Abstract
The detrimental impact of tobacco on human health is clearly recognized and despite aggressive efforts to prevent smoking, close to one billion individuals worldwide continue to smoke. People with chronic obstructive pulmonary disease (COPD) are susceptible to recurrent respiratory infections with pathogens, including non-typeable Haemophilus influenzae (NTHI), yet the reasons for this increased susceptibility are poorly understood. As mortality rapidly increases with multiple exacerbations, development of protective immunity is critical to improving patient survival. Acute NTHI infection has been studied in the context of cigarette smoke exposure, but this is the first study to investigate chronic infection and the generation of adaptive immune responses to NTHI following chronic smoke exposure. After chronic NTHI infection, mice that had previously been exposed to cigarette smoke developed increased lung inflammation and compromised adaptive immunity relative to air-exposed controls. Importantly, NTHI-specific T cells from mice exposed to cigarette smoke produced lower levels of IFN-γ and IL-4, and B cells produced reduced levels of antibodies against outer membrane lipoprotein P6, with impaired IgG1, IgG2a and IgA class-switching. However, production of IL-17, which is associated with neutrophilic inflammation, was enhanced. Interestingly, cigarette smoke exposed mice exhibited a similar defect in the generation of adaptive immunity following immunization with P6. Our study has conclusively demonstrated that cigarette smoke exposure has a profound suppressive effect on the generation of adaptive immune responses to NTHI and suggests the mechanism by which prior cigarette smoke exposure predisposes COPD patients to recurrent infections, leading to exacerbations and contributing to mortality.
Keywords: cigarette smoke, NTHI, chronic inflammation, P6, adaptive immunity
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a disease of the lungs in which the airways become narrow, thereby limiting the airflow and causing dypsnea (1,2). In contrast to asthma, the limitation of airflow is poorly reversible and usually progressively worsens (3,4). Chronic bronchitis and emphysema commonly co-exist in the lungs of COPD patients. This condition is initiated by respiratory exposure to noxious particles or gas, most commonly tobacco smoke, which triggers an inflammatory response in the lung. Cigarette smoke is recognized as a crucial factor in the development and pathogenesis of COPD (5). Prolonged cigarette smoke exposure results in increased vulnerability to many respiratory infections and predisposes to airway diseases (6,7). Chronic respiratory disease now ranks as the third most common cause of death in the US and the fifth worldwide (8).
COPD is characterized by intermittent disease exacerbation. Exacerbations account for the majority of hospitalizations for COPD patients and a significant share of direct and indirect economic costs, and are associated with a significantly increased risk of death (9). Even though the potential contributions of pulmonary infections to the etiology, pathogenesis, and clinical course of COPD have been identified, the precise role of bacterial infections to disease outcome has remained a subject of some controversy. Improvements in PCR detection of pathogen gene sequences has confirmed the causal contribution of bacterial infections to exacerbations of COPD, accounting for at least 50% of all exacerbations (10–12). Non-typeable Haemophilus influenzae (NTHI), Moraxella catarrhalis, and Streptococcus pneumoniae are the three most common bacterial pathogens that cause respiratory tract infections in COPD patients (13–15). Besides colonizing the airways when the patient is clinically stable, the acquisition of new NTHI strains is considered an important cause of lower respiratory tract infection and is associated with disease exacerbations. Because mortality rapidly increases with multiple exacerbations (16), developing an efficient immune response following a first exacerbation may be critical to improving patient survival.
Efforts to reduce the disease burden caused by repeat NTHI infections have focused on the major outer membrane proteins and lipooligosaccharide as potential candidate vaccine antigens (17–19). However, antigenic heterogeneity of these molecules in many of the NTHI strains suggests that a highly conserved, immunogenic molecule is required for formulation of an effective vaccine. Although it comprises less than 1% of the total outer membrane protein, the minor outer membrane lipoprotein P6 is highly conserved at the nucleotide and amino acid level among all tested strains of NTHI due to its integral function as an anchor between the outer membrane and the bacterial cell wall (20). Importantly, in consideration of vaccine development, P6 expresses epitopes on the outer membrane accessible for antibody binding and contains an immunodominant T cell epitope for assessing generation of cellular immunity (21–23). We have previously demonstrated that T cell responses to P6 are associated with relative protection against NTHI infection in adults with COPD (24). The immunogenic nature of this highly conserved lipoprotein makes P6 a promising vaccine candidate for NTHI (25,26). The expectation would be that vaccine-induced immunity would minimize NTHI-mediated lung damage during COPD exacerbations.
While previous studies have provided good evidence that cigarette smoke may be immunosuppressive (6,27–30), no reports have described the impact of cigarette smoke exposure on the development of adaptive immune responses to respiratory pathogens. Cigarette smoke is itself an inflammatory mediator and induces pulmonary inflammation by damaging the respiratory epithelial barrier, thereby facilitating repeated infections (31). Inflammatory mediators generated in response to these infections further accentuate a milieu of chronic inflammation in the lungs of smokers. Most models of respiratory inflammation simply evaluate the impact of either smoke exposure or infection alone, neglecting that the combination of several inflammatory mediators creates a unique microenvironment that may have an additive effect. To better understand the connections between chronic smoking, chronic pulmonary infection, chronic inflammation, and changes in adaptive immunity we developed a mouse model of these events. We have studied how chronic cigarette smoke exposure affects the generation of adaptive immune responses following chronic exposure to NTHI. Additionally, we have evaluated the vaccination efficacy of systemic P6 immunization in order to determine whether this treatment modality has the potential to alleviate respiratory inflammation and minimize lung damage resulting from combined cigarette smoke and NTHI exposure.
MATERIALS AND METHODS
Mice
Six-week old female C57BL/6J mice (Jackson Laboratory) were used in all experiments. Mice were maintained under specific pathogen-free conditions. Number of animals used in each experiment are provided in figure legends. All procedures performed on animals were IACUC-approved, and complied with all state, federal, and NIH regulations.
Cigarette smoke exposure
Mice were housed in the Inhalation Core Facility at the University of Rochester and were exposed to mainstream cigarette smoke as previously described (28,32,33). Mice were placed in individual compartments of a wire cage, which was placed inside a closed plastic box connected to the smoke source. 3R4F research cigarettes (University of Kentucky College of Agriculture Reference Cigarette Program) were smoked according to the FTC protocol (1 puff/min of 2 sec duration and 35 ml volume) in a Jaeger-Baumgartner CSM2072i cigarette smoking machine (CH Technologies). Mainstream cigarette smoke was diluted with filtered air and directed into the exposure chamber. The smoke exposure (total particulate matter per cubic meter of air, TPM) was monitored by gravimetric sampling. The smoke concentration was set at a nominal value of 250 mg/m3 TPM by adjusting the flow rate of the dilution air. The average actual exposure for these experiments was 259 ± 47 mg/m3. Mice were exposed for 5 hours per day, 5 days per week, for four weeks. Control mice were exposed to filtered air in an identical chamber according to the same schedule. Following the final smoke exposure, the mice were transported to Roswell Park Cancer Institute for infection and vaccination experiments.
Acute and chronic NTHI exposure
A frozen glycerol stock of NTHI strain 1479 (clinical isolate from a COPD exacerbation) was streaked on chocolate-agar plates and single colonies were grown in a liquid culture of brain-heart infusion media supplemented with 10 µg/ml hemin and 10 µg/ml β-nicotinamide adenine dinucleotide (Sigma). After 3–4 hrs of culture in a 37°C shaking incubator, OD600 was determined in order to dilute the required number of colony forming units (cfu) to 2×108 cfu/ml in PBS. Bacteria were pelleted in microcentrifuge tubes at 13000 × g for 10 min and washed twice in PBS. Upon completion of four weeks of air or cigarette smoke exposure, NTHI was administered by oropharyngeal instillation via the trachea. Mice were anesthetized by isoflurane inhalation and 50 µl of NTHI diluted in PBS was instilled via the trachea using a 200 µl sterile pipette tip. For initiating acute NTHI-mediated inflammation, mice received one instillation of 1×106 cfu live NTHI. For initiating chronic NTHI-mediated inflammation, mice received bi-weekly instillations of 1×106 cfu live NTHI for eight consecutive weeks.
P6 immunization
Air and cigarette smoke exposed mice were immunized i.p. with 40 µg of purified native P6 emulsified in CFA and boosted one week later with antigen in IFA and two weeks later with antigen alone (25). Sham immunized mice received PBS. Mice were bled retro-orbitally on a weekly basis. Titers of P6-specific antibodies were detected via an indirect ELISA as described below.
Lung histology
Lungs were excised and fixed in 10% formaldehyde (Polysciences Inc) in PBS, embedded in paraffin, sectioned, and stained with H&E by the Roswell Park Cancer Institute histopathology core facility. Images were obtained on an Olympus light microscope equipped with a CCD camera and Spot image analysis software (v25.4, Diagnostics Instruments). Lung pathology was evaluated by a pathologist (P.N.B.). A scoring schema was developed to quantify the extent of inflammation and immune cell infiltration in the lungs of mice exposed to NTHI chronically (34). Identity of the slides remained blinded during two independent scoring sessions by the pathologist and a consensus score of 0 to 3 was given for each of the parameters evaluated.
Bronchoalveolar lavage
On the day of euthanasia, mice were injected i.p. with 1 ml of warmed 2.5% Avertin solution (2,2,2-tribromethanol). The thoracic cavity was opened and the trachea exposed for cannulation with a 22-gauge i.v. catheter. PBS + 0.1% FCS (750 µL) was injected and withdrawn from the lung two times using a tuberculin syringe. A white blood cell count of bronchoalveolar lavage (BAL) fluid was assessed using a hemocytometer. Cells were cytocentrifuged onto clean glass slides and stained with Hema 3® (Fisher Scientific) to obtain cell differential counts of macrophages, lymphocytes, and neutrophils. Anti-P6 Ig, cytokine levels, and albumin leak in BAL fluid were measured by ELISA.
Isolation of lung lymphocytes
Lungs were excised from mice and minced into small pieces on ice using curved scissors. The lung slurry was mixed with 1 mg/ml Type IA-S collagenase + 50 U/ml DNase I (Sigma) and placed on a rotator at 37°C for 60 min. The single cell suspension was passed through a 45 µm filter to remove large debris and undigested tissue. Washed cells were resuspended in 5 ml complete RPMI tissue culture media supplemented with 10% FCS, underlaid with 4 ml of room temperature Ficoll-Paque (GE Healthcare), and centrifuged for 20 min, 1400 × g, room temperature, brake off. Immune cells at the interface were collected and washed extensively to remove residual Ficoll-Paque before use in ELISPOTs.
ELISA
Cytokine concentrations in BAL fluid and supernatants of stimulated T cells were evaluated with the eBioscience kits according to manufacturer’s instructions: IL-1β (cat # 88-7013-77), IL-6 (cat # 88-7064-77), TNF-α (cat # 88-7324-77), IL-4 (cat # 88-7044-77), IL-17 (cat # 88-7371-77), IFN-γ (cat # 88-7314-77). Quantification of albumin in BAL fluid was evaluated with a kit from Bethyl Laboratories (cat # E90–134). Titers of P6-specific antibodies were measured using ELISA plates coated with 3 µg/ml of P6 protein. Weekly serum samples and BAL fluid from individual mice were added to blocked plates and bound anti-P6 Ig were detected with HRP-conjugated goat anti-mouse Ig(H+L), IgA, IgG1, IgG2a, IgG2b (Southern Biotech). Plates were developed with 3,3’,5,5’-tetramethylbenzidine (for HRP) and absorbance read at 450 nm.
ELISPOTs
Frequency of P6-specific T cells was evaluated by ELISPOTs. Multiscreen HA plates (Millipore) were coated with 3 µg/ml of anti-IFN-γ (clone AN-18), anti-IL-4 (clone 11B11), or anti-IL-17 (clone eBio17CK15A5). Lymphocytes were co-cultured with APCs pulsed with 1 µM P641–55 peptide (Genscript) (21). After 18 hr, the plates were washed and cytokines were detected with biotinylated antibodies (anti-IFN-γ clone R4–6A2; anti-IL-4 clone BVD6-24G2; anti-IL-17 clone eBio17B7) followed by addition of streptavidin-HRP (all reagents from eBioscience). Spots were developed with TMB substrate (Vector Labs) and enumerated microscopically. Frequency of P6-specific antibody-secreting cells in bone marrow and spleen was enumerated on ELISPOT plates coated with 3 µg/ml of P6; after 18 hr, bound anti-P6 antibody was detected with HRP-conjugated secondary antibodies to mouse IgG1 and IgG2a (Southern Biotech). Spots were developed with TMB.
NTHI clearance
Lungs were excised from mice and mechanically disrupted with a tissue homogenizer in 1 ml PBS on ice. Serial dilutions of the lung homogenate were cultured on chocolate-agar plates at 37°C to enumerate cfu per gram of lung tissue.
Statistical analysis
Testing for differences between mean values was determined using either Student t-test or 2-way ANOVA with post-test comparisons. The area under the curve (AUC) for the anti-P6 antibody responses from week 0 to week 16 were estimated per animal using the standard trapezoidal approach. Direct comparisons were made between the two exposure groups using an exact-permutation Kruskal-Wallis rank test and tested at level α = 0.05 (two-sided).
RESULTS
Cigarette smoke exposure exacerbates NTHI-mediated chronic respiratory inflammation
We first examined how two important inflammatory mediators in the lung, cigarette smoke and bacterial infection, impact pulmonary inflammation. C57BL/6 mice were exposed to cigarette smoke or air for 4 weeks, followed by 8 weeks of chronic NTHI exposure (Fig. 1A). Histopathological examination of the lungs revealed that characteristic lymphocytic accumulation surrounding airways and bronchovasculature was observed in both groups (Fig. 1B); however, the extent and severity of immune cell infiltration was greatly increased in cigarette smoke exposed mice compared to control air exposed mice. Lungs of mice exposed to cigarette smoke only (i.e. no NTHI) exhibit mild inflammatory changes but completely lack marked lymphocytic infiltration or accumulations of immune cells (Sup. Fig. 1 and data not shown) (33,34). Pulmonary inflammation was scored using a blinded semi-quantitative system by a pathologist to determine whether any differences existed between air and smoke exposure groups (Fig. 1C). Bronchovascular inflammation in air exposed mice was scored primarily as moderate, whereas inflammation in cigarette smoke exposed mice was scored as marked. The extent of pleural and interstitial inflammation was equivalent in both groups. Thus, though the localization pattern of infiltrating cells was similar between air and cigarette smoke exposed mice, the extent of infiltration was substantially greater following cigarette smoke exposure, indicating that this insult plays an important role in the subsequent respiratory response to bacterial infection.
FIGURE 1.
Cigarette smoke exposure exacerbates NTHI-mediated chronic respiratory inflammation. (A) C57BL/6J female mice were exposed for 4 weeks to cigarette smoke or air, followed by 8 weeks of NTHI (n=10 air + NTHI; n=8 cigarette smoke + NTHI). (B) H&E stained lung sections prepared after combined inflammatory insult were evaluated for the extent and severity of inflammation. Lymphocytic cuffs are present adjacent to vasculature (arrows) and airways (arrowheads). Bars represent 100 µm. (C) Consensus scores from two blinded non-consecutive sessions evaluating respiratory inflammation in peribronchial, pleural, and interstitial regions of the lung.
The role and contribution of cigarette smoke to the immune cell composition (Fig. 2A) and cytokine production (Fig. 2B) from bronchoalveolar lavage (BAL) fluid was also assessed. Air exposed mice exhibited a combination of high lymphocytic accumulation and low neutrophil presence observed in the BAL following NTHI exposure, which is characteristic of a chronic inflammatory environment. In contrast, the frequency and number of neutrophils was elevated in the airways of cigarette smoke exposed mice compared to air exposed mice and lymphocyte levels were significantly decreased (Fig. 2A).
FIGURE 2.
Cigarette smoke exposure modulates airway immune cell composition and cytokine profile. (A) Bronchoalveolar lavage (BAL) fluid was evaluated for the composition of immune cells accumulating within airways following 8 weeks of NTHI-mediated chronic respiratory inflammation (n=10 air + NTHI; n=8 cigarette smoke + NTHI). Frequency and number of neutrophils, macrophages, and lymphocytes was determined by Wright-Giemsa differential staining. (B) Concentration of cytokines in BAL fluid was determined by ELISA. Line represents mean; *p<0.05, **p<0.01, ***p<0.001 two-tailed unpaired t-test.
Levels of IL-1β, IL-6, and TNF-α in BAL fluid was evaluated as hallmarks of an inflammatory response to bacterial challenge in the lung. IFN-γ, IL-4, and IL-17 were evaluated to determine the relative levels of Th1/Th2/Th17 cytokine production in response to chronic NTHI infection. Levels of pro-inflammatory IL-1β, IL-6, and TNF-α were elevated in cigarette smoke exposed mice compared to air-exposed mice (Fig. 2B), corroborating the histopathological phenotype of enhanced pulmonary inflammation. Additionally, prior smoke exposure modulated the levels of IFN-γ and IL-17, cytokines that are associated with Th1 and Th17 inflammatory responses. While IFN-γ was present at lower levels in BAL fluid from cigarette smoke exposed mice, IL-17 levels were elevated (Fig. 2B). Collectively, these findings demonstrate that the milieu in the lungs of cigarette smoke exposed mice promotes sustained chronic inflammation and hinders the accumulation of immune mediators and lymphocytes designed to combat bacterial infection.
Prior cigarette smoke exposure suppresses the adaptive immune response
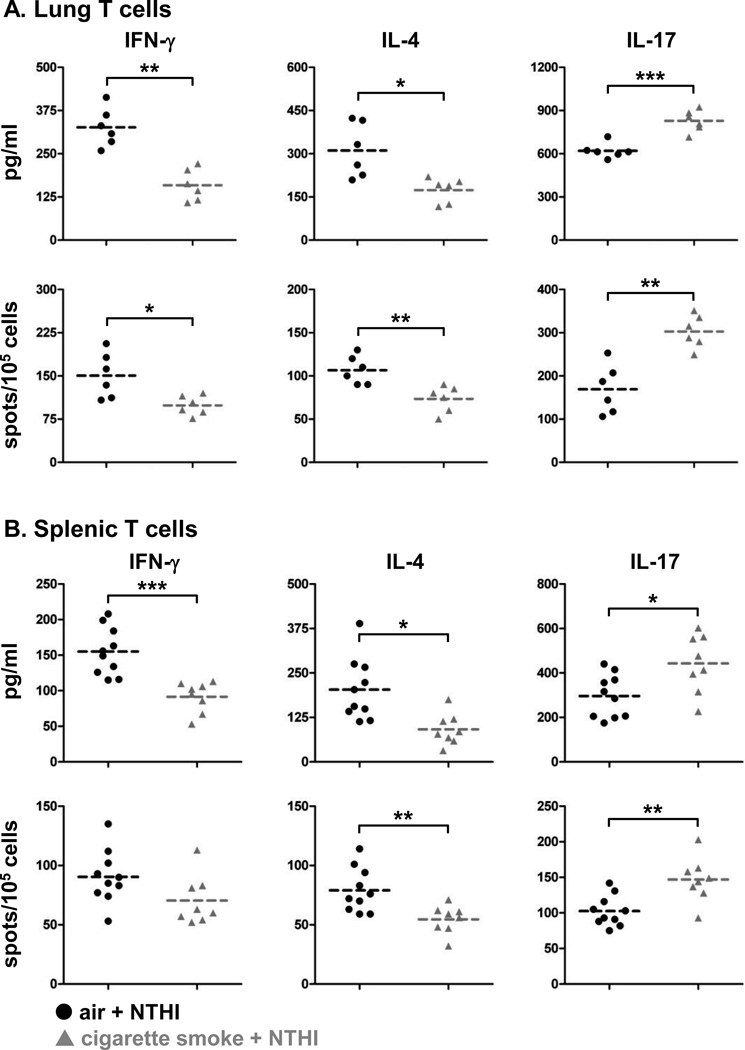
As cigarette smoke exposure increased NTHI-mediated chronic respiratory inflammation, we also assessed whether this inflammatory insult impacted the generation and function of NTHI-specific adaptive immune responses. The outer membrane lipoprotein P6 of NTHI is highly conserved and immunogenic and has an immunodominant T cell epitope. We used the P641–55 peptide to measure the frequency of cytokine-secreting P6-specific T cells and the level of cytokines produced from lung-infiltrating lymphocytes (Fig. 3A) and the spleen (Fig. 3B) of air and cigarette smoke exposed mice. T cells isolated from the lungs and spleen exhibited lower levels of IFN-γ and IL-4 secretion but higher levels of IL-17 (Fig. 3A, B). Importantly, the profile of Th1, Th2, and Th17 P6-specific T cells in the lungs of cigarette smoke exposed mice (Fig. 3A) resembled that of the BAL cytokine profile (Fig. 2B). The frequency of Th17 cells and levels of IL-17 production were significantly elevated in lung lymphocytes from cigarette smoke exposed mice compared to air exposed mice. IFN-γ and IL-4 producing T cells from the lungs and the spleen were decreased (Fig. 3A, B); demonstrating that anti-bacterial T cell immunity and development of helper cells for anti-NTHI antibody responses are dysregulated by prior cigarette smoke exposure.
FIGURE 3.
P6-specific T cells with Th17 signature are elevated in cigarette smoke exposed mice. Single-cell suspensions of (A) lung lymphocytes and (B) splenocytes were isolated from air or cigarette smoke exposed mice and incubated with P641–55 peptide pulsed APCs. Amount of cytokine secreted was measured by ELISA and frequency of cytokine secreting T cells was measured by ELISPOT. Line represents mean; *p<0.05, **p<0.01, ***p<0.001 two-tailed unpaired t-test.
T cell-derived IL-4 is required for antibody class switching to IgG1, while IFN-γ is required for class switching to IgG2a. Because the number of T cells secreting these cytokines was reduced in cigarette smoke exposed mice (Fig. 3A, B), we also evaluated B cell responses. The frequency of P6-specific IgG1- and IgG2a-secreting B cells from the bone marrow and spleen was measured by B cell ELISPOT (Fig. 4). We observed a significant decrease in the frequency of anti-P6 Ig-secreting cells for both IgG subclasses in cigarette smoke exposed mice compared to control mice. Although the presence of both IgG1 and IgG2a subclasses indicates that class switching did occur, it was clearly less efficient in mice previously exposed to cigarette smoke.
FIGURE 4.
Frequency of antibody-secreting NTHI-specific cells is decreased in cigarette smoke exposed mice. B cell ELISPOTs were performed to quantify frequency of P6-specific IgG1- and IgG2a-secreting cells from bone marrow (top row) and spleens (bottom row) of air or cigarette smoke exposed mice. Line represents mean; *p<0.05, **p<0.01 two-tailed unpaired t-test.
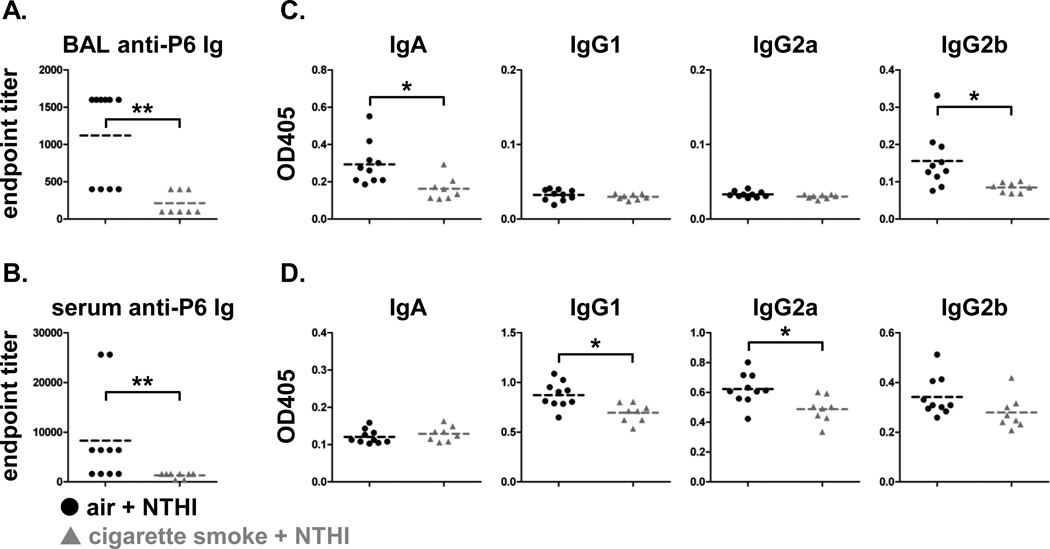
Anti-P6 antibodies in BAL fluid and sera were also measured to evaluate whether alterations in B cell frequency affected antibody responses. Total anti-P6 antibody levels were significantly lower in the BAL and sera of cigarette smoke exposed mice (Fig. 5A, B). In both groups, BAL fluid contained only IgA and IgG2b anti-P6 antibodies, and once again, these levels were significantly lower in cigarette smoke exposed mice (Fig. 5C). Serum levels of IgG1 and IgG2a anti-P6 antibodies were also significantly reduced with prior smoke exposure (Fig. 5D). Collectively, the diminished anti-NTHI T cell and B cell responses observed present a complete profile of the dysfunctional adaptive immune response to a chronic pathogen infection following cigarette smoke exposure.
FIGURE 5.
Cigarette smoke exposure modulates accumulation of anti-P6 Ig in airways and serum. Levels of anti-P6 Ig were measured in (A) BAL fluid and (B) serum from air or cigarette smoke exposed mice receiving 8 weeks of chronic NTHI exposure. OD values at 405 nm for (C) BAL fluid dilutions at 10−2.6 (1:400) and (D) sera dilutions at 10−3.2 (1:1600) were analyzed for levels of P6-specific IgA, IgG1, IgG2a, and IgG2b. Line represents mean; *p<0.05, **p<0.01 two-tailed unpaired t-test.
Efficacy of immunization is reduced following cigarette smoke exposure
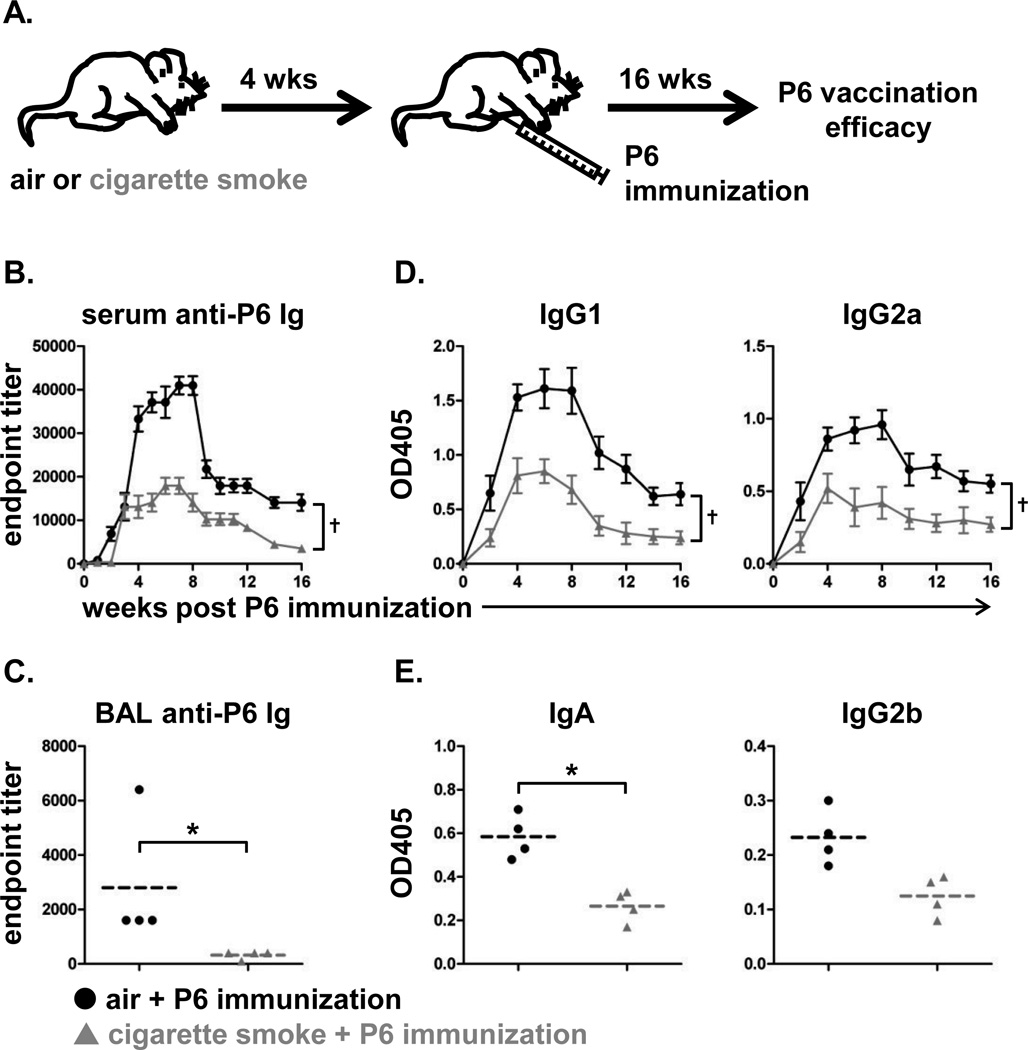
The altered immune response elicited to NTHI in smoke exposed mice prompted us to evaluate whether smoke exposure also impacted the immune response to a candidate vaccine antigen. Control and cigarette smoke exposed mice were immunized with purified P6 antigen; vaccination efficacy was measured by anti-P6 antibody titers (Fig. 6A). Not only were the kinetics of anti-P6 antibody appearance slower in cigarette smoke exposed mice, the magnitude of the antibody titers in the sera were substantially lower in comparison to control mice (Fig. 6B). Antibody titers were also significantly reduced in the BAL fluid of cigarette smoke exposed mice after 16 weeks (Fig. 6C). Serum antibodies were predominantly of the IgG1 and IgG2a subclasses in both groups of mice, but, consistent with total antibody titers, cigarette smoke exposure reduced the levels of these anti-P6 isotypes as well (Fig. 6D). Anti-P6 of IgA isotype was present in the BAL but at significantly lower levels in cigarette smoke exposed mice compared to air exposed mice (Fig. 6E).
FIGURE 6.
Immunization efficacy is compromised in cigarette smoke exposed mice. (A) C57BL/6 female mice were immunized i.p. with 40 µg lipoprotein P6 from NTHI outer membrane after receiving 4 weeks of air or cigarette smoke exposure (n=10 air + NTHI; n=9 cigarette smoke + NTHI). Vaccination efficacy was measured 16 weeks after i.p. immunization. Levels of anti-P6 Ig were measured from (B) weekly serum samples and (C) BAL fluid obtained at termination. (D) OD values at 405 nm for sera were determined for levels of P6-specific IgG1 and IgG2a. (E) OD values at 405 nm for BAL fluid was analyzed for levels of P6-specific IgA and IgG2b. Mean ± SD; †p<0.05 AUC Kruskal-Wallis rank test. Line represents mean; *p<0.05 two-tailed unpaired t-test.
Because antibody titers were reduced following immunization of cigarette smoke exposed mice, we determined the frequency of P6-specific B cells and T cells by ELISPOT. The frequency of P6-specific antibody-secreting cells was lower in the bone marrow and spleen of P6-immunized cigarette smoke exposed mice compared to control mice (Sup. Fig. 2). The frequency of P6-specific T cells secreting IFN-γ or IL-4 was also significantly reduced in cigarette smoke exposed mice, confirming that prior smoke exposure impairs Th1 and Th2 responses and class switching (Sup. Fig. 3). Interestingly, the number of IL-17 expressing T cells was unaffected by prior smoke exposure. Thus, in cigarette smoke exposed mice, the inability to elicit robust Th1 and Th2 immunity following vaccination likely hinders the generation of protective responses against pathogen infection.
We reasoned that though the immunization efficacy was substantially weaker in cigarette smoke exposed mice, this level of protection might still be effective against an acute NTHI challenge. To test this, air and cigarette smoke exposed mice were immunized with P6 and challenged with live bacteria 16 weeks later. Sham immunized air and cigarette smoke exposed mice served as controls to evaluate the effectiveness of P6 immunization on mitigating the hallmarks of acute respiratory inflammation 4 and 24 hrs after NTHI challenge (Fig. 7A). The kinetics of NTHI clearance from the lungs was more rapid in P6 immunized mice (●,▲) compared to sham immunized (○,∆) mice regardless of cigarette smoke exposure (Fig. 7B), establishing that the attenuated immune response elicited in cigarette smoke exposed mice was still effective in clearing bacteria from the lungs. However, the beneficial effect of immunization was clearly demonstrated in that bacterial clearance rates in P6 immunized cigarette smoke exposed mice were similar to those in sham immunized air exposed mice. Thus, immunization was able to mitigate the negative impact of cigarette smoke exposure.
FIGURE 7.
P6 immunization in cigarette smoke exposed mice marginally mitigates acute NTHI-mediated inflammation. (A) Hallmarks of acute inflammation were measured in air or cigarette smoke exposed mice 16 weeks after P6 immunization or sham immunization. Kinetics of acute inflammation was evaluated at baseline and 4 or 24 hrs post NTHI challenge. (B) Rate of NTHI clearance in the lung was measured by colony-plating assay. (C) Accumulation of neutrophils in BAL determined by Wright-Giemsa differential staining. (D-G) Concentration of albumin and pro-inflammatory cytokines in BAL fluid determined by ELISA. n=3 mice per group per time point; mean ± SD; p<0.01 2-way ANOVA, *p<0.05 Bonferroni post-test comparison of air vs cigarette smoke.
Although clearance rates of NTHI were compromised in cigarette smoke exposed mice, the influx of neutrophils was elevated compared to air exposed mice regardless of immunization status (Fig. 7C). Despite the increased neutrophil infiltration in the lungs, clearance of NTHI was not improved, suggesting that the phagocytic function of the neutrophils was compromised by prolonged cigarette smoke exposure. The combination of decreased bacterial clearance and increased neutrophil influx resulted in increased lung epithelial damage, which was quantified by measuring BAL fluid albumin leak as a surrogate marker of lung damage. P6 immunized and sham immunized cigarette smoke exposed mice had higher levels of albumin in the BAL compared to air-exposed mice (Fig. 7D). Evaluation of pro-inflammatory cytokines in the BAL fluid revealed that elevated levels of IL-1β, IL-6, and TNF-α were not sustained in P6 immunized mice post NTHI challenge, whereas their concentration remained elevated in sham immunized mice (Fig. 7E–G). Immunization of cigarette smoke exposed mice reduced the level of pro-inflammatory cytokines to below that of sham immunized mice but not to level of air-exposed, immunized mice. Thus, while prior smoke exposure promotes increased inflammation and impairs the immune response, P6 immunization mitigated the negative impact of cigarette smoke exposure on bacterial clearance, lung inflammation and the adaptive immune response.
DISCUSSION
COPD patients suffer from chronic inflammation resulting from prolonged cigarette smoke exposure combined with repeated bouts of respiratory infections. We have established a novel model of chronic smoke exposure followed by chronic infection to address this knowledge gap and to determine how cigarette smoke exposure impacts the generation of adaptive immunity following exposure to the bacteria and vaccination against a key antigen. A previous study by Gaschler et al evaluated the inflammatory response in the lungs of female C57BL/6 and BALB/c mice that had been exposed to air or cigarette smoke and subsequently infected with a single NTHI challenge (35). As such, their study design queried only the innate immune response, primarily mediated by neutrophils, monocytes and NK cells. They demonstrated that cigarette smoke exposure led to decreased bacterial clearance and increased acute (24 hr) inflammation. In contrast, by using multiple repeated NTHI challenges over 8 weeks, this study queries the adaptive immune response (B and T cells) in addition to the innate response. As a result, our study has conclusively demonstrated for the first time that cigarette smoke exposure has a negative effect on the generation of adaptive immune responses to NTHI, a respiratory pathogen known to colonize the lungs of smokers with COPD. In addition, NTHI-mediated lung inflammation was worsened in cigarette smoke exposed mice, a pathological feature frequently observed in COPD patients who have experienced recurrent bacterial respiratory infections. Importantly, this deficiency in adaptive immunity could be rescued by immunization. Although immunization only raised the immune response of smoke-exposed mice to that of non-exposed, non-immunized mice, that level of immunity may be sufficient to protect COPD patients from the detrimental effects of repeated exacerbation.
While it was previously reported that smoke exposure impaired acute clearance of a single bacterial infection, here we have provided convincing evidence that increased pulmonary inflammation arising from chronic cigarette smoke exposure and bacterial infection is compounded by the host’s inability to mount an effective immune response against the bacteria. Although we did not measure bacterial clearance in the initial experiment, we note that bacterial clearance was impaired in the immunization study in mice that were exposed to smoke and received sham immunization (Fig. 7). Since this challenge was 16 weeks after smoke exposure, we think it probable that clearance was also impaired in this experiment 8 weeks after exposure. In this way, our results are consistent with but also extend those of Gaschler et al, as we have demonstrated defects not only in clearance, but also in B and T cell function. Intriguingly, these deficits in adaptive immune function persisted up to 16 weeks after the final smoke exposure, demonstrating that tobacco smoke exposure has dramatic and long-term effects on immune cell function even after smoking cessation.
Cigarette smoke is known to be immunosuppressive, although the exact mechanisms are not clearly understood (6,27,28,30,36). Here we show that chronic cigarette smoke exposure followed by chronic infection results in decreased levels of Th1 and Th2 cytokines and decreased T cell and B cell function, but an increased IL-17 response; IL-17 is associated with maintenance of chronic inflammation (37,38). Thus, cigarette smoke exposure skews the T cell response away from productive responses that could clear bacterial infections and toward a response that sustains chronic inflammation. Exaggerated IL-17 production in the lung hastens bronchoconstriction and asthmatic symptoms (39). Though little is known about the role of Th17 cells in COPD, these cells have been identified in lung biopsies from COPD patients and IL-17 has been detected in the sputum from patients during disease exacerbations (40). Chronic cigarette smoke exposure can tip the balance of Th17 and Tregs by decreasing Foxp3 and IL-10 expression while simultaneously increasing ROR-γT and IL-17 expression (41). Here, we also show that prior smoke exposure increased production of IL-6, a cytokine that favors Th17 cells (35). One mechanistic explanation for the altered immune response may involve the aryl hydrocarbon receptor (AhR). Cigarette smoke contains multiple AhR ligands and activates AhR-dependent transcription (42), while Chen and colleagues have shown that AhR-deficient mice are incapable of generating Th17 cells (43). Thus, AhR ligands found in cigarette smoke may play a role in driving Th17 differentiation. Taken together, these findings point to the IL-17 signaling axis as a key player in the sustenance of chronic inflammation in COPD and demonstrates that cigarette smoke exposure skews T cell responses.
Prior smoke exposure significantly reduced levels of IFN-γ in BAL, splenic and lung P6-specific T cells, and IL-4 in splenic and lung T cells but not BAL. Although BAL fluid levels of IL-4 were similar in air and cigarette smoke exposed mice, P6-specific T cell production of this cytokine is reduced in cigarette smoke exposed mice. IL-4 measured in BAL fluid by ELISA does not allow us to elucidate the source of the cytokine; thus, it could have been produced by lymphocytes and possibly alveolar macrophages (44). As this cytokine is primarily required for B cell activation, it is likely that IL-4 is required in lymphoid organs rather than at the site of inflammation. The failure of P6-specific T cells to produce IL-4 is thus implicated as one of the causes for the deficit in antibody production, as P6-specific T cells are unable to provide appropriate helper signals to NTHI-specific B cells at the level of the immunological synapse.
In our model, mice previously exposed to cigarette smoke displayed increased levels of neutrophils after chronic infection, compared to air exposed mice. Our observation of the persistence of neutrophils in the lungs of the cigarette smoke exposed mice compared to air exposed mice is a novel finding, as neutrophils are typically short-lived and have a high turnover rate. A potential reason for their continued presence may be due to impaired cell death of the neutrophils. Cigarette smoke hinders spontaneous neutrophil cell death by blocking Akt deactivation via suppression of diphosphoinositol pentakisphosphate production (45), thus dysregulation of neutrophil cell death may account for their persistence in the lungs and contribute to the chronic inflammation mediated by cigarette smoke.
Cigarette smoke has additional immunosuppressive effects. For example, cigarette smoke inhibits bacterial phagocytosis by alveolar macrophages (46) and reduces macrophage expression of toll-like receptor 2 (TLR2) (47). We have previously shown that TLR2 is essential for the generation of NTHI-specific adaptive immune responses (48). Thus, altering the ability of phagocytic cells to sense and clear bacteria represents a second immunosuppressive mechanism of prior chronic smoke exposure. Our results show that mice exposed to chronic infection develop heightened inflammation and are unable to efficiently generate an adaptive immune response. This likely explains the susceptibility of COPD patients to exacerbations.
Repeated infections from newly acquired strains of NTHI further exacerbate the poor lung function in COPD patients, thus it is critical to induce robust, long-lived immune responses that prevent bacterial colonization and further respiratory inflammation. Here, we evaluated a candidate vaccine antigen selected based on our previous work describing the immunogenic and protective potential of the lipoprotein P6 (26). Although anti-P6 responses were diminished in the cigarette smoke exposed mice, the vaccine was still protective, and conferred immunity similar to that of unimmunized mice never exposed to smoke. An interesting aspect of this immunization was the decreased amount of P6-specific IgA in the lungs of cigarette smoke exposed mice, suggesting that the chronic cigarette smoke exposure may have altered the bioavailability or transport of this antibody in the respiratory mucosa. As the presence of specific IgA in the lungs is critical for minimizing pathogen colonization and biofilm formation, this represents a third mechanism by which chronic cigarette smoke exposure impairs effective immune responses to lung infection.
Corticosteroid treatment is used in COPD patients to reduce exacerbations and improve health outcomes (49). Unfortunately, this treatment option is immunosuppressive and is associated with increased bacterial-mediated pneumonia (50). New strategies are needed to prevent COPD exacerbations and associated increased mortality. Our results demonstrate that immunization with an appropriate antigen can overcome some of the immune defects associated with chronic cigarette smoke exposure, not only restoring T cell and B cell responses but also moderating tissue inflammation. However, in light of the clinical use of corticosteroids in this patient population, future studies will need to investigate the impact of corticosteroids on anti-NTHI immunization efficacy.
Overall, our study shows that the altered inflammatory profile induced by cigarette smoke has profound long-term negative consequences on the host’s ability to respond to respiratory pathogens and provides an understanding of the persistent and progressive nature of COPD. It also lays the groundwork for the design of therapeutic interventions in COPD patients that should take into consideration its impact on adaptive immunity against NTHI, a main cause of devastating COPD exacerbations.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Wade Narrow and Kristina Owens for technical assistance in performing the cigarette smoke exposures.
This work was supported by the RPCI/URMC Wilmot Cancer Center Joint Project funds (YT, RPP). This work was supported in part by AI069379 (YT), P50CA090440 (SPORE developmental funds to YT), P30ES01247, R01HL088325 (RPP, PJS), and by award number 8UL1TR000042 from the National Center for Advancing Translational Science to the University of Rochester (RPP, PJS, THT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ABBREVIATIONS USED IN THIS ARTICLE
- BAL
bronchoalveolar lavage
- COPD
chronic obstructive pulmonary disease
- NTHI
non-typeable Haemophilus influenzae
- P6
outer membrane lipoprotein P6
- TPM
total particulate matter
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 3.Carolan BJ, Sutherland ER. Clinical phenotypes of chronic obstructive pulmonary disease and asthma: recent advances. J. Allergy Clin. Immunol. 2013;131:627–634. doi: 10.1016/j.jaci.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Spencer P, Krieger B. The differentiation of chronic obstructive pulmonary disease from asthma: a review of current diagnostic and treatment recommendations. Open. Nurs. J. 2013;7:29–34. doi: 10.2174/1874434601307010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blanc PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ. Health. 2005;4:7. doi: 10.1186/1476-069X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat. Rev. Immunol. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 7.John G, Kohse K, Orasche J, Reda A, Schnelle-Kreis J, Zimmermann R, Schmid O, Eickelberg O, Yildirim AO. The composition of cigarette smoke determines inflammatory cell recruitment to the lung in COPD mouse models. Clin. Sci. (Lond) 2013 doi: 10.1042/CS20130117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. World Health Statistics 2012. Geneva: WHO Press; 2013. [Google Scholar]
- 9.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117:5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 10.Murphy TF. Respiratory infections caused by non-typeable Haemophilus influenzae. Curr. Opin. Infect. Dis. 2003;16:129–134. doi: 10.1097/00001432-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Murphy TF. The role of bacteria in airway inflammation in exacerbations of chronic obstructive pulmonary disease. Curr. Opin. Infect. Dis. 2006;19:225–230. doi: 10.1097/01.qco.0000224815.89363.15. [DOI] [PubMed] [Google Scholar]
- 12.Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El-Ebiary M, Hernandez C, Rodriguez-Roisin R. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am. J. Respir. Crit Care Med. 1998;157:1498–1505. doi: 10.1164/ajrccm.157.5.9711044. [DOI] [PubMed] [Google Scholar]
- 13.Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 2001;14:336–363. doi: 10.1128/CMR.14.2.336-363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 15.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 16.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostberg KL, Russell MW, Murphy TF. Mucosal immunization of mice with recombinant OMP P2 induces antibodies that bind to surface epitopes of multiple strains of nontypeable Haemophilus influenzae. Mucosal. Immunol. 2009;2:63–73. doi: 10.1038/mi.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novotny LA, Clements JD, Bakaletz LO. Kinetic analysis and evaluation of the mechanisms involved in the resolution of experimental nontypeable Haemophilus influenzae-induced otitis media after transcutaneous immunization. Vaccine. 2013;31:3417–3426. doi: 10.1016/j.vaccine.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong W, Peng D, Rivera M, Gu XX. Protection against nontypeable Haemophilus influenzae challenges by mucosal vaccination with a detoxified lipooligosaccharide conjugate in two chinchilla models. Microbes. Infect. 2010;12:11–18. doi: 10.1016/j.micinf.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy TF, Kirkham C, Lesse AJ. Construction of a mutant and characterization of the role of the vaccine antigen P6 in outer membrane integrity of nontypeable Haemophilus influenzae. Infect. Immun. 2006;74:5169–5176. doi: 10.1128/IAI.00692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon M, Murphy TF, Kyd J, Thanavala Y. Role of an immunodominant T cell epitope of the P6 protein of nontypeable Haemophilus influenzae in murine protective immunity. Vaccine. 2005;23:3590–3596. doi: 10.1016/j.vaccine.2005.01.151. [DOI] [PubMed] [Google Scholar]
- 22.Ishida Y, Abe Y, Yanai M, Kobayashi H, Harabuchi Y. Identification of human T-cell epitopes and highly immunogenic analog peptides on the non-typeable Haemophilus influenzae P6 outer membrane protein. Clin. Immunol. 2006;121:90–99. doi: 10.1016/j.clim.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Nomura Y, Abe Y, Ishida Y, Kobayashi H, Harabuchi Y. Promiscuous peptides on the nontypeable Haemophilus influenzae P6 outer membrane protein. J. Clin. Immunol. 2008;28:361–369. doi: 10.1007/s10875-008-9189-0. [DOI] [PubMed] [Google Scholar]
- 24.Abe Y, Murphy TF, Sethi S, Faden HS, Dmochowski J, Harabuchi Y, Thanavala YM. Lymphocyte proliferative response to P6 of Haemophilus influenzae is associated with relative protection from exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med. 2002;165:967–971. doi: 10.1164/ajrccm.165.7.2109009. [DOI] [PubMed] [Google Scholar]
- 25.Badr WH, Loghmanee D, Karalus RJ, Murphy TF, Thanavala Y. Immunization of mice with P6 of nontypeable Haemophilus influenzae: kinetics of the antibody response and IgG subclasses. Vaccine. 1999;18:29–37. doi: 10.1016/s0264-410x(99)00166-8. [DOI] [PubMed] [Google Scholar]
- 26.Lugade AA, Bianchi-Smiraglia A, Pradhan V, Elkin G, Murphy TF, Thanavala Y. Lipid motif of a bacterial antigen mediates immune responses via TLR2 signaling. PLoS. One. 2011;6:e19781. doi: 10.1371/journal.pone.0019781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herr C, Beisswenger C, Hess C, Kandler K, Suttorp N, Welte T, Schroeder JM, Vogelmeier C. Suppression of pulmonary innate host defence in smokers. Thorax. 2009;64:144–149. doi: 10.1136/thx.2008.102681. [DOI] [PubMed] [Google Scholar]
- 28.Thatcher TH, Benson RP, Phipps RP, Sime PJ. High-dose but not low-dose mainstream cigarette smoke suppresses allergic airway inflammation by inhibiting T cell function. Am. J. Physiol Lung Cell Mol. Physiol. 2008;295:L412–L421. doi: 10.1152/ajplung.00392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu W, Patel KB, Booth JL, Zhang W, Metcalf JP. Cigarette smoke extract suppresses the RIG-I-initiated innate immune response to influenza virus in the human lung. Am. J. Physiol Lung Cell Mol. Physiol. 2011;300:L821–L830. doi: 10.1152/ajplung.00267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsland BJ, Konigshoff M, Saglani S, Eickelberg O. Immune system dysregulation in chronic lung disease. Eur. Respir. J. 2011;38:500–501. doi: 10.1183/09031936.00103211. [DOI] [PubMed] [Google Scholar]
- 31.Heijink IH, Bruin HGde, van den Berge M, Bennink LJ, Brandenburg SM, Gosens R, Oosterhout AJvan, Postma DS. Role of aberrant WNT signalling in the airway epithelial response to cigarette smoke in chronic obstructive pulmonary disease. Thorax. 2013;68:709–716. doi: 10.1136/thoraxjnl-2012-201667. [DOI] [PubMed] [Google Scholar]
- 32.Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am. J. Pathol. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thatcher TH, Hsiao HM, Pinner E, Laudon M, Pollock SJ, Sime PJ, Phipps RP. Neu-164 and Neu-107, two novel antioxidant and anti-myeloperoxidase compounds, inhibit acute cigarette smoke-induced lung inflammation. Am. J. Physiol Lung Cell Mol. Physiol. 2013;305:L165–L174. doi: 10.1152/ajplung.00036.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lugade AA, Bogner PN, Thanavala Y. Murine model of chronic respiratory inflammation. Adv. Exp. Med. Biol. 2011;780:125–141. doi: 10.1007/978-1-4419-5632-3_11. [DOI] [PubMed] [Google Scholar]
- 35.Gaschler GJ, Skrtic M, Zavitz CC, Lindahl M, Onnervik PO, Murphy TF, Sethi S, Stampfli MR. Bacteria challenge in smoke-exposed mice exacerbates inflammation and skews the inflammatory profile. Am. J. Respir. Crit Care Med. 2009;179:666–675. doi: 10.1164/rccm.200808-1306OC. [DOI] [PubMed] [Google Scholar]
- 36.van Rijt SH, Keller IE, John G, Kohse K, Yildirim AO, Eickelberg O, Meiners S. Acute cigarette smoke exposure impairs proteasome function in the lung. Am. J. Physiol Lung Cell Mol. Physiol. 2012;303:L814–L823. doi: 10.1152/ajplung.00128.2012. [DOI] [PubMed] [Google Scholar]
- 37.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez-Velazquez C, Castillo EC, Guido-Bayardo L, Ortiz-Navarrete V. IL-17-producing peripheral blood CD177+ neutrophils increase in allergic asthmatic subjects. Allergy Asthma Clin. Immunol. 2013;9:23. doi: 10.1186/1710-1492-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Chu S, Zhong X, Lao Q, He Z, Liang Y. Increased expression of CD4+IL-17+ cells in the lung tissue of patients with stable chronic obstructive pulmonary disease (COPD) and smokers. Int. Immunopharmacol. 2013;15:58–66. doi: 10.1016/j.intimp.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Peng W, Weng Y, Ying H, Li H, Xia D, Yu W. Imbalance of Th17/Treg cells in mice with chronic cigarette smoke exposure. Int. Immunopharmacol. 2012;14:504–512. doi: 10.1016/j.intimp.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, Phipps RP. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am. J. Physiol Lung Cell Mol. Physiol. 2005;289:L391–L399. doi: 10.1152/ajplung.00062.2005. [DOI] [PubMed] [Google Scholar]
- 43.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, Zheng M. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS. One. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Flamme AC, Kharkrang M, Stone S, Mirmoeini S, Chuluundorj D, Kyle R. Type II-activated murine macrophages produce IL-4. PLoS. One. 2012;7:e46989. doi: 10.1371/journal.pone.0046989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y, Li H, Bajrami B, Kwak H, Cao S, Liu P, Zhou J, Zhou Y, Zhu H, Ye K, Luo HR. Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7726–7731. doi: 10.1073/pnas.1302906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J. Infect. Dis. 2006;194:1375–1384. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 47.Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir. Res. 2005;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lugade AA, Bogner PN, Murphy TF, Thanavala Y. The role of TLR2 and bacterial lipoprotein in enhancing airway inflammation and immunity. Front Immunol. 2011;2:10. doi: 10.3389/fimmu.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roche N, Marthan R, Berger P, Chambellan A, Chanez P, Aguilaniu B, Brillet PY, Burgel PR, Chaouat A, Devillier P, Escamilla R, Louis R, Mal H, Muir JF, Perez T, Similowski T, Wallaert B, Aubier M. Beyond corticosteroids: future prospects in the management of inflammation in COPD. Eur. Respir. Rev. 2011;20:175–182. doi: 10.1183/09059180.00004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am. J Respir. Crit Care Med. 2007;176:162–166. doi: 10.1164/rccm.200611-1630OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.