Abstract
We describe a general mass spectrometry approach to determine subunit stoichiometry and lipid binding in intact membrane protein complexes. By exploring conditions for preserving interactions during transmission into the gas phase and for optimally stripping away detergent, by subjecting the complex to multiple collisions, we release the intact complex largely devoid of detergent This enabled us to characterize both subunit stoichiometry and lipid binding in 4 membrane protein complexes.
Membrane proteins perform a wide range of biological functions including respiration, signal transduction and molecular transport. Despite their obvious importance, these proteins and their complexes remain notoriously difficult to study. High-resolution methods, such as X-ray crystallography, require recombinant expression and crystallization of proteins, both of which are difficult for membrane subunits. Low-resolution methods to characterize membrane protein complexes include blue native gel electrophoresis1, sedimentation equilibrium or sedimentation velocity measurements via analytical ultracentrifugation2 and small-angle X-ray scattering3. Although excellent results regarding the size of membrane proteins can be obtained, large quantities of protein are usually required, and it is essential to account for the contribution of the micelle to the overall mass. It can also be difficult to obtain definitive data on large or unstable complexes that do not form single oligomeric states in detergent solutions. Moreover the low accuracy of ultracentrifugation or X-ray scattering data does not normally reveal lipid binding or allow observation of post-translational modifications.
Recently, using nano-electrospray mass spectrometry, we had demonstrated transfer of the intact BtuC2D2 complex, containing both cytoplasmic and transmembrane subunits, into the gas phase, where we had observed cooperative binding of ATP4. The rationale behind our method was to project the intact membrane protein complex into the gas phase within a protective detergent micelle, by maintaining concentrations of a nonionic detergent, β-d-dodecylmaltoside (DDM), above the critical micelle concentration. We then subjected the protein micelle complex to gas phase collisions, activating the detergent and causing its release. This leaves the protein complex largely devoid of loosely associated small molecules but with protein subunit and lipid binding interactions intact. The atomic structure and nucleotide binding properties of BtuC2D2 have been previously reported using crystallography5. Here, to expand the method we reported for BtuC2D2 to make it into a generally applicable approach, we targeted three complexes whose structures are not known and one which is controversial (EmrE)6. All four are representative of the two major classes of transporters: two ATP binding cassette (ABC) transporters, MacB7 and LmrCD8, and two secondary active transporters or proton-driven antiporters, the small multidrug resistance transporter EmrE9 and the resistance-nodulation division transporter MexB10.
The ABC transporter MacB is part of a tripartite complex used by Gram-negative bacteria to translocate drugs and protein toxins across inner and outer membranes. LmrCD, an ABC transporter from Lactococcus lactis, is composed of two half transporters LmrC and LmrD. Its drug extrusion activity and ATP hydrolysis are dependent on the presence of both subunits forming a heterodimer containing two dissimilar nucleotide-binding domains11. The smallest of the transporters we examined here, EmrE (a 24 kDa dimer), confers multidrug resistance by H+-linked drug efflux across the bacterial cytoplasmic membrane. Its structure has been controversial, owing to questions about its oligomeric state and its overall topology6. The largest of the complexes we considered here, MexB, is part of the MexAB-OprM efflux system, which is central to the multidrug resistance of Pseudomonas aeruginosa. In this tripartite complex, MexB is the inner membrane–spanning drug efflux protein, of unknown stoichiometry and structure, that functions with the outer membrane factor OprM and the periplasmic membrane fusion protein, MexA.
To optimize solution conditions for our method, we examined eight membrane complexes at a concentration of ~15 μM and varied the ratio of the nonionic detergents DDM or decyl maltoside (DM) (Supplementary Table 1). Our results showed that provided that the concentration of detergent did not fall below the critical micelle concentration, the intact complex could be detected by mass spectrometry. The optimal detergent:protein molar ratio, in terms of the overall quality of the mass spectra, was ≤100:1.
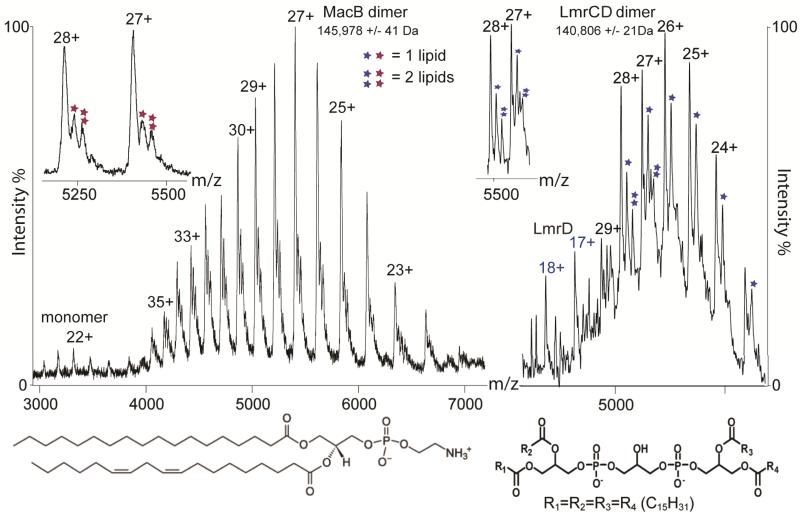
We also explored mass spectrometry parameters that are critical for controlled removal of detergent from these membrane complexes (Supplementary Fig. 1). By computing an ‘activation coefficient’, derived from the cone and collision voltages, and plotting this against the molecular mass we found that the activation required to release the complex from the micelle is proportional to the mass of the complex. Comparing this relationship with the analogous one for soluble complexes showed that this parameter is notably higher for membrane complexes than soluble complexes, in accord with a protective role for micelles in the gas phase4. We illustrated the effects of increasing activation energy for MacB (Fig. 1 and Supplementary Fig. 2). Well-resolved charge states assigned to the dimer emerged from the micelle with additional small-molecule binding (Supplementary Table 2). We analyzed this small molecule by tandem mass spectrometry, and confirmed the presence of stearic and linoleic acid moieties, allowing us to identify the lipid as phosphatidylethanolamine (Supplementary Fig. 3). The absence of small molecules associated with monomeric MacB series implies that lipid binding occurs predominantly within subunit interfaces.
Figure 1. Subunit stoichiometry and lipid binding for two ABC transporters.
Mass spectra of MacB (left) and LmrCD (right) showing almost exclusively homodimer and heterodimer formation, respectively. Insets, expansion across charge states showing that phosphatidylenthanolamine and cardiolipin bind to the MacB and LmrCD dimers, respectively. Structures of phosphatidylenthanolamine (left) and cardiolipin (right) are shown at the bottom.
We examined the specificity of subunit interactions using the heterodimer LmrCD from L. lactis, purified in DDM micelles. We assigned predominant peaks to the LmrCD heterodimer and also observed extensive small-molecule binding (Fig. 1). The mass of this small molecule (1,352 Da) is consistent with only one assignment in the lipid database, a cardiolipin. Cardiolipin is a natural membrane component of many Gram-negative and Gram-positive bacteria, including L. lactis12. Notably our mass spectrometry data showed no evidence for either LmrC2 or LmrD2 homodimers (Fig. 1). Although we expected this detergent-purified complex to be a heterodimer, formation of either homodimer could not be excluded8. The absence of homodimers in our mass spectrum highlights the fidelity of our method in preserving interactions within the micelle solution.
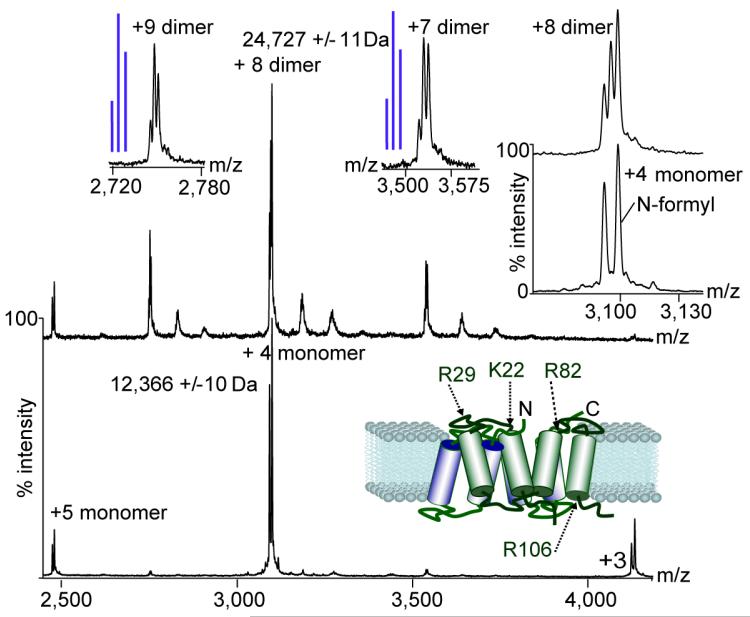
We investigated the use of nonionic detergents with different hydrophobic tails with two versions of EmrE, one with the naturally occurring 27-amino-acid C-terminal tag (EmrE-His) the other with the majority of the tag removed (EmrE-EFE(1–113)). We compared results for different detergent micelles including those formed DM, and combinations of nonylglucoside (NG) and DM. We obtained optimum spectra from micelles containing NG:DM (2.5:1) and DM alone for the truncated and full-length forms of the protein, respectively (Fig. 2 and Supplementary Fig. 4). The mass spectrum of EmrE-EFE(1–113) from NG:DM micelles showed that the dimeric complex dissociates. Expanding the monomeric peaks revealed doublets that we assigned to unmodified EmrE and protein with formylation at an N-terminal methionine (Fig. 2). At lower activation in the same NG:DM micelles, we detected three EmrE dimers: two homodimers with two N-terminally formylated or unformylated EmrE molecules and one heterodimer containing both forms. Based on the abundance of monomeric forms we observed a statistical incorporation of monomers for the 9+ charge state of the dimer (that is, one that is consistent with random incorporation of either monomer; Fig. 2). We could not investigate incorporation of subunits for the 8+ charge state because we cannot rule out contributions from the 4+ monomer with the same mass-to-charge ratio (mz) value. For the 7+ charge state, however, the formylated EmrE dimer was formed in a greater proportions than predicted statistically, consistent with preferential dimer formation from subunits with lower charge. This highlights our ability not only to fine-tune the ratio of different detergents but also to monitor the effects of post-translational modifications on complex formation.
Figure 2. Incorporation of modified subunits in the EmrE dimer.
Mass spectra of EmrE-EFE(1–113) under conditions where interactions are lost (bottom spectrum) and preserved (top spectrum). Schematic representation of the EmrE based on a structure from the Protein Data Bank (PDB) (3B5D)16 shows the location of basic residues. Insets, expansion of the monomeric and dimeric charge states reveals modified (N-terminally formylated) and free protein. Blue lines indicate schematically the peaks intensities calculated for a statistical incorporation of subunits into the dimer.
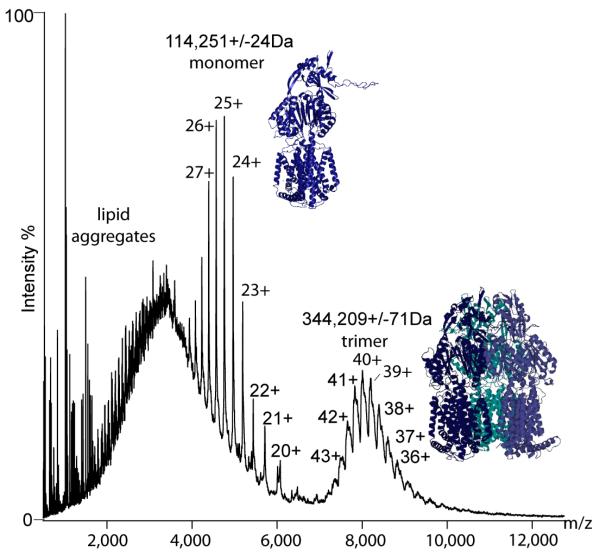
The mass spectrum of MexB showed two clear charge state series corresponding to monomers and trimers (Fig. 3). Peaks assigned to the monomer were well-resolved whereas those of the trimer were broad, suggesting heterogeneous binding of lipids within interfaces. The only previous report of the stoichiometry of MexB had been determined by analytical ultracentrifugation, in which a value of 380–450 kDa13 had been assigned to a trimer (theoretical mass, 342 kDa). This highlights the power of our direct method to determine mass (experimental mass, 344,209 Da), which demonstrates unequivocally that the oligomeric state is exclusively trimeric. Applying the knowledge of the trimeric state together with the considerable sequence identity between MexB and AcrB (70%), we homology-modeled monomeric MexB using 3D-jigsaw14 and docked trimeric MexB using the program Modeller15 (Fig. 3). Our model presents many opportunities, not only for mutagenesis to probe interaction interfaces but also for adding other components (MexA and OprM) and factors that initiate and stabilize assembly of the intact functional transporter.
Figure 3. Trimers of MexB enable homology modeling.
Mass spectrum of MexB, revealing only one oligomeric form, the trimer (right). Also present in the spectra are lipid aggregates and a well resolved monomeric form (left). Atomic models of the monomer were generated using 3D-jigsaw via homology modeling from the X-ray structure of AcrB14. The model of trimeric MexB was generated using the program Modeller15.
Overall we extended our original result4 by establishing a robust and generally applicable mass spectrometry method that enables determination of subunit stoichiometry and characterization of lipid binding to intact membrane protein complexes. Our method allows direct measurement of the mass of integral membrane proteins, devoid of the contribution from the micelle, using only very small quantities of complex, typically <10 pmol. We anticipate therefore that this mass spectrometry approach will become increasingly important, not only for determining subunit stoichiometry, but also for assessing ligand and lipid binding as well as the effects of micelles on membrane and soluble subunit interfaces.
Online methods
Mass spectrometry
Q-toF2 mass spectrometers and a Synapt HD mass spectrometer (Waters) were used, all of which were modified for transmission and detection of ions at high m/z ratios17. Aliquots of complex-containing solutions (1 μl of ~15 μM protein) in detergent (typically ~200 μM DDM) were introduced via gold-coated nanoflow electrospray capillaries, prepared in-house. Typical instrument parameters were: needle voltage 1.5 kV, MCP 2,350 V, cone voltage 150–200 V and collision voltage 100–200 V. The pressures in the quadrupole and collision cell were 4.5 × 10−5 mbar and 30–50 μbar (analyzer readback). For a detailed description of the mass spectrometry set up, see the Supplementary Protocol.
Purification and expression of protein complexes
MacB was expressed as reported previously18. For mass spectrometry experiments, DDM (Melford Laboratories) was used.
The contiguous lmrCD genes were amplified from the chromosome of Lactococcus lactis subsp. cremoris MG1363 using forward primer 5′-ATC CAT GGG GCA TCA CCA TCA CCA TCA CCA TCA CCA TCA TAT GAT TTT CAA ATC AAT CAT GAA GCA TAA ATG-3′ containing a sequence encoding a 10His tag and the reverse primer 5′-TAT CTA GAT TAT CAT TCA AAA ACG AAT TGA TTA TGA TAA AG-3′. The PCR product was cloned into the E. coli vector pGEM-5Zf using restriction sites NcoI and XbaI yielding pGEM_LmrCD_DecaHisN. Next, a linker containing the prescission protease cleavage site was cloned between the sequence encoding the 10His tag and the start of lmrC using forward primer 5′-TAT ATC ATA TGC TAG AAG TTC TGT TCC AGG GGC CGG CAG GTG CTG GAG CAA TTT TCA AAT CAA TCA TGA AGC ATA AAT G-3′ and the above reverse primer and ligating NdeI/XbaI digested PCR product and pGEM_LmrCD_DecaHisN resulting in the plasmid GEM_LmrCD_DecaHisN_Presc. The lmrCD gene containing the gene encoding the 10His tag linked with a prescission protease cleavage site upstream of lmrC was then cloned via NcoI/XbaI into the lactococcal pNZ8048 vector19, under control of a nisin A–inducible promoter, yielding pNZ8048_LmrCD_DecaHisN_Presc. LmrCD proteins were expressed in L. lactis NZ9000 ΔlmrA ΔlmrCD20 harboring pNZ8048_LmrCD_DecaHisN_Presc in M17 medium containing 0.5% glucose and 5 μg ml−1 chloramphenicol at 30 °C.
Cells were grown to an optical desnisty at 600 nm (OD660) of 0.5 and were induced for 2 h in the presence of a 1:5,000 dilution of the culture supernatant of the nisin A–producing L. lactis NZ970019, corresponding to a nisin A concentration of 2 pg ml−1. Cells were collected by centrifugation at 13,000g for 6 min. In all subsequent steps 50 mM potassium phosphate (pH 7.0) was used as the buffer. The cell pellet was washed at 4 °C in buffer and resuspended to an absorbance at 660 nm (A660) of 40 in buffer with 2 mg ml−1 lysozyme and incubated for 30 min at 30 °C. Protease inhibitor cocktail (Sigma) was added and cells were broken by passage twice through a Basic Z 0.75-kW benchtop cell disruptor (Constant Systems) at 20,000 lb in2−1. We added 10 μg ml−1 DNase (Sigma) and 10 mM MgSO4 and incubated the suspension at 30 °C for 30 min. Potassium-EDTA was added to a final concentration of 15 mM. Unbroken cells and cell debris were removed by centrifugation at 13,000g for 15 min at 4 °C. Inside-out membrane vesicles were then collected by centrifugation at 125,000g for 30 min at 4 °C. The membrane vesicles were resuspended to a protein concentration of 20 mg membrane protein per milliliter in 100 mM NaPi (pH 8), 200 mM NaCl and 10% glycerol and stored in liquid N2. LmrCD was solubilized from membrane vesicles by previously described methods21 with modifications. Membrane vesicles (3.6 ml) were incubated in 10 ml 100 mM NaPi (pH 8), 200 mM NaCl and 10% glycerol containing 1.5% (wt/vol) n-dodecyl-D-maltoside (DDM; Melford) for 1.5 h at 4 °C. The unsolubilized membrane fraction was removed by centrifugation at 125,000g for 30 min at 4 °C, and the solubilized membrane proteins were loaded onto a Ni2+-NTA gravity flow column containing 1.5 ml resin (Qiagen). The resin was washed with 30 ml of 30 mM imidazole (pH 7.5), 200 mM NaCl, 10% glycerol, 0.03% DDM, and the protein was eluted in 8 ml of the same buffer but containing 200 mM imidazole pH 7.5. The protein was concentrated to a volume of 150 μl using an Amicon Ultra 4 centrifugal filter device (50 KDa cut-off; Millipore) to a concentration of 4 mg ml−1 (0.6 mg yield). The protein was analyzed by SDS-PAGE (data not shown). For mass spectrometry experiments, DDM (Melford Laboratories) was used.
EmrE was purified from membranes as described previously22, with minor modifications. Escherichia coli cells expressing EmrE-Myc-6His were broken using a continuous flow cell disruptor (Constant Cell Systems); cell debris was removed by centrifugation (45 min, 10,000g, 4 °C) and membranes collected from the supernatant by ultracentrifugation (2 h, 100,000g, 4 °C). After solubilising the membranes in 2% dodecylmaltoside, EmrE-Myc-6His was purified using NiNTA agarose (Qiagen), and detergent exchange was performed by washing the NiNTA column with the detergent of choice and then eluting in the same detergent. The Myc-6His tag was removed by endoproteinase-GluC (Roche) cleavage, followed by negative purification on an anion exchange column. For mass spectrometry experiments of the EmrE-His and EmrE-EFE(1–113) versions, DM (Anatrace) and NG/DM (Anatrace) were used, respectively.
The mexb gene was amplified from genomic DNA of P. aeruginosa strain PAO1 (M. Welch, Cambridge University) using Accuzyme DNA polymerase (Bioline). Escherichia coli C41 (DE3) (Lucigen Corp.) was transformed with pMexBH. The protein was expressed and purified as described previously13. For mass spectrometry experiments, DDM (Alexis Biochemicals) was used.
Calculation of the activation coefficient
The activation coefficient was calculated from the product of the average charge state and the sum of the cone and collision cell voltages expressed as a fraction of their maximum values as follows:
Identification of glycerophospholipid in LmrCD preparations
A search of the lipid database (http://www.caffreylabs.ul.ie/ for lipid masses 1,350 ± 8 Da revealed only one possible molecular formula C73H140O17P2 with a mass of 1,352 Da. This corresponded to the symmetrical hexadecanoic acid, 5,8,11-trihydroxy-5,11-dioxido-4,6,10,12-tetraoxa-5,11-diphosphapentadecane-1,2,14,15-tetrayl ester; hexadecanoic acid, 5,8,11-trihydroxy-4,6,10,12-tetraoxa-5,11-diphosphapentadecane-1,2,14,15-tetrayl ester, P,P’-dioxide or isomers with two C15 and two C17 chains.
Homology modeling of the MexB trimer
The amino acid sequences and three-dimensional structures of the homologous protein used as template for comparative modelling of MexB were obtained from the Protein Data Bank (http://www.rcsb.org/pdb/). Initial alignments between target protein and its template were obtained using the program FUGUE23.
Models were produced using the program MODELLER15. Spatial restraints were obtained from statistical analysis of the relationship between pairs of homologous structures from a database of alignments of sequences of 416 proteins of known three-dimensional structure in 105 families. Comparative models were verified with validation programs including PROCHEK24 and JOY25. The alignments were then manually modified as required and the modelling and validation process repeated. The process of modelling, validation, realignment was repeated until models with good geometry, conformations and validation parameters had been obtained.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge funding from the Biotechnology and Biological Sciences Research Council, Medical Research Council, European Molecular Biology Organization, Swiss National Science Foundation, The Wellcome Trust, Royal Society, European Union PROSPECTS and Walters-Kundert trust. We thank M. Welch for P. aeruginosa genomic DNA.
References
- 1.Schagger H, von Jagow G. Anal. Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 2.Burgess NK, Stanley AM, Fleming KG. Methods Cell Biol. 2008;84:181–211. doi: 10.1016/S0091-679X(07)84007-6. [DOI] [PubMed] [Google Scholar]
- 3.Putnam CD, Hammel M, Hura GL, Tainer JA. Q. Rev. Biophys. 2007;40:191–285. doi: 10.1017/S0033583507004635. [DOI] [PubMed] [Google Scholar]
- 4.Barrera NP, Di Bartolo N, Booth PJ, Robinson CV. Science. 2008;321:243–246. doi: 10.1126/science.1159292. [DOI] [PubMed] [Google Scholar]
- 5.Locher KP, Lee AT, Rees DC. Science. 2002;296:1091–1098. doi: 10.1126/science.1071142. [DOI] [PubMed] [Google Scholar]
- 6.Tate CG. Curr. Opin. Struct. Biol. 2006;16:457–464. doi: 10.1016/j.sbi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi N, Nishino K, Yamaguchi A. J. Bacteriol. 2001;183:5639–5644. doi: 10.1128/JB.183.19.5639-5644.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubelski J, van Merkerk R, Konnings WN, Dreissen AJ. Biochemistry. 2006;45:648–656. doi: 10.1021/bi051276s. [DOI] [PubMed] [Google Scholar]
- 9.Yerushalmi H, Lebendiker M, Schuldiner S. J. Biol. Chem. 1995;270:6856–6863. doi: 10.1074/jbc.270.12.6856. [DOI] [PubMed] [Google Scholar]
- 10.Morshed SR, Lei Y, Yoneyama H, Nakae T. Biochem. Biophys. Res. Commun. 1995;210:356–362. doi: 10.1006/bbrc.1995.1669. [DOI] [PubMed] [Google Scholar]
- 11.Seeger MA, van Veen HW. Biochim. Biophys. Acta. 2009;1794:725–737. doi: 10.1016/j.bbapap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Goldfine H. Adv. Microb. Physiol. 1972;8:1–58. doi: 10.1016/s0065-2911(08)60187-3. [DOI] [PubMed] [Google Scholar]
- 13.Mokhonov V, et al. Protein Expr. Purif. 2005;40:91–100. doi: 10.1016/j.pep.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Seeger MA, et al. Science. 2006;313:1295–1298. doi: 10.1126/science.1131542. [DOI] [PubMed] [Google Scholar]
- 15.Sali A, Blundell TL. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 16.Chen YJ, et al. Proc. Natl. Acad. Sci. USA. 2007;104:18999–19004. doi: 10.1073/pnas.0709387104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobott F, et al. Anal. Chem. doi: 10.1021/ac0110552. [DOI] [PubMed] [Google Scholar]
- 18.Lin HT, et al. J. Biol. Chem. 2009;284:1145. doi: 10.1074/jbc.M806964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Ruyter PG, Kuipers OP, de Vos WM. Appl. Environ. Microbiol. 1996;62:3662. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venter H, et al. Biochem. Pharmacol. 2008;75:866. doi: 10.1016/j.bcp.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Margolles A, et al. Biochemistry. 1999;38:16298. doi: 10.1021/bi990855s. [DOI] [PubMed] [Google Scholar]
- 22.Tate CG, et al. EMBO J. 2001;20:77. doi: 10.1093/emboj/20.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi J, Blundell TL, Mizuguchi K. J. Mol. Biol. 2001;310:243. doi: 10.1006/jmbi.2001.4762. [DOI] [PubMed] [Google Scholar]
- 24.Laskowski RA, et al. J. Appl. Cryst. 1993;26:283. [Google Scholar]
- 25.Mizuguchi K, et al. Bioinformatics. 1998;14:617. doi: 10.1093/bioinformatics/14.7.617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.