Significance
Cortical neurons are often active when they are not participating in sensorimotor processes. Here we show that this baseline activity reflects a modulatory signal predicting the monkey’s success on a difficult task without specifying what the monkey perceives or will do. The activity correlates inversely with the monkey’s recent history of success, so that a period of failure is associated with an increase in the baseline and predicts an increase in the monkey’s likelihood of success. The activity is not related to the spatial locus of the monkey’s visual attention. We suggest that this activity is the cortical manifestation of an arousal or state process that controls the efficiency of an animal’s behavior without specifying the nature of that behavior.
Keywords: motivation, modulatory systems, LIP
Abstract
We recorded the activity of neurons in the lateral intraparietal area of two monkeys while they performed two similar visual search tasks, one difficult, one easy. Each task began with a period of fixation followed by an array consisting of a single capital T and a number of lowercase t’s. The monkey had to find the capital T and report its orientation, upright or inverted, with a hand movement. In the easy task the monkey could explore the array with saccades. In the difficult task the monkey had to continue fixating and find the capital T in the visual periphery. The baseline activity measured during the fixation period, at a time in which the monkey could not know if the impending task would be difficult or easy or where the target would appear, predicted the monkey’s probability of success or failure on the task. The baseline activity correlated inversely with the monkey's recent history of success and directly with the intensity of the response to the search array on the current trial. The baseline activity was unrelated to the monkey’s spatial locus of attention as determined by the location of the cue in a cued visual reaction time task. We suggest that rather than merely reflecting the noise in the system, the baseline signal reflects the cortical manifestation of modulatory state, motivational, or arousal pathways, which determine the efficiency of cortical sensorimotor processing and the quality of the monkey’s performance.
Most studies of the physiology of behavior or perception in awake, behaving monkeys begin with an epoch in which the monkey fixates on a spot, waiting for a stimulus, a discriminandum, or an action target to appear (1). The neural activity during this epoch is frequently referred to as “background,” “spontaneous,” “ongoing,” or “baseline” activity because it is not related to sensory or motor aspects of the task (2). Baseline activity is often subtracted from the measured spike rate for purposes of analysis of sensorimotor activity (2–5) on the assumption that it represents neural noise. However, under certain circumstances, baseline activity can show task-related activity. For example, when a monkey can anticipate making a saccade to a stimulus in the receptive field of a neuron, baseline activity often reflects that anticipation (6), and when monkeys attend to a spatial location in the receptive fields of neurons in V1, activity increases before the stimulus appears (7). This V1 activity is greater when the monkey is likely to succeed in the task.
In all of these cases the effect is specific to the receptive or movement field of the neuron under study. However, neural activity is not only subject to spatially specific, attentional modulation. Spatially nonspecific modulatory factors can also affect baseline activity. For example, a small percentage of neurons in monkey V1 have an enhanced response to the appearance of a visual stimulus in their receptive fields whenever the monkey makes a saccade or a hand movement, regardless of whether the saccade is made to the receptive field or away from it, and this enhancement of activity was equated with an increase in arousal (8). However, in V1 the arousal signal was not present in the baseline activity (8).
In parietal cortex, task-related enhancement of visual responses is spatially selective. It occurs only when the monkey attends to the receptive field of the neuron. Here we asked if the baseline response of parietal neurons were modulated in a task-related manner and whether this modulation was spatially selective or nonselective. We chose the lateral intraparietal area (LIP) as a cortical area to study baseline changes. LIP represents a priority map of the visual field, the peak of which both describes the locus of visual attention and also predicts the goal and latency of an impending saccade when a saccade is appropriate (9). The great bulk of its activity is related to specific sensorimotor inputs such as visual, mnemonic, and saccadic, and this activity is modulated by attention (10), motivation (11), surround suppression (12, 13), and reward (14). We used two versions of a visual search task, one easy and the other difficult. Each task began with a period of fixation, after which a search array, consisting of a single capital T and a number of lowercase t's, appeared. The monkey had to find the capital T and report its orientation, upright or inverted, with a hand movement. In the easy task the monkey could explore the array with saccades (15). In the difficult task the monkey had to continue fixating and find the capital T in the visual periphery. The monkeys succeeded nearly 100% of the time in the easy task, but only 70% of the time in the difficult task. We found that the baseline reflected the monkey’s ongoing behavior: it predicted the monkey’s probability of success in the difficult task on the current trial, correlated inversely with the monkey’s recent history of success, and predicted the intensity of the visual transient subsequently evoked by the array appearance. Baseline activity was unrelated to the monkey’s locus of spatial attention as determined by a cued visual reaction time task.
Results
Behavior.
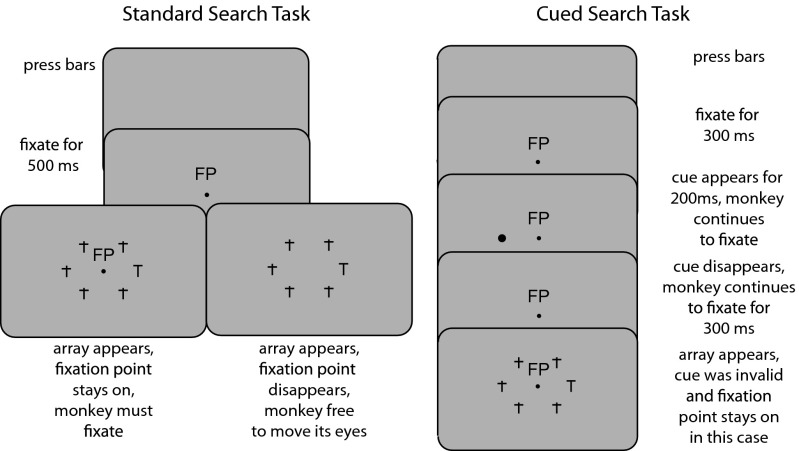
We used two variations of the standard visual search task (Fig. 1, Left). Each version began with the monkey grasping two bars, one with each hand. Then a fixation point appeared, and the monkey had to look at the fixation point within a window of 3 degrees for 500 ms, at which point the search array appeared. The array consisted of five lowercase t’s and an upright or inverted capital T, arranged symmetrically on a circle centered around the fixation point. The monkey had to find the capital T and release the left or right bar depending upon the orientation (upright or inverted) of the capital T to obtain a liquid reward. On some trials the fixation point disappeared and the monkey was free to move its eyes (15); on other trials the fixation point remained lit and the monkey had to continue to fixate and find the capital T without making a saccade to it. The free task was easy: monkey T had a success rate of 97%, monkey G had a success rate of 98%, and monkey R had a success rate of 94%. Although the free task was easy, the target did not pop out: in a previous study using the free task we found that the monkeys’ performance exhibited a set-size effect (16). The fixation task was more difficult: monkey T had a success rate of 69%, monkey G had a success rate of 81%, and monkey R had a success rate of 64%. There was no significant difference between the accuracy of left- and right-hand responses (P > 0.05 by t test) (Fig. S1). The majority of the errors in both the free and fixation tasks were trials in which the monkey fixated but made the wrong hand movement (Table S1). All further analysis will include only trials in which the monkey made correct or wrong hand movements. The monkeys’ manual reaction times were longer in the free task by about the latency of a visually guided saccade (Fig. S2). In general, the monkeys’ performance went in good and bad streaks, but the mean performance remained stable throughout the experiment, and there were no long-term trends in the average magnitude of the fixation performance (Fig. S3A) during a recording day. When we compared the success and failure rates on the fixation task for the first 150 trials of each recording day to the last 150 trials of each recording day we found no difference (Fig. S3B). The monkey’s actual locus of fixation did not differ between successful and unsuccessful trials (Fig. S4).
Fig. 1.
Tasks. (Left) The standard search task. The monkey presses a bar with each hand, and a fixation point appears. The monkey looks at the fixation point for 500 ms, and then a radially symmetric array of objects appears consisting of a capital T and five lowercase distractors. The monkey must find the pseudorandomly located capital T and signal, by releasing the left or right bar, whether the capital T is upright or inverted. On half of the trials the fixation point remains lit, and the monkey must continue to fixate, solving the task with its peripheral vision (Bottom Left). On other, randomly interleaved trials, the fixation point disappears, and the monkey is free to move its eyes (Bottom Right). The target appears pseudorandomly at each location with equal probability. (Right) The cued visual reaction time search task. The monkey fixates for 300 ms, then a cue appears for 200 ms, and the monkey continues to fixate for a total of 800 ms, after which the array appears. The cue appears at the target location 50% of the time and at each of the distractor locations 10% of the time. The monkey must then, as in the standard task, find the capital T and release the bar, with or without a saccade, depending upon whether the fixation point remained lit. We only illustrate the fixation case here.
Dataset.
We recorded extracellular spike activity in 60 neurons in the LIP of three monkeys, 27 in monkey T, 18 in monkey R, and 15 in monkey G, using standard techniques described previously (15). Thirty-seven were recorded in the standard task, 23 in the cued task. Because the results were similar in all three monkeys we pooled the data for some analyses, although in the scatter plots illustrated below, each monkey’s data have different colors. We limited our analysis to neurons with a visual response to the appearance of the array and delay period and/or presaccadic activity in the memory-guided delayed saccade task (17).
Baseline Activity Predicts Success and the Intensity of the Visual Transient.
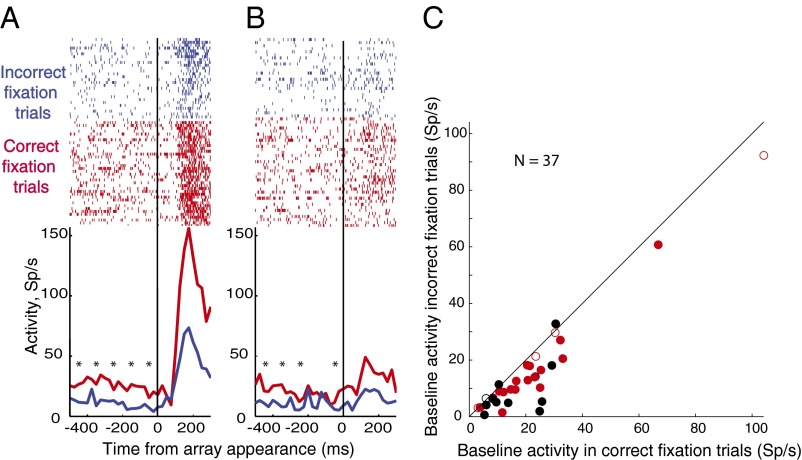
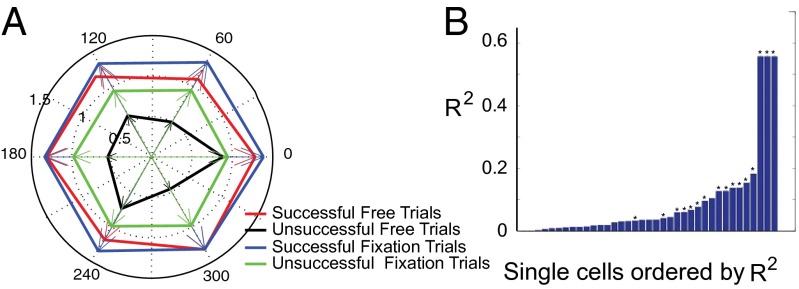
We studied 37 neurons in the standard search task. Even though the monkey could not predict the impending trial type or target location, the neural activity in the 500 ms while the monkey fixated waiting for the array was significantly higher on successful trials than for unsuccessful trials, both for trials in which the target was in the receptive field (Fig. 2A, for a single-cell example) and for trials in which a distractor was in the receptive field (Fig. 2B, for a single-cell example). This was true across the population (Fig. 2C, for fixation trials). In the standard search task, 32/37 neurons had a greater average baseline activity on successful than unsuccessful trials, of which 29 were significantly different (P < 0.05 by Mann–Whitney u test), as was the population as a whole (P < 0.0004 for the population by Wilcoxon signed-rank test). Successful fixation trials had the highest baseline activity, successful free trials had the next highest, unsuccessful fixation trials had less, and unsuccessful free trials had the lowest baseline activity of all (Fig. 3A, main effect of trial type P < 0.0001 by ANOVA). Just as the monkey could not predict where the target would be, the activity for successful and unsuccessful trials was not tuned for the actual location of the target (P > 0.05 by ANOVA, Fig. 3A).
Fig. 2.
Relationship of baseline activity to performance. (A) Relationship in a single cell for target in the receptive field. (Top) Raster diagrams. Each dot is an action potential; successive lines are successive trials synchronized on the appearance of the array (vertical line). Trials separated according to incorrect fixation trials (blue) and correct fixation trials (red). Asterisks show 100-ms epochs where there was a significant difference (Mann–Whitney u test, multiple comparison compensation by false discovery rate, P < 0.05) between correct and incorrect trials. Graphs at bottom show poststimulus histograms (bin width 20 ms), activity (ordinate, spikes per s) plotted against time (abscissa) of the rasters above, colors as in the rasters. (B) Same as A, for distractor in the receptive field. (C) Relationship across the population. Each dot is the median value for a single cell. Red, monkey T; black, monkey R; closed circles, unsuccessful trials statistically different (Mann–Whitney u test, P < 0.05); open circles, successful vs. unsuccessful trials not statistically different.
Fig. 3.
(A) Tuning of background activity as a function of target location. Mean activity in the interval 0–500 ms before the array appears plotted against target location in the array. Each cell rotated so the receptive field was at 0 and then normalized to the peak background activity. Radial axis, normalized spike frequency; circumferential axis, position of object in the array. (B) Correlation between peak of response to array onset and average of baseline activity in the 500-ms epoch immediately before array onset. Ordinate, Spearman correlation R of the peak of the visual response with the baseline; abscissa, bar graphs for each cell, ordered by degree of correlation. Asterisks (*) mark cells for which correlation is significant at P < 0.05.
There was a complicated relationship between baseline activity and manual reaction time. We found a positive relationship between baseline activity and manual reaction time for 7/37 cells [for each cell: Spearman correlation, P < 0.05; P < 0.01 by chi square test (Fig. S5)] and a negative relationship for 6/37 cells (for each cell: Spearman correlation, P < 0.05; P < 0.01 by chi square test). Baseline activity did not correlate either with saccadic latency or saccadic peak velocity in the free task.
Neural activity in the fixation epoch also affected the size of the visual transient, even for trials in which there was a distractor in the receptive field, on both the single-cell level (Fig. 2A for target in the receptive field and Fig. 2B for distractor in the receptive field) and across the population. For each cell we regressed the peak of the visual transient against the background activity on a trial-by-trial basis, using fixation trials only to avoid any effect the saccade might have on the visual response. There was a significant correlation (Spearman correlation, P < 0.05) for 16/37 cells (Fig. 3B). Because we calculated the correlations in Fig. 3B after subtracting the baseline value from the peak visual response, the effect of baseline activity on peak activity was multiplicative, not merely additive. The correlation was not due to the mean differences between successful and unsuccessful trials: the correlation was significant (Spearman correlation, P < 0.05) for 17/37 cells for successful trials alone and for 6/37 cells for failure trials alone).
Baseline Activity Is Independent of the Monkey’s Locus of Attention During the Fixation Period.
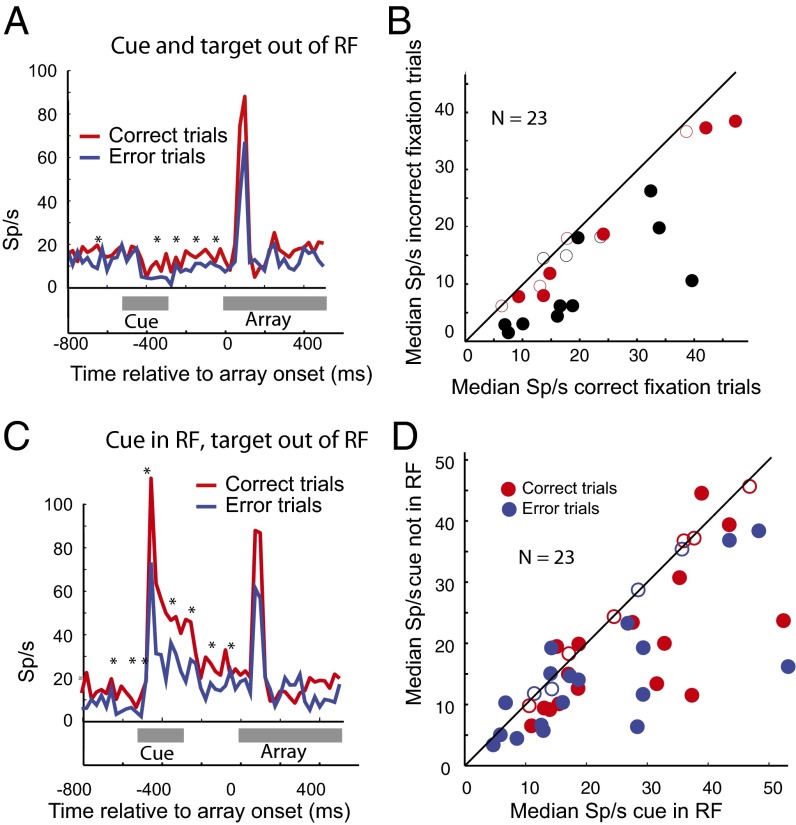
Despite the lack of spatial tuning of the baseline effect, it is possible that the monkey attended to the receptive field location more during successful trials than unsuccessful trials even though there was no stimulus in the receptive field, and this somehow affected performance. To eliminate this possibility we studied 23 cells, 8 in monkey T and 15 in monkey G, using a cued visual reaction time version (18) of the search task to pin the monkey’s attention to a specific location in space (Fig. 1, Right). In this task we increased the fixation interval to 800 ms and flashed a cue for 200 ms, beginning 300 ms after the fixation point appeared. The cue predicted the target location 50% of the time and appeared at one of the five distractor sites 10% of the time for each site. Valid cues decreased, and invalid cues increased both the saccadic and manual reaction times, indicating that the monkeys attended to the valid cue (Table 1). On trials in which the cue did not appear in the neuron’s receptive field, baseline activity in the epochs before and after the cue appeared correlated with the monkey’s probability of success, even though the monkey was not attending to the receptive field during the fixation period (Fig. 4A for a single cell and Fig. 4B for the population, P < 0.00006 by Wilcoxon signed-rank test). When the cue appeared in the receptive field, the monkey’s attention moved to the receptive field, and this resulted in a spatially selective attentional component to the baseline activity (Fig. 4C): activity was significantly greater when the cue was in the receptive field than when it was elsewhere for both successful (Fig. 4D, P < 0.03 by Wilcoxon signed-rank test) and unsuccessful (P < 0.03 by Wilcoxon signed-rank test) trials.
Table 1.
Manual and saccadic reaction times for validly and invalidly cued trials
| Cue validity and action | Monkey G (ms) | Monkey T (ms) |
| Valid, saccade | 157 ± 54 | 180 ± 39 |
| Invalid, saccade | 198 ± 37 | 202 ± 37 |
| Valid, bar release | 619 ± 177 | 551 ± 151 |
| Invalid, bar release | 677 ± 195 | 646 ± 233 |
Fig. 4.
Baseline activity in the cued visual attention trials. (A) Activity of a single neuron when the cue and target were out of the receptive field. Poststimulus histograms (25-ms bin width) when the cue (appearing 500–300 ms before array appears, gray line) and target (appearing at 0) appeared outside the receptive field. Asterisks, significant difference (P < 0.05 corrected for multiple comparisons by false discovery rate); red line, correct trials; blue line, incorrect trials. Ordinate, sp/s; abscissa, time relative to array onset. (B) Population activity in the cued visual attention task in fixation trials. Each dot is the median value for a single cell in the interval 300 ms before the array appears; activity in incorrect trials (ordinate) plotted against activity in correct trials (abscissa). Red, monkey T; black, monkey G; closed circles, successful vs. unsuccessful trials statistically different (Mann–Whitney u test, P < 0.05); open circles, successful vs. unsuccessful trials not statistically different. Population difference significant (P < 0.00006, Wilcoxon rank sum). (C) Activity of a single neuron when the cue was in the receptive field but the target outside the receptive field. Same neuron and conventions as in A. (D) Effect of the cue on the baseline activity in fixation trials. Each dot is the median value for a single cell; baseline activity when cue was outside the receptive field (ordinate) plotted against baseline activity when cue was in the receptive field (abscissa). Red dots, successful trials; blue dots, error trials; closed circles, successful vs. unsuccessful trials statistically different (Mann–Whitney u test, P < 0.05); open circles, successful vs. unsuccessful trials not statistically different. Each population is significant (P < 0.05, Wilcoxon rank sum).
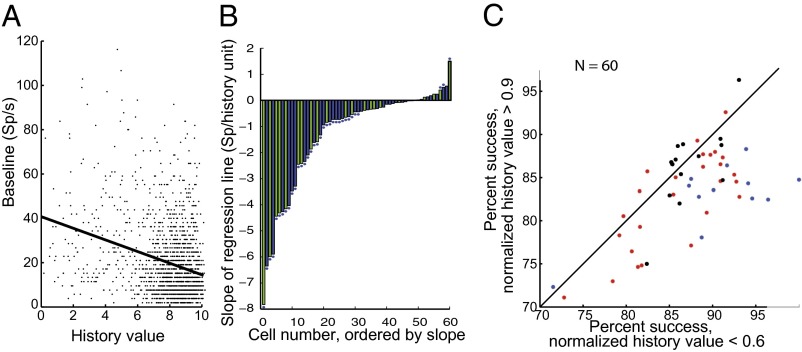
Baseline Activity Correlates Inversely with the Monkey’s Recent History of Success and Failure and the Monkey’s Performance on the Current Trial.
Although the monkeys’ average performance remained stable throughout the experiment, it did fluctuate over short intervals. The monkeys’ recent history of success or failure influenced both the baseline activity and the monkey’s success rate. We calculated a history vector for each trial by scoring every past successful trial 1 and every unsuccessful trial 0 and then computed a history value using the dot product of the history vector and a decaying exponential with a time constant, tau. We then regressed the baseline for each trial against the history value and examined for each cell the tau with the maximum significant slope (Fig. 5A for a single cell, tau = 10). Fifty out of sixty cells had negative slopes, with the minimum being −6.4 spikes per history value. The mean tau associated with maximum slope was 12, and the range was from 5 to 20. Whether or not the correlation was significant did not depend upon tau in the range that we studied (2–50). To eliminate the problem of multiple comparisons we plotted the slopes for each cell as calculated with a tau of 10 (as in Fig. 5A). Thirty-two of the negatively sloped cells had significant correlations [P < 0.05, Spearman’s rho (Fig. 5B), tau of 10 for all cells]. Ten cells had positive slopes, nine of which were less than 0.5, and three of which also had a significant correlation [P < 0.05 (Fig. 5B), tau of 10 for all cells].
Fig. 5.
Effect of performance history on baseline. (A) Single-cell example of history-slope calculation. Each dot is the mean baseline activity in sp/s in the 500 ms before the array appeared (ordinate), plotted again the history value for that trial (abscissa). Least squares regression, y = −2.6263x + 40.7655, R = 0.3, P < 0.0001. (B) Slopes of every cell in the sample, calculated with tau = 10. Blue bars, standard search task; green bars, cued search task; ordinate, slope for each cell; abscissa, cell number ordered by slope. Asterisks are cells with individual significant slopes (P < 0.05, Spearman rho). (C) History value predicts performance. Each dot is a single cell. Abscissa is the average performance for trials whose history value >0.9; ordinate is the average performance whose history value is <0.6. (P < 0.003, Wilcoxon signed-rank test; history values calculated with tau = 10).
The history value itself had behavioral significance. When the history value was high the monkey was less likely to succeed on the current trial than when the history value was low. To compare across days we normalized the history value so the best history value for a given day had a value of 1. We then compared the performance on trials when the normalized history value was >0.9 to trials in which the best history value was <0.6, a division which led to roughly equal numbers of trials in each bin. The monkeys’ performance was significantly better after low than high history values (P < 0.03 by Wilcoxon signed-rank test) (Fig. 5C). Thus, the history value independently correlated inversely with the monkey’s performance and the baseline.
Discussion
We found that the baseline activity of LIP neurons is related to the monkey’s behavior in a spatially nonspecific manner, at a time at which the neurons have no sensorimotor activity. The baseline predicts both the monkey’s performance on a difficult task and the intensity of the neuron’s response to a visual transient. The baseline activity is untuned for the impending target location and is independent of the monkey’s locus of spatial attention as determined by cue location in a cued visual reaction time task. The baseline activity is inversely correlated with the monkey’s recent history of success and failure. We will discuss these results in the context of previous studies of baseline effect and reward and the possible sources and significance of this signal.
Spatially Specific Baseline Effects.
Baseline activity can correlate with a particular part of the monkey’s behavior in task-specific ways. When monkeys can reasonably anticipate what they will do after the substantive part of the trial begins, the baseline activity often anticipates the response that the monkey will make. In the frontal eye field, when the monkey performed blocks of saccades to the same target in the neuron’s receptive field, the monkey could predict the direction of the impending saccade, and the baseline activity began to anticipate the appearance of the target (6). This anticipatory activity disappeared when the target location became unpredictable.
The baseline activity of LIP neurons predicts the monkey’s choice of a saccade target in or out of the receptive field when monkeys have to make a saccade under conditions in which a usually reliable cue has no information about the proper choice (19). When a monkey can anticipate that a stimulus perturbation in a neuron’s receptive field in a stable array will signal the appearance of the search target in the receptive field, LIP neurons respond more during the fixation period than when the monkeys know that the perturbation will signal the appearance of the target away from the receptive field (20). In a combined study of LIP, the frontal eye field, and the supplementary eye field in a free choice task, the baseline in all three areas increased when the monkey decided to make a saccade to the receptive field (21). The intensity of visual responses in V1 of the anesthetized cat correlated with the mean optical signal measured during the baseline interval (22). This signal may be related to the baseline signal that we measured and its correlation with the intensity of the response to the onset of the array, although it had, perforce, no correlation with behavior. Furthermore, in this study the optical image was gathered from cortex including the neurons whose response correlated with the signal, or at least within the range of lateral interactions from that area. The baseline signal that we have shown here has no spatial selectivity.
Spatially Nonspecific Baseline Effects.
There are several reports of spatially nonspecific baseline effects in cortical neurons, but none of these resemble the phenomenon we have described here. Baseline activity in prefrontal cortex on the first trial of a three-step self-ordered task correlated with the monkey’s performance. However, the correlation peaked minutes before and after the current trial (23), and there was no correlation with performance for the current trial. Neurons in posterior cingulate decreased their activity when a task began, seemingly inhibited by task engagement, although neurons in LIP do not track with task engagement (24).
The results reported here are very different from these previous observations of baseline activity. In our first experiment the monkey could not anticipate either where the target will be or what kind of trial will occur when the fixation point disappears, so the sensorimotor aspects of the baseline activity were constant across all trials. Nonetheless, the level of baseline activity fluctuated naturally, and its level predicted the monkey’s performance and, in a multiplicative manner, the intensity of the visual transient even when a distractor and not the target was going to appear in the cell’s receptive field. The baseline level was predicted by the monkey’s recent history of success or failure, with both the baseline and the monkey’s performance inversely related to a recency-weighted measure of the monkey’s success or failure. In a theoretical paper, Niv et al. (25) postulated that baseline activity controlled by dopamine should affect manual reaction time. We found a positive relationship between baseline activity and manual reaction time for 7/37 cells (Spearman correlation, P < 0.05; P < 0.01 by chi square test) and a negative relationship for 6/37 cells (Spearman correlation, P < 0.05; P < 0.01 by chi square test).
Signals Related to Previous Reward.
Signals related to previous reward have been seen in other studies. When monkeys learn to associate a saccade target with a reward, the intensity of the LIP response to the target increases with the expected reward (11). In a foraging task with reward probabilities fluctuating from block to block, LIP neurons tracked the probability of reward in their task-related activity but not in the baseline (14). In a task in which the monkeys used success or failure to solve a timing problem, the response of dopamine neurons to the reward increased when the difference between the current award and a recency-weighted index of past rewards was large, indicating that the monkey’s guess about timing was correct. The activity, however, was not related to the magnitude of the reward itself. Furthermore, the baseline activity, which increased when a warning sound signaled the beginning of a trial, did not have any relationship to reward or the monkey’s recent performance (26).
Attention and the Baseline Signal.
To rule out the unlikely possibility that the monkey was always attending to the neuron’s receptive field during the trial, even though there was no stimulus in the receptive field, for some cells we used a 50% valid cue during the fixation period to pin the monkey’s attention to the spatial location of the cue, which appeared in the receptive field only on 1/6 of the trials. The cue had the expected validity effect both on saccadic and bar-release reaction times, which showed that the monkey attended to the cue when it appeared. Nonetheless, the baseline activity, when the cue was not in the neuron’s receptive field and the monkey was attending elsewhere, still reflected the monkey’s probability of success. Thus, although LIP is important in the neural processes underlying attention (9), the signal in the baseline that predicts the efficiency of the monkey’s behavior is not related to the monkey’s selective attention, because the baseline effect occurred even when the monkey was not attending to the spatial location of the receptive field. However, attention can affect the baseline: when the cue appeared in the neuron’s receptive field the baseline activity was greater for both successful and unsuccessful trials—this increment was probably related to a spatially selective, attentional component of the baseline activity, similar to that described previously in LIP (19) and the frontal eye field (6).
Motivation, Arousal, and State.
It is likely that a signal with little spatial, featural, attentional, or motor selectivity modulates the baseline activity. Because the externally imposed reward is constant throughout the experiment, we suggest that the fluctuation in baseline activity is the manifestation of intrinsic state, motivational, or arousal signals. A few studies have demonstrated arousal effects on sensory responses. Thus, the response of V1 neurons to a visual stimulus in the receptive field is enhanced when a monkey makes a saccade to a stimulus anywhere in the visual field, not just to a target in the receptive field, compared with a fixation task, and also when the monkey makes a hand movement in response to a stimulus anywhere in the visual field (8). V1 neurons in the mouse have a greater response to drifting gratings when the mouse is running than when the mouse is resting but not sleeping (27). In neither of these studies was there an enhancement of the baseline activity. The V1 enhancement signals, like our LIP baseline and baseline-related visual signals, rather than containing information about the specifics of sensorimotor processing, may condition the efficiency of that processing, representing an increase in motivation or arousal. A monkey should be more aroused and more motivated to succeed after a recent history of failure and less aroused and less motivated after a recent history of success, and, in fact, both the baseline activity and the monkey’s performance itself correlate inversely with a measure of the monkey’s recent performance.
A number of ascending, presumably modulatory pathways project to the parietal lobe, including a cholinergic projection from the basal forebrain and a noradrenergic pathway from the locus ceruleus (28). These pathways may also be affected by the dopaminergic projection to prefrontal cortex, which could then excite the cholinergic neurons in the basal forebrain and set the modulatory tone of LIP (29). The cholinergic projection is a promising candidate because acetylcholine is known to increase the response of V1 neurons to attended objects (30). The baseline activity, which predicts both the gain of the visual on-response and the monkey’s motivation to perform the task in a spatially nonspecific manner, could come from these ascending pathways or from cortical areas with nonspatial activity. It is clear from our results that understanding how the brain influences primate behavior will require more than unraveling the nuances of sensorimotor processing; it will require understanding the broad strokes by which neuromodulation affects that processing, especially because of the importance of neuromodulators, their agonists, and antagonists as therapeutic and recreational agents.
Experimental Methods
Behavior.
The New York State Psychiatric Institute and Columbia University Medical Center Institutional Animal Care and Use Committees approved all animal protocols and certified them to be in compliance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (31). Behavior was controlled by a paradigm written in the REX programming language (32), running under the QNX operating system on a Dell OptiPlex computer. The monkey sat 72 cm in front of a tangent screen upon which computer-generated visual stimuli were back-projected. Target and invalid cue positions were randomized using the QNX random seed and not adjusted to balance the number of locations within a block. Cue and target locations were entirely unpredictable: the probability that any target location (including that of the current trial) would appear on the previous trial or the following trial was 1/6. The fixation point, array objects, and cues were 400 cd/m2. The screen background was 2 cd/m2.
The multiple conditions used in this study required more than 1,000 trials per cell to get adequate data. Because we could not record more than one cell a day we usually had to supplement the monkey’s fluid intake after the recording sessions, and as a result the monkeys performed in a relatively stable manner during the recording sessions.
Physiology.
Animals were prepared for study by the implantation of subconjunctival eye coils (33), a head-holding device (Crist Instruments), and a recording chamber with the dura left intact, through which electrodes could be inserted into the brain for recording. Eye position was measured using a Crist Instruments phase detector. Neural activity was measured using a resin-coated tungsten electrode driven by a Narishige microdrive, connected to an FHC Neurocraft amplifier. Neurons were discriminated online using the MEX spike sorter system (Laboratory of Sensorimotor Research, National Eye Institute). Eye position and neural pulses were recorded at 1Khz by the REX system. The intraparietal sulcus was located by a T1 MRI (GE 1.5T Signa scanner). LIP was located by its position in the posterolateral bank of the intraparietal sulcus, where we found cells active in all three phases of the memory-guided delayed saccade task. Cells studied in this report were active in the memory-guided delayed saccade task. Offline data analysis was done using MATLAB.
Supplementary Material
Acknowledgments
We are grateful to Yana Pavlova for veterinary technical help, Drs. Girma Asfaw and Moshe Shalev for veterinary care, John Caban and Matthew Hasday for machining, Glen Duncan for computer and electronic assistance, and Latoya Palmer and Holly Kline for facilitating everything. Dr. Annegret Falkner developed the method of quantifying success and failure for another study. Drs. C. Daniel Salzman and Jacqueline Gottlieb read an early version of this paper. This research was supported, in part, by grants from the Zegar, Keck, Kavli, and Dana Foundations and the National Eye Institute [P30EY019007, R24EY015634, R21EY017938, R01EY014978, R01EY017039 (M.E.G., Principal Investigator)] and 973 program (2100CBA00400); by the Chinese government; the Hundred Talent Program, Chinese Academy of Sciences; the Pujiang Program, Shanghai government; and the State Key Laboratory of Neuroscience, Chinese government [M.Z., Principal Investigator].
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407540111/-/DCSupplemental.
References
- 1.Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol. 1972;35(4):560–574. doi: 10.1152/jn.1972.35.4.560. [DOI] [PubMed] [Google Scholar]
- 2.Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. J Neurophysiol. 1991;66(3):1095–1108. doi: 10.1152/jn.1991.66.3.1095. [DOI] [PubMed] [Google Scholar]
- 3.Bushnell MC, Goldberg ME, Robinson DL. Behavioral enhancement of visual responses in monkey cerebral cortex. I. Modulation in posterior parietal cortex related to selective visual attention. J Neurophysiol. 1981;46(4):755–772. doi: 10.1152/jn.1981.46.4.755. [DOI] [PubMed] [Google Scholar]
- 4.Platt ML, Glimcher PW. Response fields of intraparietal neurons quantified with multiple saccadic targets. Exp Brain Res. 1998;121(1):65–75. doi: 10.1007/s002210050438. [DOI] [PubMed] [Google Scholar]
- 5.Rao V, DeAngelis GC, Snyder LH. Neural correlates of prior expectations of motion in the lateral intraparietal and middle temporal areas. J Neurosci. 2012;32(29):10063–10074. doi: 10.1523/JNEUROSCI.5948-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53(3):603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- 7.Supèr H, van der Togt C, Spekreijse H, Lamme VA. Internal state of monkey primary visual cortex (V1) predicts figure-ground perception. J Neurosci. 2003;23(8):3407–3414. doi: 10.1523/JNEUROSCI.23-08-03407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wurtz RH, Mohler CW. Enhancement of visual responses in monkey striate cortex and frontal eye fields. J Neurophysiol. 1976;39(4):766–772. doi: 10.1152/jn.1976.39.4.766. [DOI] [PubMed] [Google Scholar]
- 9.Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76(5):2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- 11.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400(6741):233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 12.Falkner AL, Krishna BS, Goldberg ME. Surround suppression sharpens the priority map in the lateral intraparietal area. J Neurosci. 2010;30(38):12787–12797. doi: 10.1523/JNEUROSCI.2327-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louie K, Grattan LE, Glimcher PW. Reward value-based gain control: Divisive normalization in parietal cortex. J Neurosci. 2011;31(29):10627–10639. doi: 10.1523/JNEUROSCI.1237-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304(5678):1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- 15.Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J Neurosci. 2006;26(14):3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisley JW, Ipata AE, Krishna BS, Gee AL, Goldberg ME. The lateral intraparietal area: A priority map in posterior parietal cortex. In: Jenkin M, Harris L, editors. Cortical Mechanisms of Vision. Cambridge, U.K.: Cambridge Univ Press; 2009. pp. 5–30. [Google Scholar]
- 17.Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70(1):216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- 18.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 19.Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci. 2002;22(21):9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balan PF, Gottlieb J. Integration of exogenous input into a dynamic salience map revealed by perturbing attention. J Neurosci. 2006;26(36):9239–9249. doi: 10.1523/JNEUROSCI.1898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coe B, Tomihara K, Matsuzawa M, Hikosaka O. Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J Neurosci. 2002;22(12):5081–5090. doi: 10.1523/JNEUROSCI.22-12-05081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273(5283):1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa RP, Blitz AM, Geller NL, Goldberg ME. Neurons in monkey prefrontal cortex that track past or predict future performance. Science. 2000;290(5497):1786–1789. doi: 10.1126/science.290.5497.1786. [DOI] [PubMed] [Google Scholar]
- 24.Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci USA. 2009;106(14):5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: Opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007;191(3):507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- 26.Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47(1):129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65(4):472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saper CS. Brain stem modulation of sensation, movement, and consciousness. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4th Ed. New York: McGraw Hill; 2001. pp. 889–909. [Google Scholar]
- 29.Ghashghaei HT, Barbas H. Neural interaction between the basal forebrain and functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience. 2001;103(3):593–614. doi: 10.1016/s0306-4522(00)00585-6. [DOI] [PubMed] [Google Scholar]
- 30.Herrero JL, et al. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454(7208):1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC), 8th Ed.
- 32.Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc. 1982;2(1):1–10. [Google Scholar]
- 33.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20(6):535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.