Abstract
Background
Functional electrical stimulation (FES) has been found to be effective in restoring voluntary functions after spinal cord injury (SCI) and stroke. However, the central nervous system (CNS) changes that occur in as a result of this therapy are largely unknown.
Objective
To examine the effects of FES on the restoration of voluntary locomotor function of the CNS in a SCI rat model.
Methods
SCI rats were instrumented with chronic FES electrodes in the hindlimb muscles and were divided into two groups: (a) FES therapy and (b) sedentary. At day 7 post-SCI, the animals were assessed for locomotion performance by using a Basso, Beattie and Bresnahan (BBB) scale. They were then anesthetized for a terminal in vivo experiment. The lumbar spinal cord and somatosensory cortex were exposed and the instrumented muscles were stimulated electrically. Associated neurovascular responses in the CNS were recorded with an intrinsic optical imaging system.
Results
FES greatly improved locomotion recovery by day 7 post-SCI, as measured by BBB scores (P < 0.05): (a) FES 10 ± 2 and (b) controls 3 ± 1. Furthermore, the FES group showed a significant increase (P < 0.05) of neurovascular activation in the spinal cord and somatosensory cortex when the muscles were stimulated between 1 and 3 motor threshold (MT).
Conclusion
Hind limb rehabilitation with FES is an effective strategy to improve locomotion during the acute phase post-SCI. The results of this study indicate that after FES, the CNS preserves/acquires the capacity to respond to peripheral electrical stimulation.
Keywords: Spinal cord injuries, Locomotion, Ambulation, Neurorecovery, Optical imaging, Electrophysiology, Rats, Neuronal plasticity, Functional electrical stimulation, Paraplegia, Tetraplegia, Rehabilitation
Introduction
Although improvements in function have been observed in patients with spinal cord injury (SCI) after months of physical and occupational therapy, the outcomes vary and are frequently not clinically relevant. Recent discoveries have demonstrated substantial recovery of voluntary grasping1–4 and walking5 functions in individuals post-SCI or stroke undergoing functional electrical stimulation (FES) therapy. Evidence is emerging that FES enhances central nervous system (CNS) plasticity in humans. FES of the common peroneal nerve during walking has been shown to temporarily increase the excitability of corticospinal tracts pertaining to ankle flexor and extensor muscles, measured by the amplitude of motor-evoked potentials.6–8 In addition, intensive FES during exercise periods with synchronous voluntary contraction of the same muscles modifies motor cortex excitability and advances sensorimotor remodeling.9
The mechanisms underlying the therapeutic benefits of FES combined with voluntary drive are unknown. It has been speculated that CNS plasticity may reside in the spinal cord and/or cerebrum. Indeed, CNS plasticity may be driven by increased afferent feedback coming directly from the stimulation of muscle afferents and/or indirectly by natural feedback from the joints, tendons, and proprioceptive fields during limb movement. Rather than relying only on direct stimulation of efferent fibers, simultaneous afferent stimulation provides a more natural locomotion pattern over time.10 Indeed, direct stimulation of lumbar dorsal roots in rat models has been found to produce a variety of ipsilateral and bilateral locomotor responses.11 The afferent stimulation during FES therapy is similar and probably even more potent than direct stimulation of lumbar dorsal roots, as FES is more targeted and properly channeled to the relevant spinal cord circuitry for the induction and maintenance of locomotion. From this perspective, the specificity and volume of training provided by FES after SCI may be crucial in restoring proper voluntary locomotor function/pattern.10 Moreover, the appropriate timing to administer a rehabilitation therapy after SCI is an important aspect to consider. It has been shown that functional recovery was reduced by delaying motor training after a SCI and was more effective when administered immediately after the injury.12 Also, forced locomotor training in wheel cages has been found to be beneficial for locomotion recovery after SCI, when initiated at 5 days after injury.13
To mimic the clinical condition, a SCI rat model with FES of the semi-paralyzed muscles is used here, to appreciate the beneficial effects of FES therapy. Since the spinal cord lesion location and severity in well controlled in rats, it reduces this important bias and thus improve the data interpretation. The originality of this experiment stems from the fact that neurovascular activation in the CNS is examined using an in vivo intrinsic optical imaging (IOI) and electrophysiological techniques14–16 to investigate changes in neurovascular responses post-FES therapy that is performed during the acute phase (i.e. from day 2 to day 6 post-SCI). Since proper neuronal drive into the lumbar spinal cord is crucial for central pattern generator activation and the induction of locomotion after thoracic SCI,17 we hypothesized that the neuronal link between peripheral afferents and the CNS will strength after short-term FES therapy. Indeed, the FES therapy can cause synaptic and neuronal reorganization within the lumbar spinal cord, to compensate for a decrease in supraspinal input from the spinal lesion, allowing the reestablishment/reactivation of the voluntary locomotor function.
Methods
Spinal cord lesion and FES electrode implantation
Twelve adult female Sprague-Dawley rats (225–250 g, Charles River, St-Constant, Quebec, Canada) were tested. Surgical procedures, performed according to guidelines of the Canadian Council on Animal Care, were approved by the animal ethics committee of Hôpital du Sacré-Coeur de Montréal. The rats were anesthetized with ketamine/xylazine mixture (90/10 mg/kg, intramuscularly) and placed on a thermal blanket until recovery. They were laminectomized to expose the T10 spinal cord segment, and a severe contusion injury (40 g·cm) was delivered at the T-10 level of the spinal cord. Then, the rats were implanted with REL-09-001 muscle electrodes (Rehabilitation Engineering Laboratory, Toronto Rehab, Canada) designed for chronic experiments in rodents, with the connector located behind the neck. Two leads were implanted bilaterally in the quadriceps and hamstring muscles. A Compex™ motion stimulator (Compex SA, Switzerland) verified the quality of electrode insertion in all hind limb muscles, which were sutured and the skin closed with wound clips. The animals received antibiotics (Duplocillin, 0.2 mg/kg, intramuscularly) immediately, and 2 and 4 days after surgery. The bladder was voided after SCI by gently massaging the lower abdomen twice daily until reflexive or voluntary emptying occurred. The animals were kept on a 12:12-hour light/dark cycle, with water and food ad libitum. They were also given softened food in their cages, and long sipper tubes on the water bottles ensured easy access to water.
Locomotion assessment
Limb function was measured according to the Basso, Beattie and Bresnahan (BBB) locomotor scale at day 2 post-SCI by two investigators blinded to the study, to verify the severity of the contusion injury.18 BBB scoring was also performed on day 7 post-SCI to evaluate the locomotion performance of all rats.
FES therapy regiment
Starting on day 2 post-SCI, the 12 SCI rats were randomly assigned to two groups: (1) FES therapy plus daily living in plastic cages (FES group, n = 6), and (2) daily living in plastic cages (control group, n = 6). Compex™ motion stimulator parameters were: (a) biphasic asymmetric pulses, (b) pulse duration of 300 µs, (c) stimulation frequency of 75 Hz, and (d) animal- and muscle-specific pulse amplitude set by trial and error to obtain proper limb flexion and extension. The above stimulation parameters19,20 have been found to produce the most natural and reproducible limb movement. The stimulation protocol simultaneously generated a complete hip flexion (quadriceps) on one side of the body and a complete hip extension (hamstring) on the other side for 2 seconds. Then, the stimulation sequence was changed so that the first side of the body generated hip extension and the other side simultaneously produced hip flexion for 2 seconds (i.e. 4 seconds is the time interval required from the moment a leg lift off occurs until the same limb performs a consecutive lift off). This stimulation sequence was optimized to generate a “walking type” range of motion of the hindlimbs. A time interval of 4 seconds has been used in this study during the FES therapy sessions, because the higher current intensity that is needed to perform a complete flexion-extension of the hip in less than 4 seconds induced signs of stress in all SCI rats and was avoided. Transitions between stimulation sequences were achieved by reducing (ramping down) stimulation intensity to zero in the first set of muscles, followed by increasing it (ramping up) in the next set of muscles. Each FES period was delivered to the rats three times a day for 10 minutes, and this therapy was administered daily from day 2 to 6 post-SCI. During the FES exercise bouts, the hind limbs were sweeping on the ground surface and rats were unable to achieve weight-bearing stepping, because the involuntary movement of their hind limbs during the electrical stimulations did alter their balance consistently.
Intrinsic optical imaging
At day 7 post-SCI, the rats were anesthetized with isoflurane (5%), and body temperature was maintained at 37°C with a heating blanket (Harvard, Canada). They were artificially ventilated (Kent Scientific, USA) by tracheotomy for ambient air supplemented with pure oxygen. Their breathing rate was maintained between 60 and 80 cycles/min, with 2 ml tidal volume and set to obtain an end tidal expired CO2 concentration of 3%. Both heart rate (Nihon Kohden, Japan) and CO2 level (Capstar 100, GENEC, Canada) were monitored. The rats were then positioned on a custom-made stereotaxic frame, and lumbar spinal cord segments from the thoracic (T10) to the sacral (S1) area were exposed by laminectomy. In addition, the skull was thinned to 100 µm with a rotating drill bit to expose the somatosensory cortex representing the hind limb area on both sides (antero-posterior: −0.5 to 1 mm; medio-lateral: ±2 to ±2.5 mm from the bregma). Two sets of clamps stabilized the spinal cord, and the head was secured with ear bars to eliminate physiological movements. Two baths were given with skin flaps placed over the skull and spinal cord, and light mineral oil was added to prevent tissue drying during the experiment and to improve optical recordings. Isoflurane administration was stopped after surgery, and the rats were anesthetized with alpha-chloralose (first by 50 mg/kg bolus and then by 25 mg/kg/h). Thirty minutes were allowed for animal stabilization. Then, the Compex™ motion stimulator determined the motor threshold (MT) of each instrumented muscle.
Optical intrinsic images were acquired with a 12-bit CCD camera (CS3960DCL, Toshiba Teli, Canada) under 1392 × 1040 resolution and 6.45 µm pixel size. A custom-made LabView interface (National Instrument, USA) controlled the camera, recorded images, synchronized data acquisition and electrical stimulation, and changed the illumination. A Nikkor Macro lens (f = 50 mm) gave sufficiently small focal depth (350 µm). Functional images of the spinal cord were recorded under multiple hashing illuminations (525, 590, and 637 nm) produced by high-power light-emitting diodes (Optek Technologies, USA). Illumination was set so that no part of the spinal cord or somatosensory cortex was under- or over-saturated by any of the wavelengths. For laser speckle imaging, a red 780-nm laser was positioned, and the camera aperture was set to 5.6 so that pixel size matched speckle size. Images were captured at a 15-Hz sampling rate with shutter speed at 10 ms.
Each stimulation onset had a 2-second duration and 18-second inter-stimulus interval. The inter-stimulus interval was set for proper return to baseline, which is necessary to correctly average neurovascular responses. The stimulus consisted of biphasic, asymmetric current pulses with pulse duration of 300 µs and stimulation frequency of 75 Hz for 2 seconds, delivered by the Compex™ motion stimulator. These stimulation parameters were equivalent to those investigated in randomized controlled trials on SCI subjects in the past,1,2,5 but scaled to fit the animal model at hand. Stimulation intensity (i.e. pulse amplitude) was adjusted, depending on the value of the previously measured MT. During the imaging part of the study, stimulation current intensity (pulse amplitude) was randomly set to values of 0, 1, 2, and 3 times the MT during the 20-minute time period. While the stimulus was delivered, the average response for each stimulation intensity was recorded (for additional details, see ref.16). We systematically discarded the results if MT changed more than 20% during the day, which is normally related to degradation of the animals' physiological condition.
The evolution of measured light intensity was related to absorbance by modified Beer-Lambert law.21 With the illumination wavelengths used, deoxyhemoglobin (HbR) and oxyhemoglobin (HbO) were the principal chromophores, and absorption was measured in connection with hemoglobin concentrations. A change in optical imaging absorbance was calculated by comparing the ratio of the activation state with a period of 3 seconds prior to stimulation onset (baseline). Blood flow was evaluated by computing spatial speckle fluctuations produced by random mutual interferences of coherent light originating from a laser diode. Moving scatters created a time-varying speckle pattern. When integrated over the exposure time of the camera, these fluctuations induced a blur in the raw image that was quantified by spatial contrast.22 After optical acquisition, the dura matter was cut at the level of the spinal cord and cortex, and a tungsten microelectrode (∼1 MΩ) was stereotaxically inserted into specific neuronal pools for electrophysiological recordings. Localization of the neuronal responses observed by IOI was correlated by electrophysiology.23,24
Spinal cord histology
After electrophysiological recordings were completed, the thoracic spinal cords were removed, post-fixed overnight in 4% paraformaldehyde and transferred to 30% sucrose solution for 2 days. Thereafter, they were blocked in optical coherence tomography tissue-freezing medium, cut into 20-μm slices on a cryostat and mounted on charged glass slides. Sections from the injury epicenter of each spinal cord were stained according to the cresyl echt violet method.25,26 The slides were photographed with a digital camera (Nikon Coolpix, Japan) attached to a microscope (Leica, Germany). Dorsal column and total white matter at the level of the injury epicenter was measured from cross-sectional areas (mm2) and then converted to relative white matter-sparing, compared to uninjured (no-SCI) rats. Spared white matter was identified as being evenly and lightly stained. The length of the injury was determined by identifying the lesion border by an absence of healthy neurons and was always distant from the injury epicenter.
Statistical analysis
IOI, electrophysiology, locomotion, and hhistology were compared among groups by analysis of variance with a P < 0.05 level of significance. When appropriate, a post hoc test (Tukey or Least Significant Difference) was performed.
Results
Recovery of locomotion after FES therapy
All rats (n = 12) had BBB locomotor scores of 21/21 before SCI. At day 2 post-SCI, their BBB score was 1 ± 1 (slight movement of one or two joints, no weight-bearing). At day 7 post-SCI, the BBB score of the FES group was significantly higher (10 ± 2, occasional weight-supported plantar steps) than that of the control group (3 ± 1.6, extensive movement of two joints, no weight support) (Table 1).
Table 1 .
BBB score, area of spared dorsal column white matter, total spared white matter, and lesion length at day 7 post-SCI
| BBB score at day 2 post-SCI (21 points scale) | BBB score at day 7 post-SCI (21 points scale) | Percentage of spared dorsal column white matter, compared to uninjured rats | Percentage of spared total white matter, compared to uninjured rats | Spinal cord lesion length (mm) | |
|---|---|---|---|---|---|
| Controls (n = 6) | 1 ± 1 | 3 ± 1.6 | 32 ± 12% | 50 ± 9% | 8.8 ± 1.2 |
| FES trained (n = 6) | 1 ± 1 | 10 ± 2* | 40 ± 14% | 57 ± 12% | 9.1 ± 1.5 |
*Significant differences between groups with P < 0.05.
Neuronal activity recording in the lumbar spinal cord
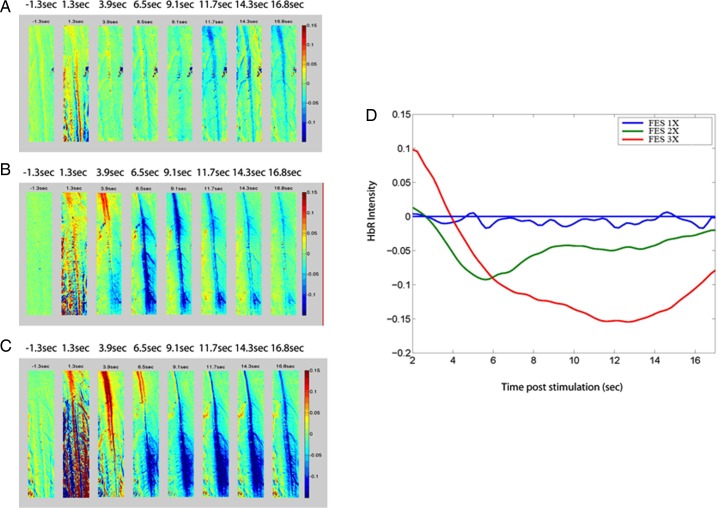
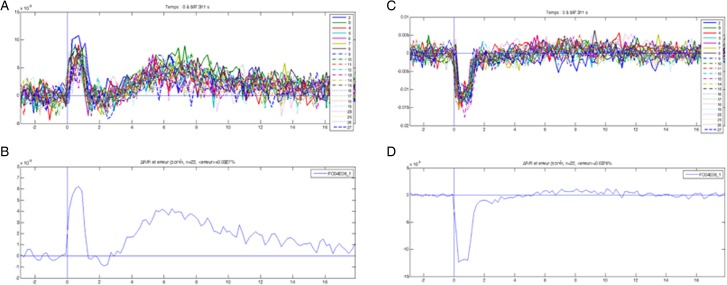
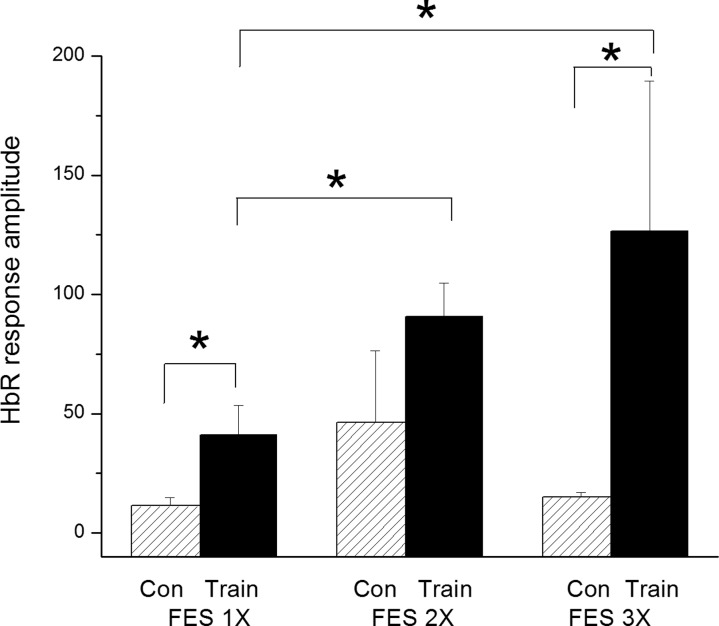
Neurovascular activation in the lumbar spinal cord was compared between the FES and control groups. As seen on Fig. 1, stimulation of the hamstring and quadriceps muscles on the right side with the Compex™ motion stimulator generated neurovascular responses between 3.9 and 16.8 seconds post-stimulation at 1, 2, and 3 MT, mostly on the ipsilateral side (in blue). Response intensity was plotted against time to obtain neuronal activation volume (Fig. 1D). Final data were generated from 20 raw responses, and representative examples on the ipsilateral and contralateral sides are shown in Fig. 2A and 2C, respectively. Average responses generating the final data are illustrated in Fig. 2B and 2D. The FES group manifested a significant increase in neurovascular responses at 1 and 3 MT compared to the control group. The neurovascular responses in the FES group were significantly higher at 2 and 3 MT than at 1 MT. On the other hand, the control group did not present any differences in response amplitude between 1, 2, and 3 MT (Fig. 3). Neurovascular activation recorded by IOI was validated electrophysiologically by recording local filed potentials (LFP). Maximal LFP and IOI amplitudes were observed exactly at the same location in the lumbar spinal cord, which is consistent with our previous reports.14,15
Figure 1 .
Optical recording of neuronal activation of deoxyhemoglobin (HbR) in the lumbar spinal cord after right hind limb muscle stimulation with the FES apparatus at an intensity of: (A) 1 MT, (B) 2 MT, and (C) 3 MT. (D) Neurovascular activation recorded by intrinsic optical imaging in the spinal cord of a rat in the FES group was proportional to stimulus intensity of 1 MT (blue line), 2 MT (green line), and 3 MT (red line). Control rats showed similar responses between 1 and 3 MT that were similar to those in graph (A) (low amplitude). Note: Since muscle stimulation lasted 2 seconds, the 1.3-second post-stimulus images are not valid, because of spinal cord movement artifacts induced by muscle contraction. Therefore, we removed the first 2 seconds in graph D for clarity.
Figure 2 .
(A) Twenty raw responses showing light-reflecting deoxyhemoglobin (HbR) intensity in the lumbar spinal cord on the ipsilateral side of the stimulated muscle. (B) Average response on the ipsilateral side. (C) Twenty raw responses representing light-reflecting HbR intensity in the lumbar spinal cord on the contralateral side of the stimulated muscle. (D) Average responses on the contralateral side. Note: The first large deflection between 0 and 2 seconds in each graph is a movement artifact caused by hind limb muscle contraction and is not considered a physiological signal. Thus, physiological optical responses were recorded between 2 and 16 seconds.
Figure 3 .
Lumbar spinal cord deoxyhemoglobin (HbR) response amplitude on the ipsilateral side after FES stimulation of the hind limb muscles at 1, 2, and 3 MT between the control (con) and FES (train) groups. *Significant at P < 0.05. n = 6 per group.
Recording in the somatosensory cortex
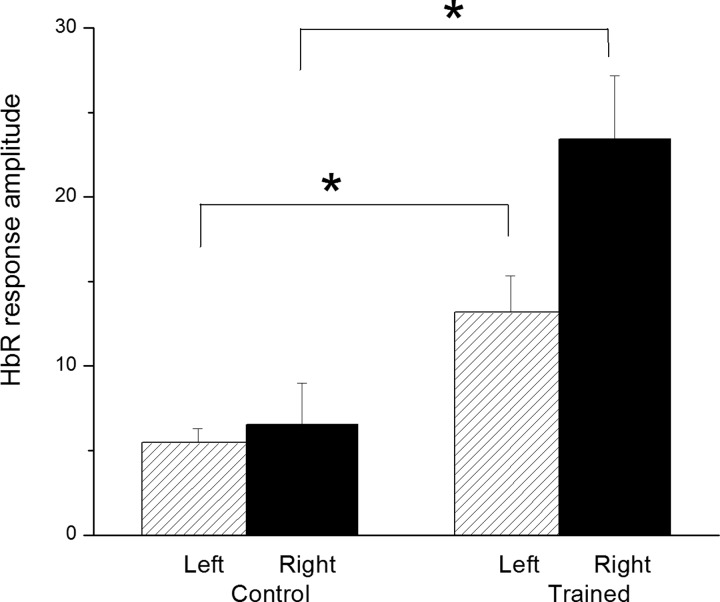
IOI responses were recorded simultaneously from both sides of the somatosensory cortex during stimulation of the left hind limb muscles. Since the SCI lesion was symmetrical at T10, we stimulated the left muscles to discriminate ipsilateral and contralateral responses at the level of the somatosensory cortex, as depicted in Fig. 4. Somatosensory amplitude response showed a significant increase in activation of about three-fold on both sides of the somatosensory cortex in the FES compared to the control group, as reported in Fig. 5. Neurovascular activation recorded by IOI was validated electrophysiologically by LFP; maximal LFP and IOI amplitudes were registered in the same areas (antero-posterior: −0.5 to 1 mm; medio-lateral: ±2 to ±2.5 mm from the bregma).
Figure 4 .
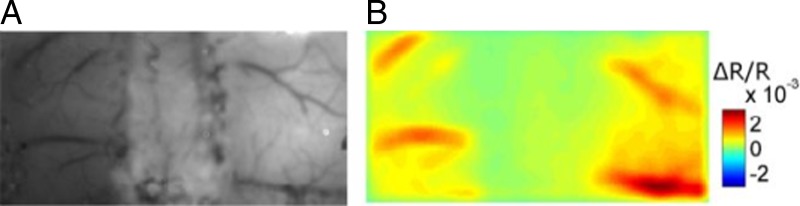
(A) Picture of the exposed cortex after thinning the skull bilaterally to 100 µm. (B) Maximum intrinsic optical imaging signal from the somatosensory cortex.
Figure 5 .
HbR responses on both sides of the somatosensory cortex after stimulation of the left hind limb muscles at a stimulation intensity of 1 MT between control and FES groups. *Significant at P < 0.05. n = 6 per group.
Spinal cord histology
The dorsal column white matter and total white matter sparing at the level of the injury epicenter was measured in both SCI groups. No significant difference was apparent between them: (1) 32 ± 12% of dorsal column white matter sparing in the control group and 40 ± 14% in the FES group; and (2) 50 ± 9% of total white matter sparing in the control group and 57 ± 12% in the FES group, in comparison to the white matter area measured in healthy rats (Table 1). The gray matter at the level of the injury epicenter was degraded at 7 days post-SCI. The lesion expansion was similar between groups and covered 8.8 ± 1.2 mm in the control group and 9.1 ± 1.5 mm in the FES group.
Discussion
Locomotion recovery with FES therapy
FES, applied acutely to semi-paralyzed muscles from day 2 to day 6 post-SCI, was effective in restoring voluntary weight supported stepping after a severe spinal cord contusion injury. Indeed, the BBB locomotor score was significantly higher in the FES compared to the control group at day 7 post-SCI. As an explanation, at least partially, FES therapy could have supplemented neuronal drive into the lumbar spinal cord by recruiting peripheral afferents during exercise bouts, which could have generated locomotor-like movements. This strategy supplements neuronal drive in the lumbar spinal cord to compensate for a decrease in descending drive from supraspinal neurons after incompletely contused SCI. Over time, FES therapy could improve connectivity and function between peripheral afferents, the spinal cord and the supraspinal structures above the SCI site, enhancing locomotion. During the establishment of the FES training procedure, by using a separate group of rats, we found that the FES rats were able to performed weight supported steps on day 7 post-SCI and they kept the ability to perform stepping over time, by possibly retraining themselves in their cage up to 4 weeks post-SCI. On the other hand, control rats improved their BBB locomotor score over time, since they also moved in their cage, but they never recovered weight-supported plantar stepping, even after 4 weeks post-SCI.
Plasticity in the lumbar spinal cord
IOI data clearly disclosed increased neurovascular response amplitude in the lumbar spinal cord from 1 to 3 MT in the FES group compared to controls. Moreover, the control group lost the ability to respond to peripheral stimulation by day 7 post-SCI, since the neurovascular amplitudes recorded were very low and showed similar values between 1 and 3 MT. In fact, in healthy (non-SCI) rats, neurovascular response amplitudes are proportional to stimulation intensity,14 as in the FES group in this study. These findings reveal that FES has the potential to preserve the neurovascular responses in the lumbar spinal cord when stimulating the instrumented hind limb muscles. A limitation to this study is that IOI is recording the neurovascular response following the activation of neuronal populations. Indeed, the hemodynamic (vascular) component that is intricately implied in the neurovascular response could mislead our interpretation of the neuronal component. To solve this issue, at least partially, we did record the local field potentials in the spinal cord using a single sharp electrode. The maximal amplitude of the field potentials and the IOI responses in the spinal cord and the somatosensory cortex were proportional and were located in the same areas. Therefore, we concluded that IOI is an adequate tool to properly assess the capacity of the CNS to respond to peripheral stimulations in a SCI rat model.
On a separate note, we injected a bolus of pancuronium intravenously to paralyze all muscles at the end of the terminal experiment in two rats in the FES group and two rats in the control group (data not reported). After drug administration, the instrumented muscles were unable to generate contraction when stimulated, and neurovascular responses in the spinal cord were completely abolished. Since this drug's effect is reversible over time, activation in the spinal cord was re-established progressively along with the capacity of the hind limb muscles to contract. Moreover, pancuronium injection did not decrease neuronal activation in the spinal cord if the sciatic nerve was directly stimulated by bipolar electrodes, instead of the instrumented muscles. These results clearly indicate that the muscle contraction induced by FES in this model is crucial for the activation of peripheral afferent neurons that have projections in the CNS.
Plasticity in the somatosensory cortex
IOI responses were recorded from both sides of the somatosensory cortex when stimulating instrumented muscles at 1 to 3 MT. The neurovascular findings indicated significantly increased response amplitude in the FES group in both right and left somatosensory cortices compared to controls. One possible explanation is that FES training could preserve the integrity of ascending neuronal tracts in the spinal cord dorsal column white matter having projections in the cuneate nucleus and somatosensory cortex. However, histological cross-sections at the level of the injury did not show any significant difference in spared dorsal column white matter, spared total white matter or in the lesion length between the FES and control groups. Another possibility is that FES training altered the properties and/or the amount of intact ascending neurons that cross the injury site. It has been demonstrated that somatosensory stimulation in humans improves hand function and increases corticomotor excitability after SCI, by augmenting the size and localization of cortical motor map areas.27,28 Recovery of locomotion in our FES group could also be promoted by amelioration of the motor system by chronic sensory stimulation. Another possible explanation for higher neurovascular response amplitudes in the FES group is the potential neuroprotective effect of chronic electrical stimulation. Indeed, if more afferents are crossing the injury site (T10) to reach supraspinal neuronal structures, it is reasonable to suppose that FES therapy will improve neuronal connectivity between the lower limb and the cortex. In fact, electrical stimulation is beneficial in enhancing the survival of transplanted neural stem cells in the spinal lesion cavity,29 induce the proliferation of oligodendrocyte progenitor cells in the corticospinal tractus30 and promote axonal elongation and neuroprotection of the optical nerve after injury.31,32
Conclusions
Randomized controlled trials in individuals with SCI suggest that FES therapy is effective for recovery of grasping and locomotion in this patient population.1–4 However, the mechanisms behind these improvements are not fully understood. To further ameliorate and better target this therapeutic intervention, it is important to understand its recuperative mechanisms by investigating FES effects in the animal model. It is clear that FES performed during the acute phase post-SCI improves locomotion. A possible explanation for this is that the spinal cord maintains the capacity to respond to peripheral stimulation, which can replace the lack of descending neuronal drive having projections in the lumbar spinal cord. Second, afferent neuronal drive projecting to supraspinal structures across the injury site is also enhanced. While more studies are needed to better understand the neurological outcomes of FES therapy in SCI, the present experiment represents an important step toward identifying the mechanisms behind FES therapy.
References
- 1.Kapadia NM, Zivanovic V, Furlan J, Craven BC, McGillivray C, Popovic MR. Functional electrical stimulation therapy for grasping in traumatic incomplete spinal cord injury: randomized control trial. Artif Organs 2011;35(3):212–6 [DOI] [PubMed] [Google Scholar]
- 2.Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord 2006;44(3):143–51 [DOI] [PubMed] [Google Scholar]
- 3.Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehab Neural Repair 2008;22(6):706–14 [DOI] [PubMed] [Google Scholar]
- 4.Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair 2011;25(5):433–42 [DOI] [PubMed] [Google Scholar]
- 5.Thrasher TA, Flett HM, Popovic MR. Gait training regimen for incomplete spinal cord injury using functional electrical stimulation. Spinal Cord 2006;44(6):357–61 [DOI] [PubMed] [Google Scholar]
- 6.Thompson AK, Stein RB, Chen XY, Wolpaw JR. Modulation in spinal circuits and corticospinal connections following nerve stimulation and operant conditioning. Conf Proc IEEE Eng Med Biol Soc 2006;1:2138–41 [DOI] [PubMed] [Google Scholar]
- 7.Rushton DN Functional electrical stimulation and rehabilitation–an hypothesis. Med Eng Phys 2003;25(1):75–8 [DOI] [PubMed] [Google Scholar]
- 8.Thompson AK, Doran B, Stein RB. Short-term effects of functional electrical stimulation on motor-evoked potentials in ankle flexor and extensor muscles. Exp Brain Res 2006;170(2):216–26 [DOI] [PubMed] [Google Scholar]
- 9.Khaslavskaia S, Sinkjaer T. Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Exp Brain Res 2005;162(4):497–502 [DOI] [PubMed] [Google Scholar]
- 10.Dean JC, Yates LM, Collins DF. Turning on the central contribution to contractions evoked by neuromuscular electrical stimulation. J Appl Physiol 2007;103(1):170–6 [DOI] [PubMed] [Google Scholar]
- 11.Barthelemy D, Leblond H, Rossignol S. Characteristics and mechanisms of locomotion induced by intraspinal microstimulation and dorsal root stimulation in spinal cats. J Neurophysiol 2007;97(3):1986–2000 [DOI] [PubMed] [Google Scholar]
- 12.Norrie BA, Nevett-Duchcherer JM, Gorassini MA. Reduced functional recovery by delaying motor training after spinal cord injury. J Neurophysiol 2005;94(1):255–64 [DOI] [PubMed] [Google Scholar]
- 13.Sandrow-Feinberg HR, Izzi J, Shumsky JS, Zhukareva V, Houle JD. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J Neurotrauma 2009;26(5):721–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brieu N, Beaumont E, Dubeau S, Cohen-Adad J, Lesage F. Characterization of the hemodynamic response in the rat lumbar spinal cord using intrinsic optical imaging and laser speckle. J Neurosci Methods 2010;191(2):151–7 [DOI] [PubMed] [Google Scholar]
- 15.Lesage F, Brieu N, Dubeau S, Beaumont E. Optical imaging of vascular and metabolic responses in the lumbar spinal cord after T10 transection in rats. Neurosci Lett 2009;454(1):105–9 [DOI] [PubMed] [Google Scholar]
- 16.Ouakli N, Guevara E, Dubeau S, Beaumont E, Lesage F. Laminar optical tomography of the hemodynamic response in the lumbar spinal cord of rats. Opt Express 2010;18(10):10068–77 [DOI] [PubMed] [Google Scholar]
- 17.Rossignol S, Barriere G, Frigon A, Barthelemy D, Bouyer L, Provencher J, et al. . Plasticity of locomotor sensorimotor interactions after peripheral and/or spinal lesions. Brain Res Rev 2008;57(1):228–40 [DOI] [PubMed] [Google Scholar]
- 18.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 1996;139(2):244–56 [DOI] [PubMed] [Google Scholar]
- 19.Fairchild MD, Kim SJ, Iarkov A, Abbas JJ, Jung R. Repetetive hindlimb movement using intermittent adaptive neuromuscular electrical stimulation in an incomplete spinal cord injury rodent model. Exp Neurol 2010;223(2):623–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung R, Ichihara K, Venkatasubramanian G, Abbas JJ. Chronic neuromuscular electrical stimulation of paralyzed hindlimbs in a rodent model. J Neurosci Methods 2009;183(2):241–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohl M, Lindauer U, Royl G, Kuhl M, Gold L, Villringer A, et al. . Physical model for the spectroscopic analysis of cortical intrinsic optical signals. Phs Med Biol 2000;45(12):3749–64 [DOI] [PubMed] [Google Scholar]
- 22.Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. Neuroimage 2005;27(2):279–90 [DOI] [PubMed] [Google Scholar]
- 23.Beaumont E, Kaloustian S, Rousseau G, Cormery B. Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci Res 2008;62(3):147–54 [DOI] [PubMed] [Google Scholar]
- 24.Franceschini MA, Nissila I, Wu W, Diamond SG, Bonmassar G, Boas DA. Coupling between somatosensory evoked potentials and hemodynamic response in the rat. Neuroimage 2008;41(2):189–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnuson DS, Lovett R, Coffee C, Gray R, Han Y, Zhang YP, et al. . Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J Neurotrauma 2005;22(5):529–43 [DOI] [PubMed] [Google Scholar]
- 26.Powers MM, Clark G. An evaluation of cresyl echt violet acetate as a Nissl stain. Stain Technol 1955;30(2):83–8 [DOI] [PubMed] [Google Scholar]
- 27.Hoffman LR, Field-Fote EC. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther 2007;87(2):208–23 [DOI] [PubMed] [Google Scholar]
- 28.Hoffman LR, Field-Fote EC. Functional and corticomotor changes in individuals with tetraplegia following unimanual or bimanual massed practice training with somatosensory stimulation: a pilot study. J Neurol Phys Ther 2010;34(4):193–201 [DOI] [PubMed] [Google Scholar]
- 29.Chen YY, Zhang W, Chen YL, Chen SJ, Dong H, Zeng YS. Electro-acupuncture improves survival and migration of transplanted neural stem cells in injured spinal cord in rats. Acupunct Electrother Res 2008;33(1–2):19–31 [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Brus-Ramer M, Martin JH, McDonald JW. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci let 2010;479(2):128–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyake K, Yoshida M, Inoue Y, Hata Y. Neuroprotective effect of transcorneal electrical stimulation on the acute phase of optic nerve injury. Invest Ophthalmol Vis Sci 2007;48(5):2356–61 [DOI] [PubMed] [Google Scholar]
- 32.Tagami Y, Kurimoto T, Miyoshi T, Morimoto T, Sawai H, Mimura O. Axonal regeneration induced by repetitive electrical stimulation of crushed optic nerve in adult rats. Jpn J Ophthalmol 2009;53(3):257–66 [DOI] [PubMed] [Google Scholar]