Significance
Although infectious disease is now recognized as a major threat to wild gorillas and chimpanzees, safety fears have stifled the use of a powerful disease control tool, vaccination. To illustrate that safety can be rigorously evaluated before vaccines are used on wild apes, we conducted what is, to our knowledge, the first conservation-oriented vaccine trial on captive chimpanzees. We tested an experimental virus-like particle (VLP) vaccine against Ebola virus, a leading killer of wild apes. Our trial illustrates both the ape conservation value that will be lost if efforts to end vaccine trials on captive chimpanzee are successful and the broader potential that noninfectious VLP vaccines have in other wildlife applications.
Keywords: conservation, wildlife disease, filovirus
Abstract
Infectious disease has only recently been recognized as a major threat to the survival of Endangered chimpanzees and Critically Endangered gorillas in the wild. One potentially powerful tool, vaccination, has not been deployed in fighting this disease threat, in good part because of fears about vaccine safety. Here we report on what is, to our knowledge, the first trial in which captive chimpanzees were used to test a vaccine intended for use on wild apes rather than humans. We tested a virus-like particle vaccine against Ebola virus, a leading source of death in wild gorillas and chimpanzees. The vaccine was safe and immunogenic. Captive trials of other vaccines and of methods for vaccine delivery hold great potential as weapons in the fight against wild ape extinction.
There is growing recognition that infectious diseases pose a threat to the survival of African apes: a threat on par with poaching and habitat loss. Heightened awareness is due both to better data on rates of disease mortality in gorillas and chimpanzees and to new molecular diagnostic assays that pinpoint the cause of death. These assays tell us that wild apes are regularly infected by a variety of virulent pathogens, including simian immunodeficiency virus (SIV) (1), anthrax (2), and malaria (3). The ethical finger has been pointed squarely and quantitatively at researchers and conservationists with the discovery that “spillover” of human respiratory viruses cause about half of deaths among chimpanzees (4, 5) and gorillas (6) habituated to human approach for research or tourism. Even more widely recognized have been massive Ebola virus (EBOV) outbreaks in gorillas and chimpanzees (7, 8), which have killed roughly one-third of the world gorilla population and led to the 2007 upgrading of western gorillas to Critically Endangered status on the World Conservation Union’s Red List of Threatened Species (9).
The ability to accurately diagnose diseases that afflict wild apes has opened the door to an active management response: vaccination (10). The door has been pushed further open by recent advances in vaccinology, including experimental vaccines against several previously unpreventable disease threats to wild apes, new vaccine platforms that reduce or eliminate the risk of infection or vaccine spillover into nontarget species, and new adjuvants that enhance vaccine efficacy (11). Although recognition of the magnitude of the disease threat has made a historically noninterventionist ape conservation community increasingly receptive to vaccination, park managers are still adamant that any experimental vaccine be tested for safety and immunogenicity in captive apes before being used on apes in the wild.
Both to address a salient disease threat and to evaluate whether captive testing of vaccines is feasible with the meager budgets typically available to ape conservationists, we decided to test an experimental vaccine against EBOV. During a consultative process lasting several years, vaccine experts, veterinarians, and park managers persistently expressed concerns about the safety of using live (replicating) vaccines on immunologically stressed wild animals. Some cited the case of SIV, which is typically benign in well-cared-for captive chimpanzees but virulent in environmentally challenged wild chimpanzees (1). Therefore, we chose to test a new virus-like particle (VLP) that does not contain an entire replicating virus but only a fragment of viral coat protein. Because they do not cause infection, VLP-based vaccines are particularly safe and several have been recently approved for human use (12). We tested a VLP vaccine based on the virulent Zaire species of EBOV that previously has been given to more than 80 captive macaques without serious health complications (13). In the present study, we did not challenge vaccinated chimpanzees with EBOV. Rather, we simply evaluated whether the vaccine caused health complications that had not been observed in macaques and whether the vaccine induced immune responses comparable to those observed in macaques who survived Ebola challenge. We also tested whether antibodies harvested from vaccinated chimpanzees could protect mice against EBOV challenge.
Results
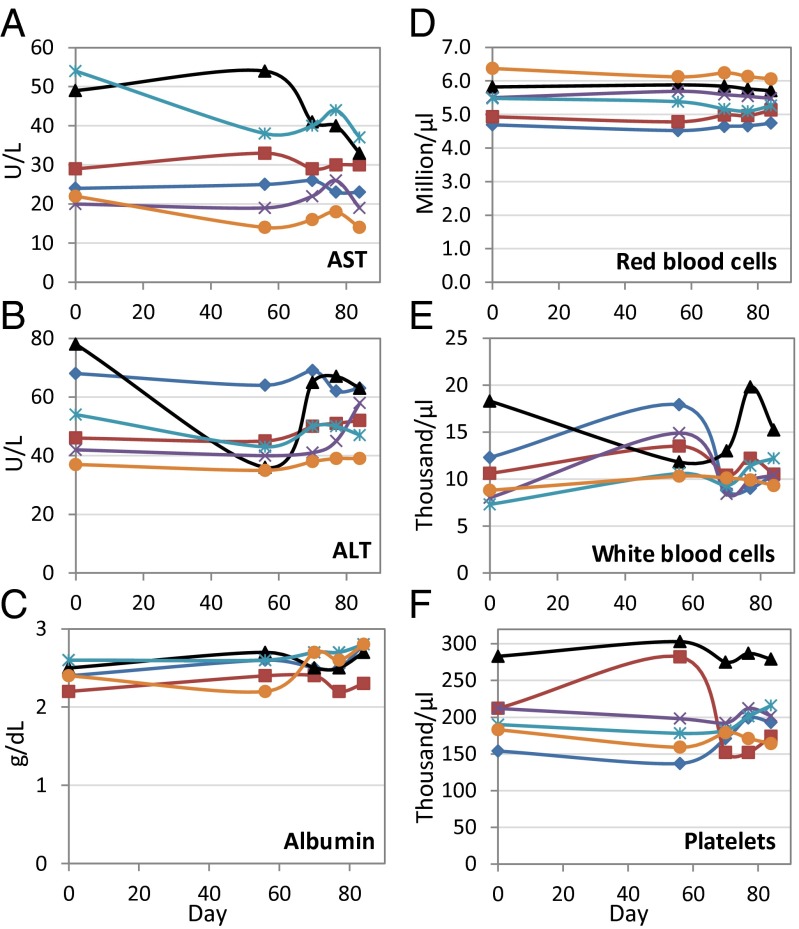
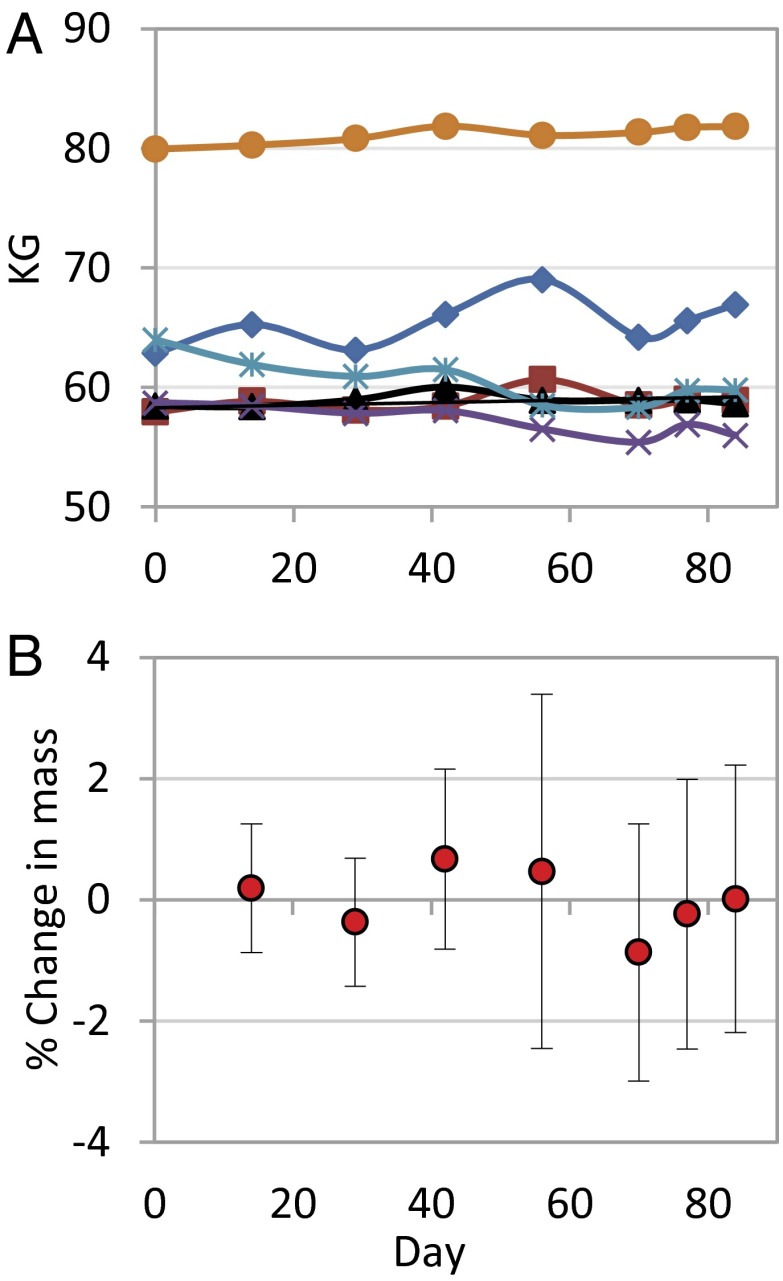
Our observations suggest that the EBOV VLP vaccine is generally safe in chimpanzees. None of the vaccinated chimpanzees exhibited clinical symptoms of disease. Nor did vaccination result in blood chemistry changes characteristic of EBOV infection. For example, serum concentrations of the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) typically rise by two orders-of-magnitude during the first week of EBOV infection, whereas albumin concentration drops precipitously (14). No such responses were evident in vaccinated chimpanzees (Fig. 1 A–C). Vaccinated chimpanzees also did not show hematological responses characteristic of EBOV infection (e.g., decreased number of red blood cells, white blood cells, or platelets) (14) (Fig. 1 D–F). Finally, vaccination did not result in the drastic weight loss typical of Ebola infection. Although the body masses of two chimpanzees did fall significantly during the trial (least-squares regression, P < 0.05), the masses of two other chimpanzees rose significantly and by a comparable amount (Fig. 2A). Thus, mean percent change in body mass (relative to prevaccination mass) never exceeded 1% during the trial (Fig. 2B).
Fig. 1.
Hematology and blood chemistry measurements in chimpanzees after VLP vaccination. Serum concentrations (units/liter) of the liver enzymes (A) AST and (B) ALT, and (C) the protein albumin are plotted separately for each of six vaccinated chimpanzees. Also plotted are cells per liter for (D) red blood cells, (E) white blood cells, and (F) platelets. Chimpanzees were vaccinated with on days 0, 29, and 56.
Fig. 2.
Body mass of chimpanzees after VLP vaccination. (A) Body mass time series for each of six VLP-vaccinated chimpanzees. (B) Mean percentage change in body mass relative to prevaccination body mass. Error bars are Gaussian 95% confidence intervals.
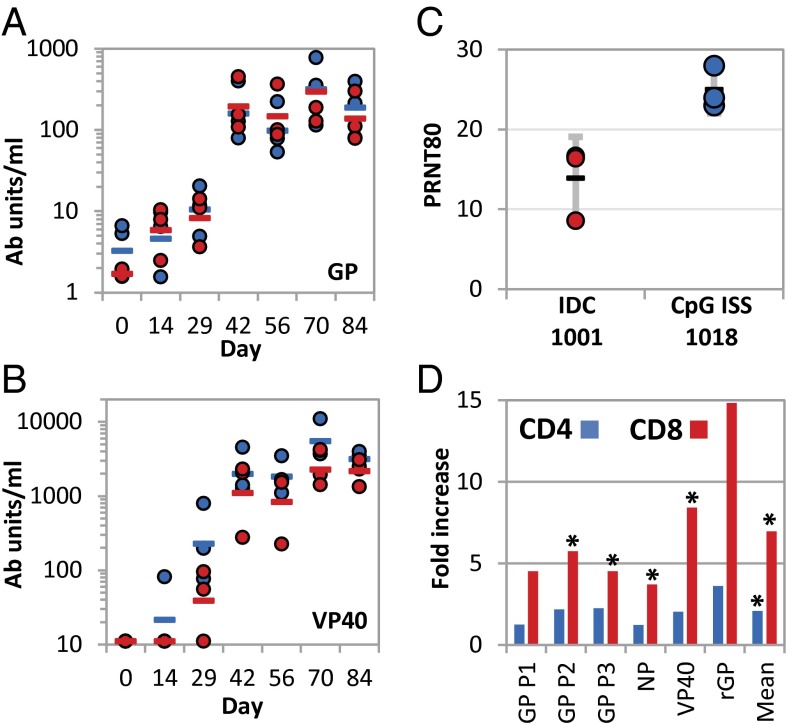
Vaccinated chimpanzees also developed robust immune responses following VLP vaccination. Virus-specific antibodies directed against glycoprotein (GP) and VP40 were detected using ELISAs as early as 2–4 wk after the first vaccination and not later than 2 wk after the second vaccination (Fig. 3 A and B). At the final study time point (day 84), the GLA-SE 1001 and CpG ISS 1018 adjuvant groups did not differ significantly in antibody titres (GP and VP40 pooled, paired t test, P = 0.34). However, VLP vaccination induced neutralizing antibodies (Fig. 3C) in serum from the CpG ISS 1018 adjuvant group achieved 80% plaque reduction neutralizing titers (PRNT80) at about twice the dilution (1:25) as serum of the GLA-SE 1001 group (paired t test, P = 0.022). CD4 and CD8 IFN-γ responses to EBOV antigens including peptide pools (GP1-3 and NP) and recombinant proteins (rGP and rVP40) were assessed 2 wk after the last vaccination (study day 70) (Fig. 3D) (13, 15). Stimulation of peripheral blood mononuclear cell (PBMCs) with six EBOV antigens produced mean twofold and sixfold increases in production of IFN-γ in CD4 and CD8 T cells compared with no stimulation (15). Asterisks in Fig. 3D indicate IFN-γ responses significantly stronger than the control (unstimulated) in paired t tests. The χ2 probability of four significant results for six antigens is 4 × 10−12 (Fig. 3D).
Fig. 3.
Immune responses in chimpanzees after VLP vaccination. ELISAs of chimpanzee serum antibodies to EBOV (A) GP and (B) VP40 proteins. Lines indicate group geometric mean antibody units at each time point for IDC-1001 (red) and CpG (blue) and individual animals are shown as closed circles. (C) PRNT80 on study day 84 for chimpanzees vaccinated with CpG and IDC-1001 adjuvants. Error bars are Gaussian 95% confidence intervals. Neutralization was not detected at 1:5 dilution in prevaccination samples. (D) IFN-γ production in CD4 and CD8 cells from PBMCs stimulated with six EBOV antigens including pools of 15-mer peptides from GP (GP1-3) or NP or recombinant (r) GP or VP40. Stars indicate IFN-γ responses significantly stronger than control (unstimulated cells) at P = 0.05 level in paired t tests. χ2 probability of four significant results for six antigens is 4 × 10−12. Asterisks indicate IFN-γ responses significantly stronger than the control (unstimulated) in paired t tests.
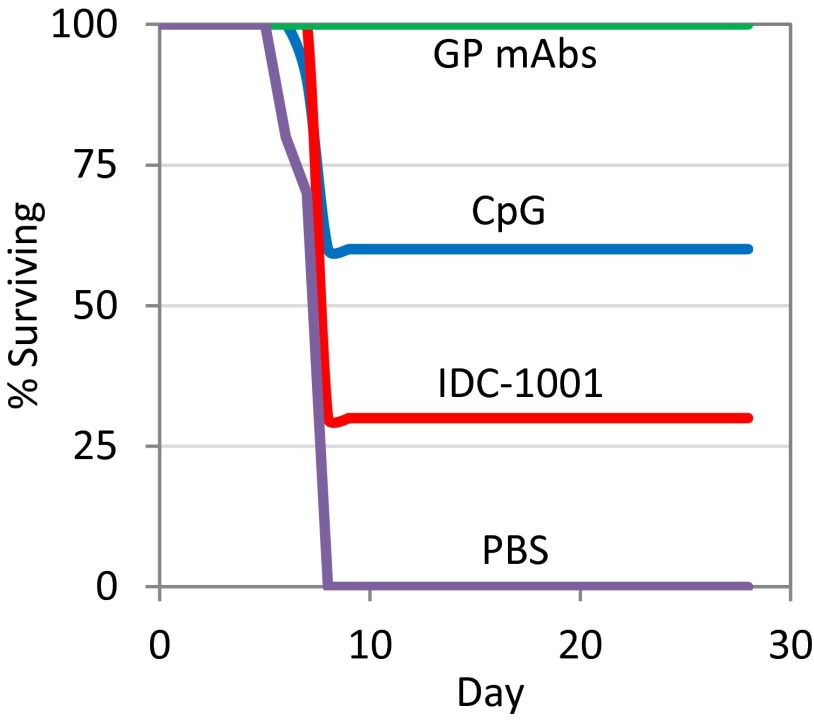
Importantly, passive transfer of total IgG fractions from VLP-vaccinated chimpanzees had a protective effect on mice challenged with mouse-adapted EBOV (Fig. 4) (16). Treatment of BALB/c mice with purified total IgG from CpG or IDC-1001 groups resulted in 60% and 30% survival (χ2 test P = 0.18) respectively, after challenge by EBOV (n = 10 per group). All mice receiving a mixture of GP-specific monoclonal antibodies (17) survived. No mice injected only with the vehicle, PBS, survived. Because of limited sample volumes, unvaccinated serum-derived IgG fractions from chimpanzees were not included in the passive transfer studies and thus remain a limitation of the experimental design of the study and data interpretation. Prior studies with nonspecific IgG, monoclonal antibodies or naive serum from mice, macaques, and humans have not provided protection in the mice model (16, 18, 19). Given that purified IgG from the vaccinated chimpanzees provided protection in mice, and previous work demonstrating that macaques vaccinated with VLPs develop strong antibody and T-cell responses that are protective against lethal challenge with EBOV (13), these data suggest that protective responses may be induced in VLP vaccinated chimpanzees.
Fig. 4.
Passive transfer efficacy study using vaccinated chimpanzee IgG. The percentage of mice surviving challenge with the Zaire strain of EBOV is graphed for BALB/c mice (n = 10 per group) treated with 0.5 mg of either a mixture of GP-specific monoclonals, purified total IgG from three chimpanzees vaccinated with the CpG adjuvant, purified total IgG from three chimpanzees vaccinated with the IDC-1001 adjuvant, or PBS. Mice were infected 1 h before antibody administration with 1,000 pfu of mouse-adapted EBOV.
Discussion
Our study illustrates both the safety and immunogenicity of the Ebola vaccine we tested and the broader potential of noninfectious VLP vaccines for wildlife applications. The enhanced safety of VLP vaccines does come at a cost in that they may require multiple administrations to reach full potency. Thus, VLP vaccines are likely to be most valuable for species that are highly endangered or immunologically fragile but also easy to vaccinate.
The study also sets a precedent. The process for licensing human vaccines is so expensive that only a small number of well-funded vaccines ever come to market. This leaves a large pool of experimental vaccines that show excellent safety and immunogenicity profiles in nonhuman primate trials but are never licensed for human use. Our study demonstrates that it is feasible even for modestly funded ape conservationists to adapt such orphan vaccines as conservation tools by confirming their safety and immunogenicity using trials on captive chimpanzees. Similar potential lies in testing experimental vaccines (e.g., against SIV or malaria) whose immunogenicity may be inadequate for human licensing but might be a godsend for an endangered species at imminent risk. An even better example may be experimental vaccines against a major culprit in disease spillover from field researchers and tourists, respiratory syncytial virus (4), which have performed poorly in humans but quite well in chimpanzees (20). Captive chimpanzees also hold the best potential for adapting vaccines to oral formulations that could greatly expand the number of wild apes protected and for developing noninvasive assays for verifying immunogenicity under field conditions.
To our knowledge, our study was the first conservation-related vaccine trial on captive chimpanzees. It may be the last. US Government policy is now headed toward an end to biomedical testing on captive chimpanzees in the United States, the only developed country to allow such research. Although Congress specifically instructed the National Institutes of Health to consider the conservation value of captive chimpanzee research, neither a blue ribbon panel convened by the Institute of Medicine (21) nor an internal policy review by National Institutes of Health’s Council of Councils (22) presents any findings on such impact. Additionally, there is no mention of either respiratory disease spillover or the conservation value of captive testing in the pending US Fish and Wildlife Service’s proposal to list captive chimpanzees under the Endangered Species Act (23), which would prohibit invasive medical procedures on chimpanzees except “to enhance the propagation or survival of the species.” Unfortunately, if the biomedical laboratories that have the facilities and inclination to conduct controlled vaccine trials “liquidate” their chimpanzee populations, there will be nowhere left to do conservation-related trials on chimpanzees. Thus, in an effort to pay back an ethical debt to captive chimpanzees, the US Government is poised to renege on an even larger debt to wild chimpanzees: both the countless wild chimpanzees that were killed to originally stock US biomedical laboratories and the wild chimpanzees that will die from viruses transmitted by American tourists and researchers (many supported by US Government grants). The US Government should pay this debt by establishing a humanely housed captive chimpanzee population specifically dedicated to conservation research, with funding at a level comparable to the tens of millions of dollars the US Congress has already allocated to house “retired” research chimpanzees.
Methods
Animals.
Chimpanzees were randomly assigned to two groups, each having two females and one male, with starting weights of 64.2 (±7.4) kg and ranging in age from 17 to 31 y. Research was conducted under protocols approved by Institutional Animal Care and Use Committees at the University of Louisiana Lafayette and the US Army Medical Institute for Infectious Disease in compliance with the Animal Welfare Act, PHS Policy, and other Federal statutes and regulations relating to animals and experiments involving animals. The facilities where this research was conducted are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and adheres to principles stated in the eighth Edition of the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011 (24). Animals were weighed and checked for general health on study days 0, 14, 29, 56, 70, 77, and 84.
Vaccinations.
We vaccinated two groups of three chimpanzees, each vaccinated study days 0, 29 and 56. Each group received a different vaccine adjuvant (Glucopyranosyl Lipid Adjuvant-Stable Emulsion “GLA-SE” from Immune Design Corporation IDC-1001 or CpG ISS 1818 from Dynavax) (25, 26). Male and female chimpanzees received injections containing 3 mg (total protein) of VLPs and 25 µg of either IDC-1001 or CpG ISS 1818. We monitored chimpanzees for clinical changes and collected blood samples at 1- to 4-wk intervals for 3 mo. VLPs were produced using a recombinant baculovirus expressing EBOV GP, VP40, and NP in an insect cell system, recovered from the culture supernatants by high-speed concentration and subsequent purification on sucrose gradients and characterized, as published elsewhere (27).
Safety Assessments.
Blood was drawn on days 0, 56, 70, 77, and 84 postvaccination to assess safety of the vaccine by monitoring standard hematologic and blood chemistry readouts. The blood chemistry panel included Ca, Cl, K, Na, P, total CO2, glucose, blood urea nitrogen, creatinine, total protein, albumin, globulin, A/G ratio, total bilirubin, lactate dehydrogenase, γ-glutamyltransferase, alkaline phosphatase, ALT, AST, and hemolysis. The hematology panel included white blood cell count, red blood cell count, hemoglobin concentration, hematocrit percentage, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular Hb concentration, red blood cell distribution width, platelet count, mean platelet volume, percent neutrophils, percent lymphocytes, percent monocytes, percent eosinophils, percent basophils, number of neutrophils (xE3/mm3), number of lymphocytes, number of monocytes, number of eosinophils, and number of basophils. Full tables of hematology (Table S1) and blood chemistry (Table S2) results are provided in Supporting Information.
Immune Responses After Vaccination.
To assess the immunological impact of vaccination, blood samples were taken from each chimpanzee on study days 0, 14, 29, 56, 70, 77, and 84 postvaccination. Levels of antibodies specific to EBOV were determined by an ELISA using purified GP and VP40 proteins made in 293T cells and Escherichia coli, respectively (28–30). The antibodies in unknown samples were determined based on a standard curve of positive control sera derived from hyperimmunized macaques and antibodies were detected using anti-human IgG-HRP. The positive control sera, also referred to as the Reference Detection Antibody (RDA), have been well-characterized using 4-parameter (4PL) curve fit. The value (dilution) at the 4PL inflection point (50% maximum response) was used to establish the number of antibody units for the RDA. The RDA with an assigned value of antibody units was tested as a reference standard curve on every ELISA plate, to quantify the antibody units of the unknown (test) samples. Neutralizing antibody were determined as previously described using twofold serial dilutions of the serum samples, starting at 1:5, tested in duplicate and incubated with a target of 100 pfu per well followed by a standard plaque assay (30). Control sera (day 0) from the chimpanzees did not demonstrate any neutralizing activity at the highest concentration tested (1:5 dilution). Live EBOV was propagated and enumerated by a standard plaque assay on Vero or Vero E6 cells (31). Filovirus-infected cells and animals were handled by qualified personnel under maximum containment in a biosafety level-4 laboratory at the US Army Medical Research Institute of Infectious Diseases. Phenotype and functional cellular responses to the viral proteins GP, VP40, or NP were performed in a manner similar to those described by Sullivan et al., 2006 (32). Briefly, ficoll-separated PBMCs were stimulated ex vivo and stained with pooled ebolazaire GP or NP peptides or purified, recombinant GP or VP40 proteins in the presence of CD49d-specific mAb (BD Biosciences) and antibody to CD28 (BD Biosciences, clone 28.2) for 6–8 h. A minimum of 106 cells per well were used and stimulations were performed in triplicate. Actin peptides and SEB (USAMRIID) were used as negative and positive controls, respectively. Staining was performed using mAbs specific for CD3 (conjugated to PerCP, Cy5.5, clone SP34-2; BD Biosciences), CD8 conjugated with Pacific Blue (clone H4A3; BioLegend), and CD4 QD605 (clone M-T477) or CD8 (PE-Cy7, clone RPA-T8; BD Biosciences), CD69 (conjugated APC-H7, clone FN50; BD Biosciences), and IFN-γ–specific antibody conjugated to APC (clone 4S.B3; BioLegend). The cells were analyzed using a BD FACSCanto II. Live and dead percentages were calculated following stimulation. The percentage of CD4+ and CD8+ total cytokine responses were determined for each peptide pool or protein and NHP and compared with medium alone or the actin peptides.
Passive Transfer.
BALB/c mice (6–8 wk of age) were challenged intraperitoneally with ∼1,000 pfu of mouse-adapted EBOV (33) 1 h before administration of 0.5 mg of purified IgG pooled from a pool the three chimpanzees in each group, a mixture of three monoclonal anti-EBOV glycoprotein antibodies as a positive control (17), or PBS vehicle only.
Supplementary Material
Acknowledgments
We thank the New Iberia Research Center for a no-cost extension to our research, Kansas State University for administrative assistance, and The Paul G. Allen Family Foundation for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316902111/-/DCSupplemental.
References
- 1.Keele BF, et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460(7254):515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leendertz FH, et al. Anthrax kills wild chimpanzees in a tropical rainforest. Nature. 2004;430(6998):451–452. doi: 10.1038/nature02722. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, et al. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature. 2010;467(7314):420–425. doi: 10.1038/nature09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Köndgen S, et al. Pandemic human viruses cause decline of endangered great apes. Curr Biol. 2008;18(4):260–264. doi: 10.1016/j.cub.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Williams JM, et al. Causes of death in the Kasekela chimpanzees of Gombe National Park, Tanzania. Am J Primatol. 2008;70(8):766–777. doi: 10.1002/ajp.20573. [DOI] [PubMed] [Google Scholar]
- 6.Palacios G, et al. Human metapneumovirus infection in wild mountain gorillas, Rwanda. Emerg Infect Dis. 2011;17(4):711–713. doi: 10.3201/eid1704.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh PD, et al. Catastrophic ape decline in western equatorial Africa. Nature. 2003;422(6932):611–614. doi: 10.1038/nature01566. [DOI] [PubMed] [Google Scholar]
- 8.Bermejo M, et al. Ebola outbreak killed 5000 gorillas. Science. 2006;314(5805):1564. doi: 10.1126/science.1133105. [DOI] [PubMed] [Google Scholar]
- 9.Walsh PD, et al. 2007. in 2007 IUCN Red List of Threatened Species, (IUCN) Available at www.iucnredlist.org.
- 10.Ryan SJ, Walsh PD. Consequences of non-intervention for infectious disease in African great apes. PLoS ONE. 2011;6(12):e29030. doi: 10.1371/journal.pone.0029030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh PD. 2012. in Key Topics in Conservation Biology, eds MacDonald DW, Willis KJ (Wiley, Hoboken), pp 452–466.
- 12.Roldão A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9(10):1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 13.Warfield KL, et al. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196(Suppl 2):S430–S437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 14.Fisher-Hoch SP, et al. Haematological and biochemical monitoring of Ebola infection in Rhesus monkeys: Implications for patient management. Lancet. 1983;2(8358):1055–1058. doi: 10.1016/s0140-6736(83)91041-3. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: Implications for regulatory approval by the animal rule. Nat Rev Microbiol. 2009;7(5):393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson JA, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287(5458):1664–1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 17.Olinger GG, Jr, et al. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc Natl Acad Sci USA. 2012;109(44):18030–18035. doi: 10.1073/pnas.1213709109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olinger GG, et al. Protective cytotoxic T-cell responses induced by venezuelan equine encephalitis virus replicons expressing Ebola virus proteins. J Virol. 2005;79(22):14189–14196. doi: 10.1128/JVI.79.22.14189-14196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeitlin L, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci USA. 2011;108(51):20690–20694. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng MN, et al. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J Virol. 2000;74(19):9317–9321. doi: 10.1128/jvi.74.19.9317-9321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institute of Medicine . Chimpanzees in Biomedical and Behavioral Research: Assessing the Necessity. Washington, DC: National Academies; 2011. [PubMed] [Google Scholar]
- 22. National Institutes of Health (2012) Report of the Council of Councils Working Group on the Use of Chimpanzees in NIH-Supported Research. Available at http://dpcpsi.nih.gov/council/pdf/FNL_Report_WG_Chimpanzees.pdf. Accessed May 8, 2014.
- 23.US Fish and Wildlife Service 2013. Endangered and threatened wildlife and plants; Listing all chimpanzees as Endangered. Federal Register 78:35201–35217.22.
- 24.National Research Council 2011. Guide for the Care and Use of Laboratory Animals, 8th ed, (National Academies Press, Washington, DC)
- 25.Coler RN, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS ONE. 2010;5(10):e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall JD, et al. Novel chimeric immunomodulatory compounds containing short CpG oligodeoxyribonucleotides have differential activities in human cells. Nucleic Acids Res. 2003;31(17):5122–5133. doi: 10.1093/nar/gkg700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warfield KL, et al. Filovirus-like particles produced in insect cells: Immunogenicity and protection in rodents. J Infect Dis. 2007;196(Suppl 2):S421–S429. doi: 10.1086/520612. [DOI] [PubMed] [Google Scholar]
- 28.Lee JE, et al. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature. 2008;454(7201):177–182. doi: 10.1038/nature07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kallstrom G, et al. Analysis of Ebola virus and VLP release using an immunocapture assay. J Virol Methods. 2005;127(1):1–9. doi: 10.1016/j.jviromet.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Moe JB, Lambert RD, Lupton HW. Plaque assay for Ebola virus. J Clin Microbiol. 1981;13(4):791–793. doi: 10.1128/jcm.13.4.791-793.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J Infect Dis. 1998;178(3):651–661. doi: 10.1086/515386. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan NJ, et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 2006;3(6):e177. doi: 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408(6812):605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.