Abstract
Objective
To describe the relationship of advancing age in persons with chronic spinal cord injury (SCI) on the prevalence of low testosterone in men with SCI compared to historical normative data from able-bodied men in the general population.
Design
Retrospective, cross-sectional study. Two hundred forty-three healthy, non-ambulatory outpatient men with chronic SCI from age of 21 to 78 years were included in this retrospective analysis.
Results
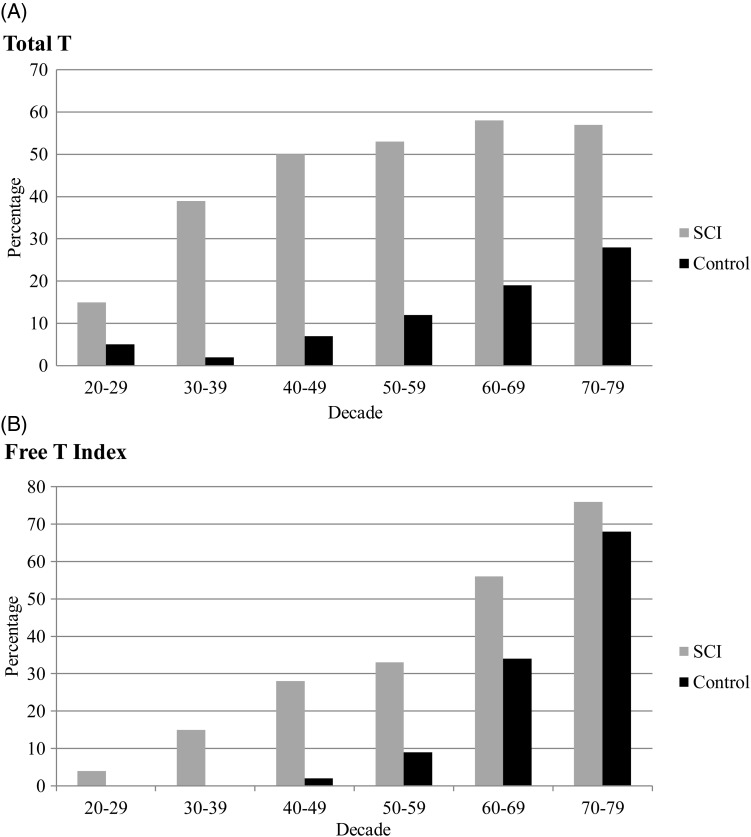
Forty-six percent of men with SCI were identified as having low serum total testosterone concentrations (total testosterone <11.3 nmol/l). The age-related decline in SCI for total serum testosterone concentration was 0.6%/year compared to 0.4%/year in the Massachusetts Male Aging Study. Between the third and eighth decade of life, men with SCI had a 15, 39, 50, 53, 58, and 57% prevalence rate of low serum total testosterone, which is higher than values reported for each decade of life for able-bodied men in the Baltimore Longitudinal Study on Aging.
Conclusion
Compared with the general population, low serum total testosterone concentration occurs earlier in life in men with SCI, at a higher prevalence by decade of life, and their age-related decline in circulating total testosterone concentration is greater. Studies of T replacement therapy in men with SCI should assist in determining the possible functional and clinical benefits from reversing low serum total testosterone concentration.
Keywords: Spinal cord injuries, Testosterone replacement therapy, Paraplegia, Tetraplegia, Aging
Introduction
Cross-sectional1–3 and longitudinal studies4–6 have demonstrated that the concentration levels of total and free serum testosterone (T) decline with increasing age in able-bodied men. The etiology of this progressive fall in serum T is multi-factorial and, in part, attributable to various combinations of lifestyle choices, illness, medications, and activity of the hypothalamic–pituitary–gonadal axis.1,7 Decreased serum T levels in the general population are associated with lowered libido, fatigue, insomnia, hot flashes, anxiety, depression, poor memory, irritability, impotence, and diminished bone and muscle mass,7–10 as well as an increased risk of non-vertebral fractures and cardiovascular disease.6,11
Spinal cord injury (SCI) has been referred to as a model of accelerated aging, with reported morbidities of cardiovascular disease, osteoporosis, and reduced lean tissue mass occurring earlier in life, and with a greater prevalence of these morbidities than that occurring in age-matched, able-bodied individuals.12–17 Previous reports in men with SCI have demonstrated abnormalities of hormones and function of the hypothalamic–pituitary–gonadal axis.18–27 Similar to the causes of hypogonadism in able-bodied persons, individuals with SCI have reduced serum concentrations of T from a number of etiologies. A recent report in veterans with SCI found that 43.3% of the cohort had serum T levels <325 ng/dl (11.3 nmol/l), with significant associations to injury severity and opioid use.28 To date, no reports in the SCI population have evaluated whether there is a greater prevalence of low values for T across the lifespan compared to those in the general male population. The purpose of this study is to describe the relationship of advancing age in persons with SCI, as well as other anthropometric and demographic data, on the prevalence of low serum T in men with SCI compared to normative data that has been published in men from the general population.
Subjects and methods
Subjects
Two hundred forty-three otherwise healthy, medically stable, non-ambulatory outpatient men between the ages of 21 and 78 years who had participated in prior studies with our center were identified. To be considered eligible for this retrospective analysis, subjects were required to be at least 1 year after acute SCI, use a wheelchair as the primary means of mobility, be free of any acute or chronic medical conditions (e.g. chronic obstructive pulmonary disease, chronic renal failure, congestive heart failure, inflammatory bowel disease, and peripheral vascular disease), or be taking medications known to affect pituitary or testicular secretory function, including major or minor tranquilizers, calcium channel antagonists, alpha-adrenergic agonists, beta-adrenergic blockers, and cholinergic agonists; however, opioid prescription was not an exclusion criterion. The parent study was approved by the Institutional Review Board and written informed consent was obtained from each subject prior to study participation. The rate of decline in serum total T, free T, and sex hormone binding globulin (SHBG) in healthy men in the Massachusetts Male Aging Study was compared to the respective rates of decline in the measures of T in our SCI cohort.1 The normative data on men with low serum total T concentration and free T index from the Baltimore Longitudinal Study on Aging was compared to our SCI subjects, both cohorts categorized by decade of life.4
Procedures
All participants had blood drawn between 8:00 and 10:00 a.m. from the antecubital vein for determination of serum T, albumin, and SHBG concentrations. Blood samples were placed on ice prior to being spun down in a refrigerated centrifuge, separated, and frozen at −28°C until batch processing at the completion of study sample collection (e.g. T, SHBG) or sent to the hospital laboratory for immediate determination (albumin). Subjects provided demographic data (e.g. age, height, weight, and ethnicity) and injury-specific data (i.e. level of neurological injury, duration of injury (DOI), and the extent of motor deficit below the level of injury).
Laboratory analysis
The total serum T concentration was determined in duplicate by radioimmunoassay, in accordance with the manufacturer's guidelines (ICN Biomedicals, Inc., Costa Mesa, CA, USA). Similarly, the serum SHBG level was determined in duplicate by immunoradiometric assay (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). The sensitivity for T assay was 0.08 ng/ml; the intra-assay coefficient of variation (CV) were 9.6, 8.1, and 7.8% for T concentrations of 0.9, 7.0, and 20 ng/ml, respectively; the inter-assay CV were 8.6, 9.1, and 8.4% for T concentrations of 0.7, 6.0, and 16 ng/ml, respectively. The sensitivity of the SHBG assay was 0.75 nmol/l; the intra-assay CV were 4.2, 6.9, and 2.5% for SHBG concentrations of 29, 68, and 178 nmol/l, respectively; the inter-assay CV were 7.0, 4.9, and 5.1% for SHBG concentrations of 26, 88, and 308 nmol/l, respectively. The serum albumin concentration was determined by an automatic analyser in the general chemistry laboratory of the James J Peters Veterans Affairs Medical Center.
Free T and bioavailable T were calculated using serum albumin and SHBG concentrations, employing previously described equations.29 The free T index was determined by dividing total T concentration by SHBG value.4 Low T was defined as a serum total T concentration <11.3 nmol/l (325 ng/dl), and low T by free T index, as <0.153 nmol/l, which are the identical laboratory value cut-off points for these measures that were used by the investigators of the Baltimore Longitudinal Study of Aging, thereby permitting comparison of their findings with ours. The normal range employed for bioavailable T was corrected for decade of life.
Statistical analysis
Values are expressed as group mean ± standard deviation, unless otherwise specified; the range of values was reported for continuous demographic data. Categorical demographic and injury-specific data are presented for the measurements as a percentage. Chi-square analyses were used to examine the distribution of low T across the total sample and within the following categories: level of injury (paraplegia vs. tetraplegia), ethnicity (Caucasian, Black, Hispanic, and Asian), and the extent of motor injury (complete vs. incomplete). The prevalence of abnormal laboratory values for albumin, SHBG, total T, bioavailable T, and free T was determined. A simple regression analysis was performed with the respective measure plotted against age to estimate yearly change; the intercept was divided by the slope of the regression equation and multiplied by 100, with the resultant value representing a yearly percent change. Logistic regression analyses were used to identify independent factors (demographic and injury-specific data) in men with SCI that predict the increased likelihood of low T, as determined by the total serum T concentration. For this analysis, serum total T concentration was categorized by age (<39 and ≥40 years), DOI (<24 and ≥25 years), and body mass index (BMI: <29.9 and ≥30 kg/m2). Because of the demographics of our original cohort, no subjects under the age of 39 years had injury durations ≥25 years; thus, only six concatenated groups were formed. Statistical analyses were performed using Stat-View 5.0 (SAS Institute, Inc., NC, USA).
Results
The mean values and range for age, height, weight, BMI, and DOI for the men with SCI are provided (Table 1). The percentage of total subjects is reported across the following categories: T status, level of injury, completeness of motor injury, and ethnicity. In addition, the percentage of low T is reported for each descriptive sub-category. Chi-square analysis revealed that only the category of ethnicity had a significant distribution difference among sub-groups (Caucasian, Black, Hispanic, and Asian) for the incidence of low T (χ2 = 8.491, P = 0.03).
Table 1 .
Demographic and spinal cord injury-specific information
| Continuous variables | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Age (years) | 48 | 15 | 21 | 78 |
| Height (cm) | 178.5 | 7.7 | 152.4 | 205.7 |
| Weight (kg) | 80.3 | 16.1 | 43.1 | 140.6 |
| BMI (kg/m2) | 25.2 | 4.8 | 15.8 | 51.7 |
| Duration of injury (years) | 17 | 13 | 1 | 56 |
| Classification variables | Percentage of total subjects | Percentage of group who have low T | ||
| T status | ||||
| Low T* | 46 (113) | |||
| Normal | 54 (130) | |||
| Level of injury** | ||||
| Paraplegia | 57 (138) | 48 (67) | ||
| Tetraplegia | 43 (105) | 44 (46) | ||
| Completeness of motor injury*** | ||||
| Incomplete | 53 (125) | 41 (52) | ||
| Complete | 47 (113) | 51 (58) | ||
| Ethnicity† | ||||
| Causasian | 59 (138) | 51 (70) | ||
| Black | 26 (62) | 36 (22) | ||
| Hispanic | 14 (32) | 41 (13) | ||
| Asian | 1 (3) | 100 (3) | ||
Number in parenthesis reflects the observed n for each cell.
*Hypogonadal = T < 11.3 nmol/l; **P = 0.5; ***P = 0.1; †χ2 = 8.491, P = 0.03.
T, testosterone; BMI, body mass index.
The median (95% confidence interval) laboratory values and percent of abnormal observations for the respective measures of serum T (total, free, and bioavailable), SHBG, albumin, and the free T index in the SCI cohort are provided (Table 2). The concentration of SHBG was elevated in 51% of the subjects, and the serum albumin level was normal in 70% of the subjects. Bioavailable T concentration is the non-protein bound T, which is the serum T fraction not precipitated by 50% ammonium sulfate concentration; the free T concentration employs both albumin and SHBG concentrations in the calculation to approximate the “true” free T concentration obtained by the equilibrium dialysis method; 80% of the subjects had a normal bioavailable T concentration, whereas 79% had a low free T concentration (Table 2). Forty-six percent of the total sample was identified as having a low serum total T concentration (total T <11.3 nmol/l), and 27% of the total sample, a serum total T concentration <10 nmol/l (288 ng/dL).
Table 2 .
Serum laboratory values of the subjects with spinal cord injury
| Continuous variables | Normal range | Median (95% RI) | Minimum | Maximum | Low* | Normal | Elevated* |
|---|---|---|---|---|---|---|---|
| Albumin (g/l) | 35–50 | 40.0 (21.0–53.9) | 9.00 | 58.0 | 24 (58) | 70 (172) | 6 (13) |
| SHBG (nmol/l) | 10–60 | 61.6 (3.4–172.6) | 1.4 | 224.8 | 5 (12) | 44 (106) | 51 (125) |
| Total T (nmol/l) | 10–35 | 12.0 (2.4–27.4) | 0.6 | 30.4 | 39 (94) | 61 (149) | — |
| Bioavailable T (nmol/l) | 1.4–8.9*** | 3.4 (0.5–16.4) | 0.07 | 22.4 | 15 (36) | 85 (207) | — |
| Free T (nmol/l) | 0.3–1.05 | 0.16 (0.03–0.67) | 0.003 | 1.05 | 79 (191) | 21 (52) | — |
| Free T index | 0.20 (0.04–3.4) | 0.003 | 12.99 | — |
Number in parenthesis reflects the observed n for each cell.
*Low and elevated values are relative to the normal range.
**Age-dependent ranges: 20–29 years: 2.9–8.9 nmol/l; 30–39 years: 2.5–8.2 nmol/l; 40–49 years: 2.1–7.4 nmol/l; 50–59 years: 1.7–6.6 nmol/l; 60–69 years: 1.4–5.8 nmol/l.
SHBG, sex hormone binding globulin; T, testosterone; RI, reference interval.
For descriptive and comparative purposes, the age-related changes in the serum T and SHBG from our cohort are provided with values from the Massachusetts Male Aging Study (Table 3).1 The decline in serum total T in the SCI group was 50% greater than that of the normative data in the able-bodied population. There was a 13.1% annual increase of SHBG in men with SCI compared to 1.2% in the healthy general population (Table 3).1 The prevalence of low T across the decades in men with SCI and in the normative data in the general population from the Baltimore Longitudinal Study on Aging are provided (Fig. 1).4 Between the third and eighth decade of life, men with SCI have a 15, 39, 50, 53, 58, and 57% incidence rate of low serum total T concentration if determined for values of the serum total T concentration; the incidence of hypogonadism is 4, 15, 28, 33, 56, and 76% by the free T index. Thus, for each decade of life, there is a substantial increase in the prevalence of low T in men with SCI compared to the historical general population sample.4
Table 3 .
Percent change with age in testosterone and SHBG values in the group with spinal cord injury and those of the Massachusetts Male Aging Study
| Variables | SCI | Control* |
|---|---|---|
| Total T (nmol/l) | −0.6 | −0.4 |
| Free T (nmol/l) | −1.1 | −1.2 |
| SHBG (nmol/l) | 13.1 | 1.2 |
*Control values from reference1
T, testosterone; SHBG, sex-binding globulin hormone.
Figure 1 .
The prevalence of low testosterone in men with SCI (gray bars) and a control (black bars) population.4 Bar height indicates the percent of observations, with low testosterone defined as total T (A) (<11.3 nmol/l) or free T index (B) (<0.153 nmol T/nmol SHBG). For Fig. 1A, there were 27, 54, 60, 36, 45, and 21 men with SCI in the third through eighth decades, respectively, for our analysis. Men with SCI, irrespective of indicator, have a greater percentage of low testosterone than those of the control group in the corresponding decade.
To further evaluate significant interactions, a risk profile for the development of low T by the serum total T concentration was determined by logistic regression analysis to enable identification of the demographic categories associated with the highest risk (Table 4). Men with SCI who were <39 years old did not have an increased risk of low serum total T concentration, regardless of BMI and/or categorization for DOI. Men with SCI who were >40 years old had considerable increased likelihood of having a low serum total T concentration, dependent upon the categorization of BMI and/or DOI. Those who had higher BMIs (≥30 kg/m2) and longer DOI (≥25 years) had an odds ratio of having a low serum total T concentration of 8.9 times higher than those with the lower BMIs (<29.9 kg/m2) and shorter DOI (<24 years) (Table 4).
Table 4 .
Logistic regression coefficients for low testosterone by the concatenated age/BMI/DOI and completeness of injury variables in men with SCI
| Age | BMI | DOI | Exponential coefficient |
|---|---|---|---|
| ≥40 | <29.9 | <24 | 1.7* |
| ≥40 | <29.9 | ≥25 | 4.5** |
| ≥40 | ≥30 | <24 | 6.2*** |
| ≥40 | ≥30 | ≥25 | 8.9† |
| Motor complete injury | 1.8†† |
*P = 0.12; **P < 0.0001; ***P < 0.01; †P = 0.059; ††P < 0.05.
BMI, body mass index; DOI, duration of injury.
Discussion
Almost half of our total population of men with SCI had low serum total T concentration using the same threshold criteria as that employed by Harman et al.4 in their general population-based study. Men with SCI had a higher percentage of low values for each decade of life for the serum total T concentration and for the serum free T index than those that were reported in the Baltimore Longitudinal Study on Aging.4 Of note, the prevalence of low serum total T concentration in those with SCI rose strikingly in the third decade of life and thereafter, a finding that may be associated with deleterious physical and psychological effects of androgen deficiency throughout the latter decades of life. Compared to the findings of able-bodied men reported by Gray et al.1 the decline in serum total T concentration for the group with SCI was 50% greater than that in healthy able-bodied persons. The annual increase in SHBG in the group with SCI was markedly greater than in healthy able-bodied persons, raising the prevalence rates for low free T index for those with SCI in the earlier decades of life (i.e. from the 20 to 50s).
Clark et al.19 found the prevalence of low serum total T concentration (<241 ng/dl or <8.4 nmol/l) to be about 60% in 102 men with SCI with a mean age of 46 years who were participating in inpatient or outpatient rehabilitation. Of note, 61% of the subjects had sub-acute SCI (<4 months) and only 35% had injury for >12 months. Those subjects who were of shorter DOI (i.e. sub-acutely injured) tended to have lower serum total T values. The lower serum total T concentrations were associated with higher prolactin levels, suggesting dysfunction of the hypothalamic–pituitary–gonadal axis.19 Safarinejad25 studied 76 men with chronic SCI (mean DOI of 16 years; range 13–20 years) compared to able-bodied controls and demonstrated that those with SCI had lower serum total T concentrations (12.0 ± 6.4 vs. 16.1 ± 4.7 nmol/l) and lower luteinizing hormone (LH) levels (3.3 ± 2.4 vs. 5.7 mIU/ml), again suggesting a dysfunction of central regulation. Huang et al.22 found significantly elevated LH responses to LH releasing hormone (LHRH) in subjects with SCI compared to controls. Of those studied with LHRH stimulation, 16 of 30 subjects with SCI had exaggerated LH responses and 6/30 had elevated FSH responses.22 Previously, our group reported that persons with SCI tend to have increased gonadotropin release to standard provocative stimulation to LHRH compared with able-bodied controls.30 In a preliminary report that addressed the end-organ responsiveness, testicular stimulation with hCG was similar in subjects with SCI and able-bodied controls.30
The authors have referred to participants as having “low T” rather than “hypogonadism” because symptoms of hypogonadism were not determined in this retrospective report. There has been little consistency in prior reports that had determined serum T or gonadotropin levels in persons with SCI, probably, in large measure, because of illness, medications, nutritional factors, and the lack of controlling for DOI (i.e. cohorts comprised of those with acute/sub-acute injury and chronic injury). Impotence and infertility have been commonly noted in the SCI population.18,31 Of the many possible explanations of poor semen quality,32 one possible etiology is dysfunction of the hypothalamic–pituitary–testicular axis,20,24 as previously discussed. Early reports have been inconclusive with regard to testicular function,18,21,24,31 discrepancies that could be attributed to varying factors in population selection, including chronic disease, level of injury, completeness of lesion, duration of SCI, or, in part, differences in study methodology.
In our study, SHBG levels were elevated in about half of our subjects. The elevation in SHBG values contributed to 79% of our subjects having below normal serum free T values. Generally, the SHBG concentration is inversely related to the serum T concentration;33 as such, because a high prevalence of T deficiency was noted, the elevation in values for SHBG was to be expected. There exists difficulty assessing androgen status because there is no validated, independent marker of androgen action in vivo, and, as such, the clinical relevance of a specific laboratory value is open to question. Thus, it is important to appreciate that any one method of T measurement (i.e. total, free, or bioavailable) had not been shown to be superior to than any other in the determination of gonadal status. Because Harman et al.4 used total T and the free T index to categorize their population, we also felt that such an approach has value, and it served to provide a general able-bodied population sample for the purpose of comparison to our SCI cohort.
The BMI and DOI were predictive of low serum total T concentration in older men with SCI (≥40 years old), but these factors do not appear to influence risk of low serum total T concentration in younger men. It is well appreciated that obesity is associated with lower serum total T concentrations in the general population.34 However, the odds of low serum total T concentration in the sub-group of older men with SCI who had a BMI >30 kg/m2 was quite remarkable, 6.2 times greater than that of the sub-group with a lower BMI, a finding that may be partially explained by a greater degree of adiposity per unit BMI in those with SCI.14,15 Potential confounders to the ability to reliably assess the effect of advancing age on gonadal status include general health, nutrition, medication usage, alcohol consumption, and, in those with SCI, level, completeness of neurological lesion, and DOI. In the analysis herein, subjects with SCI were excluded from participation if they had a known chronic illness or were prescribed one of several medications known to affect pituitary or testicular secretory function. The possibility of alcohol abuse was not determined in our research record, and this could potentially confound interpretation of our results.35 Of note, the prevalence of alcoholism in veterans with SCI has been reported to be far less than in the general population.36 Another possible confounder to interpretation of our results may have been opioid use for pain relief. It is appreciated that opioids depress the pituitary–gonadal axis and lower the circulating T concentration.9 The SCI population has neuropathic and muscle-skeletal pains that are often not responsive to other medications than narcotics. Thus, narcotic use may have played a role in our findings, albeit the magnitude of the effect will have to be defined in future studies.
Cross-sectional studies have shown a decrease in serum total T with age.4 From a population-based cross-sectional survey of men 39–70 years old from the Massachusetts Male Aging Study, Gray et al.1 evaluated the effects of aging independent of specific chronic disease states or medications for total T, bioavailable T, and free T. To more accurately define the effects of normal aging from those of disease or drug administration on endpoints, the population was divided by health status. Group 1 consisted of 415 subjects who were healthy not obese, non-alcoholic, not diagnosed with a chronic illness (cancer, coronary heart disease, hypertension, diabetes, and ulcer), and not being treated with prescription drugs; Group 2 consisted of 1294 men who had one or more of the disqualifiers for membership in Group 1. Concentrations of measures of T (serum total T (0.4 %/year), bioavailable T (1.0 %/year), and free T (1.2 %/year) had declined with age in Group 1 and Group 2, but values were consistently lower by 10–15% for serum total and free T determinations across age in Group 2 (i.e. the group with health issues).1 The SHBG increased each year by 1.2%/year, serving to increase the decline in serum total T concentration.1
Harman et al. 4 studied the effect of aging on total and the free T index in 890 men who participated in the Baltimore Longitudinal Study on Aging. These investigators observed an effect of aging on both total T (−0.124 nmol/l/year) and the free T index (−0.0049 nmol T/nmol SHBG/year). Hypogonadism for serum total T or free T index was defined as below the 2.5th percentile for young men, albeit the authors discussed the limitations of such a laboratory approach, which neglected the clinical realities of varying individual thresholds for hormone deficiency states and the difficulty in attempting to extrapolate clinical relevance from a laboratory finding. These limitations being appreciated, the prevalence of low serum total T increased steadily above age 60 (approximately 20% 60–69 years, 30% 70–79 years, and 50% >80 years).4 The percentages by decade for low serum total T concentrations in an able-bodied cohort were lower than those observed in our subjects with SCI.
In an identical twin study, one twin discordant for SCI, increasing age resulted in a global loss of lean body tissue, suggesting a systemic, possibly hormonal, etiology.15 Thus, the possibility should be considered that an androgenic-deficiency state may contribute to accelerated muscle loss in those with SCI, both above and below the level of lesion. In a relatively young able-bodied population, producing a hypogonadal state by hormonal manipulation resulted in a loss of lean body tissue.37 Reversal of hypogonadism in younger adults has been shown to improve muscle mass and strength.38 A recent study has demonstrated that T replacement therapy for 12 months significantly improved lean body tissue in persons with SCI.39 A decrement in muscle mass and strength as a consequence of hypogonadism in persons aging with SCI may, eventually, contribute to lack of function and independence.
This was a retrospective report that focused on describing the effects of increasing age on concentrations of T in men with SCI. Because there are numerous factors that may have had the potential to influence T concentrations, it is important to appreciate that, regardless of the etiology, low T has consistently been observed at higher prevalence rates in SCI than non-SCI men. Efforts were made at the outset to identify confounders in the subject's research records with known influence on T concentration and to exclude these potential subjects from the analysis. However, patients who were taking opioids were not excluded from our population sample, and this is a potential reason for a sub-group of those with SCI to have lower T values. A limitation to our design was the use of historical able-bodied men from large, general population samples, with the possibility for differences between cohorts as a result of geography, socioeconomic status, and being a veteran or non-veteran status.
Conclusion
Persons with SCI appear to have a higher prevalence of low T concentration for each decade of life than those in the general population. Of note, and of potential clinical relevance, the low T values tend to occur earlier in life in persons with SCI. The decline in serum total T concentration over time in the group with SCI was 50% greater than that for the able-bodied controls. The percent of low T in older men with SCI is predicted to be greater in those with higher BMIs and longer DOI. The functional and clinical significance of our laboratory finding of low T concentrations in the SCI population has yet to be determined. Additional studies of hormone replacement therapy should assist in determining a possible benefit from reversing a hypogonadal state in the individuals with SCI. Clinicians who care for men with SCI should consider routine annual screening for depressed measures of serum T because physical findings and psychological symptoms that are often observed in the SCI population may also be speculated to be the result of, or contributed to, low T concentrations.
Acknowledgments
The authors thank the James J Peters VA Medical Center, Bronx, NY, and the Department of Veterans Affairs Rehabilitation Research & Development Service for their support. This work was funded by a Rehabilitation Research & Development (RR&D) Center of Excellence for the Medical Consequences of Spinal Cord Injury (#B2648C and B9212C).
References
- 1.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 1991;73(5):1016–25 [DOI] [PubMed] [Google Scholar]
- 2.Longcope C, Goldfield SR, Brambilla DJ, McKinlay J. Androgens, estrogens, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab 1990;71(6):1442–6 [DOI] [PubMed] [Google Scholar]
- 3.Simon D, Preziosi P, Barrett-Connor E, Roger M, Saint-Paul M, Nahoul K, et al. The influence of aging on plasma sex hormones in men: the Telecom Study. Am J Epidemiol 1992;135(7):783–91 [DOI] [PubMed] [Google Scholar]
- 4.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001;86(2):724–31 [DOI] [PubMed] [Google Scholar]
- 5.Morley JE, Kaiser FE, Perry HM, III, Patrick P, Morley PM, Stauber PM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 1997;46(4):410–3 [DOI] [PubMed] [Google Scholar]
- 6.Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol 1997;146(8):609–17 [DOI] [PubMed] [Google Scholar]
- 7.Vermeulen A, Kaufman JM. Ageing of the hypothalamo-pituitary-testicular axis in men. Horm Res 1995;43(1–3):25–8 [DOI] [PubMed] [Google Scholar]
- 8.Tenover JL Testosterone and the aging male. J Androl 1997;18(2):103–6 [PubMed] [Google Scholar]
- 9.Reddy RG, Aung T, Karavitaki N, Wass JA. Opioid induced hypogonadism. BMJ 2010;341:c4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95(6):2536–59 [DOI] [PubMed] [Google Scholar]
- 11.LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab 2009;94(9):3337–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994;43(6):749–56 [DOI] [PubMed] [Google Scholar]
- 13.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005;43(7):408–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spungen AM, Wang J, Pierson RN, Jr, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol 2000;88(4):1310–5 [DOI] [PubMed] [Google Scholar]
- 15.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95(6):2398–407 [DOI] [PubMed] [Google Scholar]
- 16.Qin W, Bauman WA, Cardozo C. Bone and muscle loss after spinal cord injury: organ interactions. Ann N Y Acad Sci 2010;1211:66–84 [DOI] [PubMed] [Google Scholar]
- 17.Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Increased cardiovascular disease risk in Swedish persons with paraplegia: The Stockholm spinal cord injury study. J Rehabil Med 2010;42(5):489–92 [DOI] [PubMed] [Google Scholar]
- 18.Bors E, Engle ET, Rosenquist RC, Holliger VH. Fertility in paraplegic males: a preliminary report of endocrine studies. J Clin Endocrinol Metab 1950;10(4):381–98 [DOI] [PubMed] [Google Scholar]
- 19.Clark MJ, Schopp LH, Mazurek MO, Zaniletti I, Lammy AB, Martin TA, et al. Testosterone levels among men with spinal cord injury: relationship between time since injury and laboratory values. Am J Phys Med Rehabil 2008;87(9):758–67 [DOI] [PubMed] [Google Scholar]
- 20.Cortes-Gallegos V, Castaneda G, Alonso R, Arellano H, Cervantes C, Parra A. Diurnal variations of pituitary and testicular hormones in paraplegic men. Arch Androl 1982;8(3):221–6 [DOI] [PubMed] [Google Scholar]
- 21.Nance PW, Shears AH, Givner ML, Nance DM. Gonadal regulation in men with flaccid paraplegia. Arch Phys Med Rehabil 1985;66(11):757–9 [PubMed] [Google Scholar]
- 22.Huang TS, Wang YH, Chiang HS, Lien YN. Pituitary-testicular and pituitary-thyroid axes in spinal cord-injured males. Metabolism 1993;42(4):516–21 [DOI] [PubMed] [Google Scholar]
- 23.Huang TS, Wang YH, Lien IN. Suppression of the hypothalamus-pituitary somatotrope axis in men with spinal cord injuries. Metabolism 1995;44(9):1116–20 [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi TA, Skowsky WR, El-Toraei I, Swerdloff R. The pituitary-gonadal axis in spinal cord injury. Fertil Steril 1976;27(10):1142–5 [DOI] [PubMed] [Google Scholar]
- 25.Safarinejad MR Level of injury and hormone profiles in spinal cord-injured men. Urology 2001;58(5):671–6 [DOI] [PubMed] [Google Scholar]
- 26.Tsitouras PD, Zhong YG, Spungen AM, Bauman WA. Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm Metab Res 1995;27(6):287–92 [DOI] [PubMed] [Google Scholar]
- 27.Wang YH, Huang TS, Lien IN. Hormone changes in men with spinal cord injuries. Am J Phys Med Rehabil 1992;71(6):328–32 [DOI] [PubMed] [Google Scholar]
- 28.Durga A, Sepahpanah F, Regozzi M, Hastings J, Crane DA. Prevalence of testosterone deficiency after spinal cord injury. PMR 2011;3(10):929–32 [DOI] [PubMed] [Google Scholar]
- 29.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84(10):3666–72 [DOI] [PubMed] [Google Scholar]
- 30.Bauman WA, Emmons RR, Cirnigliaro CM, Kirshblum SC, Spungen AM. Provocative stimulation of the pituitary-gonadotropin axis in hypogonadal and eugonadal persons with spinal cord injury. 91st Annual Meeting of the Endocrine Society; 2009; Washington, DC [Google Scholar]
- 31.Ver Voort SM Infertility in spinal-cord injured male. Urology 1987;29(2):157–65 [DOI] [PubMed] [Google Scholar]
- 32.Linsenmeyer TA, Perkash I. Infertility in men with spinal cord injury. Arch Phys Med Rehabil 1991;72(10):747–54 [PubMed] [Google Scholar]
- 33.Anderson DC Sex-hormone-binding globulin. Clin Endocrinol (Oxf) 1974;3(1):69–96 [DOI] [PubMed] [Google Scholar]
- 34.Zumoff B, Strain GW, Miller LK, Rosner W, Senie R, Seres DS, et al. Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab 1990;71(4):929–31 [DOI] [PubMed] [Google Scholar]
- 35.Bertello P, Gurioli L, Faggiuolo R, Veglio F, Tamagnone C, Angeli A. Effect of ethanol infusion on the pituitary-testicular responsiveness to gonadotropin releasing hormone and thyrotropin releasing hormone in normal males and in chronic alcoholics presenting with hypogonadism. J Endocrinol Invest 1983;6(6):413–20 [DOI] [PubMed] [Google Scholar]
- 36.Kirubakaran VR, Kumar VN, Powell BJ, Tyler AJ, Armatas PJ. Survey of alcohol and drug misuse in spinal cord injured veterans. J Stud Alcohol 1986;47(3):223–7 [DOI] [PubMed] [Google Scholar]
- 37.Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab 1998;83(6):1886–92 [DOI] [PubMed] [Google Scholar]
- 38.Sonmez A, Haymana C, Bolu E, Aydogdu A, Tapan S, Serdar M, et al. Metabolic syndrome and the effect of testosterone treatment in young men with congenital hypogonadotropic hypogonadism. Eur J Endocrinol 2011;64(5):59–64 [DOI] [PubMed] [Google Scholar]
- 39.Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, et al. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res 2011;43(8):574–9 [DOI] [PubMed] [Google Scholar]