Significance
The auxiliary α2δ-1 subunits of voltage-gated calcium (CaV) channels are important therapeutic targets, representing the receptor for gabapentinoid drugs in neuropathic pain therapy. It is therefore important to understand their function. Because α2δ subunits augment calcium currents, it is believed that they increase cell-surface expression of these channels. Here, using exofacially tagged CaV2.2 constructs, we now show this to be the case. However, recent proteomic analysis found that α2δ subunits are associated only loosely and nonquantitatively with CaV2 channels, challenging their role as calcium channel subunits. In contrast, we find that CaV2.2 and α2δ-1 are intimately and completely associated at the plasma membrane and that this is not disrupted by the α2δ-1 ligand gabapentin, which reduces cell-surface expression of both CaV2.2 and α2δ-1.
Abstract
CaV1 and CaV2 voltage-gated calcium channels are associated with β and α2δ accessory subunits. However, examination of cell surface-associated CaV2 channels has been hampered by the lack of antibodies to cell surface-accessible epitopes and of functional exofacially tagged CaV2 channels. Here we report the development of fully functional CaV2.2 constructs containing inserted surface-accessible exofacial tags, which allow visualization of only those channels at the plasma membrane, in both a neuronal cell line and neurons. We first examined the effect of the auxiliary subunits. Although α2δ subunits copurify with CaV2 channels, it has recently been suggested that this interaction is easily disrupted and nonquantitative. We have now tested whether α2δ subunits are associated with these channels at the cell surface. We found that, whereas α2δ-1 is readily observed at the plasma membrane when expressed alone, it appears absent when coexpressed with CaV2.2/β1b, despite our finding that α2δ-1 increases plasma-membrane CaV2.2 expression. However, this was due to occlusion of the antigenic epitope by association with CaV2.2, as revealed by antigen retrieval; thus, our data provide evidence for a tight interaction between α2δ-1 and the α1 subunit at the plasma membrane. We further show that, although CaV2.2 cell-surface expression is reduced by gabapentin in the presence of wild-type α2δ-1 (but not a gabapentin-insensitive α2δ-1 mutant), the interaction between CaV2.2 and α2δ-1 is not disrupted by gabapentin. Altogether, these results demonstrate that CaV2.2 and α2δ-1 are intimately associated at the plasma membrane and allow us to infer a region of interaction.
Purification of L-type voltage-gated calcium (CaV) channels from skeletal muscle shows that they consist of a pore-forming α1 subunit, CaV1.1, associated with three accessory subunits, β1, α2δ-1, and γ1 (1, 2). Cardiac L-type channels have a similar subunit composition, although the α1 subunit is α1C and the γ subunit is not present (3). However, the study of cell surface-associated N-type (CaV2.2) and P/Q-type (CaV2.1) calcium channels has been hampered by the lack both of antibodies to cell-surface epitopes and of functional exofacially tagged CaV2 channels. Here we report the development of fully functional CaV2.2 constructs containing inserted surface-accessible exofacial tags, which allow visualization of only those channels at the cell surface, in both cell lines and neurons. Using this methodological advance, we can now examine directly the effect of the auxiliary subunits on cell-surface expression of CaV2 channels.
Although α2δ subunits have been shown to be associated with CaV2.1 and CaV2.2 following purification (4, 5), it has recently been suggested that the α2δ subunits are associated only very loosely and nonquantitatively with CaV2 channels (6), calling into question their role as calcium channel subunits. This study found that the α2δ proteins α2δ-1, α2δ-2, and α2δ-3 could only be copurified using digitonin for tissue solubilization, and not with other detergents; even with digitonin, the study found α2δ present at less than 10% of the molar ratio of Cav2 α1 and β subunits (6). One feature of such proteomic techniques is that they capture calcium channel complexes at all stages of maturation as they are trafficked and degraded, as well as the mature proteins, in this case the channels at the plasma membrane. Furthermore, glycosyl phosphatidylinositol anchoring of α2δ subunits (7) may result in their partial separation from CaV2.2 during purification procedures (6).
Functionally, the main effect of α2δ subunits on both CaV1 and CaV2 channels is to increase macroscopic calcium currents, which is likely to involve an effect on trafficking of the channels to the plasma membrane or on endocytosis, because no effect has been observed on single-channel conductance and little effect on open probability (8–10). However, there are effects of α2δ subunits on voltage dependence and kinetics of inactivation (7, 11, 12), indicating that the channel complex is likely to remain intact at the plasma membrane, although these effects might also relate to altered maturation of the channel induced by transient interaction with α2δ subunits. Thus, evidence that α2δ remains tightly associated with the channels at the plasma membrane is sparse, and it is possible that the primary function of α2δ subunits may be as trafficking proteins for calcium channels.
Here we have directly tested whether α2δ subunits represent trafficking chaperone proteins for CaV2 channels, rather than remaining associated with the channels at the cell surface. Our evidence indicates conclusively that CaV2.2 and α2δ-1 are intimately associated at the plasma membrane, and that this interaction is not disrupted by the α2δ-1 ligand gabapentin.
Results
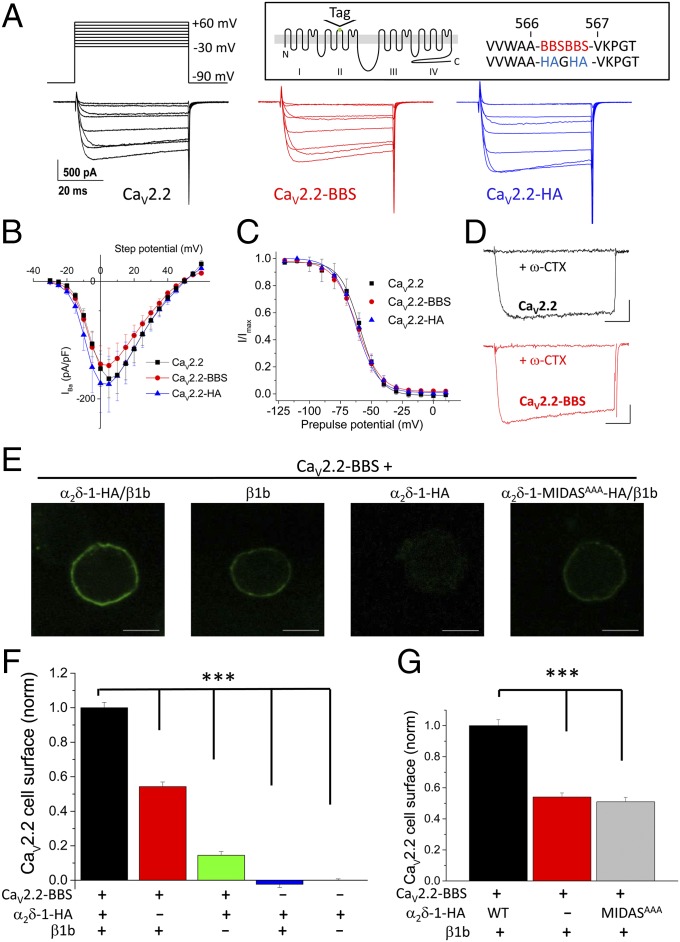
A tandem HA tag (CaV2.2-HA) or a tandem bungarotoxin-binding site (BBS) tag (CaV2.2-BBS) was inserted in an extracellular loop in domain II of CaV2.2 (Fig. 1A, Inset). These constructs exhibited Ba2+ current density–voltage (IV) relationships with no significant differences compared with wild-type (WT) CaV2.2 when expressed with α2δ-1 and β1b in tsA-201 cells (Fig. 1 A and B and Table S1), and also showed identical steady-state inactivation parameters (Fig. 1C). Furthermore, CaV2.2-BBS was also inhibited by ω-conotoxin GVIA to the same extent as WT CaV2.2 (Fig. 1D). Moreover, the tags of both constructs were available on the cell surface (Fig. 1E and Fig. S1). Single tags in the same position were not cell surface-accessible, although the channels were otherwise functional, in terms of generating Ba2+ currents.
Fig. 1.
Properties of CaV2.2-HA and CaV2.2-BBS constructs. (A) Examples of IBa currents (the voltage protocol is shown at the top). Data shown are steps between −20 and +50 mV in 10-mV steps, for tsA-201 cells expressing CaV2.2 (Left; black traces), CaV2.2-BBS (Center; red traces), or CaV2.2-HA (Right; blue traces), all with α2δ-1/β1b. (Scale bars refer to all panels.) (Inset) Schematic diagram of CaV2.2 with the location of the tag site (HA and BBS) identified. (B) Mean IV plots for CaV2.2/α2δ-1/β1b (black squares; n = 30), CaV2.2-BBS/α2δ-1/β1b (red circles; n = 17), and CaV2.2-HA/α2δ-1/β1b (blue triangles; n = 13). Individual IV relationships were fit by a modified Boltzmann function. Mean Gmax, V50, act, Vrev, and k values showed no significant differences (Table S1). (C) Mean steady-state inactivation data for CaV2.2/α2δ-1/β1b (black squares; n = 9), CaV2.2-BBS/α2δ-1/β1b (red circles; n = 6), and CaV2.2-HA/α2δ-1/β1b (blue triangles; n = 5). Mean data were fit by Boltzmann functions, with V50, inact values of −59.2, −61.6, and −60.4 mV, respectively. (D) Application of ω-conotoxin GVIA (1 µM for 2 min) produced a complete block of both WT CaV2.2 (Upper; black traces) and CaV2.2-BBS (Lower; red traces) IBa (both representative of n = 5). Currents were elicited by a 50-ms test pulse to +10 mV from −80 mV holding potential. (Scale bars, 200 pA and 10 ms.) Tail current transients have been curtailed for clarity. (E) Representative images showing cell-surface expression of CaV2.2-BBS in Neuro2A cells visualized with α-BTX-AF 488. CaV2.2 +α2δ-1-HA/β1b, +β1b, +α2δ-1-HA, and +α2δ-1-MIDAS-HA/β1b. (Scale bars, 10 μm.) (F) Bar chart of mean (±SEM) cell-surface CaV2.2-BBS density for CaV2.2-BBS/α2δ-1-HA/β1b (black bar; n = 612), CaV2.2-BBS/β1b (red bar; n = 493), CaV2.2-BBS/α2δ-1-HA (green bar; n = 238), α2δ-1-HA/β1b (blue bar; n = 45), and α2δ-1-HA alone (rightmost bar; n = 265). Data are from five separate transfections. Statistical differences were determined by one-way ANOVA and Bonferroni post hoc tests. ***P < 0.001 for CaV2.2-BBS/α2δ-1-HA/β1b compared with all other conditions. Cells were selected that were positive for internal CaV2.2. (G) Bar chart of mean (±SEM) cell-surface CaV2.2-BBS density for CaV2.2-BBS/α2δ-1-HA/β1b (black bar; n = 133), CaV2.2-BBS/β1b (red bar; n = 111), and CaV2.2-BBS/α2δ-1-MIDAS-HA (gray bar; n = 107). Data were obtained from three separate transfections. Statistical differences were determined by one-way ANOVA and Bonferroni post hoc tests. ***P < 0.001 for CaV2.2-BBS/α2δ-1-HA/β1b compared with the other two conditions.
α2δ-1 Increases Cell-Surface Expression of CaV2.2.
We used the neuronal cell line Neuro2A for subsequent imaging experiments to provide a relevant environment for the neuronal CaV2.2 channels. In initial studies using CaV2.2-HA, we found that, as expected (13), β subunits are essential for expression of CaV2.2 at the plasma membrane, and that almost no surface staining was obtained with CaV2.2-HA alone, indicating there are no endogenous β subunits in these cells (Fig. S1 A and B).
In subsequent experiments in Neuro2A cells, we used the equivalent CaV2.2-BBS, which was detected at the cell surface with α-bungarotoxin (BTX)-AF 488. When CaV2.2-BBS was expressed only with β1b, the amount of CaV2.2-BBS at the cell surface was 54% of that in the presence of all subunits (Fig. 1 E and F). Surprisingly, when CaV2.2-BBS was coexpressed with α2δ-1 alone, it showed some surface expression, reaching 14% of the control plus β and α2δ-1 (Fig. 1 E and F). These results demonstrate clearly that α2δ-1 does increase the amount of CaV2.2 protein at the plasma membrane, and although its effect is particularly exerted after the β subunit interacts with the channel, surprisingly α2δ-1 does have some effect alone.
We have previously shown that an intact metal ion-dependent adhesion site (MIDAS) in the Von Willebrand factor A (VWA) domain of α2δ-1 and α2δ-2 is essential for the enhancement of CaV1 and CaV2 calcium currents (12, 14). We found that an α2δ-1 construct with three MIDAS residues mutated to alanine (α2δ-1-MIDASAAA) did not increase the amount of CaV2.2-BBS at the cell surface (Fig. 1 E and G), which parallels its inability to enhance CaV2.2 calcium currents (14). We also found that in this neuronal cell line, α2δ-1-MIDASAAA itself exhibits reduced cell-surface density compared with WT α2δ-1 when expressed alone (Fig. S2), indicating that this site may be involved in the interaction with a protein that is important for trafficking α2δ-1.
Paradoxical Loss of α2δ-1 Labeling at the Cell Surface When Coexpressed with CaV2.2 and β.
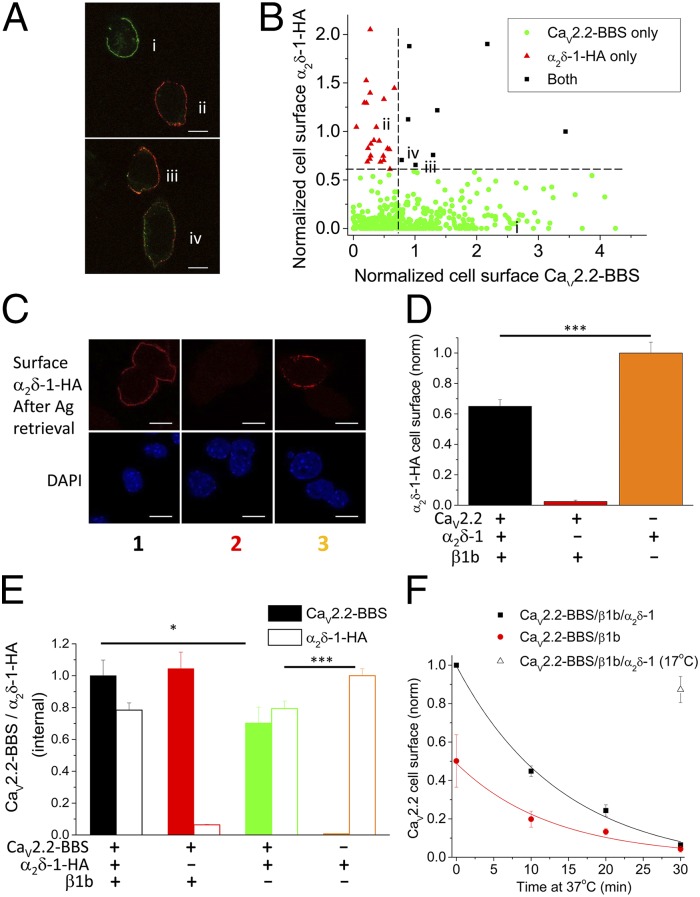
Our studies have shown previously that α2δ subunits can reach the cell surface when expressed alone (12). We therefore examined the cell-surface expression of α2δ-1-HA, and the effect of CaV2.2 coexpression on this, to gain insight into the interaction site of the auxiliary subunits, with the aim of determining whether they remained associated as judged by colocalization at the cell surface. We used α2δ-1-HA for these studies, as we have shown previously that it supports calcium channel currents with identical properties to WT α2δ-1 (15).
Surprisingly, we found that the amount of α2δ-1-HA detected on the cell surface was strongly reduced by coexpression of CaV2.2-BBS, both in the presence and absence of the β subunit (Fig. 2 A and B, conditions 1 and 3 compared with 5). In contrast, α2δ-1-HA cell-surface expression was not reduced by coexpression with only β1b, with which it does not interact directly (Fig. 2 A and B, condition 4 compared with 5). The low detection of α2δ-1-HA on the cell surface, when coexpressed with CaV2.2-BBS, is surprising, because it is effective to increase the amount of CaV2.2 at the cell surface (Fig. 1 E–G). A possible contributing factor is that there is likely to be intracellular interaction between α2δ-1 and CaV2.2, particularly in the absence of β, which results in complex formation and intracellular retention (Fig. 2 A and B, condition 3). However, this would not be the explanation in condition 1, when all three subunits are transfected. The same result, loss of cell-surface staining for α2δ-1 in cells transfected with CaV2.2-BBS/α2δ-1/β1b, was also obtained when using α2δ-1 without an HA epitope tag (Fig. 2C) and when using WT CaV2.2 without an epitope tag (Fig. S3).
Fig. 2.
Cell-surface localization of α2δ-1: effect of CaV2.2 and β1b. (A) Representative images showing cell-surface expression of CaV2.2-BBS (row 1, green), internal CaV2.2 following permeabilization (row 2, red), and cell-surface α2δ-1-HA (row 3, white) in Neuro2A cells. The transfected subunits in conditions 1–5 correspond to those in B. (Scale bars, 10 μm.) Cells were selected that were positive for internal CaV2.2. (B) Bar chart of mean (±SEM) cell-surface α2δ-1-HA density for CaV2.2-BBS/α2δ-1-HA/β1b (1, black bar; n = 612), CaV2.2-BBS/β1b (2, red bar; n = 493), CaV2.2-BBS/α2δ-1-HA (3, green bar; n = 238), α2δ-1-HA/β1b (4, blue bar; n = 64), and α2δ-1-HA alone (5, orange bar; n = 265). Data are from five separate transfections. Statistical differences were determined by one-way ANOVA and Bonferroni post hoc tests for the conditions shown ±CaV2.2-BBS. ***P < 0.001. (C) Representative images showing cell-surface expression of α2δ-1 (red, using α2δ-1 mAb; Left) and CaV2.2-BBS (green; Center) and nuclear staining with DAPI (Right) in Neuro2A cells transfected with α2δ-1 (without an HA tag) either together with CaV2.2-BBS and β1b (Upper) or alone (Lower). (Scale bars, 10 μm.) N/D, not determined.
We therefore examined the relationship between CaV2.2-BBS expression and α2δ-1-HA expression in 522 individual cells (Fig. 3 A and B). Cell-surface CaV2.2-BBS and α2δ-1-HA staining were negatively correlated (Fig. 3B), with cells exhibiting the highest surface CaV2.2 staining showing very low surface α2δ-1 staining, and vice versa. It is possible that many of the cells with elevated staining for α2δ-1 [>0.6 normalized arbitrary units (a.u.)] were transfected with only low levels or no CaV2.2, as this cDNA is the largest and most difficult to cotransfect. However, for the cells exhibiting strong cell-surface staining for CaV2.2 (>0.7 normalized a.u.), only 8/522 (1.53%) showed staining for α2δ-1 of >0.6 normalized a.u., and in these cells there was little colocalization with CaV2.2 (Fig. 3A). It is unlikely that this population of cells did not become transfected with α2δ-1, because we have shown that α2δ-1 promotes the cell-surface expression of CaV2.2 (Fig. 1 E–G).
Fig. 3.
α2δ-1 epitope occlusion by CaV2.2 and lack of effect on endocytosis. (A) Representative images following transfection of CaV2.2-BBS/α2δ-1-HA/β1b showing cell-surface expression of CaV2.2-BBS (i, green) or cell-surface α2δ-1-HA (ii, red) in Neuro2A cells. A few cells showed staining for both subunits, either colocalized (yellow staining) or in separate domains (red and green) (iii and iv). (Scale bars, 10 μm.) (B) Scatter plot of cell-surface staining for both α2δ-1-HA and CaV2.2-BBS in individual cells (n = 522 from four experiments). Cells are categorized as described in SI Materials and Methods. The dashed lines represent the criteria used for the symbols. All cells were included that exhibited surface staining above the background in cells not transfected with the relevant subunit. (C) Representative images showing cell-surface expression of α2δ-1-HA following antigen retrieval as described in SI Materials and Methods (red; Upper) and nuclear staining with DAPI (Lower) in Neuro2A cells transfected with CaV2.2-BBS/α2δ-1-HA/β1b (Left), CaV2.2-BBS/β1b (Center), or α2δ-1-HA alone (Right). (Scale bars, 10 μm.) (D) Bar chart of mean (±SEM) cell-surface α2δ-1-HA density following antigen retrieval, for cells such as those shown in C, for CaV2.2-BBS/α2δ-1-HA/β1b (black bar; n = 82), CaV2.2-BBS/β1b (red bar; n = 6), and α2δ-1-HA alone (orange bar; n = 60). Data were obtained from two separate transfections. Statistical differences were determined by one-way ANOVA and Bonferroni post hoc tests for the conditions shown. ***P < 0.001. (E) Bar chart of mean normalized (±SEM) internal CaV2.2 (identified by II-III loop Ab; solid bars) and α2δ-1-HA (open bars) density measured in the same cells, for the subunit combinations CaV2.2-BBS/α2δ-1-HA/β1b (1, black bars; n = 169), CaV2.2-BBS/β1b (2, red bars; n = 205), CaV2.2-BBS/α2δ-1-HA (3, green bars; n = 156), and α2δ-1-HA alone (4, orange bars; n = 213). Data were obtained from three separate transfections. Statistical differences were determined by one-way ANOVA and Bonferroni post hoc tests for the conditions shown ±CaV2.2-BBS or ±β1b. *P < 0.05, ***P < 0.001. (F) CaV2.2-BBS was measured on the cell surface after 0–30 min incubation at 37 °C for cells expressing CaV2.2-BBS/β1b/α2δ-1-HA (black squares; n = 3 experiments) or CaV2.2-BBS/β1b (red circles; n = 3 experiments). In each experiment, 50–90 cells were analyzed per time point. The decay time constant, τ, for the plotted fits was 14.1 min for CaV2.2-BBS/β1b/α2δ-1-HA (black line) and 12.8 min for CaV2.2-BBS/β1b (red line). As a control, cells were incubated at 17 °C for 30 min (open triangle; n = 139).
Interaction with CaV2.2 Occludes the Detection of the α2δ-1 Antigenic Epitope at the Cell Surface.
Two possibilities might therefore explain this unexpected finding. First, α2δ-1 might deliver CaV2.2 to the plasma membrane but not remain closely associated with it, being rapidly endocytosed and recycled separately. It has been described previously that α2δ subunits are only loosely associated with CaV2 channels during purification (6, 16), and that α2δ subunits partition into lipid raft domains (7, 17), and also undergo endocytosis (18). A second possibility is that the epitopes for both the internal HA tag in α2δ-1 and the α2δ-1 monoclonal antibody used in these experiments are occluded by a tight association between α2δ-1 and CaV2.2.
With the aim of examining whether the antigenic epitope for α2δ-1-HA was hidden by association with the CaV2.2 α1 subunit on the cell surface, we used an antigen retrieval method (7, 19). We found that the level of α2δ-1-HA epitope detected on the cell surface is markedly increased from 15% of the level seen for α2δ-1 alone, without antigen retrieval (Fig. 2B), to 65% with antigen retrieval (Fig. 3 C and D). This result clearly shows that when α2δ-1 and CaV2.2 are coexpressed together, they must be closely and almost completely associated at the cell surface, sufficient to occlude both the HA antibody from binding to its epitope tag and the binding site of the monoclonal antibody on α2δ-1. We then mapped the epitope on α2δ-1 recognized by the monoclonal antibody used in this study. We found that its recognition site requires amino acids 751–755 in the α2 moiety of α2δ-1 (Fig. S4). This epitope is downstream of the VWA domain, and is within the bacterial chemosensory-like domains (20), as is the HA epitope in α2δ-1-HA (which is inserted between amino acids 549 and 550). Therefore, this region may be involved in interaction with CaV2.2. In agreement with this, when α2δ-1-MIDASAAA was coexpressed with CaV2.2 and β1b, the level of α2δ-1-MIDASAAA on the cell surface was significantly lower than when it was expressed alone (Fig. S2), which indicates that CaV2.2 is still able to interact with α2δ-1-MIDASAAA intracellularly. Therefore, the MIDAS motif on the VWA domain of α2δ-1 is unlikely to be key to the interaction between these two proteins.
Effect of CaV2.2 on Intracellular Detection of α2δ-1.
To determine whether an intracellular interaction of α2δ-1 with CaV2.2 also occluded the detection of intracellular α2δ-1, we permeabilized cells and quantified internal CaV2.2 and α2δ-1 in parallel experiments to those in which staining for cell-surface CaV2.2-BBS was determined. We found that detection of intracellular α2δ-1 was not reduced in the CaV2.2/β1b/α2δ-1 condition, compared with α2δ-1 alone (Fig. 3E and Fig. S5, condition 1 compared with 4). Thus, the interaction between CaV2.2 and α2δ-1 in intracellular trafficking compartments is either sufficiently flexible that the α2δ-1-HA epitope is not occluded or the interaction is loosened by the permeabilization procedure.
Recalling the result that coexpression of CaV2.2-BBS with α2δ-1-HA in the absence of β reduced cell-surface expression of α2δ-1 (Fig. 2 A and B, condition 3), which indicates that there must be intracellular interaction between these two moieties leading to their intracellular retention, we found that in the same condition (Fig. 3E, condition 3: CaV2.2-BBS/α2δ-1-HA) there was a significant reduction of intracellular α2δ-1-HA compared with cells transfected with α2δ-1-HA alone (condition 4) and also a significant reduction of CaV2.2-BBS compared with coexpression of all three subunits (Fig. 3E, condition 1: CaV2.2-BBS/β1b/α2δ-1-HA). Thus, when CaV2.2 and α2δ-1 are cotransfected, they may both be subjected to degradation in the absence of the β subunit (21, 22). Together, these results indicate that α2δ-1 is likely to interact intracellularly with CaV2.2, even in the absence of β, but that trafficking out of the endoplasmic reticulum to the plasma membrane is promoted by the β subunit.
We then examined endocytosis of CaV2.2-BBS from the cell surface by labeling with α-BTX at 17 °C and then incubating the cells for 10–30 min at 37 °C. We found that the presence of α2δ-1 had no significant effect on the rate of removal of CaV2.2-BBS from the cell surface at 37 °C over the time period measured, the decay time constant (τ) being 12.2 ± 1.8 min for CaV2.2/β1b and 15.2 ± 3.2 min for CaV2.2-BBS/β1b/α2δ-1 (n = 3 experiments, P > 0.05; Fig. 3F). As a control, there was only 13% reduction of labeling when cells were incubated for 30 min at 17 °C, a temperature at which endocytosis does not occur (18), indicating that unbinding of α-BTX is negligible over this time course.
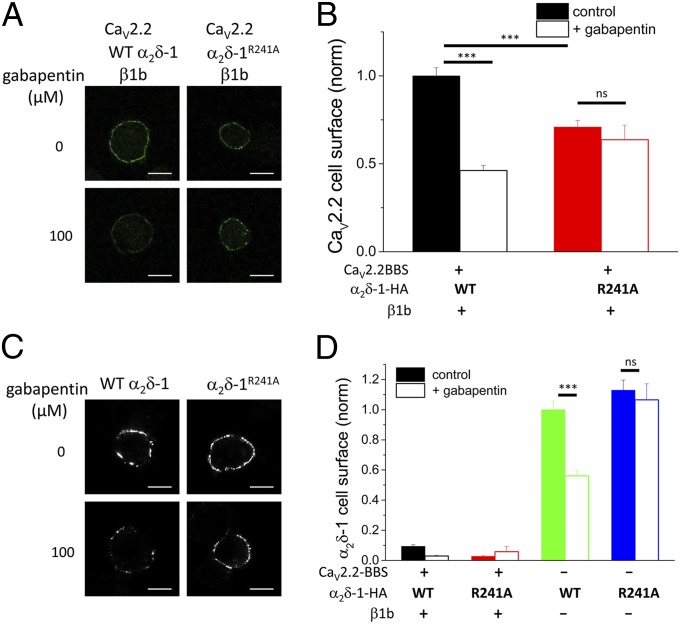
Gabapentin Reduces Cell-Surface Expression of CaV2.2 and α2δ-1.
As further evidence that α2δ-1 influences CaV2.2 trafficking and cell-surface expression, we investigated the effect of the α2δ-1 ligand gabapentin. We found that incubation of Neuro2A cells with gabapentin (100 µM for 24 h) significantly reduced cell-surface expression of CaV2.2-BBS by 54% for the CaV2.2-BBS/β1b/α2δ-1-HA combination (Fig. 4 A and B). This result is in agreement with our previous electrophysiological results for CaV2.2 channels (23). In contrast, when a mutant α2δ-1 that does not bind gabapentin (α2δ-1R241A) (24) was used in place of WT α2δ-1, gabapentin had no effect on cell-surface expression of CaV2.2-BBS (Fig. 4 A and B). Furthermore, cell-surface expression of CaV2.2-BBS in the presence of α2δ-1R241A was reduced compared with that in the presence of WT α2δ-1 (Fig. 4B), in agreement with the reduced functionality of this construct to support CaV2.2 currents noted previously (25). In this experiment, cell-surface CaV2.2 was increased by α2δ-1 alone, in the absence of β subunits, as also seen in Fig. 1F, to a level that was 27.0 ± 4.1% (n = 106) of the control plus both auxiliary subunits, and we found that this effect was completely prevented by gabapentin.
Fig. 4.
Effect of gabapentin on cell-surface expression of CaV2.2 and α2δ-1 and α2δ-1R241A. (A) Cell-surface expression of CaV2.2-BBS in Neuro2A cells transfected with CaV2.2-BBS/α2δ-1-HA (WT)/β1b (Left) or CaV2.2-BBS/α2δ-1R241A-HA/β1b (Right). (Upper) Control cells. (Lower) Cells incubated with gabapentin (100 µM). (Scale bars, 10 μm.) Cells positive for internal CaV2.2 were analyzed. (B) Bar chart of mean (±SEM) cell-surface CaV2.2-BBS density in the absence (solid bars) and presence (open bars) of 100 µM gabapentin, for CaV2.2-BBS/α2δ-1-HA (WT)/β1b (black bars; n = 259, 306) and CaV2.2-BBS/α2δ-1R241A-HA/β1b (red bars; n = 136, 56). Data were obtained from two to four separate transfections. Statistical differences ±gabapentin were determined by Student t test. ***P < 0.001; not significant (ns), P > 0.05. (C) Representative images showing cell-surface expression of α2δ-1-HA (Left) and α2δ-1R241A-HA (Right) in Neuro2A cells transfected with the α2δ-1 subunit alone. (Upper) Control cells. (Lower) Cells incubated with gabapentin (100 µM). (Scale bars, 10 μm.) (D) Bar chart of mean (±SEM) cell-surface α2δ-1-HA density in the absence (solid bars) and presence (open bars) of 100 µM gabapentin for the same experiments quantified in B, with the subunit combinations CaV2.2-BBS/α2δ-1-HA (WT)/β1b (black bars; n = 259, 306), CaV2.2-BBS/α2δ-1R241A-HA/β1b (red bars; n = 136, 56), α2δ-1-HA (WT) (green bars; n = 175, 201), and α2δ-1R241A-HA (blue bars; n = 147, 80). Statistical differences ±gabapentin were determined by Student t test. ***P < 0.001; ns, P > 0.05. Cells were selected that were positive for internal CaV2.2, or α2δ-1 when CaV2.2 was not transfected.
Furthermore, gabapentin reduced cell-surface staining of α2δ-1-HA (WT) when it was expressed alone (by 44%; Fig. 4 C and D) but had no effect on the cell-surface expression of α2δ-1R241A-HA (Fig. 4 C and D). In addition, gabapentin did not counteract the occluded cell-surface detection of α2δ-1-HA, or α2δ-1R241A-HA, when it was coexpressed with CaV2.2-BBS and β1b (Fig. 4D), indicating that it does not prevent the interaction between α2δ-1 and CaV2.2 subunits on the cell surface. If this interaction were disrupted by gabapentin, then increased detection of α2δ-1-HA on the cell surface might have been expected.
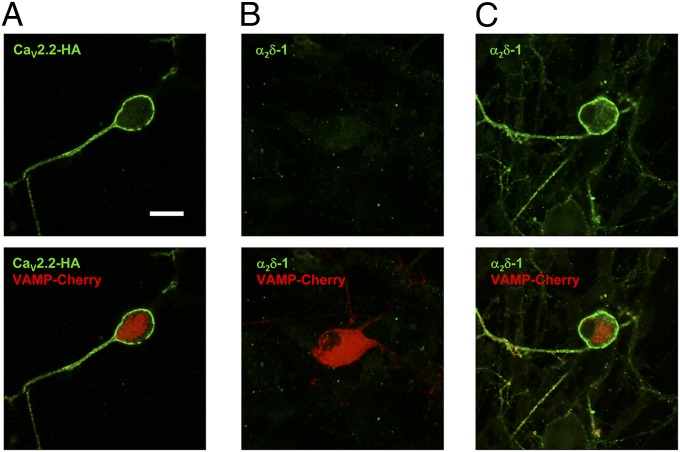
Expression of CaV2.2-HA in Neurons.
When CaV2.2-HA was expressed in cultured dorsal root ganglia (DRG) neurons, together with α2δ-1 and β1b, it could be visualized on the plasma membrane of nonpermeabilized DRG neuron somata, and extended down the neurites (Fig. 5A). Furthermore, similar to our finding in Neuro2A cells, the epitope for α2δ-1 was hidden in all transfected DRG neurons examined (Fig. 5B), unless they were subjected to antigen retrieval (Fig. 5C).
Fig. 5.
Cell-surface localization of CaV2.2 and α2δ-1 in DRG neurons. Cell-surface expression of CaV2.2-HA (A) and α2δ-1 (B and C) in nonpermeabilized DRG neurons transfected with CaV2.2-HA/α2δ-1/β1b and VAMP-mCherry. Transfected cells were identified by VAMP-mCherry (red). (Lower) Merged images). (A) CaV2.2-HA immunostaining (green); 58/71 mCherry-positive DRG examined (81.7%) had surface HA signal in this condition. (B) α2δ-1 immunostaining (green); 0/20 mCherry-positive DRG had surface α2δ-1 signal. (C) α2δ-1 immunostaining (green) after antigen retrieval; 52/62 mCherry-positive DRG (85.5%) had surface α2δ-1 signal in this condition. (Scale bar, 20 μm.) Representative of two separate transfections.
Discussion
Development of an Exofacially Tagged CaV2.2.
To examine the factors affecting the plasma-membrane expression and trafficking of CaV2.2, the development of fully functional exofacially tagged CaV2.2 constructs was essential. In previous studies, tagged CaV2.2 constructs have been used that were not described as functional (26), and the uncertainty remains that partial or complete lack of function may either result in, or be the result of, altered channel trafficking. The functional exofacially tagged CaV2.2 constructs described here thus represent important tools for the examination of CaV2.2 distribution and trafficking and the effect of auxiliary subunits and other factors. Expression of CaV2.2-HA in DRG neurons also results in robust expression on the cell surface, unlike the finding for an HA-tagged CaV2.1 construct (27), providing evidence that these constructs represent important tools for studying CaV2.2 trafficking and localization in these neurons.
Mechanism of Action of α2δ-1 to Increase Cell-Surface Expression of CaV2.2.
Although it is believed that the major mechanism whereby α2δ subunits increase the functional expression of calcium channels is due to an increase of the amount of channel protein at the plasma membrane (12), definitive evidence that this is the case has been lacking, particularly for CaV2 channels, with measurements for L-type channels mainly relying on determination of gating charge (28, 29). However, the single-channel conductance and open probability of CaV2.2, which are two other mechanisms whereby macroscopic current could be increased without affecting the number of channels in the plasma membrane, are little affected by α2δ subunits (8, 10). Nevertheless, there are minor effects of α2δ subunits on kinetic and voltage-dependent properties of the currents to increase voltage-dependent inactivation and to hyperpolarize the voltage dependence of steady-state inactivation, which might be attributed, either to an effect of α2δ proteins on calcium channel folding and maturation or to ongoing association of the channels with α2δ subunits, to form functional channel complexes on the plasma membrane (7, 12, 30).
We now provide definitive evidence for the increase by α2δ-1 of cell-surface expression of CaV2.2, and also demonstrate that CaV2.2 and α2δ-1 are completely associated at the cell surface when they are coexpressed (together with a β subunit), which is sufficient to occlude the binding of both the HA antibody and the monoclonal antibody to α2δ-1. We also show that in cultured DRG neurons, antigen retrieval is required to detect α2δ-1 when it is overexpressed with CaV2.2 and β1b, indicating that the epitope is also hidden in these neurons, as is also true for endogenous α2δ-1 (7, 19). Furthermore, we demonstrate that α2δ-1 has no effect on endocytosis, and is therefore likely to increase forward trafficking of the channels.
Site of Interaction Between α2δ-1 and CaV2.2.
The epitope for the α2δ-1 antibody and the inserted HA tag are both within the region spanning the two chemosensory-like domains of α2δ-1, downstream of the VWA domain (20). It is tempting to speculate that this region forms part of the interaction site with the α1 subunit, which results in masking of these epitopes when the two subunits interact. In agreement with this, our evidence also indicates that although α2δ-1-MIDASAAA does not increase CaV2.2 cell-surface density, there is still an association of this mutant with CaV2.2, sufficient to completely prevent α2δ-1-MIDASAAA cell-surface expression. Thus, an intact VWA domain may not be required for interaction with CaV2.2, but is required for correct trafficking of both α2δ-1 and its complex with the pore-forming subunit, possibly via interaction with a trafficking protein(s). This domain of α2δ-1 has also previously been shown to interact with secreted extracellular matrix proteins of the thrombospondin family (31).
Our results indicate that CaV2.2 can interact intracellularly with α2δ-1, possibly before its β subunit-mediated exit from the endoplasmic reticulum, because the cell-surface expression of α2δ-1 (which alone can readily reach the cell surface) is markedly reduced by coexpression with CaV2.2 in the absence of β, indicating that CaV2.2 must be causing α2δ-1 to be retained intracellularly. Surprisingly, we also find a small but significant effect of α2δ-1 to increase the amount of CaV2.2 on the cell surface, even in the absence of β subunits. This is unlikely to be a result of the influence of endogenous β, because no CaV2.2 reaches the cell surface in the absence of both β and α2δ-1. As expected, the presence of a β subunit alone increased CaV2.2 cell-surface expression markedly from a very low level; this is in agreement with indirect evidence for CaV1.2 channels from most (32, 33) but not all (34) other studies.
Mechanism of Action of Gabapentin on N-Type Calcium Channel Cell-Surface Expression.
Gabapentin reduced the cell-surface expression of both CaV2.2 and α2δ-1 in all conditions in which α2δ-1 was coexpressed. Furthermore, our finding that gabapentin does not increase the detection of cell surface-expressed α2δ-1 when it is occluded by coexpression with CaV2.2/β1b indicates that the interaction between CaV2.2 and α2δ-1 is not disrupted by gabapentin, and that this does not therefore form part of its mechanism of action. All of the effects of gabapentin are via binding to α2δ-1, as evidenced by the lack of effect of gabapentin when the α2δ-1R241A subunit is used in place of WT α2δ-1. This mutation abrogates the binding and function of gabapentinoids in experimental models of neuropathic pain and epilepsy (24, 25, 35).
In conclusion, this study has identified CaV2.2-HA and CaV2.2-BBS to be important tools for research into factors affecting N-type calcium channel trafficking (36). It has allowed us to show that α2δ-1 increases the plasma-membrane expression of N-type channels and remains closely associated with these channels on the cell surface, with the interaction possibly involving the α2δ-1 chemosensory-like domains. This study has also increased our understanding of the mechanism of action of the gabapentinoid drugs on N-type calcium channel trafficking.
Materials and Methods
Molecular biology, cell culture, immunocytochemistry, imaging, electrophysiology and immunoblotting methods are given in SI Materials and Methods. The primers used for molecular biology and the full tag sequences are given in Table S2. The details of analysis for electrophysiology and imaging are also given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Kerry Dickens (supported by a Wellcome Trust Vacation Scholarship) and Zhe Li for initial studies leading to the result shown in Fig. S4, and Kanchan Chaggar for technical assistance. This work was supported by a Wellcome Trust Senior Investigator Award (098360/Z/12/Z, to A.C.D.). J.S.C. was supported by a Medical Research Council Co-operative Award in Science and Engineering PhD studentship in collaboration with Pfizer (Sandwich)/Neusentis.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403731111/-/DCSupplemental.
References
- 1.Takahashi M, Seagar MJ, Jones JF, Reber BFX, Catterall WA. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci USA. 1987;84(15):5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis SB, et al. Sequence and expression of mRNAs encoding the α 1 and α 2 subunits of a DHP-sensitive calcium channel. Science. 1988;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 3.Walsh CP, Davies A, Butcher AJ, Dolphin AC, Kitmitto A. Three-dimensional structure of CaV3.1: Comparison with the cardiac L-type voltage-gated calcium channel monomer architecture. J Biol Chem. 2009;284(33):22310–22321. doi: 10.1074/jbc.M109.017152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witcher DR, et al. Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science. 1993;261(5120):486–489. doi: 10.1126/science.8392754. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, et al. Identification of three subunits of the high affinity omega-conotoxin MVIIC-sensitive Ca2+ channel. J Biol Chem. 1996;271(23):13804–13810. [PubMed] [Google Scholar]
- 6.Müller CS, et al. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci USA. 2010;107(34):14950–14957. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies A, et al. The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci USA. 2010;107(4):1654–1659. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakamori M, Mikala G, Mori Y. Auxiliary subunits operate as a molecular switch in determining gating behaviour of the unitary N-type Ca2+ channel current in Xenopus oocytes. J Physiol. 1999;517(Pt 3):659–672. doi: 10.1111/j.1469-7793.1999.0659s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barclay J, et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21(16):6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodbeck J, et al. The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated alpha 2 delta-2 protein with abnormal function. J Biol Chem. 2002;277(10):7684–7693. doi: 10.1074/jbc.M109404200. [DOI] [PubMed] [Google Scholar]
- 11.Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel alpha2delta subunit. J Neurosci. 1997;17(18):6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantí C, et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci USA. 2005;102(32):11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leroy J, et al. Interaction via a key tryptophan in the I-II linker of N-type calcium channels is required for beta1 but not for palmitoylated beta2, implicating an additional binding site in the regulation of channel voltage-dependent properties. J Neurosci. 2005;25(30):6984–6996. doi: 10.1523/JNEUROSCI.1137-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoppa M, Lana B, Margas W, Dolphin AC, Ryan TA. α2δ couples calcium channels to neurotransmitter release sites to control release probability. Nature. 2012;486(7401):122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadurin I, et al. Calcium currents are enhanced by α2δ-1 lacking its membrane anchor. J Biol Chem. 2012;287(40):33554–33566. doi: 10.1074/jbc.M112.378554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jay SD, et al. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J Biol Chem. 1991;266(5):3287–3293. [PubMed] [Google Scholar]
- 17.Davies A, et al. The calcium channel α2δ-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: Implications for localization and function. J Neurosci. 2006;26(34):8748–8757. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran-Van-Minh A, Dolphin AC. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J Neurosci. 2010;30(38):12856–12867. doi: 10.1523/JNEUROSCI.2700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer CS, et al. The increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J Neurosci. 2009;29(13):4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolphin AC. Calcium channel auxiliary α2δ and β subunits: Trafficking and one step beyond. Nat Rev Neurosci. 2012;13(8):542–555. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- 21.Waithe D, Ferron L, Page KM, Chaggar K, Dolphin AC. β-Subunits promote the expression of Ca(V)2.2 channels by reducing their proteasomal degradation. J Biol Chem. 2011;286(11):9598–9611. doi: 10.1074/jbc.M110.195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altier C, et al. The Cavβ subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat Neurosci. 2011;14(2):173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- 23.Hendrich J, et al. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc Natl Acad Sci USA. 2008;105(9):3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Offord J, Oxender DL, Su TZ. Structural requirement of the calcium-channel subunit α2δ for gabapentin binding. Biochem J. 1999;342(Pt 2):313–320. [PMC free article] [PubMed] [Google Scholar]
- 25.Field MJ, et al. Identification of the α2-δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA. 2006;103(46):17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altier C, et al. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9(1):31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- 27.Watschinger K, et al. Functional properties and modulation of extracellular epitope-tagged Ca(V)2.1 voltage-gated calcium channels. Channels (Austin) 2008;2(6):461–473. doi: 10.4161/chan.2.6.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangalore R, Mehrke G, Gingrich K, Hofmann F, Kass RS. Influence of L-type Ca channel alpha 2/delta-subunit on ionic and gating current in transiently transfected HEK 293 cells. Am J Physiol. 1996;270(5 Pt 2):H1521–H1528. doi: 10.1152/ajpheart.1996.270.5.H1521. [DOI] [PubMed] [Google Scholar]
- 29.Qin N, Olcese R, Stefani E, Birnbaumer L. Modulation of human neuronal α1E-type calcium channel by α2δ-subunit. Am J Physiol. 1998;274(5 Pt 1):C1324–C1331. doi: 10.1152/ajpcell.1998.274.5.C1324. [DOI] [PubMed] [Google Scholar]
- 30.Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16(2):431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 31.Eroglu C, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Josephson IR, Varadi G. The β subunit increases Ca2+ currents and gating charge movements of human cardiac L-type Ca2+ channels. Biophys J. 1996;70(3):1285–1293. doi: 10.1016/S0006-3495(96)79685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altier C, et al. Trafficking of L-type calcium channels mediated by the postsynaptic scaffolding protein AKAP79. J Biol Chem. 2002;277(37):33598–33603. doi: 10.1074/jbc.M202476200. [DOI] [PubMed] [Google Scholar]
- 34.Neely A, Wei X, Olcese R, Birnbaumer L, Stefani E. Potentiation by the β subunit of the ratio of the ionic current to the charge movement in the cardiac calcium channel. Science. 1993;262(5133):575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- 35.Lotarski S, et al. Anticonvulsant activity of pregabalin in the maximal electroshock-induced seizure assay in α2δ1 (R217A) and α2δ2 (R279A) mouse mutants. Epilepsy Res. 2014;108(5):833–842. doi: 10.1016/j.eplepsyres.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Ferron L, Nieto-Rostro M, Cassidy JS, Dolphin AC. Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat Commun. 2014;5:3628. doi: 10.1038/ncomms4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.