean of the individual genus scores: MI = Σνifi, where νi is the c–p (colonizers–persisters) value of species i (53–55), and fi is the frequency of that species.
Modifications of the Sediment Column.
Sediment samples from both the fishing grounds and the control sites at ∼500-m water depth were subsampled (every centimeter down to 10 cm in depth) and freeze-dried, to obtain the vertical profiles of the dry bulk density (ratio of dry weight to volume of the wet sediment slice), as a proxy for sediment compaction. To estimate net accumulation of recently deposited sediments, the activities of the radionuclide 210Pb (half-life, 22.3 y) were determined by counting the α-emission of its radioactive product 210Po (56). To obtain the total organic C sedimentary contents (as percentages of total dry weight), aliquots of dried sediment from the top 10 cm of selected sediment were first decalcified by fuming with concentrated HCl for 48 h at 40 °C. Then the remaining C in the sample was determined with a LECO CN-2000 autoanalyzer. Blanks and standards (analytical grade EDTA; Sigma) were intercalated between replicate samples for calibration. Although the acidification of sediments with HCl could have eliminated the more volatile components of organic carbon (OC), we used OC profiles as geochemical tracers for illustrating the effects of intensive bottom trawling on the vertical distribution of OC deep in the sediment.
Statistical Analyses.
Differences among the trawled and untrawled sediments were assessed using univariate and multivariate distance-based permutational nonparametric analyses of variance (PERMANOVA) (57). The design included four fixed levels: trawled sediments at 500 m in depth (n = 2–5 stations) and untrawled sediments at 500, 800, and 2,000 m in depth (n = 2–4, n = 3, and n = 1 stations, respectively), with n = 3 as the combination of factors (i.e., true replicates coming from independent deployments of the corer at each station). The analyses were carried out on Euclidean distances (organic matter) or Bray–Curtis similarity matrices (fauna data) of previously normalized data (organic matter) or on fourth-root transformed data, using unrestricted permutations of the raw data (univariate tests) or 999 permutations under a reduce model (multivariate test). When significant differences were observed, pair-wise comparison tests were also carried out. The multivariate differences in the compositions of the sedimentary organic matter, meiofauna communities, and nematode assemblages between trawled and untrawled areas are illustrated using the biplots produced after canonical analysis of the principal coordinates (CAP) using the same distance/similarity matrices used for PERMANOVA. Turnover diversity (β diversity) of nematodes between pairs of contrasted stations (e.g., trawled vs. untrawled sediments) have been calculated as Bray–Curtis dissimilarity percentage using the SIMPER (similarity percentages) tool. The PERMANOVA tests, CAP analyses, and SIMPER tests were carried out using the homonymous routines included in the software PRIMER 6+.
Estimation of Trawling-Induced Organic C Removal from the Seabed.
To estimate the amount of organic C removed daily by the trawling activities from a single square meter in the study area, we first calculated the mean difference in the sedimentary organic C quantity (as estimated from biopolymeric C contents) (43) between the trawled and untrawled sediments at 500 m in depth. This amount was extrapolated to the total amount of organic C removed annually by trawling from the entire area, using the volume estimate of 2.40 × 10−4 km3 of sediment per year, normalized per square meter (using 4.2 km2 as an estimate of the surface of the trawled area) (18) and per single day (dividing by 365). This amount of C removed by the trawling was compared with estimates of particle flux in the north-western Mediterranean Sea during a two-decade time-series study at the Dynamique des Flux Atmosphériques en Méditerranée (DYFAMED) site (58).
Supplementary Material
Acknowledgments
Assistance at sea by the crews of research vessel García del Cid is acknowledged. The Vessel Monitoring System data were provided by the Spanish General Secretariat of Maritime Fishing. This study was conducted within the framework of the Hotspot Ecosystem Research and Man's Impact On European Seas European Union Collaborative Project (EC Contract 226354). The oceanographic cruise was funded by the Spanish Research Plan (Project CTM2010-11084-E). This study was supported by the Project Ricerca Italiana per il Mare. J.M. received funding from a Junta para la Ampliación de Estudios contract granted by Consejo Superior de Investigaciones Científicas and cofinanced by the European Social Fund. P.M. acknowledges funding from the Government of Catalonia through the Institució Catalana de Recerca i Estudis Avançats Academia prize. We are grateful to two anonymous reviewers for their constructive criticism on an early version of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 8704.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1405454111/-/DCSupplemental.
References
- 1.Thrush SF, Dayton PK. Disturbance to marine benthic habitats by trawling and dredging: Implications for marine biodiversity. Annu Rev Ecol Syst. 2002;33:449–473. [Google Scholar]
- 2.Roberts C. The Unnatural History of the Sea. Chicago, IL: Island Press; 2007. [Google Scholar]
- 3.Graham M. The trawl fisheries: A scientific and national problem. Nature. 1938;142(3609):1143–1146. [Google Scholar]
- 4.Myers RA, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423(6937):280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 5.Thurstan RH, Brockington S, Roberts CM. The effects of 118 years of industrial fishing on UK bottom trawl fisheries. Nat Commun. 2010;1:15. doi: 10.1038/ncomms1013. [DOI] [PubMed] [Google Scholar]
- 6.Worm B, Tittensor DP. Range contraction in large pelagic predators. Proc Natl Acad Sci USA. 2011;108(29):11942–11947. doi: 10.1073/pnas.1102353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts CM. Deep impact: The rising toll of fishing in the deep sea. Trends Ecol Evol. 2002;17(5):242–245. [Google Scholar]
- 8.Morato T, Watson R, Pitcher TJ, Pauly D. Fishing down the deep. Fish Fish. 2006;7(1):24–34. [Google Scholar]
- 9.Costello MJ, et al. A census of marine biodiversity knowledge, resources, and future challenges. PLoS ONE. 2010;5(8):e12110. doi: 10.1371/journal.pone.0012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rex MA, Etter RJ. Deep-Sea Biodiversity: Pattern and Scale. Cambridge, MA: Harvard Univ Press; 2010. [Google Scholar]
- 11.Norse EA, et al. Sustainability of deep-sea fisheries. Mar Policy. 2012;36(2):307–320. [Google Scholar]
- 12.Althaus F, et al. Impacts of bottom trawling on deep-coral ecosystems of seamounts are long-lasting. Mar Ecol Prog Ser. 2009;397:279–294. [Google Scholar]
- 13.Giere O. 2009. Meiobenthology. The Microscopic Motile Fauna of Aquatic Sediments (Springer, Berlin) [Google Scholar]
- 14.Lambshead PJD. 2004. Marine nematode biodiversity. Nematology: Advances and Perspectives: Nematode Morphology, Physiology and Ecology, Tsinghua University Press (TUP) Book Series, eds Chen ZX, Chen SY, Dickson DW (CABI Publishing, Wallingford, UK), Vol 1, pp. 436–467.
- 15.Heip C, Vincx M, Vranken G. The ecology of marine nematodes. Oceanogr Mar Biol. 1985;23:399–489. [Google Scholar]
- 16.Danovaro R, et al. Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss. Curr Biol. 2008;18(1):1–8. doi: 10.1016/j.cub.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 17.Palanques A, et al. Evidence of sediment gravity flows induced by trawling in the Palamós (Fonera) submarine canyon (northwestern Mediterranean) Deep-Sea Res. 2006;53(2):201–214. [Google Scholar]
- 18.Puig P, et al. Ploughing the deep sea floor. Nature. 2012;489(7415):286–289. doi: 10.1038/nature11410. [DOI] [PubMed] [Google Scholar]
- 19.Martín J, Puig P, Palanques A, Ribó M. Trawling-induced daily sediment resuspension in the flank of a Mediterranean submarine canyon. Deep Sea Res Part 2 Top Stud Oceanogr. 2014 doi: 10.1016/j.dsr2.2013.05.036. [DOI] [Google Scholar]
- 20.Sañé E, Martín J, Puig P, Palanques A. Organic biomarkers in deep-sea regions affected by bottom trawling: Pigments, fatty acids, amino acids and carbohydrates in surface sediments from the La Fonera (Palamós) Canyon, NW Mediterranean Sea. Biogeosciences. 2013;10:8093–8108. [Google Scholar]
- 21.Levin LA, Dayton PK. Ecological theory and continental margins: Where shallow meets deep. Trends Ecol Evol. 2009;24(11):606–617. doi: 10.1016/j.tree.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 22.McClain CR, Barry JP. Habitat heterogeneity, disturbance, and productivity work in concert to regulate biodiversity in deep submarine canyons. Ecology. 2010;91(4):964–976. doi: 10.1890/09-0087.1. [DOI] [PubMed] [Google Scholar]
- 23.Company JB, et al. Climate influence on deep sea populations. PLoS ONE. 2008;3(1):e1431. doi: 10.1371/journal.pone.0001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witte U, et al. In situ experimental evidence of the fate of a phytodetritus pulse at the abyssal sea floor. Nature. 2003;424(6950):763–766. doi: 10.1038/nature01799. [DOI] [PubMed] [Google Scholar]
- 25.Mayor DJ, et al. Resource quality affects carbon cycling in deep-sea sediments. ISME J. 2012;6(9):1740–1748. doi: 10.1038/ismej.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Oevelen D, et al. Canyon conditions impact carbon flows in food webs of three sections of the Nazaré canyon. Deep Sea Res Part 2 Top Stud Oceanogr. 2011;58(23-24):2461–2476. [Google Scholar]
- 27.Aller R. Bioturbation and remineralization of sedimentary organic matter: Effects of redox oscillation. Chem Geol. 1994;114(3-4):331–345. [Google Scholar]
- 28.Meysman FJR, Middelburg JJ, Heip CHR. Bioturbation: A fresh look at Darwin’s last idea. Trends Ecol Evol. 2006;21(12):688–695. doi: 10.1016/j.tree.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Pusceddu A, Fiordelmondo C, Danovaro R. Sediment resuspension effects on the benthic microbial loop in experimental microcosms. Microb Ecol. 2005;50(4):602–613. doi: 10.1007/s00248-005-5051-6. [DOI] [PubMed] [Google Scholar]
- 30.Martín J, et al. Effect of commercial trawling on the deep sedimentation in a Mediterranean submarine canyon. Mar Geol. 2008;252(3-4):150–155. [Google Scholar]
- 31.Schratzberger M, Jennings S. Impacts of chronic trawling disturbance on meiofaunal communities. Mar Biol. 2002;141(5):991–1000. [Google Scholar]
- 32.Jennings S, et al. Trawling disturbance can modify benthic production processes. J Anim Ecol. 2001;70(3):459–475. [Google Scholar]
- 33.Bongiorni L, et al. Deep-water scleractinian corals promote higher biodiversity in deep-sea meiofaunal assemblages along continental margins. Biol Conserv. 2010;143(7):1687–1700. [Google Scholar]
- 34.Liu X-S, Cheung SG, Shin PKS. Meiofauna with special reference to nematodes in trawling ground of subtropical Hong Kong. Mar Pollut Bull. 2009;58(4):607–615. doi: 10.1016/j.marpolbul.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Gray JS. Effects of environmental stress on species rich assemblages. Biol J Linn Soc Lond. 1989;37(1-2):19–32. [Google Scholar]
- 36.Warwick RM. A new method for detecting pollution effects on marine macrobenthic communities. Mar Biol. 1986;92(4):557–562. [Google Scholar]
- 37.Pimentel D, et al. Environmental and economic costs of soil erosion and conservation benefits. Science. 1995;267(5201):1117–1123. doi: 10.1126/science.267.5201.1117. [DOI] [PubMed] [Google Scholar]
- 38.Lal R. Soil erosion and the global carbon budget. Environ Int. 2003;29(4):437–450. doi: 10.1016/S0160-4120(02)00192-7. [DOI] [PubMed] [Google Scholar]
- 39.Cramer VA, Hobbs RJ, Standish RJ. What’s new about old fields? Land abandonment and ecosystem assembly. Trends Ecol Evol. 2008;23(2):104–112. doi: 10.1016/j.tree.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Palanques A, et al. General patterns of circulation, sediment fluxes and ecology of the Palamós (La Fonera) submarine canyon, north western Mediterranean. Prog Oceanogr. 2005;66(2-4):89–119. [Google Scholar]
- 41.Martín J, Palanques A, Puig P. Composition and variability of downward particulate matter fluxes in the Palamós submarine canyon (NW Mediterranean) J Mar Syst. 2006;60(1-2):75–97. [Google Scholar]
- 42.Martín J, Palanques A, Puig P. Near-bottom horizontal transfer of particulate matter in the Palamós Submarine Canyon (NW Mediterranean) J Mar Res. 2007;65(2):193–218. [Google Scholar]
- 43.Pusceddu A, Dell’Anno A, Fabiano M, Danovaro R. Quantity and bioavailability of sediment organic matter as signatures of benthic trophic status. Mar Ecol Prog Ser. 2009;375:41–52. [Google Scholar]
- 44.Danovaro R. Methods for the Study of Deep-Sea Sediments, Their Functioning and Biodiversity. Boca Raton, FL: CRC; 2010. [Google Scholar]
- 45.Pinckney J, Papa R, Zingmark R. Comparison of high-performance liquid chromatographic, spectrophotometric, and fluorometric methods for determining chlorophyll a concentrations in estuarine sediments. J Microb Met. 1994;19(1):59–66. [Google Scholar]
- 46.Fabiano M, Danovaro R. Enzymatic activity, bacterial distribution, and organic matter composition in sediments of the ross sea (Antarctica) Appl Environ Microbiol. 1998;64(10):3838–3845. doi: 10.1128/aem.64.10.3838-3845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feller RJ, Warwick RM. Energetics. In: Higgins RP, Thiel H, editors. Introduction to the Study of Meiofauna. Washington, DC: Smithsonian Institution Press; 1988. [Google Scholar]
- 48.Seinhorst JW. A rapid method for the transfer of nematodes from fixative to anhydrous glycerine. Nematologica. 1959;4(1):67–69. [Google Scholar]
- 49.Sanders HL. Marine benthic diversity: A comparative study. Am Nat. 1968;102(925):243–282. [Google Scholar]
- 50.Hurlbert SH. The nonconcept of species diversity: A critique and alternative parameters. Ecology. 1971;52(4):577–586. doi: 10.2307/1934145. [DOI] [PubMed] [Google Scholar]
- 51.Soetaert K, Heip C. Sample-size dependence of diversity indices and the determination of sufficient sample size in a high-diversity deep-sea environment. Mar Ecol Prog Ser. 1990;59:305–307. [Google Scholar]
- 52.Danovaro R, Bianchelli S, Gambi C, Mea M, Zeppilli D. α-, β-, γ-, δ-, and ε-diversity of deep-sea nematodes in canyons and open slopes of Northeast Atlantic and Mediterranean margins. Mar Ecol Progr Ser. 2009;396:197–209. [Google Scholar]
- 53.Bongers T, Alkemade R, Yeates GW. Interpretation of disturbance-induced maturity decrease in marine nematode assemblages by means of the Maturity Index. Mar Ecol Prog Ser. 1991;76:135–142. [Google Scholar]
- 54.Bongers T, Bongers M. Functional diversity of nematodes. Appl Soil Ecol. 1998;10(3):239–251. [Google Scholar]
- 55.Gambi C, Vanreusel A, Danovaro R. Biodiversity of nematode assemblages from deep-sea sediments of the Atacama Slope and Trench (South Pacific Ocean) Deep Sea Res Part I Oceanogr Res Pap. 2003;50(1):103–117. [Google Scholar]
- 56.Sanchez-Cabeza JA, Masqué P, Ani-Ragolta I. 210Pb and 210Po analysis in sediments and soils by microwave acid digestion. J Radioanal Nucl Chem. 1998;227(1-2):19–22. [Google Scholar]
- 57.Anderson MJ. Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci. 2001;58(3):626–639. [Google Scholar]
- 58.Miquel JC, et al. Dynamics of particle flux and carbon export in the northwestern Mediterranean Sea: A two-decade time-series study at the DYFAMED site. Prog Oceanogr. 2011;91(4):461–481. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.