Significance
During embryonic development, multipotent stem cells progressively acquire specific cell fates. The somite is an embryological structure that gives rise to different mesodermal cell types, including skeletal muscle and vascular cells of blood vessels. We show by genetic manipulation that the Notch signaling pathway promotes a vascular cell-fate choice at the expense of skeletal muscle in the mouse somite. Pax3+ cells in the adjacent somites give rise to myogenic and endothelial cells in the limbs. Gain-of-function or inhibition of Notch signaling affects this cell-fate choice prior to the migration of these somite-derived cells into the limb. This embryological role of Notch is of potential therapeutic relevance to deriving stem cells for tissue repair.
Abstract
Multipotent Pax3-positive (Pax3+) cells in the somites give rise to skeletal muscle and to cells of the vasculature. We had previously proposed that this cell-fate choice depends on the equilibrium between Pax3 and Foxc2 expression. In this study, we report that the Notch pathway promotes vascular versus skeletal muscle cell fates. Overactivating the Notch pathway specifically in Pax3+ progenitors, via a conditional Pax3NICD allele, results in an increase of the number of smooth muscle and endothelial cells contributing to the aorta. At limb level, Pax3+ cells in the somite give rise to skeletal muscles and to a subpopulation of endothelial cells in blood vessels of the limb. We now demonstrate that in addition to the inhibitory role of Notch signaling on skeletal muscle cell differentiation, the Notch pathway affects the Pax3:Foxc2 balance and promotes the endothelial versus myogenic cell fate, before migration to the limb, in multipotent Pax3+ cells in the somite of the mouse embryo.
During development, the segmented paraxial mesoderm of the somites gives rise to different mesodermal derivatives. As somites mature, cells delaminate from the dorsal dermomyotome to form the skeletal muscle of the myotome and later trunk muscles, or migrate from the hypaxial dermomyotome into the early limb bud to form limb muscles (1). Vascular progenitors also derive from this part of the dermomyotome. In the chicken embryo, a subpopulation of endothelial cells and myogenic progenitors in the trunk (2) and the limb (3) arise from the same multipotent cells in the somite, as do skeletal muscle and vascular smooth muscle of some blood vessels in the trunk (2). Clonal analysis in the mouse has shown that smooth muscle cells of the dorsal aorta and the myotome have a common origin (4). Dermomyotomal cells are marked by Pax3, which is essential for the migration of myogenic progenitors to sites of skeletal muscle formation, such as to the limb (1). Genetic tracing experiments confirm that some endothelial cells in the mouse limb derive from Pax3+ cells in the somite (5).
Reciprocal inhibition between Pax3 and Foxc2 in the somite, when perturbed genetically in the mouse embryo, affects vascular versus myogenic cell fate choices (6). Signaling molecules impact the somite, potentially changing the Pax3:Foxc2 equilibrium. In the chicken embryo, manipulation of bone morphogenetic protein signaling showed that it promotes an endothelial cell fate, whereas Notch signaling promotes the formation of vascular smooth muscle at the expense of skeletal muscle (2). However, in another report on the chicken embryo, overactivation of Notch signaling was shown to increase the migration of vascular endothelial cells from the somite to the dorsal aorta (7). Notch signaling is active in the hypaxial region of the chick somite (2) and also in somites and in endothelial cells of blood vessels at embryonic day (E) 9.5 in the mouse embryo (7, 8).
To examine the role of Notch signaling in the myogenic versus endothelial fate choice in the mouse embryo, we have targeted one allele of Pax3 with a sequence coding for NICD, the constitutively active intracellular domain of Notch receptor 1. In the trunk of such Notch gain-of-function embryos, both vascular smooth and endothelial cells derived from the somite are increased, whereas myogenesis is diminished. In the limbs, fewer Pax3+ cells are present initially, reflecting the promotion of an endothelial versus skeletal muscle cell fate. Somite explant experiments confirm this shift in cell fate, which is accompanied by an increase in Foxc2 expression, whereas when Notch signaling is inhibited, the reverse is observed with a relative increase in myogenic cells. We conclude that the endothelial/myogenic cell fate choice takes place in Pax3+ cells in the somite, before their migration to the limbs, and is regulated by the Notch signaling pathway which affects the Pax3:Foxc2 genetic equilibrium.
Results
The Notch Pathway Promotes a Vascular Fate in the Trunk.
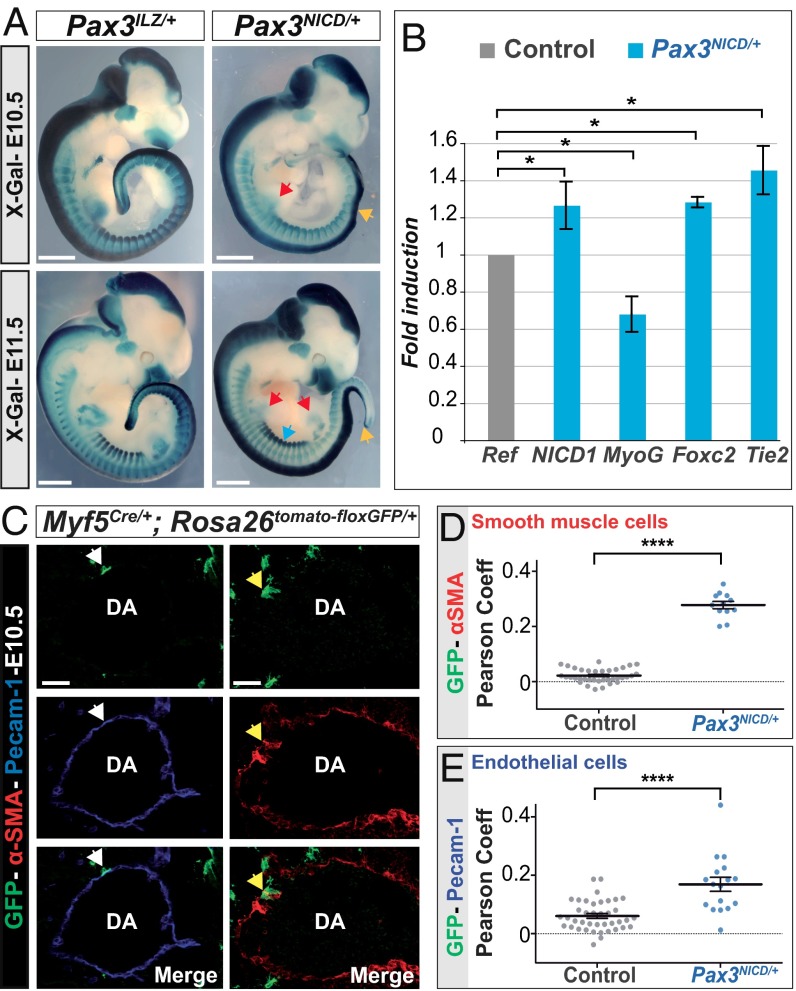
To determine the role of the Notch pathway in cell-fate decisions in Pax3+ cells in the mouse somite, we designed a mouse model where a sequence encoding the activated intracellular domain of Notch receptor 1 (NICD) was introduced into the first exon of the Pax3 gene (Fig. S1A). The floxed Pax3NICD-IRESnLacZ allele was activated by crossing with a PGK-Cre transgenic line, unless otherwise stated. Expression of NICD1 and Notch target genes, Hey1 and Lunatic fringe, was increased at sites of myogenesis, notably in the hypaxial somite, in Pax3NICD-IRESnLacZ/+ (Pax3NICD/+) compared with control embryos (Fig. S1 B and C). Comparison of β-Galactosidase (β-Gal) activity between Pax3IRESnLacZ/+ (Pax3ILZ/+) (9) and Pax3NICD-IRESnLacZ/+ (Pax3NICD/+) embryos showed expression at similar sites, reflecting the expression of Pax3 (Fig. 1A). Pax3NICD/+ embryos have embryonic defects at sites of Pax3 expression in the neural tube and somites, resulting in developmental defects, which are different from those of Pax3-null embryos. We detected differences in thoracic somites at E11.5, when the organization of Pax3+ cells in the hypaxial dermomyotome is perturbed (Fig. S2A).
Fig. 1.
Somite defects and an increase in somite-derived vascular cells in the trunk of Pax3NICD/+ embryos. (A) X-Gal staining of Pax3NICD-IRESnLacZ/+ (Pax3NICD/+) compared with Pax3IRESnLacZ/+ (Pax3ILZ/+), at E10.5 (Upper) and E11.5 (Lower). Yellow arrowheads: neural tube defects; red arrowheads: reduction in X-Gal staining in the limbs; blue arrowhead: structural perturbation in the hypaxial dermomyotome (Scale bar, 2 mm). (B) qRT-PCR analysis on Pax3NICD/+ somite explants after 3 d in culture, compared with those from control (Pax3+/+) embryos, taken as 1, relative to transcripts for gapdh (*P < 0.05, error bars indicate SEM, n = three experiments with six pooled embryos). (C) Immunohistochemistry on sections of Myf5Cre/+;Rosa26tomato-floxGFP/+, at E10.5, with GFP, Pecam-1 (Left) and α-smooth muscle actin (α-SMA) (Right) antibodies, at the level of the dorsal aorta (DA). White arrows: GFP+;Pecam-1+ cells; yellow arrows: GFP+;α-SMA+ cells (Scale bar, 50 μm). (D and E) Quantification of the contribution of somitic cells (GFP+) to smooth muscle cells (α-SMA+) (D) and endothelial cells (Pecam-1+) (E) of the dorsal aorta, was automatically measured using the Pearson coefficient as a colocalization tool, in Pax3NICD/+ (Pax3NICD/+;Myf5Cre/+;Rosa26tomato-floxGFP/+) and control (Pax3+/+;Myf5Cre/+;Rosa26tomato-floxGFP/+) embryos. ****P < 0.0001, error bars indicate SEM (n > 12 aorta sections).
We first examined the effect of the Pax3NICD allele on vascular cell versus myogenic cell fates in the trunk in somite explant cultures (6). After 3-d culture of presomitic mesoderm and immature somites isolated from E9.5 embryos in the presence of NICD, transcripts of Myogenin (Myog) were lower, whereas transcripts of Tie2 and Foxc2 that mark vascular cells were significantly higher (Fig. 1B), as was the expression of Pecam-1 (Fig. S3A), a marker of differentiated endothelial cells. In vivo, quantitative RT-PCR (qRT-PCR) analysis of immature posterior somites of E11.5 embryos shows up-regulation of Foxc2 and down-regulation of Pax3, as well as the myogenic regulatory genes Myf5 and Myog, in Pax3NICD/+ embryos compared with controls (Fig. S3B).
Using genetic tracing with Pax3Cre and Rosa26tomato-floxGFP alleles, we show that cells that have expressed Pax3 give rise to both endothelial and smooth muscle cells of the intersomitic vessels (Fig. S4A) that are formed from sprouting of the aorta. This finding is in keeping with GFP perduration seen in all smooth muscle cells of the dorsal aorta in Pax3GFP/+ embryos (4). To avoid any contribution of neural crest cells, which also express Pax3 (1) and can contribute to the formation of vascular cells, we used the Myf5Cre allele, which is first activated in a subset of cells in the presomitic mesoderm (10). Myf5Cre/+;Rosa26tomato-floxGFP/+ embryos have scattered GFP+ cells within the dermomyotome (Fig. S4B). At E10.5, a subpopulation of endothelial and smooth muscle cells of the dorsal aorta are labeled in this experiment (Fig. 1C). Endothelial cells of the aorta are laid down before the smooth muscle component and are less marked by Pax3- and Myf5-Cre drivers, expressed in late presomitic mesoderm. To analyze the role of the Notch pathway in vivo at the cellular level, we quantified the number of endothelial and smooth muscle cells in the dorsal aorta, labeled with the Rosa26tomato-floxGFP and Myf5Cre alleles, as in Fig. 1C, in control and Pax3NICD/+ embryos. There is a significant increase of the contribution to the aorta of smooth muscle (Fig. 1D) and endothelial cells (Fig. 1E) derived from the somite. This accumulation of vascular cells induces intersomitic vessel disorganization later as shown by Pecam-1 whole-mount staining in the tail of Pax3NICD/+ embryos at E12.5 (Fig. S5).
In conclusion, these results show that in the mouse embryo, the Notch pathway promotes acquisition of a vascular cell fate in Pax3+ cells. The choice of cell fate probably occurs early during development, because we have observed GFP+ cells at E9.25 in the dorsal aorta of Myf5Cre/+;Rosa26tomato-floxGFP/+ embryos (Fig. S4B).
Somite-Derived Endothelial and Myogenic Cells in the Limbs.
We then analyzed the contribution of Pax3+ cells of the somite to the limb. As previously described (5), cells that have expressed Pax3 give rise to endothelial cells, of the superficial vessels (Fig. S4C) as well as to skeletal muscles of the limb. No smooth muscle cells in the limb were labeled in this experiment, indicating that in the mouse embryo they do not derive from the somite (Fig. S4C). Cell labeling with GFP in the limbs of Myf5Cre/+;Rosa26tomato-floxGFP/+ embryos at E10.5 confirms that endothelial as well as myogenic cells derive from the somite, excluding the implication of neural crest cells (Fig. S4D).
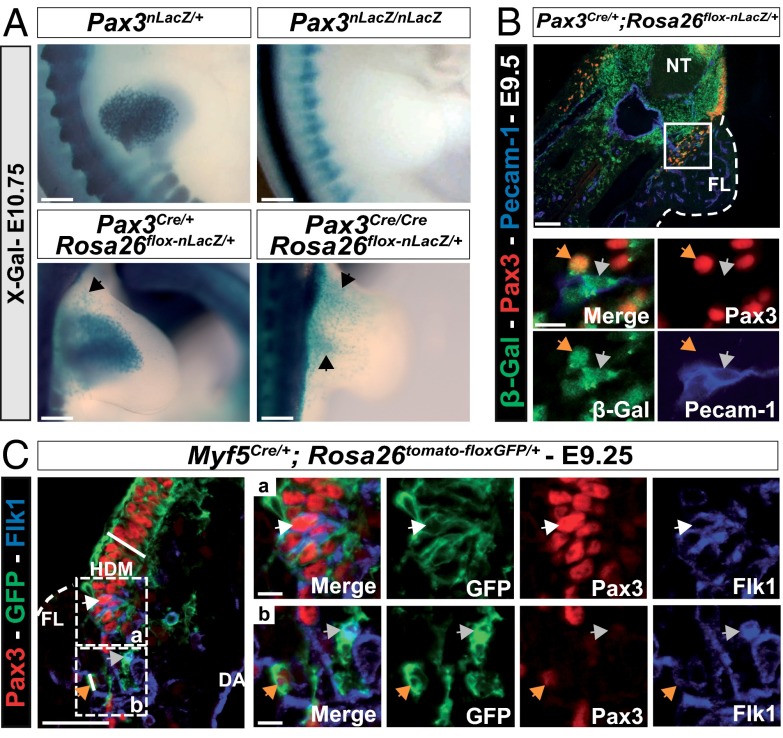
In the absence of Pax3, myogenic progenitors do not migrate to the limbs and no β-Gal+ cells are present in Pax3nLacZ/nLacZ embryos compared with Pax3nLacZ/+ embryos (Fig. 2A). However, in Pax3Cre/Cre;Rosa26floxnLacZ/+ embryos, cells that had expressed Pax3 are present (Fig. 2A). In addition to showing that migration of endothelial cells is Pax3-independent, this result also indicates that endothelial cells have migrated from the somites to the forelimbs before apoptosis of cells in the hypaxial dermomyotome, seen by E10.5 in the Pax3 mutant (1).
Fig. 2.
Endothelial cells of somitic origin, in the limb. (A) X-Gal staining of Pax3nLacZ/+ and Pax3Cre/+;Rosa26flox-nLacZ/+ embryos compared with Pax3nLacZ/nLacZ and Pax3Cre/Cre;Rosa26flox-nLacZ/+ mutant embryos, at E10.75. Black arrowheads indicate nonmyogenic cells that had expressed Pax3 (Scale bar, 1 mm). (B) Immunohistochemistry on sections of Pax3Cre/+;Rosa26flox-nLacZ/+ embryos at E9.5, with Pax3 (red), β-Galactosidase (β-Gal; green), and Pecam-1 (blue) antibodies, at the forelimb (FL) level (Scale bars, 100 μm). Close up of the boxed region in the Upper panel of the two Lower panels (Scale bars, 10 μm), shows myogenic progenitors (orange arrows: β-Gal+, Pax3+, Pecam-1−) and endothelial cells derived from Pax3+ progenitor cells (gray arrows: β-Gal, Pax3−, Pecam-1+). NT, neural tube. (C) Immunohistochemistry on sections of Myf5Cre/+;Rosa26tomato-floxGFP/+ embryos at E9.25, with Pax3 (red), GFP (green), and Flk1 (blue) antibodies, in the hypaxial somite (Scale bars, 50 μm). Close ups of the boxed region of the hypaxial dermomyotome (HDM) (a) and of delaminating cells (b) are represented in panels on the right (Scale bars, 10 μm). White arrowheads in (a) (Pax3+, GFP+, Flk1+, potential bipotent progenitors); orange arrowheads in (b) (Flk1−; Pax3+, myogenic progenitors); gray arrows in b (Flk1+; Pax3−, endothelial cells).
Once myogenic progenitors are specified in the dermomyotome, they begin to delaminate at E9.25 and migrate into the forelimb at E9.5. We observed at the beginning of the migration that somite-derived endothelial cells, labeled by β-Gal, have already acquired an endothelial cell fate, as shown by Pecam-1 labeling and the absence of Pax3 (Fig. 2B). At E9.25, we observed by using genetic tracing with Myf5Cre and Rosa26tomato-floxGFP alleles, that GFP+ cells localized in the hypaxial dermomyotome express both Pax3 and Flk1 proteins (Fig. 2 C, a). Flk1 is an early marker of endothelial cells. We therefore propose that these cells are bipotent progenitors able to give rise to both endothelial and myogenic cells. When somitic cells leave the hypaxial dermomyotome, cells that maintain Pax3 expression are no longer Flk1+, whereas cells maintaining Flk1 are no longer Pax3+ (Fig. 2 C, b). These observations suggest that Flk1 is extinguished in Pax3+ myogenic cells once they have left the somite and that the loss of Pax3 expression is a characteristic of cells that have entered the endothelial lineage.
In conclusion, these results suggest that bipotent progenitors in the hypaxial dermomyotome, which give rise to both endothelial and myogenic cells in the limb, undergo a cell fate choice before they leave the somite.
Inhibition of Myogenic Differentiation by the Notch Pathway.
In Pax3NICD/+ embryos there is a strikingly reduced number of Pax3+ cells in the limbs, as shown by X-Gal staining at E10.5 or E11.5 compared with control Pax3ILZ/+ embryos (Fig. 1A and Fig. S6A), also seen for transcripts of Pax3 and Lbx1 (see, for example, Fig. S6 B and C), which label migrating myogenic progenitors. In keeping with previous reports on the role of Notch signaling in myogenesis (11, 12), we observed that Myod1 was not activated initially in the remaining Pax3+ cells in the limbs of Pax3NICD/+ embryos (Fig. S6D). Because Pax3 is down-regulated at E12.5, myogenic cells that are now Pax7+ (Fig. S6E) show hyperproliferation rates and also accelerated myogenic differentiation in Pax3NICD/+ embryos, compared with controls (Fig. S6 F and G). By E18.5 skeletal muscle masses are indistinguishable from controls (Fig. S6H). These observations complement those on Notch loss-of-function embryos, illustrating the inhibitory effect of Notch signaling on the entry of progenitor cells into the myogenic program. The findings also demonstrate the plasticity of the myogenic process, so that skeletal muscle formation recovers once a developmental perturbation is lifted.
The Notch Pathway Promotes an Endothelial Cell Fate.
This effect on skeletal muscle differentiation occurs once myogenic progenitors have reached the limbs and does not explain why the number of these progenitors is initially reduced in Pax3NICD/+ embryos. At E9.5–10.5, there is no detectable cell death or structural defect in the hypaxial dermomyotome of these embryos (Fig. S2B). We therefore investigated whether there had been a corresponding increase in somite-derived endothelial cells in the limbs. The decrease in Pax3 and Myf5 transcripts is accompanied by an increase in Foxc2 transcripts, in keeping with an increase of vascular cells in the forelimbs of Pax3NICD/+ embryos (Fig. 3A). Immunohistochemistry on whole embryos with a Pecam-1 antibody showed increased labeling with this endothelial marker at E11.5 compared with the control (Fig. 3B). Serial confocal images from the dorsal superficial layer to the center of the forelimb showed that this increase is most marked in the superficial region of Pax3NICD/+ embryos (Fig. S7), where genetic tracing experiments had shown preferential location of somite-derived endothelial cells (5). For quantification of the contribution of somitic cells to both endothelial and myogenic cell types, we used Myf5Cre and Rosa26tomato-floxGFP alleles to analyze GFP+Pax3+ (myogenic progenitor cells) and GFP+Pecam-1+ (endothelial cells) in the forelimbs of Pax3NICD/+ and control embryos at E10.5 (Fig. 3C). There is a significant increase of the contribution of somitic progenitors to the endothelial, at the expense of the myogenic lineage, when Notch signaling is stimulated.
Fig. 3.
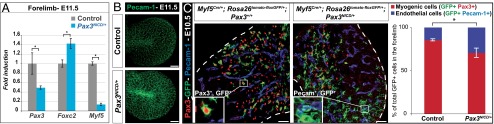
Increase of the number of endothelial cells derived from the somite, in Pax3NICD/+ embryos. (A) qRT-PCR analysis of transcripts of Pax3, Foxc2, and Myf5 compared with gapdh, on forelimbs of Pax3NICD/+ embryos, at E11.5, compared with the controls (Pax3+/+), taken as 1. *P < 0.05, error bars indicate SEM (n = 3 embryos). (B) Whole-mount immunostaining with an antibody to Pecam-1 on the forelimbs of control (Pax3IRES-nLacZ/+) and Pax3NICD/+ embryos, at E11.5 showing the 3D projection (mean value) of the images obtained with confocal sectioning of the limb buds (Scale bar, 100 μm). (C) Immunohistochemistry on sections from control (Pax3+/+;Myf5Cre/+;Rosa26tomato-floxGFP/+) and Pax3NICD/+;Myf5Cre/+;Rosa26tomato-floxGFP/+ embryos, with Pax3 (red), Pecam-1 (blue), and GFP (green) antibodies, at E10.5, at the forelimb level. Insets show enlargements of GFP+Pax3+ (Left) and GFP+Pecam-1+ (Right) cells (Scale bar, 50 μm). The graphs represent quantification of the contribution of somitic cells (GFP+) in the myogenic lineage (GFP+ cells labeled by Pax3) and in the endothelial lineage (GFP+ cells labeled by Pecam-1), expressed as a percentage of total GFP+ cells. *P < 0.05, error bars indicate SEM (n = 3 embryos).
Notch Affects the Cell Fate of Limb Progenitors in the Somite.
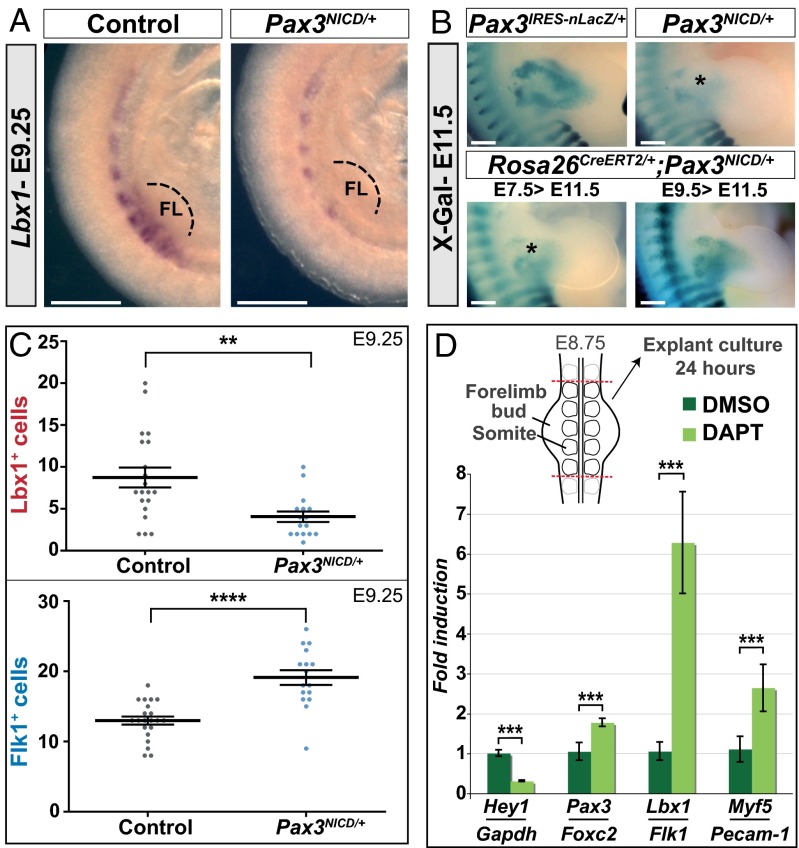
Lbx1 activation in the hypaxial dermomyotome of the somite marks myogenic progenitor cells that will migrate into the limbs (1). In Pax3NICD/+ embryos at E9.25, Lbx1 transcripts are notably reduced compared with the control, indicating an early defect of myogenic specification in the somite (Fig. 4A). To determine when this defect occurs during development, instead of using the PGK-Cre line, we crossed Pax3NICD/+ and Rosa26Cre-ERT2/Cre-ERT2 mice to induce the overexpression of Notch by tamoxifen induction, specifically in Pax3+ cells, at different times during development (Fig. 4B). When Notch overexpression was induced after E7.5, Pax3NICD/+;Rosa26CreERT2/+ embryos present the same reduction of Pax3+ cells in the limbs, as in Pax3NICD/+ embryos. However, when this induction occurred after E9.5, Pax3NICD/+;Rosa26CreERT2/+ embryos were similar to the control (Fig. 4B). This result indicates that the reduced number of myogenic progenitors expressing Pax3 in the limb of Pax3NICD/+ embryos is because of an effect of NICD in this time window.
Fig. 4.
Endothelial, versus myogenic cell fate in the somite is promoted by the Notch pathway. (A) Whole-mount in situ hybridization for Lbx1 transcripts on control (Pax3+/+) and Pax3NICD/+ embryos at E9.25. Close ups at the forelimb (FL) level are shown (Scale bar, 500 μm). (B) X-Gal staining of Pax3IRES-nLacZ/+, Pax3NICD/+ and Pax3NICD/+;Rosa26CreERT2/+ embryos, at E11.5. Enlargements at forelimb level are shown. Tamoxifen was injected into pregnant mice at E7.5 or E9.5, as indicated (Scale bar, 2 mm). (C) Quantification of the total number of endothelial progenitors (Flk1+) and myogenic progenitors (Lbx1+) in the hypaxial region at E9.25. **P < 0.01 and ****P < 0.0001. Error bars indicate SEM (n > 16 limb bud sections). (D, Upper) A schema of the explant procedure. (Lower) qRT-PCR results expressed as ratios of Hey1/Gapdh, Pax3/Foxc2, Lbx1/Flk1, and Myf5/Pecam-1 transcripts on explants after 24 h of culture, in DMSO and DAPT (50 µM). ***P < 0.001, error bars indicate SEM (pool of n > 2 explants by experiment, n > 3 experiments).
We have quantified the number of Lbx1+ cells and Flk1+ cells in the hypaxial region near the limb bud at E9.25 in Pax3NICD/+ embryos. There is a significant reduction of the number of Lbx1+ cells and more Flk1+ cells in and around the hypaxial dermomyotome, than in the control embryo (Fig. 4C). Furthermore, in Pax3NICD/+ embryos the percentage of cells expressing Foxc2 and Flk1 is significantly higher (Fig. S8). These results indicate that the balance between specification of endothelial cells and myogenic cells in the hypaxial dermomyotome at forelimb level is affected by the Notch pathway, which promotes the endothelial fate.
Finally, to complement these Notch gain-of-function experiments, we used loss-of-function strategies. To inactivate the Notch pathway, we conditionally deleted RBPJ-κ using Pax3Cre/+, but because of RBPJ-κ stability, protein depletion was not efficient in the critical time window when the cell-fate decision occurs between myogenic and endothelial lineages (Fig. S9). Deletion by Cre-recombinase under the control of genes expressed earlier than Pax3 results in somite defects. We therefore developed the strategy of Notch depletion by the γ-secretase inhibitor, DAPT, on explants of forelimb buds and adjacent somites isolated from wild-type embryos at E8.75 (Fig. 4D). After 24-h culture, qPCR analysis showed a down-regulation of the Notch target gene, Hey1, and an increase in the ratio of transcripts for Pax3/Foxc2, Lbx1/Flk1, and Myf5/Pecam-1, compared with control explants (Fig. 4D). These loss-of-function experiments therefore confirm that the Notch signaling pathway acts on the balance between Pax3 and Foxc2 and promotes an endothelial, at the expense of a myogenic cell fate.
Discussion
In the experiments reported here, we characterize somite-derived endothelial cells in the limb and go on to show that increased Notch signaling in Pax3+ progenitors in the somite promotes the endothelial versus myogenic cell fate of these cells.
Endothelial, but not smooth muscle, cells that have migrated from the somite are present in the limb and contribute to blood vessels, particularly to those located superficially, consistent with previous findings (5). On the other hand, in the trunk smooth muscle cells of the dorsal aorta derive from the somites (4), and we now show for the mouse embryo that endothelial cells of the dorsal aorta are also somite-derived, as previously reported for the chick. Endothelial cells of somitic origin in the limb already express Pecam-1, indicating that they are no longer multipotent and indeed they no longer express Pax3 and do not depend on Pax3 for their migration, in contrast to myogenic progenitors. This finding is in keeping with the observation that endothelial (5), unlike myogenic, cell migration does not depend on the expression of the c-met gene, which is a Pax3 target (1). In the Pax3 mutant, the hypaxial domain of the somite is compromised by apoptosis; however, this occurs later (1), after endothelial cells are detected in the limb at E9.5. Before expression of the endothelial cell-marker Pecam-1, most cells in the hypaxial somite express Flk1. These cells are also Pax3+ and express Lbx1. Subsequently, Flk1 is present in cells that do not express Pax3, whereas cells that retain Pax3 as they leave the somite are Flk1−. These observations suggest that Flk1 marks multipotent Pax3+ cells in the somite, as well as endothelial cells.
We have examined the effects of Notch signaling in Pax3+ cells by overexpression of NICD targeted to a Pax3 allele. Stimulation of Notch signaling in Pax3+ myogenic progenitors inhibits muscle differentiation, as might be expected from previous results for Notch loss-of-function embryos (11, 12) where myogenesis was accelerated, with depletion of the progenitor cell population. As Pax3NICD is down-regulated during development, compensatory mechanisms operate, providing a striking example of developmental plasticity that permits the skeletal muscle mass to attain a normal size.
In the somites of Pax3NICD/+ embryos, we observe up-regulation of Foxc2 and of vascular markers with an increased contribution of somite-derived smooth muscle and endothelial cells to the dorsal aorta. In the chicken embryo, Notch signaling was reported to promote a smooth-muscle cell fate in multipotent progenitors in the dermomyotome (2). Our results indicate that the endothelial versus myogenic cell fate is also Notch-dependant in the mouse somite. This finding does not exclude that bone morphogenetic protein signaling, known to inhibit myogenesis (13), is not also implicated in this cell-fate choice, as shown in the chick (2). There is a striking reduction in Pax3+ myogenic progenitors in the limbs of Pax3NICD/+ embryos and an increased contribution of somite-derived endothelial cells. In the somites at forelimb level and in somite explants, changes in endothelial versus myogenic markers are already detectable. This finding points to a cell-fate choice, influenced by Notch signaling that occurs already in the somite, contrary to what had previously been suggested (5). The early labeling with endothelial markers and loss of Pax3, as well as the time frame in which activation of the NICD allele affects somite-derived cells in the limb, are in keeping with an early cell-fate choice in the somite, where bipotent endothelial and myogenic progenitors are present (Fig. 5). These observations, based on Notch gain-of-function, are complemented by experiments on explants where inhibition of the Notch pathway results in converse effects at the level of endothelial versus myogenic cell fate choices, accompanied by changes in Pax3/Foxc2 transcripts, consistent with the pivotal role of the equilibrium in cell-fate choices (6). Downstream effects on myogenesis are also consistent with the impact of down-regulation of Notch signaling on muscle cell differentiation (11, 12). We therefore conclude that the Pax3NICD allele is affecting an existing process rather than creating an artificial situation.
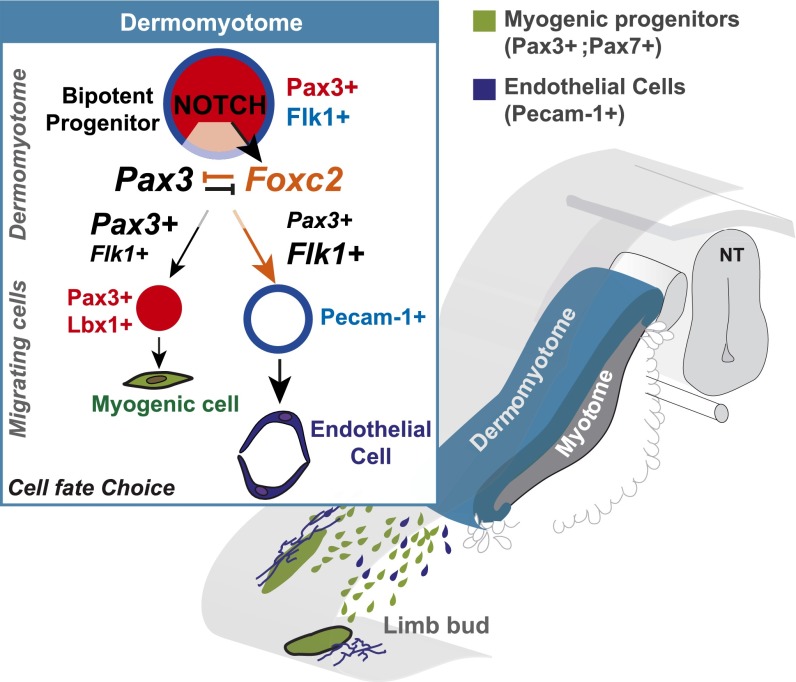
Fig. 5.
Model. Schematic representation of the role of the Notch pathway in cell-fate decisions in the somite.
In conclusion, stimulation of Notch signaling in Pax3+ cells results in an increase in the number of endothelial cells and a reduction in the number of myogenic cells that migrate into the limbs. This cell-fate change takes place in the Pax3+ cells of the somite that can give rise to both derivatives, and is accompanied by a change in the expression of Pax3 versus Foxc2 which promote myogenic versus endothelial cell fates, respectively.
Methods
Targeting Vectors and the Generation of the Pax3AP(NICD-IRESnLacZ) Allele.
The targeting construct is derived from Relaix et al. (9). The Pax3AP(NICD-IRESnLacZ) allele contains 2.4 kb of the 5′ and 4 kb of the 3′ flanking region of Pax3. A floxed Alkaline Phosphatase–polyA (APpA)-Puromycin cassette preceded by an NICD sequence (14), and an IRES-nLacZ cassette with an SV40 pA sequence was introduced into exon1 immediately after the 5′ UTR (Fig. S1A).
Mouse Strains and Tamoxifen Administration.
PGK-Cre transgenic mice (15), Myf5Cre/+ mice (16), and Rosa26CreERT2 mice [from Lars Grotewold and Austin Smith (Wellcome Trust/Medical Research Council Stem Cell Institute, Cambridge, United Kingdom)] were used to generate a Pax3NICD-IRES-nLacZ allele (Pax3NICD). Pax3Cre/+ mice (17) and Myf5Cre/+ mice (15) were crossed with Rosa26tomato-floxGFP/tomato-floxGFP (18) and Rosa26floxnLacZ/floxnLacZ mice [gift of J. F. Nicolas (Institut Pasteur, Paris)]. Pax3Cre/+ mice were also used for the deletion of the RBPJ-κflox allele (19). Pax3nLacZ/+ and Pax3IRES-nLacZ/+ mice lines were described in Relaix et al. (20). Pax3IRES-nLacZ/+ embryos were used as controls for Pax3NICD/+ embryos or, as indicated in the figure legends, Pax3+/+ embryos from the same litter were sometimes used. No difference was observed at any stage between Pax3IRES-nLacZ/+ and Pax3+/+ embryos. Embryos were dated taking E0.5 as the day after the vaginal plug and somite number was used for precise comparison. Experiments on mice were carried out in accordance with regulations of the French Ministry of Agriculture.
Tamoxifen (Sigma; T5648-1G) dissolved in ethanol and emulsified in sunflower oil (Sigma) at a final concentration of 10 mg/mL was intraperitoneally injected in Rosa26CreERT2/+ mice at 2 mg per pregnant female (30 g).
X-Gal Staining, in Situ Hybridization, and Immunodetection.
X-Gal staining, after 15-min fixation and whole-mount in situ hybridization with digoxigenin-labeled probes, was carried as previously described (6). Probes were kindly provided by C. Birchmeier (Max Delbrück Centrum für Molekulare Medizin, Berlin) for Lbx1, O. Pourquié (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France) for Lunatic fringe, and A. Gossler (Hannover University Medical School, Hannover, Germany) for Notch1 (NICD) and Hey1. Whole-mount immunodetection was performed for myosin heavy chain and Pecam-1 (21), as previously described. A Confocal Zeiss LSM 700 Laser Scanning Microscope was used for vessel section surface acquisition (Imagopole, Institut Pasteur). The intensity of labeling was quantified using ImageJ 1.44 Software (National Institutes of Health, Bethesda). Fluorescent coimmunohistochemistry, after 2-h embryo fixation, was performed on 16-µm sections, as previously described (6). Primary antibodies used and their respective concentrations are listed in SI Methods. Secondary antibodies were coupled to fluorochromes: Alexa 488 (Molecular Probes; 1/500), Alexa 647 (Molecular Probes; 1/500) and Alexa 546 (Molecular Probes; 1/500). For EdU staining, pregnant mice were injected intraperitoneally with EdU (50 mg/kg) and dissected after 30 min. The Click-iT EdU Imaging Kit (Invitrogen) was used on slides, after secondary antibodies. Images were acquired with Apotome Zeiss and Axiovision 4.6 software (Imagopole, Institut Pasteur).
Quantification by Acapella Software.
Image analysis was performed using scripts developed in Acapella Image analysis software (v2.3; Perkin-Elmer Technologies). Scripts successively segment in the appropriate channels (red and green), with their respective associated features (number, size, intensity). From this segmentation, two binary masks were performed to retain the location of each cell. The script then compares the cells present in both channels by locating the recovery of the two binary masks (for the proliferation study) or the nonrecovery (for the differentiation study).
Quantification of Contribution of Somitic Cells to Different Lineages.
For genetic tracing experiments with the Myf5Cre/+ mice, the image analysis was performed using the JACoP (Just Another Colocalization Plugin) function of ImageJ 1.44 Software (National Institutes of Health, Bethesda, MD) as described in Bolte and Cordelières (22). The Pearson coefficient, which estimates colocalization, was measured for each image (taken with the apotome).
For quantification of the number of cells in the hypaxial region (Fig. 4C), pictures were taken for each slide, with Apotome Zeiss and Axiovision 4.6 software (Imagopole, Institut Pasteur). After deconvolution, applied by computer batch run with Huygens Professional Software (v4.3.0p7, Scientific Volume Imaging B.V.), Object Analyzer Module (v4.3.0p7, Scientific Volume Imaging B.V.) was used to characterize objects and quantify the number of each category of cells. The χ2 test was used to compare the distribution of cells expressing different markers in Fig. S8.
Explant Culture.
Explant experiments with presomitic mesoderm/early somites (interlimb level at E9.5) or with somites and forelimb buds (E8.75) were carried out as described in refs. 6 and 23, respectively. DAPT in DMSO (Calbiochem) was added at 50-µM final concentration, with DMSO alone added to control cultures.
qRT-PCR Analysis.
Total RNA was prepared by using TRIzol reagent (Invitrogen) or with the RNeasy Mini Kit (Qiagen). RNA was reverse transcribed using the SuperScript II kit (Invitrogen) or the QuantiTect Reverse Transcription Kit (Qiagen) and real-time PCR, as described in ref. 6. Primer sequences are listed in SI Methods.
Statistics.
All experiments were carried out on a minimum of three embryos. Data have been analyzed with the Mann–Whitney nonparametric test, using Anastats files (http://www.anastats.fr). The Student t test was applied, preceded by the Fisher’s test. Data are presented as the mean and SEM.
Supplementary Material
Acknowledgments
We thank C. Bodin and S. Coqueran for technical help; E. Perret and P. Roux for imaging; R. Kopan for the Notch intracellular domain construct; and M. Lopes, P. Mourikis, and I. Le Roux for advice. A.M. was supported in part by a PhD fellowship awarded by the Université Paris VI and in part by OptiStem Grant 223098, with additional support from the French Society of Myology. The M.B. laboratory was supported by the Pasteur Institute and the Centre National de la Recherche Scientifique, with grants from the Association Française Contre les Myopathies, and the European Union programs EuroSyStem (Grant 200720) and OptiStem (Grant 223098), which also financed M.L..
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407606111/-/DCSupplemental.
References
- 1.Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Yair R, Kalcheim C. Notch and bone morphogenetic protein differentially act on dermomyotome cells to generate endothelium, smooth, and striated muscle. J Cell Biol. 2008;180(3):607–618. doi: 10.1083/jcb.200707206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kardon G, Campbell J-K, Tabin C-J. Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Dev Cell. 2002;3(4):533–545. doi: 10.1016/s1534-5807(02)00291-5. [DOI] [PubMed] [Google Scholar]
- 4.Esner M, et al. Smooth muscle of the dorsal aorta shares a common clonal origin with skeletal muscle of the myotome. Development. 2006;133(4):737–749. doi: 10.1242/dev.02226. [DOI] [PubMed] [Google Scholar]
- 5.Hutcheson D-A, Zhao J, Merrell A, Haldar M, Kardon G. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin. Genes Dev. 2009;23(8):997–1013. doi: 10.1101/gad.1769009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagha M, et al. Pax3:Foxc2 reciprocal repression in the somite modulates muscular versus vascular cell fate choice in multipotent progenitors. Dev Cell. 2009;17(6):892–899. doi: 10.1016/j.devcel.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, et al. Notch mediates the segmental specification of angioblasts in somites and their directed migration toward the dorsal aorta in avian embryos. Dev Cell. 2008;14(6):890–901. doi: 10.1016/j.devcel.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Nowotschin S, Xenopoulos P, Schrode N, Hadjantonakis A-K. A bright single-cell resolution live imaging reporter of Notch signaling in the mouse. BMC Dev Biol. 2013;13:15. doi: 10.1186/1471-213X-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Relaix F, et al. The transcriptional activator PAX3-FKHR rescues the defects of Pax3 mutant mice but induces a myogenic gain-of-function phenotype with ligand-independent activation of Met signaling in vivo. Genes Dev. 2003;17(23):2950–2965. doi: 10.1101/gad.281203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckingham M, Mayeuf A. 2012. Skeletal muscle development. Muscle: Fundamental Biology and Mechanisms of Disease, Chapter 52, pp. 749–762, eds Hill J, Olson E (Elsevier, Inc.), Vol 2.
- 11.Vasyutina E, et al. RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. Proc Natl Acad Sci USA. 2007;104(11):4443–4448. doi: 10.1073/pnas.0610647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster-Gossler K, Cordes R, Gossler A. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc Natl Acad Sci USA. 2007;104(2):537–542. doi: 10.1073/pnas.0608281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pourquié O, et al. Lateral and axial signals involved in avian somite patterning: A role for BMP4. Cell. 1996;84(3):461–471. doi: 10.1016/s0092-8674(00)81291-x. [DOI] [PubMed] [Google Scholar]
- 14.Kopan R, Nye J-S, Weintraub H. The intracellular domain of mouse Notch: A constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994;120(9):2385–2396. doi: 10.1242/dev.120.9.2385. [DOI] [PubMed] [Google Scholar]
- 15.Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7(2):105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- 16.Tallquist M-D, Weismann K-E, Hellström M, Soriano P. Early myotome specification regulates PDGFA expression and axial skeleton development. Development. 2000;127(23):5059–5070. doi: 10.1242/dev.127.23.5059. [DOI] [PubMed] [Google Scholar]
- 17.Engleka K-A, et al. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol. 2005;280(2):396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Muzumdar M-D, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 19.Han H, et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14(6):637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- 20.Relaix F, Rocancourt D, Mansouri A, Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004;18(9):1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes M, et al. Msx genes define a population of mural cell precursors required for head blood vessel maturation. Development. 2011;138(14):3055–3066. doi: 10.1242/dev.063214. [DOI] [PubMed] [Google Scholar]
- 22.Bolte S, Cordelières F-P. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(Pt 3):213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 23.Molyneaux K-A, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240(2):488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.