Significance
Advancing our understanding of how and why forests dynamically change in their productivity is important to predict the future change. The traditional view of forest dynamics originated by Kira, Shidei, and Odum suggests a decline in net primary productivity [or gross primary productivity (GPP) − autotrophic respiration (Ra)] in aging forests due to stabilized GPP and continuously increased Ra. We found that, in contrast to the traditional view, both GPP and Ra decline in aging forests while GPP decreases more rapidly than Ra does, and thus generalize the alternative hypothesis initiated by Ryan and colleagues with a large dataset. We presented a new quantitative model to describe forest dynamics that can be incorporated into ecosystem models.
Keywords: chronosequence, plant respiration, carbon use efficiency, ecosystem development, eddy covariance
Abstract
The traditional view of forest dynamics originated by Kira and Shidei [Kira T, Shidei T (1967) Jap J Ecol 17:70–87] and Odum [Odum EP (1969) Science 164(3877):262–270] suggests a decline in net primary productivity (NPP) in aging forests due to stabilized gross primary productivity (GPP) and continuously increased autotrophic respiration (Ra). The validity of these trends in GPP and Ra is, however, very difficult to test because of the lack of long-term ecosystem-scale field observations of both GPP and Ra. Ryan and colleagues [Ryan MG, Binkley D, Fownes JH (1997) Ad Ecol Res 27:213–262] have proposed an alternative hypothesis drawn from site-specific results that aboveground respiration and belowground allocation decreased in aging forests. Here, we analyzed data from a recently assembled global database of carbon fluxes and show that the classical view of the mechanisms underlying the age-driven decline in forest NPP is incorrect and thus support Ryan’s alternative hypothesis. Our results substantiate the age-driven decline in NPP, but in contrast to the traditional view, both GPP and Ra decline in aging boreal and temperate forests. We find that the decline in NPP in aging forests is primarily driven by GPP, which decreases more rapidly with increasing age than Ra does, but the ratio of NPP/GPP remains approximately constant within a biome. Our analytical models describing forest succession suggest that dynamic forest ecosystem models that follow the traditional paradigm need to be revisited.
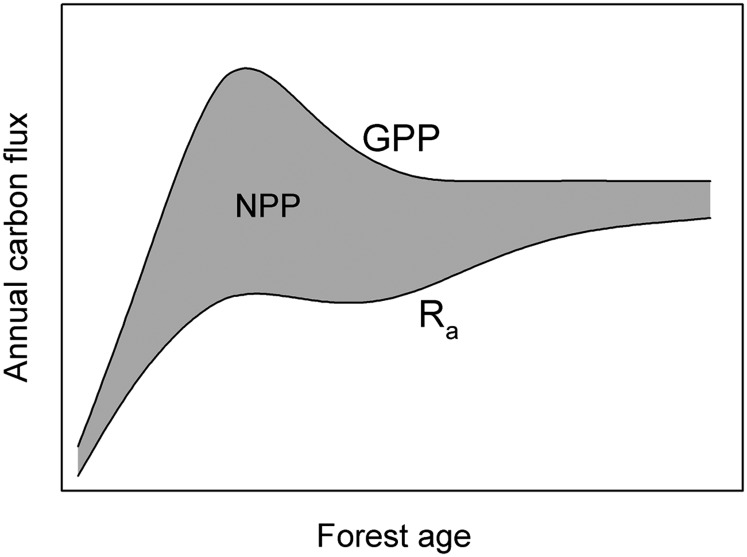
It has been long observed and well established that forest net primary production (NPP), particularly aboveground NPP, increases during initial stand development, peaks at maturity, and then gradually declines as forests age (1–8). Kira and Shidei (9) were the first to analyze 10 y of empirical data and developed the long-accepted theory that forest NPP declines with age because wood respiration increases in response to the accumulating wood biomass, whereas gross primary production (GPP) remains quasiconstant (Fig. 1). Similarly, in his theory of ecosystem succession, Odum (10) postulated that ecosystem respiration (i.e., the sum of autotrophic and heterotrophic respiration) increases with age and eventually balances GPP such that the net ecosystem carbon balance approaches zero at a dynamic steady state. Odum did not specify the successional pattern of autotrophic respiration (Ra) and NPP, but the underlying assumption is similar to that of Kira and Shidei, i.e., the difference between carbon uptake and release declines with age primarily because respiratory losses increase.
Fig. 1.
The traditional conceptual model of GPP, NPP, and Ra as a function of forest age [modified from Kira and Shidei (9) and Odum (10)]. The gray area indicates NPP, the difference between the GPP line and Ra line.
Ryan et al. have disproved these earlier hypothesized patterns and contended that total stem respiration, including growth and maintenance respiration, in a chronosequence of subalpine lodgepole pine (Pinus contorta ssp. latifolia) stands in Colorado did not increase with forest age (11), and that the observed decrease in aboveground NPP with increasing age in an experimental forest of Eucalyptus saligna in Hawaii originated from a decrease in GPP that overshadows a simultaneous decrease in the sum of all carbon that is not used for aboveground NPP (i.e., aboveground respiration plus belowground allocation) (3). Ryan et al. concluded that, with forest aging, canopy photosynthesis decreases in company with a decline in aboveground production, aboveground respiration, and belowground allocation (2). More recently, Drake et al. (6) supported Ryan et al. (2, 3) by concluding that the decrease in NPP with age was driven by the decrease in GPP and the companion decrease in Ra, a conclusion drawn from their work conducted across a chronosequence of forest stands at the Duke Forest in North Carolina. However, with the widely used eddy covariance method and its companion measurements of NPP and heterotrophic respiration, the classical hypothesis was supported recently that the increasing Ra, rather than GPP, drove the decrease in NPP along a chronosequence of boreal forest stands (12). Therefore, to empirically test Odum’s established theory and the generality of the alternative hypothesis proposed by Ryan et al. (2, 3, 11) and supported by Drake et al. (6) through site-specific studies, more measurement data across different biomes and ecosystems are needed to support if it is because (i) GPP decreases but Ra increases with forest age, or (ii) both GPP and Ra decrease with age that results in the observed decrease in NPP with age.
Advancing our mechanistic understanding of the control of forest age on ecosystem production and respiration has become more crucial during the past decades because improving our ability to predict long-term ecosystem responses to global change is urgently needed for making sound climate change policy. Although most second-growth forests are in dynamic succession, lacking the ability to incorporate forest age into ecosystem and carbon cycle models as an important driver makes prediction of future ecosystems unrealistic. As a result, few ecosystem models have been able to include forest age or succession as an important variable (7). How to quantify the age effect on carbon dynamics is challenging in model development. Recent advancement of technology in carbon flux measurement has dramatically expanded our ability to quantify the ecosystem carbon budget from individual plants (e.g., ref. 13) to the landscape scale (14). As a result, more and more GPP, NPP, and Ra datasets are becoming available.
To revisit the classical ecological paradigm and generalize the alternative paradigm on the age-driven decline of forest productivity, we use a recently assembled extensive global dataset (15) to test (i) whether forest NPP declines in aging forests as a general trend across biomes, and (ii) whether the age-related decline in forest NPP is due to increasing respiration, as assumed in the paradigm, or is instead primarily due to decreasing GPP. We intend to propose an analytical model to explain the age-driven change in forest productivity and respiration.
Results and Discussion
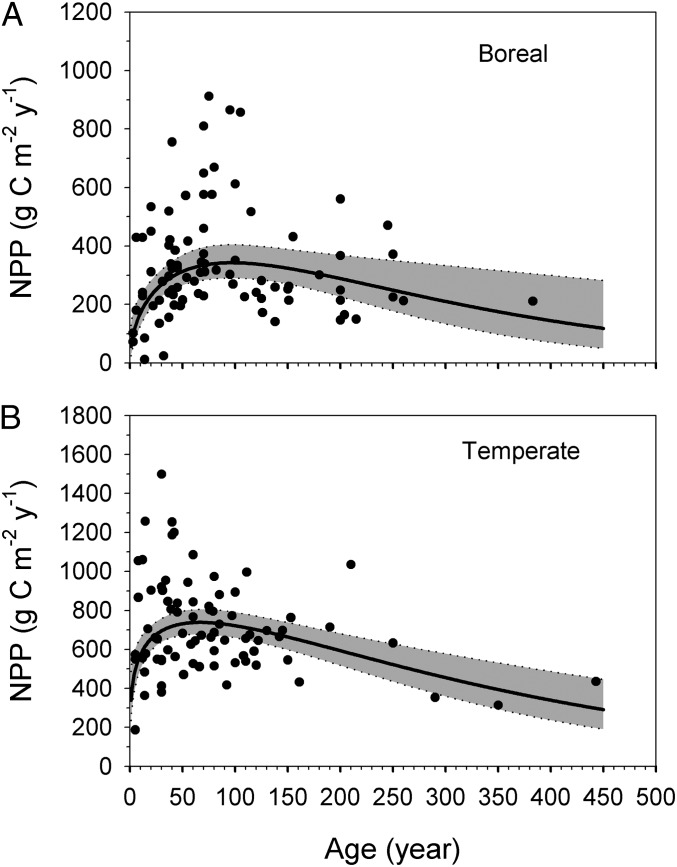
The age patterns of NPP for temperate and boreal forests agree with the classical model, i.e., following a stand-replacing disturbance, NPP initially increases, reaches a maximum at maturity, and then decreases with further forest aging (Fig. 2). The fitted Γ functions are highly statistically significant (t tests and F tests), but exhibit low R2 (Table 1). These low R2 values were expected given the strong dependence of NPP on other factors such as climate, soil fertility, disturbances, management history, etc. (15, 16). Among the five models that were compared (the second-degree polynomial function, the third-degree polynomial function, the logarithmic function, the Michaelis–Menten function, and the Γ function), the Γ function had the best overall performance (the lowest Akaike information criterion, or AICc) across the four datasets (temperate NPP vs. age, boreal NPP vs. age, temperate GPP vs. age, and boreal GPP vs. age). The rare NPP or GPP values from old forests (age > 300 y) were found to be important points in the regression lines, but none of them was statistically highly influential (Di > 1, Di is the Cook’s distance), and all were within the 95% confidence interval areas of the regressions. However, we are aware that the parameters of the functions may change if we have more data, particularly in older forests.
Fig. 2.
The NPP patterns with forest age in boreal (A) and temperate (B) forests. The dots are NPP measurements. The solid lines are fitted from measurement data (Eq. 1, Table 1). The gray shades indicate 95% confidence interval of fitted NPP.
Table 1.
Parameters in the gamma (Γ) function estimated for the boreal and temperate forests
| k0 | k1 | k2 | n | p | R2 | |
| Boreal GPP | 235.152 | 0.426 | −0.00484 | 29 | <0.01 | 0.58 |
| NPP | 56.202 | 0.505 | −0.00521 | 86 | <0.01 | 0.15 |
| Temperate GPP | 975.914 | 0.173 | −0.00295 | 44 | <0.02 | 0.14 |
| NPP | 339.612 | 0.243 | −0.00364 | 80 | <0.01 | 0.21 |
In boreal forests, the fitted regression model showed a maximum NPP at 340 (290–400, 95% confidence interval) g C⋅m−2⋅y−1 around 100 y old. In temperate forests, the fitted maximum NPP amounted to 740 (680–800, 95% confidence interval) g C⋅m−2⋅y−1 at ∼70 y old. The higher maximum NPP and earlier maturity in temperate compared with boreal forests may be the consequence of the higher metabolic rates due to the higher mean annual temperature and also higher soil fertility in temperate forests.
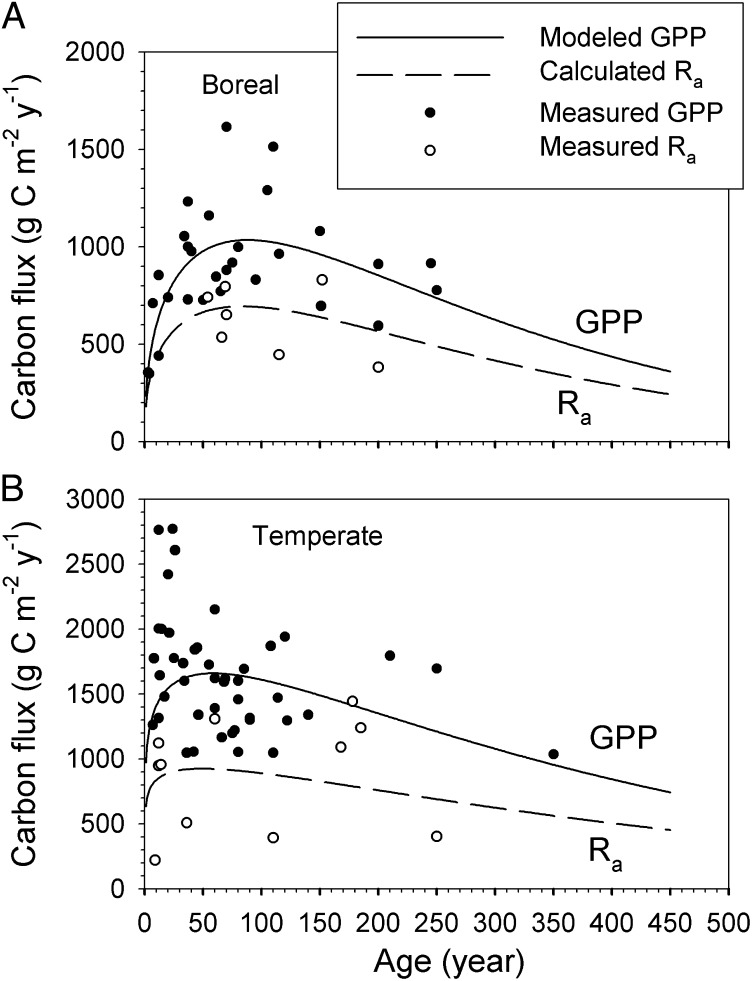
Although the NPP data in our dataset agree with the observations upon which Kira and Shidei (9) developed their original hypothesis, the field observations of GPP and the derived Ra (calculated as GPP – NPP) do not follow the pattern assumed in the classical model (Fig. 3). In contrast to the earlier theorem, GPP does not stabilize at levels slightly below the maximum value at maturity, but declines far more than previously assumed. Potential mechanisms to explain the age-related decrease in GPP primarily include (i) nutrient limitation: soil nutrients, especially nitrogen, become increasingly immobilized in the organic surface horizon and in the N-rich, humified soil organic matter due to the accumulation of woody biomass (1); (ii) hydraulic limitation: hydraulic resistance increases with tree height, resulting in decreased stomatal conductance (3, 17, 18); and (iii) genetic control: reduced photosynthetic rates could be controlled by gene expression programmed in the meristematic cells in plant stems, resulting in diminishing metabolism rates in aging plants (19, 20). Although these mechanisms have been discussed and tested in a limited number of studies (1, 3), no consensus has yet been reached (21, 22).
Fig. 3.
The GPP and Ra patterns with forest age in boreal (A) and temperate (B) forests. The dots are measurement data. The solid lines indicate the modeled GPP using Eq. 1 and parameters from Table 1. The dashed lines indicate the calculated Ra as GPP – NPP.
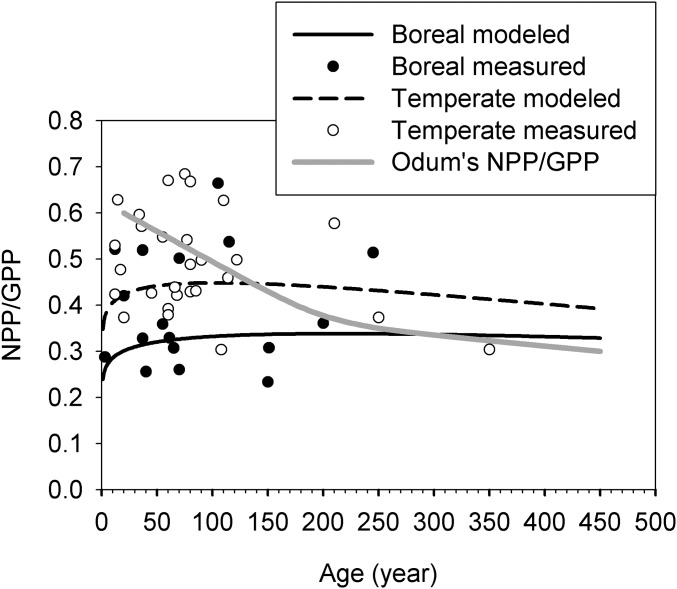
Our results suggest that the NPP/GPP ratio varies considerably among forest biomes (Fig. 4). The difference in the NPP/GPP ratio between mature boreal and temperate forests is significant, as NPP/GPP ranging from 0.30 to 0.34 in boreal forests and 0.40 to 0.45 in temperate forests, analytically derived from the ratio of NPP/GPP with the parameters from Table 1 and Eq. 1. Paired GPP–NPP measurement data verified these results. NPP/GPP is significantly different between temperate and boreal forests (ANOVA, P < 0.01) with mean values of 0.39 (±0.13 SD, n = 17) in boreal forests and 0.49 (±0.11 SD, n = 28) in temperate forests. Vicca et al. (16) postulated that this large difference in NPP/GPP between boreal and temperate forests is mainly due to the typically higher fertility in temperate forests, where trees invest less photosynthates in nutrient acquisition mechanisms such as exudation or symbionts, leaving a higher fraction of GPP available for wood production.
Fig. 4.
Changes in NPP/GPP with age in boreal and temperate forests. The dots are measurements from paired GPP–NPP data. The black lines are derived from the modeled GPP and NPP using Eq. 1 and parameters from Table 1. The gray line is conceptual following Odum’s traditional model in Fig. 1.
Within both the boreal and temperate biomes, we observed a stable NPP/GPP ratio across age (Fig. 4). Paired GPP–NPP data indicate that the correlation between GPP/NPP and age is not significant (slope P > 0.05) in both temperate and boreal forests. This constancy with age (two black lines in Fig. 4) contradicts with the original theorem, where the NPP/GPP ratio is expected to decrease with age because NPP declines and GPP stabilizes (the gray line in Fig. 4). Our observed trend of insensitivity of NPP/GPP to age within a biome but significant difference between boreal and temperate forests disagrees with previous publications that NPP/GPP decreases with age (23, 24). We also disagree with that NPP/GPP tends to be conservative across age classes and ecosystems (25–28) and agrees with the variability of NPP/GPP across ecosystems (23). Recent studies has shown that the NPP/GPP ratio could by influenced more by temperature (29) or by nutrient availability (16) than by age.
In the classical model, Ra would be expected to continue to increase as forests age (Fig. 1). Both our calculated Ra lines and measurement data contradict this classical hypothesis, with a decrease in Ra after maturity (Fig. 3), in agreement with findings of Ryan et al. (2, 3, 11). The primary explanation for the hypothesized continuous increase in respiration was that, although leaf respiration stabilizes with maximum leaf area index after canopy closure, wood respiration continues to increase with the accumulation of woody biomass in aging forests (9). In recent years, many studies have indicated the tight coupling between ecosystem GPP and respiration (30–33), which is not surprising given that plants cannot respire more than the available supply of recent or stored photosynthate. This tight coupling between GPP and respiration not only substantiates the indication for a constant NPP/GPP ratio with age within a biome but also suggests that the decrease in GPP is the prime reason for the decline in Ra. If the respiratory carbon cost is indeed a fixed proportion of GPP, then a reduction in GPP with age would be the main cause of the widely observed age-driven decline in NPP.
There are other mechanisms that uncouple respiration from accumulated biomass stocks in aging forests and could explain the reduction in Ra more mechanistically. If autotrophic respiration is partitioned into growth and maintenance respiration, the latter may increase with age due to the increase in standing biomass, but growth respiration would decline with the age-related decrease in NPP, perhaps offsetting the increase in maintenance respiration, and thus potentially resulting in a decrease in total autotrophic respiration (2). Moreover, leaf and root biomass are unlikely to increase with aging, and the respiratory sapwood only accounts for a small fraction of the accumulated woody biomass in aging forests. Hence, any age-related increase in maintenance respiration with increasing forest age is expected to be very limited. An empirical study has indeed shown that stem respiration in an old-growth hardwood stand decreased compared with young and mature stands in the Great Lakes area (13).
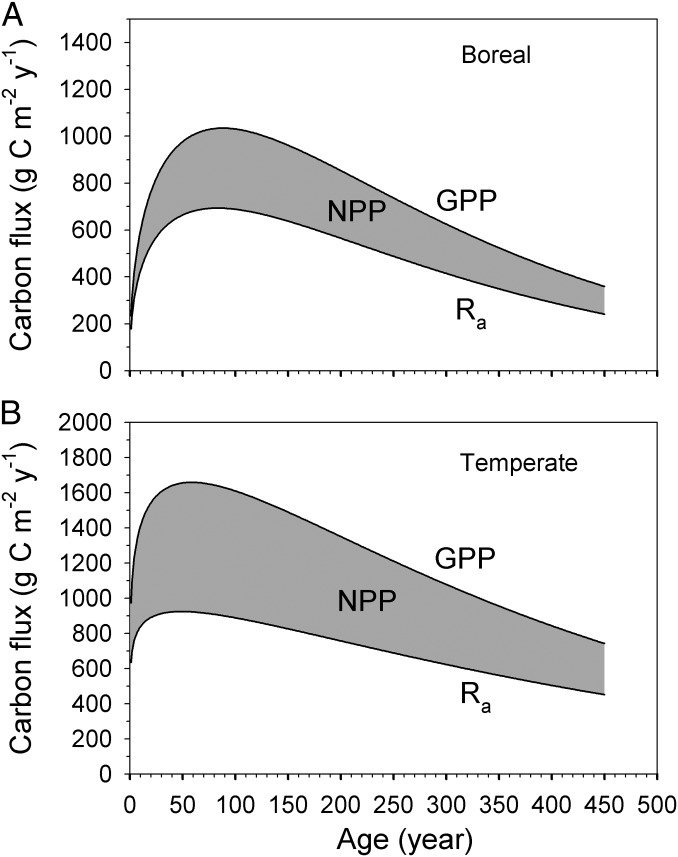
Because our results for age-related trends in GPP and Ra for both boreal and temperate forests oppose the traditional view of the mechanism underlying the age-related decline in forest NPP, we propose here a previously unidentified conceptual model (Fig. 5). This conceptual model, presented in terms of parameterized Γ functions, builds on that proposed earlier by Ryan et al. (2) but differs from both the classical model and this newer view in that we specify that the decrease in NPP results from the combined effects of (i) a decline in GPP with increasing age and (ii) an approximately constant NPP/GPP ratio independent of age, i.e., Ra follows the change in GPP but decreases more slowly with increasing age than GPP in aging forests. Our results suggest that the age effect on forest GPP, Ra, and NPP, independent of climatic and edaphic controls and management practices, is of fundamental importance for understanding patterns of forest growth and carbon dynamics. Long-term forest ecosystem models must therefore also consider age as a dominant control on spatial and temporal patterns of forest productivity to realistically simulate carbon cycles. The ratio of NPP/GPP (and thus of Ra/GPP) is highly variable across biomes but appears independent of age in mature forests within a biome. The decline in NPP in aging forests is mainly driven by the reduction in GPP. The mechanism of reduced GPP with age needs further studies.
Fig. 5.
Our model showing the decrease in NPP resulting from both decreases in GPP and Ra in boreal (A) and temperate (B) forests, in contrast to Fig. 1. The GPP line and the gray area of NPP are fitted from measurement data (Eq. 1 and Table 1), and the Ra line is derived from fitted GPP and NPP as GPP – NPP.
Materials and Methods
We used an established global database (15) of forest GPP and NPP, obtained from eddy covariance measurement (34, 35), chamber-based flux measurement, biometric inventories, modeling results, and corresponding site properties and climatic parameters to investigate the age effect on GPP, NPP, Ra, and NPP/GPP. The database was assembled from published literature, existing computer databases (36, 37), and the AmeriFlux and CarboEurope networks within the FLUXNET (38). NPP was typically measured by the sum of incremental biomass of forest woods and annual production of leaves and roots. Estimating NPP for roots is challenging (39) and allometric equations (e.g., ref. 6), root in-growth cores (40), or minirhizotrons (e.g., ref. 41) are often used. GPP was derived from eddy covariance measurements (34, 35), modeling results, or biometric (for NPP) plus chamber measurement (for Ra). Chamber-based measurement of Ra is the sum of respiration from individual components (woods, leaves, and roots) (e.g., ref. 13).
We compiled a subset of the database by using only annual sums of GPP, NPP, Ra, and age (years since regeneration after a major disturbance, e.g., harvest, fire, or land-use shift). We excluded any forest sites subject to major experimental or management treatments (e.g., elevated ambient CO2 concentration, fertilization, thinning, and/or irrigation). We also averaged multiple years, where available, of GPP, NPP, and Ra to obtain mean annual values. For those sites with different GPP values originating from multiple methods, we used only eddy covariance-derived GPP. Although reporting uncertainty is important (42), most individual datasets do not contain uncertainty levels. Based on the literature reports on eddy flux uncertainty (14, 43) and expert judgment (15), the uncertainty, varying among individual sites, was less than 30% for GPP and 20% for NPP for our dataset. We have not found any evidence that the uncertainty is correlated with forest age. We also acknowledge the uncertainty associated with the chronosequence approach (e.g., ref. 5) that we used to study the age effect on GPP, NPP, and Ra, given the variability of site conditions. Therefore, we grouped datasets by major biomes and assumed that this uncertainty associated with the chronosequence method is randomly distributed within a biome.
We categorized all forest sites into boreal, temperate, Mediterranean, and tropical biomes based on climatic characteristics and species mixtures. Due to limited measurement data in Mediterranean and tropical sites for regression analysis, in this study we only analyzed boreal and temperate sites. As a result, we obtained 73 sites with GPP data, 166 sites with NPP data, 17 sites with independently measured Ra data (not calculated from GPP − NPP), and 45 paired GPP–NPP datasets all accompanied with age data (see Table S1 for details). Paired GPP–NPP data mean that both GPP and NPP were independently measured at one site at the same or close to the same year so that we could directly calculate the NPP/GPP ratio for this site.
We used a gamma (Γ) function (Eq. 1) to fit the age effect of GPP and NPP for both boreal and temperate biomes. This type of function has been used to describe the age-driven change of forest floor organic matter (44) even though the simplicity of the pattern has been challenged by field-based data (45):
| [1] |
where y is GPP or NPP (in grams of carbon per square meter per year), t is the forest age (in years since the last major disturbance such as harvest or fire), and k0, k1, and k2 are parameters.
Eq. 1 could be log-transformed to a linear equation. Parameters in Eq. 1 were then estimated by conducting multivariate linear regression. Two sets of the Γ function for GPP and NPP, respectively, allowed us to analytically derive Ra (GPP – NPP) and the ratio of NPP/GPP as a function of age. Independently measured NPP/GPP and Ra data were used to validate the modeled results of NPP/GPP and Ra.
We determined the age at which maximum values of GPP and NPP were obtained by differentiating Eq. 1 (Eq. 2):
| [2] |
We also tested different functions to fit the data, including a second-degree polynomial function, a third-degree polynomial function, a logarithm function, and a Michaelis–Menten function [y = ax/(b + x)]. We used the root-mean-squared error (RMSE) to evaluate the model accuracy and efficiency. We also used the Akaike information criterion (AIC) to compare models as AIC considers both the lowest RMSE and the fewest model parameters, accounting for both goodness-of-fit and the complexity of the model (46, 47). When the number of parameters (p) is large comparing with the sample size (n) (generally n/P < 40), we used AICc (Eq. 3) (47). The model with the lowest AICc is the best candidate:
| [3] |
where n is the number of observations, σ is the RMSE, and p is the number of parameters.
Because the age data are not evenly distributed with less data in older forests, we used the “Cook’s distance” to estimate the influence of a data point (48), for example, in old-growth forests:
| [4] |
where Di is the Cook’s distance for data point i, Ŷj is the predicted value from the model for data point j, Ŷj(i) is the predicted value for point j from the model in which point i has been omitted, p is the number of fitted parameters in the model, and MSE is the mean square error (deviation), or RMSE2. The Cook’s distance considers both the x value and y value to evaluate if a data point is highly influential (an outlier). Although controversial, The point with Di > 1 is considered a highly influential point (49). If there are points with substantially high Di than others, we carefully recheck the points by comparing regression with and without these points and remove the points if they are outliers.
Statistical analyses were conducted with the statistical package Stata (Stata Corporation).
Supplementary Material
Acknowledgments
We thank all site investigators, their funding agencies, and the various regional flux networks (Afriflux, AmeriFlux, AsiaFlux, CarboAfrica, CarboEurope Integrated Project, ChinaFlux, Fluxnet–Canada, KoFlux, Large-Scale Biosphere–Atmosphere Experiment in Amazonia, Nordic Centre for Studies of Ecosystem Carbon Exchange and Its Interactions with the Climate System, OzFlux, Terrestrial Carbon Observation System–Siberia, and US–China Carbon Consortium), and the Fluxnet project, whose support is essential for obtaining the measurement data without which this synthesis analysis would not be possible. We thank Xi Yang for statistical assistance, Matteo Campioli for maintaining and updating the original database, and Dennis Baldocchi for constructive comments on the earlier version of the manuscript. The collection of the original global database was funded by the Research Foundation–Flanders (FWO-Vlaanderen) who supported S.L. with a postdoctoral fellowship and a research grant (FWO 1.5037.07N). J.T. was partially supported by US Department of Energy Office of Biological and Environmental Research Grant DE-SC0006951 and National Science Foundation Grants DBI-959333 and AGS-1005663.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320761111/-/DCSupplemental.
References
- 1.Gower ST, McMurtrie RE, Murty D. Aboveground net primary production decline with stand age: Potential causes. Trends Ecol Evol. 1996;11(9):378–382. doi: 10.1016/0169-5347(96)10042-2. [DOI] [PubMed] [Google Scholar]
- 2.Ryan MG, Binkley D, Fownes JH. Age-related decline in forest productivity: Pattern and process. Adv Ecol Res. 1997;27:213–262. [Google Scholar]
- 3.Ryan MG, Binkley D, Fownes JH, Giardina CP, Senock RS. An experimental test of the causes of forest growth decline with stand age. Ecol Monogr. 2004;74(3):393–414. [Google Scholar]
- 4.Luyssaert S, et al. Old-growth forests as global carbon sinks. Nature. 2008;455(7210):213–215. doi: 10.1038/nature07276. [DOI] [PubMed] [Google Scholar]
- 5.Kashian DM, Romme WH, Tinker DB, Turner MG, Ryan MG. Postfire changes in forest carbon storage over a 300-year chronosequence of Pinus contorta-dominated forests. Ecol Monogr. 2013;83(1):49–66. [Google Scholar]
- 6.Drake JE, Davis SC, Raetz LM, DeLucia EH. Mechanisms of age-related changes in forest production: The influence of physiological and successional changes. Glob Change Biol. 2011;17(4):1522–1535. [Google Scholar]
- 7.He LM, Chen JM, Pan YD, Birdsey R, Kattge J. Relationships between net primary productivity and forest stand age in U.S. forests. Global Biogeochem Cy. 2012;26(3) [Google Scholar]
- 8.Pregitzer KS, Euskirchen En S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob Change Biol. 2004;10(12):2052–2077. [Google Scholar]
- 9.Kira T, Shidei T. Primary production and turnover of organic matter in different forest ecosystems of the Western Pacific. Jap J Ecol. 1967;17:70–87. [Google Scholar]
- 10.Odum EP. The strategy of ecosystem development. Science. 1969;164(3877):262–270. doi: 10.1126/science.164.3877.262. [DOI] [PubMed] [Google Scholar]
- 11.Ryan MG, Waring RH. Maintenance respiration and stand development in a sub-alpine lodgepole pine forest. Ecology. 1992;73(6):2100–2108. [Google Scholar]
- 12.Goulden ML, et al. Patterns of NPP, GPP, respiration, and NEP during boreal forest succession. Glob Change Biol. 2011;17(2):855–871. [Google Scholar]
- 13.Tang J, et al. Ecosystem respiration and its components in an old-growth northern forest. Agric For Meteorol. 2008;148:171–185. [Google Scholar]
- 14.Baldocchi DD. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob Change Biol. 2003;9(4):479–492. [Google Scholar]
- 15.Luyssaert S, et al. CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob Change Biol. 2007;13(12):2509–2537. [Google Scholar]
- 16.Vicca S, et al. Fertile forests produce biomass more efficiently. Ecol Lett. 2012;15(6):520–526. doi: 10.1111/j.1461-0248.2012.01775.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoder BJ, Ryan MG, Waring RH, Schoettle AW, Kaufmann MR. Evidence of reduced photosynthetic rates in old trees. For Sci. 1994;40(3):513–527. [Google Scholar]
- 18.Koch GW, Sillett SC, Jennings GM, Davis SD. The limits to tree height. Nature. 2004;428(6985):851–854. doi: 10.1038/nature02417. [DOI] [PubMed] [Google Scholar]
- 19.Haffner V, Enjalric F, Lardet L, Carron MP. Maturation of woody plants: A review of metabolic and genomic aspects. Ann Sci For. 1991;48:615–630. [Google Scholar]
- 20.Day ME, Greenwood MS, White AS. Age-related changes in foliar morphology and physiology in red spruce and their influence on declining photosynthetic rates and productivity with tree age. Tree Physiol. 2001;21(16):1195–1204. doi: 10.1093/treephys/21.16.1195. [DOI] [PubMed] [Google Scholar]
- 21.Ryan MG, Phillips N, Bond BJ. The hydraulic limitation hypothesis revisited. Plant Cell Environ. 2006;29(3):367–381. doi: 10.1111/j.1365-3040.2005.01478.x. [DOI] [PubMed] [Google Scholar]
- 22.Kutsch WL, et al. 2009. Ecophysiological characteristics of mature trees and stands—consequences for old-growth forest productivity. Old Growth Forests, Ecological Studies, ed Cea W (Springer, Berlin), Vol 207, pp 57–79.
- 23.Mäkelä A, Valentine HT. The ratio of NPP to GPP: Evidence of change over the course of stand development. Tree Physiol. 2001;21(14):1015–1030. doi: 10.1093/treephys/21.14.1015. [DOI] [PubMed] [Google Scholar]
- 24.DeLucia EH, Drake JE, Thomas RB, Gonzalez-Meler M. Forest carbon use efficiency: Is respiration a constant fraction of gross primary production? Glob Change Biol. 2007;13(6):1157–1167. [Google Scholar]
- 25.Ryan MG, Lavigne MB, Gower ST. Annual carbon cost of autotrophic respiration in boreal forest ecosystems in relation to species and climate. J Geophys Res-Atmos. 1997;102(D24):28871–28883. [Google Scholar]
- 26.Waring RH, Landsberg JJ, Williams M. Net primary production of forests: A constant fraction of gross primary production? Tree Physiol. 1998;18(2):129–134. doi: 10.1093/treephys/18.2.129. [DOI] [PubMed] [Google Scholar]
- 27.Gifford RM. Plant respiration in productivity models: Conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol. 2003;30(2):171–186. doi: 10.1071/FP02083. [DOI] [PubMed] [Google Scholar]
- 28.Litton CM, Raich JW, Ryan MG. Carbon allocation in forest ecosystems. Glob Change Biol. 2007;13(10):2089–2109. [Google Scholar]
- 29.Piao SL, et al. Forest annual carbon cost: A global-scale analysis of autotrophic respiration. Ecology. 2010;91(3):652–661. doi: 10.1890/08-2176.1. [DOI] [PubMed] [Google Scholar]
- 30.Högberg P, et al. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature. 2001;411(6839):789–792. doi: 10.1038/35081058. [DOI] [PubMed] [Google Scholar]
- 31.Janssens IA, et al. Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob Change Biol. 2001;7(3):269–278. [Google Scholar]
- 32.Tang J, Baldocchi DD, Xu L. Tree photosynthesis modulates soil respiration on a diurnal time scale. Glob Change Biol. 2005;11(8):1298–1304. [Google Scholar]
- 33.Hopkins F, et al. Ecosystem-level controls on root-rhizosphere respiration. New Phytol. 2013;199(2):339–351. doi: 10.1111/nph.12271. [DOI] [PubMed] [Google Scholar]
- 34.Falge E, et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric For Meteorol. 2001;107(1):43–69. [Google Scholar]
- 35.Reichstein M, et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob Change Biol. 2005;11(9):1424–1439. [Google Scholar]
- 36.Olson RJ, Johnson KR, Zheng DL, Scurlock JMO. 2001. Global and Regional Ecosystem Modeling: Database of Model Drivers and Validation Measurements (Oak Ridge National Laboratory, Oak Ridge, TN), Tech Rep ORNL/TM-2001/196.
- 37.Papale D, et al. Towards a standardized processing of Net Ecosystem Exchange measured with eddy covariance technique: Algorithms and uncertainty estimation. Biogeosciences. 2006;3(4):571–583. [Google Scholar]
- 38.Baldocchi D, et al. FLUXNET: A new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull Am Meteorol Soc. 2001;82(11):2415–2434. [Google Scholar]
- 39.Hendricks JJ, et al. Assessing the patterns and controls of fine root dynamics: An empirical test and methodological review. J Ecol. 2006;94(1):40–57. [Google Scholar]
- 40.Vogt KA, Persson H. Measuring growth and development of roots. In: Lassoie JP, Hinckley TM, editors. Techniques and Approaches in Forest Tree Ecophysiology. Boca Raton, FL: CRC; 1991. pp. 477–501. [Google Scholar]
- 41.Norby RJ, Ledford J, Reilly CD, Miller NE, O’Neill EG. Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc Natl Acad Sci USA. 2004;101(26):9689–9693. doi: 10.1073/pnas.0403491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanai RD, et al. Estimating uncertainty in ecosystem budget calculations. Ecosystems (N Y) 2010;13(2):239–248. [Google Scholar]
- 43.Reichstein M, et al. Determinants of terrestrial ecosystem carbon balance inferred from European eddy covariance flux sites. Geophys Res Lett. 2007;34:L01402. [Google Scholar]
- 44.Covington WW. Changes in forest floor organic matter and nutrient content following clear cutting in northern hardwoods. Ecology. 1981;62(1):41–48. [Google Scholar]
- 45.Yanai RD, Currie WS, Goodale CL. Soil carbon dynamics after forest harvest: An ecosystem paradigm reconsidered. Ecosystems (N Y) 2003;6(3):197–212. [Google Scholar]
- 46.Yang X, Mustard JF, Tang JW, Xu H. Regional-scale phenology modeling based on meteorological records and remote sensing observations. J Geophys Res Biogeosci. 2012;117:G03029. [Google Scholar]
- 47.Migliavacca M, et al. On the uncertainty of phenological responses to climate change, and implications for a terrestrial biosphere model. Biogeosciences. 2012;9(6):2063–2083. [Google Scholar]
- 48.Cook RD. Detection of influential observation in linear regression. Technometrics. 1977;19(1):15–18. [Google Scholar]
- 49.Cook RD, Weisberg S. Residuals and Influence in Regression. New York: Chapman & Hall; 1982. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.