Abstract
Objective
Management of patient distress is a critical task in cancer nursing and cancer practice. Here we describe two examples of how an electronic patient-reported outcome (ePRO) measurement system implemented into routine oncology care can practically aid clinical and research tasks related to distress management.
Methods
Tablet personal computers were used to routinely complete a standardized ePRO review of systems surveys at point of care during every encounter in the Duke Oncology outpatient clinics. Two cases of use implementation are explored: (1) triaging distressed patients for optimal care, and (2) psychosocial program evaluation research.
Results
Between 2009 and 2011, the ePRO system was used to collect information during 17,338 Duke Oncology patient encounters. The system was used to monitor patients for psychosocial distress employing an electronic clinical decision support algorithm, with 1,952 (11.3%) referrals generated for supportive services. The system was utilized to examine the efficacy of a psychosocial care intervention documenting statistically significant improvements in distress, despair, fatigue, and quality of life (QOL) in 50 breast cancer patients.
Significance of results
ePRO solutions can guide best practice management of cancer patient distress. Nurses play a key role in implementation and utilization.
Keywords: Patient-reported outcomes, Oncology, Distress management
INTRODUCTION
According to the Institute of Medicine, distress is any psychological, behavioral, or social problem that interferes with a patient’s ability to participate fully in their healthcare and manage their illness and its consequences (Institute of Medicine, 2008). Monitoring for distress along a continuum ranging from common and normal feelings of vulnerability, sadness and fears, to disabling problems (e.g., depression, anxiety), and identifying people at need for psychosocial care services has been advocated for over a decade (National Comprehensive Cancer Network, 2011), with increasing demand for consideration of psychological distress as the “sixth vital sign.”
In 2004, the Canadian federal government’s public health agency, Health Canada–Canadian Strategy for Cancer Control, approved the designation of emotional distress as a vital sign (Bultz & Carlson, 2006). The United States Commission on Cancer accredits cancer centers that provide management for more than 70% of Americans with newly diagnosed cancers; as of 2015, the commission will require distress screening and management in order to maintain accreditation. Requirements include screening using a standardized instrument at least once per pivotal visit (e.g., diagnosis, transitions in treatment) and triage to appropriate care providers when distress is moderate or severe (American College of Surgeons, 2012). In addition, it is recommended that the encounter (e.g., screening, referral/provision of care, follow-up) be documented in the medical record. Furthermore, messaging to the appropriate clinician (e.g., oncologist, nurse, social worker) may help to improve daily workflow and time-management processes in addition to providing good clinical care.
However, barriers to implementation threaten to undermine the distress management requirements set forth by the Commission on Cancer. While there is a growing interest in collecting and using patient-reported outcomes (PROs) in support of the principles of patient-centered care (U.S. Food and Drug Administration, 2011) and PRO assessment within routine clinical care was found to improve communication and patient well-being (Velikova et al., 2004), the historical paper-based PRO collection system employed during clinical visits by healthcare staff is often costly and inefficient. For example, this process can interrupt workflow and has an associated data entry expense, which introduce inefficiency and extra cost into clinical and research operations. Dissemination and integration of significant PRO findings (e.g., psychological distress, pain, nausea) to the multidisciplinary healthcare team (e.g., nurse, physician, social worker, marriage and family counselor) and into clinical care practices often is delayed, leading to missed opportunities in addressing issues during a patient’s clinical visit. The costs associated with a paper-based collection process can impede efforts to process and analyze the PRO data for research purposes and quality-related initiatives (Thompson et al., 2003; Llieva et al., 2002).
To help address these quality- and cost-related barriers, efforts are underway nationally to implement electronic patient-reported outcome (ePRO) systems. The present paper proposes to describe the use of an ePRO system within an academic medical setting to: (1) understand patient concerns and symptoms, (2) triage patients who need psychosocial care support services, and (3) study a new psychosocial care intervention.
USE OF AN ePRO SYSTEM TO UNDERSTAND PATIENT CONCERNS
In an effort to address the inefficiencies inherent in paper-based data collection, a system for collecting ePRO was tested in the course of routine clinical care and implementation research at Duke University Medical Center. The ePRO data collection system comprises four components: (1) software, (2) PRO survey instrument(s) administered, (3) analytics/reporting, and (4) a process for integrating into care. The software chosen was the PACE software developed by a group of community oncology practices in the United States and distributed through Supportive Oncology Services Inc. (Memphis, Tennessee). As hardware, we chose wireless tablet personal computers (e/tablets), available in all participating cancer clinic waiting rooms. The e/tablet wirelessly feeds PROs collected via the PACE software into a database system, which longitudinally warehouses data locally and/or across multiple sites. The ePRO survey used was a review of a systems instrument (Patient Care Monitor v2.0; PCM) that was confirmed to be both reliable and valid for clinical and research purposes (Abernethy et al., 2010c; Fortner et al., 2003; 2006). The ePRO software generates a real-time color-coded report reflecting an individual patient’s survey results over time and efficiently depicts how different symptom items change in relation to each other, so it was employed in the Duke clinics. Finally, the process of care was organized to support distress screening and general outpatient oncology care as outlined herein.
Before undertaking the projects described, we conducted a validation study to confirm that the paper and electronic methods for collecting PROs were sufficiently similar to consider ePRO data usable for research purposes and that the use of e/tablets did not disrupt the clinic flow (Abernethy et al., 2008).We locally validated PCM v2.0 with breast, lung, and gastrointestinal cancer patients to confirm its psychometric properties and responsiveness to change when new interventions were introduced into our clinics (Abernethy et al., 2010c). We conducted local interviews to confirm that the PCM report generated by the ePRO system would be well received by the multidisciplinary team of nurses, physicians, and social workers. Finally, feasibility testing of the collection of ePROs as a routine part of clinical care demonstrated that this was a practical approach in our multidisciplinary oncology setting and acceptable to our cancer patients (Abernethy et al., 2009).
Given these encouraging psychometric and feasibility findings, a series of related projects were undertaken—for example, ePRO data employed to identify distressed patients for referral to psychosocial support services in an oncology clinic and examine relationships between psychosocial resources and outcomes in a piloted intervention study (Smith et al., 2011; Abernethy et al., 2010a).
USE OF AN ePRO SYSTEM TO TRIAGE PATIENTS WHO NEED PSYCHOSOCIAL CARE SERVICES
Could an ePRO system be used both to meet the Commission on Cancer distress screening requirement and to support patient care? Using the ePRO system, Duke Oncology patients and their healthcare providers monitored cancer-related symptoms and psychosocial well-being. PCM v2.0 reports were generated at each visit, which highlight areas of concern (e.g., pain, fatigue, distress) and present recent history/trend data in a user-friendly format. PCM v2.0 established scoring algorithms for the Distress and Despair subscales that can be normalized against a national cancer population standard. Each PCM report efficiently presents individual item reports of component concerns such as worry, anxiety, sadness, and guilt, as well as normalized t scores for the Distress and Despair subscales. A t score of 50 reflects that a person meets the midpoint of the general population; each 10-point increment reflects one standard deviation from the norm.
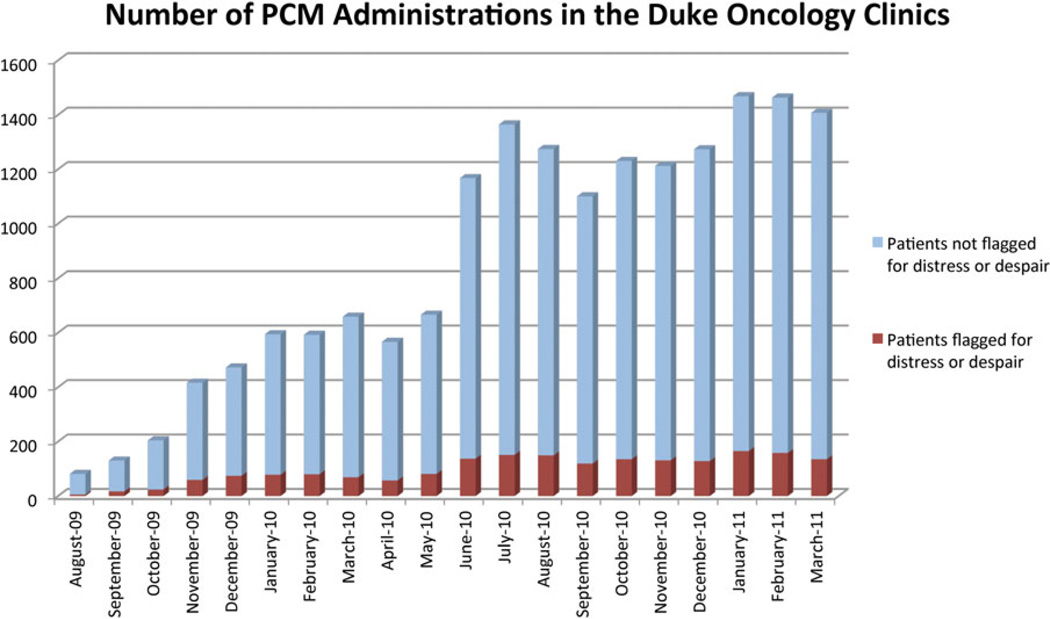
This full triage process (i.e., collecting ePRO data and identifying distressed patients) was informed by the National Comprehensive Cancer Network guidelines for distress management (2011) and was disseminated in coordination with implementation of new disease-based medical team models in outpatient oncology clinics. Benchmarking against scores on other validated instruments such as the FACT-B, we established a t score of 65 on either the Distress or Despair subscales as meeting the Commission on Cancer requirement for needing referral to a psychosocial care provider. We also designated any score greater than zero on the question asking whether a patient felt that they would be “better off dead” (derived from the Patient Health Questionnaire [PHQ-9]) as needing referral for psychosocial care. When these criteria were met, a clinic nurse organized the referral. In addition, e-mail notifications were sent to the psychosocial provider (e.g., social worker, marriage and family therapist, nurse). A patient’s clinician could always override the triage system, calling for a psychosocial referral at any point regardless of distress scores documented by the ePRO system. In order to ensure that the available psychosocial care resources were not overwhelmed, we monitored volume of care provider time, number of referrals, and outcomes, adjusting triage algorithms as needed. Within Duke Oncology clinics, approximately 11.3% of patients met the referral criteria (Fig. 1).
Fig. 1.
Electronic Patient-reported Outcome (ePRO) System statistics for Duke Oncology outpatient clinics showing PCM encounters triggering a distress screen flag.
The distress management application of the ePRO system has been recognized for the following strengths: (1) assessment of the “whole person,” with distress symptoms embedded within the PCM (i.e., biopsychosocial) measure; (2) cost-effective assessment and collection of symptom scores, which eliminates the need for manual collection (via paper and pencil); and (3) multidisciplinary approach to distress management, with enhanced communication and involvement of the medical and psychosocial providers via automated notifications.
USE OF AN ePRO SYSTEM TO STUDY A NEW PSYCHOSOCIAL CARE INTERVENTION
Pathfinders, a new psychosocial care program, was introduced to our clinic, and we wanted to determine whether it merited further resources and implementation, and so we employed the ePRO system to evaluate it (Abernethy et al., 2010b). Pathfinders is a strengths-based coping skills model that integrates psychosocial assessment and care for cancer patients through the guidance of a program manual. Such tools and techniques as cognitive restructuring, guided imagery, and self-care planning are used to bolster a sense of hope and spirit, to promote recognition of inner strengths, to improve self-care practices, and enhance one’s support system. A central goal of the Pathfinders program is to create a common language of personal recovery among the multidisciplinary healthcare team and patients by using themes presented in seven unique domains or “pillars” (Fig. 2).
Fig. 2.
Pathfinders common language: seven pillars of personal recovery.
A phase II research protocol to evaluate the Pathfinders program was prepared and approved by Duke’s institutional review board, which is responsible for the ethical conduct of research with human subjects. Eligible study participants were women with metastatic breast cancer willing to participate in monthly surveys using the ePRO system for at least six months. The standard PCM v2.0 used with the ePRO system was supplemented with additional survey instruments such as the Functional Assessment of Cancer Therapy General-Breast scale (FACT-B; Brady et al., 1997) in order to add the quality of life (QOL), psychological, and other PRO information needed to support programmatic evaluation. Pathfinders study participants were surveyed across multiple timepoints to assess changes in QOL-related outcomes and correlations between resources targeted by the Pathfinders intervention and the outcomes. In our pilot study, we found statistically significant improvements in an individual’s QOL, distress, and despair after three months (Abernethy et al., 2010b) as well as correlations between psychosocial resources (e.g., social support, coping skills, learned optimism) and outcomes (e.g., distress, despair; Smith et al., 2011). Study findings were generated within two months of completing data collection because the ePRO system allowed electronic warehousing of all information and easy retrieval of organized data ready for analysis.
This evaluation framework and its related technology- based infrastructure provide an efficient model allowing for evaluation of new patient-centered healthcare programs introduced into the clinic setting and deeper exploration into potential mediator effects on outcomes. Specifically, by repeatedly querying patients about hypothesized Pathfinders resource targets and outcomes in a sequential framework across time and planning analyses to correlate changes in targets to subsequent changes in outcomes, the evaluation approach allowed exploration of the relationship between average change in intervention targets and average change in outcomes.
IMPLICATIONS FOR NURSING PRACTICE
Screening cancer patients for psychosocial distress can facilitate identification of high-risk patients who need further evaluation and intervention. As frontline providers, nurses play a key role in implementation of the ePRO system at Duke and referral of oncology patients to appropriate mental health providers. This program opened the doors of communication within oncology services and strengthened the partnership between the frontline nursing staff and individual disease-based teams. Nurses are now encouraged to be proactive and to embrace new methods for communicating what our “gut” often tells us for the betterment of the care we provide to our patients. Future work will focus on the development of evidence-based guidelines for distress management.
SUMMARY AND CONCLUSIONS
We have demonstrated how the application of an ePRO system in three areas within an oncology setting (clinical research, psychosocial, and quality patient-centered care) can enhance multidisciplinary care. Introduction of the ePRO system for distress management was shown to help bring together resources to create a comprehensive process for screening, service provision, and data management. For example, healthcare providers, patients, and family members are more aware of available support services and there is increased collaboration and communication among psychosocial (i.e., counseling, social work, nursing) providers. In the Pathfinders pilot study, the ePRO system enhanced the data collection process by moving from a paper- to a computer-based format to measure outcomes. This ePRO implementation resulted in better clinic workflow (so that the process was better able to support multiple-timepoint data collection) and cost efficiencies (e.g., reduced data entry expense).
The limitations of the ePRO system include a lack of detailed psychosocial screening questions (e.g., practical, relational, spiritual issues) in the PCM. In addition, the current system does not electronically upload PRO data into the patient’s medical record. However, the benefits of the process outweigh its limitations. Following establishment of the feasibility, acceptability, and validity of this system, current efforts are focused on enhancing the ePRO process and maximizing shared resources to better provide patient-centered care and expansion into other disease groups. For example, a web-based ePRO system is being developed that will expand our reach outside the hospital (i.e., it will be accessible via a web browser).
ACKNOWLEDGMENTS
We gratefully acknowledge the Duke Comprehensive Cancer Center (Director’s Fund) and Pathfinders National (a not-for-profit organization) for their generous support. We are also fortunate to have an Outcomes Research service agreement with Pfizer Inc. and the National Cancer Institute (Cancer Care Quality Training Grant, CA116339; Comparative Effectiveness Research Grant, KM1-CA156687-01).
REFERENCES
- Abernethy AP, Herndon JE, Wheeler JL, et al. Improving health care efficiency and quality using tablet personal computers to collect research-quality, patient-reported data. Health Services Research. 2008;43:1975–1991. doi: 10.1111/j.1475-6773.2008.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abernethy AP, Herndon JE, Wheeler JL, et al. Feasibility and acceptability to patients of a longitudinal system for evaluating cancer-related symptoms and quality of life: Pilot study of an e/tablet data collection system in academic oncology. Journal of Pain and Symptom Management. 2009;37:1027–1038. doi: 10.1016/j.jpainsymman.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Abernethy AP, Ahmad A, Zafar SY, et al. Electronic patient-reported data capture as a foundation of rapid learning cancer care. Medical Care. 2010a;48(Suppl 6):S32–S38. doi: 10.1097/MLR.0b013e3181db53a4. [DOI] [PubMed] [Google Scholar]
- Abernethy AP, Herndon JE, Coan A, et al. Phase 2 pilot study of Pathfinders: A psychosocial intervention for cancer patients. Supportive Care in Cancer. 2010b;18:893–898. doi: 10.1007/s00520-010-0823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abernethy AP, Zafar SY, Uronis H, et al. Validation of the Patient Care Monitor (version 2.0), a review of a systems instrument for cancer patients. Journal of Pain and Symptom Management. 2010c;40:545–558. doi: 10.1016/j.jpainsymman.2010.01.017. [DOI] [PubMed] [Google Scholar]
- American College of Surgeons. Commission on Cancer. 2012 October 2012 update. http://www.facs.org/cancer/
- Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. Journal of Clinical Oncology. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- Bultz BD, Carlson LE. Emotional distress: The sixth vital sign—future directions in cancer care. Psycho-Oncology. 2006;15(2):93–95. doi: 10.1002/pon.1022. [DOI] [PubMed] [Google Scholar]
- Fortner B, Okon T, Schwartzberg L, et al. The cancer care monitor: Psychometric content evaluation and pilot testing of a computer administered system for symptom screening and quality of life in adult cancer patients. Journal of Pain and Symptom Management. 2003;26:1077–1092. doi: 10.1016/j.jpainsymman.2003.04.003. [DOI] [PubMed] [Google Scholar]
- Fortner B, Baldwin S, Schwartzberg L, et al. Validation of the cancer care monitor items for physical symptoms and treatment side effects using expert oncology nurse evaluation. Journal of Pain and Symptom Management. 2006;31:207–214. doi: 10.1016/j.jpainsymman.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Adler NE, Page AEK, editors. Institute of Medicine. Cancer care for the whole patient: Meeting psychosocial health needs. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- Llieva J, Baron S, Healey NM. Online surveys in marketing research: Pros and cons. International Journal of Marketing Research. 2002;44:361–367. [Google Scholar]
- National Comprehensive Cancer Network. Distress management clinical practice guidelines in oncology. 2011 doi: 10.6004/jnccn.2003.0031. November 2011 update. http://www.nccn.org/professionals/physician_gls/f_guidelines.aspsupportive. [DOI] [PubMed]
- Smith SK, Herndon JE, Lyerly HK, et al. Correlates of quality-of-life-related outcomes in breast cancer patients participating in the Pathfinders pilot. Psycho-Oncology. 2011;20:559–564. doi: 10.1002/pon.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LF, Surface EA, Martin DL, et al. From paper to pixels: Moving personnel surveys to the Web. Personnel Psychology. 2003;56(1):197–227. [Google Scholar]
- U.S. Food and Drug Administration. Guidance for industry. Patient-reported outcome measures: Use in medical product development to support labeling claims. 2011 doi: 10.1186/1477-7525-4-79. November 2011 update. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. [DOI] [PMC free article] [PubMed]
- Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well being: A randomized controlled trial. Journal of Clinical Oncology. 2004;22(4):714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]