Abstract
The primary embryonic axes in flies, frogs and fish are formed through translational regulation of localized transcripts before fertilization1. In Drosophila, the axes are established through the transport and translational regulation of gurken (grk) and bicoid (bcd) messenger RNA (mRNA) in the oocyte and embryo1. bcd and grk mRNA are both translationally silent while being localized within the oocyte along microtubules by cytoplasmic Dynein1-4. Once localized, grk is translated at the dorsoanterior of the oocyte to send a TGF-alpha signal to the overlying somatic cells5. In contrast, bcd is translationally repressed in the oocyte until its activation in early embryos to form an anteroposterior morphogenetic gradient6. How this differential translational regulation is achieved is not fully understood. Here, we address this question using ultrastructural analysis, super-resolution microscopy and live cell imaging. We show that grk and bcd ribonucleoprotein (RNP) complexes associate with electron dense bodies that lack ribosomes and contain translational repressors, characteristic of Processing bodies (P bodies), which are regions of cytoplasm where translational decisions are made. Endogenous grk mRNA forms dynamic RNP particles that become docked and translated at the periphery of P bodies, where we show that the translational activator Orb/CEPB and the anchoring factor Squid (Sqd) are also enriched. In contrast, an excess of grk mRNA becomes localized inside the P bodies, where endogenous bcd mRNA is localized and translationally repressed. Interestingly, bcd mRNA dissociates from P bodies in embryos following egg activation, when it is known to become translationally active. We propose a general principle of translational regulation during axis specification involving remodeling of transport RNPs and dynamic partitioning of different transcripts between the translationally active edge of P bodies and their silent core.
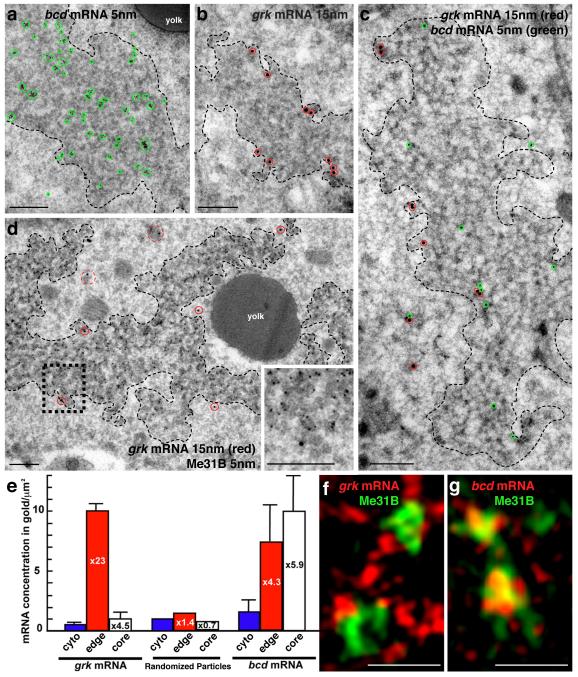
At the dorsoanterior corner of the oocyte, localized grk mRNA is associated with electron dense structures7, which were originally characterized in nurse cells8 and later in the oocyte as sponge bodies7. These structures have subsequently been shown to share at least some components with yeast and mammalian P bodies9-11. We, therefore, refer to sponge bodies as P bodies. To test whether, like grk, bcd mRNA is associated with P bodies, we used in situ hybridization with antisense bcd probe combined with immuno-electron microscopy on frozen sections (ISH-IEM)12. We found that bcd mRNA is also associated with P bodies in mid-oogenesis (Fig. 1a and Supplemental Fig. 1). Closer examination revealed that grk mRNA is present along the periphery of the P body (Fig.1b,c), whereas bcd is mostly present within its interior (Fig. 1a,b). To characterize this further, we covisualized endogenous grk mRNA and a DEAD-Box helicase Maternal expression at 31B (Me31B), a well established P body marker13. We found that grk mRNA appears to localize just outside a ‘core’ zone of concentrated Me31B labeling (Fig. 1d). We then performed a quantitative analysis of the density of gold as a function of the distance from the border of the P body. This analysis applied to Me31B and Orb (Supplemental Fig. 2b,b′, Supplemental methods), shows that P bodies are organized into two different regions: a ‘core’ containing the majority of Me31B and ‘edge’, the outermost 60-80nm of electron dense material, defined as 70nm for convenience of analysis (Fig. 1e, Supplementary Fig. 2c). We found that, relative to the surrounding cytoplasm, grk mRNA is 23 times more concentrated at the edge of the P body whereas bcd is 6 fold more concentrated in the P body core (Fig. 1e).
Figure 1. Differential association of bcd and grk with P bodies.
(a-d) mRNA detection at the dorsoanterior corner (for orientation, see Fig. 2a) by ISH-IEM on wild-type (WT) ultra-thin frozen sections of stage 9 oocytes. (a) bcd mRNA (5nm, green circles) is present both inside and at the edge of electron dense P bodies (dashed black line). Gold particles here cluster due to the use of a bridging antibody. (b) grk mRNA (15nm, red circles) is enriched at the edge of P bodies (dashed black line). (c) bcd mRNA (5nm, green circles) and grk mRNA (15nm, red circles) can associate with the same P body but bcd is enriched inside. (d) grk mRNA (15nm, red circles) docks at the edge of the P body (black dashed box magnified, inset bottom right), just outside of the Me31B dense core region (5nm gold), while grk transport particles, as described in Delanoue et. al., 2007(7), are detected in the cytoplasm at a short distance from the P body (15nm, red dashed circles). (e) mRNA density (gold/um2) in the P body sub-regions when compared to the surrounding cytoplasm. For comparison, randomized particles were analyzed in an identical way to RNAs. Error bars show SEM of gold density per scan (grk n=13, bcd n=11). (f,g) Fixed Me31B::GFP stage 8/9 expressing oocytes imaged using the OMX structured illumination super-resolution mode (3D-SIM) with double labeling of either (f) grk*mCherry, that mainly interdigitates with Me31B (green) while showing some colocalization at the edge (yellow) or (g) bcd*RFP grk mRNA (red), that shows significant colocalization (yellow) with Me31B (green). Scale bars, 200nm (a-d); 0.5μm (f, g).
While ISH-IEM provides excellent ultrastructural preservation and resolution, it only represents a two dimensional view and has limited sensitivity of detection of RNA, since gold conjugates only penetrate the first 5nm of the ultrathin 60nm frozen section12. We, therefore, used the complementary method of structured illumination microscopy (3D-SIM), which provides double the conventional resolution of light microscopy in each dimension14. To covisualize P bodies and mRNA, we detected P bodies with a GFP fusion to the DEAD-Box helicase Maternal expression at 31B (Me31B::GFP)13 at the same time as tagging grk mRNA with 12 copies of the MS2 binding loop or bcd mRNA with 6 copies of MS2, both encoding functional genes2,15,16. The tagged mRNA was decorated with MS2-coat protein (MCP) fused to fluorescent proteins2,15-17, subsequently referred to as grk*GFP, RFP or mCherry). We found that grk*mCherry is excluded from the core of the P bodies and interdigitates with Me31B::GFP, with a slight overlap at the interface, which we interpret as consistent with the ISH-IEM results (Fig. 1f). In contrast, bcd*RFP overlaps significantly with the P body core in both techniques (Fig. 1g).
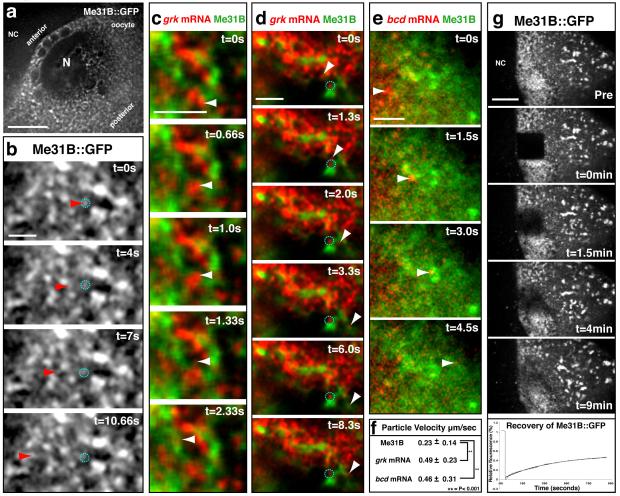
We next tested whether the differences we observe in the distribution of grk and bcd mRNAs relative to P bodies can be explained by their distinct transport and anchoring dynamics. We performed rapid single and multicolor wide-field fluorescence microscopy in living stage 8/9 oocytes (Fig. 2a-e, Supplementary Movie 1-6). We observed three distinct populations of mRNA particles: ‘active transport particles’ moving along linear paths characteristic of transport by molecular motors; ‘paused transport particles’ showing a complex constrained motion; and ‘docked grk’ statically associated with P bodies (Supplemental Fig. 3a,b). Active transport particles of grk mRNA and bcd mRNA do not colocalize with Me31B, but rather move in the space between P bodies with an average velocity of 0.5 μm/s (Fig. 2c,e,f, Supplementary Movie 4,6). 41% of grk particles dock at the edge of P bodies (Fig. 2d, Supplementary Movie 5). In contrast, out of 155 bcd transport particles, none showed grk-like docking at the edge of the P bodies in stage 8/9 oocytes. A quantitative analysis of particle motility, by mean squared displacement confirmed the difference between motile and docked particles (Supplemental materials and methods). We suggest that bcd mRNA rapidly integrates into the P body core upon contact with the P bodies, whereas grk mRNA particles remain docked on their outside.
Figure 2. Dynamics of grk, bcd and Me31B particles in live oocytes.
(a) Low magnification wide-field image of the DA corner of a stage 8-9 egg chamber expressing Me31B::GFP. (b) Me31B::GFP expressing oocytes show small faint dynamic particles of Me31B (red arrowheads) moving between and fusing together with other often larger, bright and static (dashed cyan circles) Me31B bodies. (Supplemental Movie 1, 2). (c,d) grk*mCherry and Me31B::GFP expressing oocytes show grk mRNA particles (white arrowheads) moving independently of Me31B (Supplemental Movie 3). (d) Dynamic particles of grk (white arrowhead) are visualized docking and remaining on the edge of Me31B rich zones. Other small grk particles are seen in association with the Me31B throughout the time course (dashed cyan circles) (Supplemental Movie 4). (e) bcd*RFP and Me31B::GFP expressing oocytes show bcd particles (white arrowheads) moving independently of Me31B. (Supplemental Movie 5). (f) Average particle velocities for dynamic Me31B (n=30), grk (n=37) and bcd (n=31) particles in μm/second +/− SEM. P-values from student’s t-tests (two tails), P<0.001. (g) Fluorescence Recovery After Photobleaching of Me31B::GFP at the DA corner shows recovery to approximately 50% of total fluorescence with a half time of 4 minutes (n= 5). NC, Nurse Cell; N, nucleus. Scale bars, 10μm (a); 2μm (b-e); 20μm (g).
Our covisualization of mRNA and Me31B showed that mRNA particles move independently of Me31B. To determine whether Me31B is also dynamic and to establish how its distribution in P bodies is maintained, we first visualized Me31B-GFP at high time resolution. We observed Me31B-GFP particles moving with an average velocity of approximately 0.25 μm/s (Fig. 2b,f, Supplementary Movie 2,3), significantly slower than RNA particles (Fig. 2f). This suggests that Me31B is in continual flux as small dynamic particles that leave or join larger Me31B rich P bodies. We then tested the degree of exchange of Me31B in and out of P bodies using Fluorescent Recovery After Photobleaching (FRAP) experiments. We found that Me31B::GFP recovers to approximately 50% of total fluorescence with a half time of approximately 4 minutes after photobleaching (Fig. 2g). In contrast, control experiments using freely diffusing GFP fused to a nuclear localization signal (nlsGFP) recovered over 90% of total fluorescence in less than 5s (n=5, data not shown). We conclude that Me31B::GFP is not freely diffusible, but instead is maintained by relatively slow exchange of dynamic particles that move independently of grk and bcd mRNA.
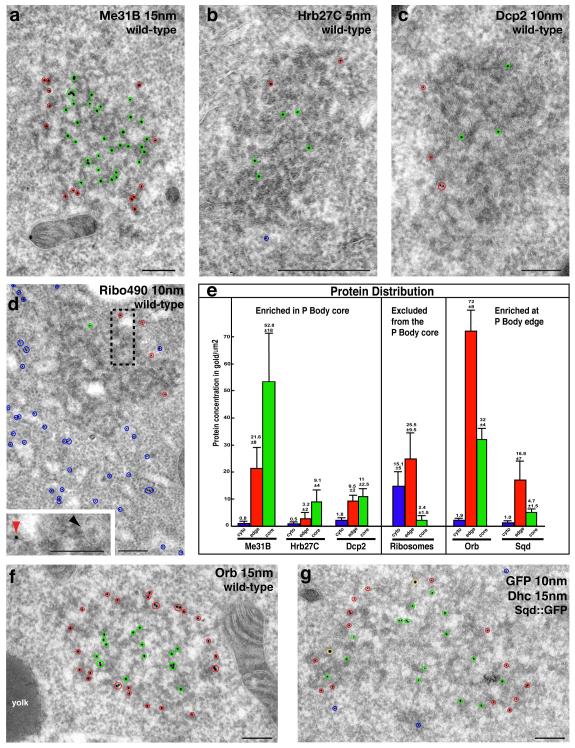
To begin to understand how this organization could influence translation, we characterized the distribution of key translation components in the oocyte at mid-oogenesis (Fig. 3a-g, Supplemental Fig. 3c-l). Our results show that many factors involved in mRNA localization and translational regulation including Exuperantia (Exu), Trailer hitch (Tral) and Growl/Lost do not colocalize with grk mRNA but display the same distribution as Me31B (Supplemental Fig. 3c-e, Fig. 1f). Similarly, Cup and Eukaryotic initiation factor 4E (eIF4E) colocalize with P bodies (Supplemental Fig. 3g-k). Furthermore, using IEM, we show that Me31B, Heterogeneous nuclear ribonucleoprotein at 27C (Hrb27C/Hrp48/p50) and the mRNA degradation enzyme Decapping protein 2 (Dcp2), a hallmark of P bodies in yeast and mammalian cells9, are significantly enriched in the P body core when compared to the surrounding cytoplasm, suggesting that the P body core is translationally silent (Fig. 3a-c,e, Supplemental Fig. 3f).
Figure 3. Oocyte P bodies exhibit zones differentially enriched in RNA associated proteins.
(a-d,f,g) IEM localization of RNA associated proteins in stage 9 oocytes at the DA corner. Protein in the core of the electron dense P bodies indicated by green circles, at the edge of P bodies by red circles and in the cytoplasm by blue circles. (a) WT egg chamber, anti-Me31B (15nm) enriched inside P bodies. (b) Hrb27C (5nm) predominantly inside of P bodies. (c) Dcp2 (10nm) inside and at the edge of P bodies. (d) WT egg chamber, anti-Ribo 490 (10nm) shows ribosomes predominantly in the cytoplasm some at the edge but mostly excluded from inside of P bodies. The pool of cytoplasmic ribosomes corresponds to polysomes and those present on the ER membrane (Rough ER), some of which is present near the edge of a P body (black dashed box, inset: red arrowhead). Smooth ER is detected inside of the P body (inset: black arrowhead). (e) Graph showing protein concentration in gold/μm2 in the cytoplasm, edge of P bodies and inside of P bodies; proteins are organized into three distinct categories. Error bars are ± standard deviation (between n=10-15 scans in each case). (f) WT egg chamber, anti-orb (15nm) enriched at the edge of P bodies. (g) Sqd-GFP in Sqd::GFP expressing egg chamber using anti-GFP (10nm) and anti-Dhc (15nm, yellow circles). Sqd is enriched inside compared to at the edge of P bodies. Scale bars, 200nm (a-d,f,g).
In line with the role of P body in translational inhibition, previous work showed that P bodies lack ribosomes, as judged by morphological analysis and by a GFP tagged ribosomal component8,18. We confirmed this by labeling endogenous ribosomes by IEM using an antibody against a specific ribosomal protein (Fig. 3d,e, Supplemental Fig. 4, Supplemental Fig. 2d and Supplemental methods). We found that 91% of ribosomes are not associated with P bodies. Interestingly, the 9% of ribosomes associated with the P bodies are almost exclusively enriched at the P body edge (Fig. 3e), and some of them are on ER strands closely apposed to P bodies (Fig. 3d). We conclude that the core of oocyte P bodies cannot support translation, whereas the edge of P bodies could support translation, for instance, of grk mRNA.
To further test this, we examined the distribution of the translational activator Orb/CEPB. Although Orb has not been shown to bind directly to grk mRNA, it is known to be required for grk mRNA localization and translation19. Our results show that Orb is enriched at the P body edge (Fig. 3e,f, Supplemental Fig. 2b), suggesting that the edge of P bodies could be an Orb mediated site of translational activation. We also determined the distribution of Squid (Sqd) in P bodies, as it is required to maintain grk mRNA localization and we previously showed that it is present in P bodies and within grk RNP transport particles7. We used an anti-GFP antibody to detect Sqd::GFP and showed that Sqd is enriched 3.5 times at the P body edge compared to its core (Fig. 3e,g). We conclude that in the oocyte, there is a distinct edge region of the P body that is competent for translation while the core is a site of translational repression. This is the first evidence suggesting a relationship between the P body stratified organization and the translational fates of distinct mRNAs.
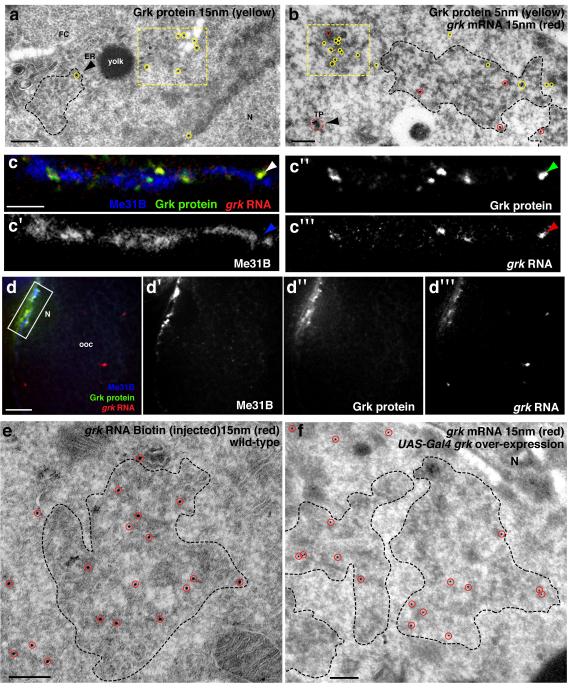
To test directly whether the edge of P bodies is also the site of grk translation, we covisualized grk mRNA and protein at the ultrastructural level. Our results show that, at steady state, the intracellular pool of endogenous Grk protein is found in the early secretory pathway, within the ER and tER-Golgi units. This distribution is in line with the fact that Grk protein is first synthesised as a transmembrane protein, processed and secreted into the intracellular space between the oocyte and dorsoanterior follicle cells (Fig. 4a)12. Although Grk protein is largely found at a different location from its mRNA, we found a small amount of Grk protein is also present on rough endoplasmic reticulum (RER) at the edge of P bodies (Fig. 4a,b, Fig. 3d) where grk mRNA is docked (Fig. 1b-d). We also observed grk mRNA transport particles lacking Grk protein (see arrowhead in Fig. 4b) that are present at the dorsoanterior corner but are not docked to P bodies. Given that grk mRNA is repressed during transport5,20, we favour the interpretation that translational activation of grk mRNA occurs when transport particles dock at the edge of P bodies from where Grk protein enters the secretory pathway.
Figure 4. RNA translation fate is regulated through association with P bodies.
(a) IEM using anti-Grk (15nm, yellow circles) on a WT stage 9 egg chamber shows Grk protein highly enriched in the tER-Golgi unit (yellow dashed box) and detected on ER (black arrowhead) at the edge of a P body (black dashed line). (b) ISH-IEM detection of grk mRNA and Grk protein on a WT stage 9 egg chamber shows grk mRNA (15nm, red circles) mostly present at the edge of the P body (black dashed line) and Grk protein (5nm, yellow circles) on a tER-Golgi unit (yellow dashed box) but also present at the edge of the P body (black dashed line). Note the grk mRNA transport particle (dashed red circle) does not contain any detectable Grk protein (black arrowhead). (c) Injection of in vitro synthesized grk RNA (mixed solution of approximately one part Alexa dye labeled and 4 parts unlabeled) into a grk null egg chamber expressing Me31B::GFP. Images shown are a 4μm projection. Localization and translation was allowed for 40 minutes before the egg chamber was fixed and labeled with anti-Grk. (c,c″) Grk protein (green) and (c,c‴) grk RNA (red) colocalize, but do not colocalize with (c,c′) Me31B (blue). Instead, grk RNA and protein interdigitates with Me31B (arrowheads). (d-d‴) Low magnification of panel c. Injected grk RNA is localized to the dorsoanterior corner where it is translated. (e) IEM on a WT stage 8/9 egg chamber injected with a high concentration of grk RNA Biotin (500 ng/μl). Injected RNA (15nm, red circles) is detected at the core of and at the edge of the P body (dashed black line). (f) IEM on a stage 8/9 egg chamber over-expressing grk mRNA using the UAS-Gal4 system. grk mRNA (15nm) is enriched at the core of and at the edge of the P body (dashed black line). FC, follicle cell; TP, transport particle; ER, endoplasmic reticulum; N, nucleus. Scale bars, 200nm (a,b,e,f); 3μm (c); 10μm (d).
At steady state, the majority of Grk protein is located in the secretory pathway12. Therefore, it is difficult to determine where grk mRNA is first translated. To overcome this, we injected a mixture of AlexaFluor labeled RNA and unlabeled RNA encoding a full length Grk protein into grk mRNA null oocytes5 (Fig. 4c,d). Despite potentially not being subject to the normal translational repression while being transported, our results show that a pulse of Grk protein translation is colocalized with grk RNA, and both were interdigitated with Me31B (Fig. 4c, arrowheads). We interpret these results as showing that grk mRNA is translated at the P body edge.
Precise regulation of the dosage level of Grk protein in the oocyte is essential for correct dorsoventral patterning20. To test whether an excess of grk mRNA is translationally repressed, we injected varying amounts of grk RNA and characterized its distribution after localization. Injection is a well established assay for monitoring RNA localization3,21. Despite being additional to endogenous grk mRNA, injected grk RNA assembles into transport particles that become localized at the DA corner22. We show that moderate concentrations of injected grk RNA (~120 ng/μl) localizes and interdigitates with Me31B, at the edge of the P bodies (Supplemental Fig. 5a, b). In contrast, injecting four fold more grk RNA leads a proportion of RNA localizing to the core of P bodies (Fig. 4e, Supplemental Fig. 5c,d). We conclude that an excess of injected grk RNA is directed to the P body core.
To test whether an excess of endogenous grk mRNA behaves like injected RNA, we used the UAS-Gal4 system to drive grk over-expression. We confirmed that over expression occurred with anti-Grk antibodies and grk FISH (Supplemental Fig. 5e,f), and found dorsoventral patterning defects in eggs to varying degrees23. We used ISH-IEM to determine where over expressed grk mRNA is localized and found a clear increase in the relative proportion of grk mRNA found in the core of P bodies when compared to wild-type (Fig. 4f, Supplemental Fig. 5g,h). Together, these results support a model where excess grk mRNA is directed into the core of the P body, which lacks ribosomes and maintains translational repression.
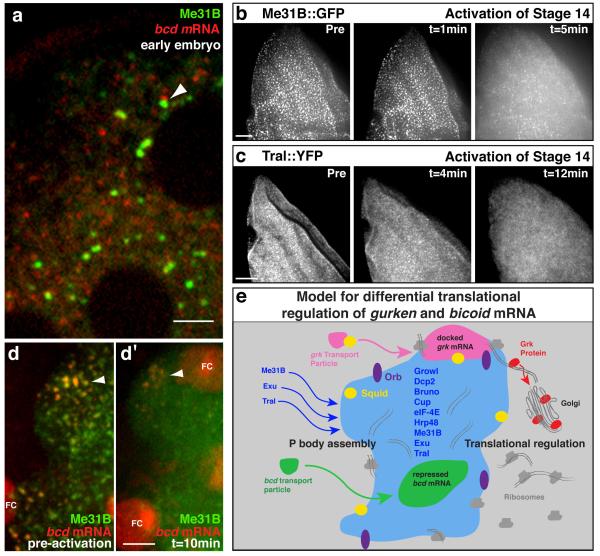
To further test the relationship between P body sub-localization and differential translation, we analyzed translationally repressed bcd mRNA in oocytes and translationally active bcd mRNA in embryos6. At stage 9 of oogenesis, a population of bcd is enriched in the P body core (Fig.1), whereas the remainder is in highly dynamic particles that are known to also be translationally repressed by a mechanism that is not well understood. At stage 14 of oogenesis, when bcd mRNA is known to be anchored and repressed6,24, all of it colocalizes with Me31B (Supplemental Fig. 5i,j). In contrast, in early embryos, at a time when it is translated, bcd mRNA is outside of P bodies (Fig. 5a). It is not known when and how bcd translation is activated in embryos, but we hypothesize that this may be related to egg activation, a major transition point between oocytes and embryos.
Figure 5. RNA association with P bodies in the early embryo.
(a) Anterior region of a live early embryo expressing bcd*RFP and Me31B::GFP. The majority of bcd mRNA is detected outside of Me31B labeling, sometimes associating with the edge (arrowhead). (b,c) Time points from a stage 14 egg chamber expressing (b) Me31B::GFP or (c) Tral::YFP, prior to and following activation. Activation buffer added at t = 0 minutes. Immediately after activation, Me31B (or Tral) labeled P bodies disperse. (d) Stage 14 egg chamber expressing bcd*RFP and Me31B::GFP (d) Before treatment with activation buffer and (d′) 10 minutes after activation buffer is applied. Pre-activation, bcd mRNA and Me31B colocalize at the anterior (arrowhead). Immediately after activation, Me31B disperses and bcd no longer colocalizes with Me31B (arrowhead). (e) Model of how P bodies regulate differential translation of bcd and grk mRNA at the DA corner in a stage 8/9 oocyte. grk mRNA transport particles move independently of Me31B. grk associates at the edge of the P body where enrichment of Sqd is involved in anchoring, Orb activates translation and ribosomes support translation. grk is translated on rough ER at the edge of P bodies and Grk protein is trafficked through the secretory pathway (tER-Golgi unit) before being secreted. bcd mRNA transport particles move independently of Me31B and associate with the core of the P body where translation is not supported. Dynamic Me31B, Exu and Tral particles assemble and maintain the P bodies. Excess grk RNA is targeted into the core of P bodies where it is translationally repressed and likely degraded. FC, follicle cell. Scale bars, 5μm (a,d); 20μm (b,c).
We previously showed that bcd mRNA is released from its anterior cytoskeletal anchor by egg activation24. To test whether egg activation is also the trigger for bcd mRNA redistributing outside the P bodies, we covisualized labeled P bodies and grk*MS2, before and after activating eggs in vitro. We found that upon egg activation, Me31B (Fig. 5b) and Tral (Fig. 5c) become dispersed, and results in a loss of colocalization between bcd mRNA and Me31B (Fig. 5d,d′). Although our data does not address directly whether the translational activation of bcd mRNA is caused by P body disassembly, we previously showed that in sarah mutant embryos, where activation does not occur, bcd mRNA is not released from the anterior or translated24,25. Therefore, we propose that translational activation of bcd mRNA results from the disassembly of P bodies upon egg activation.
Our results are the first to demonstrate the stratification of P bodies and suggest how localized transcripts could be differentially translated by distinct associations with P body zones. We propose a model for the spatio-temporal regulation of the translation of developmentally important mRNAs in the oocyte (Fig. 5e). Translationally repressed RNP transport particles associate with the edge of P bodies where anchoring and translation activation factors, such as Sqd and Orb, are enriched. We previously showed that disrupting Sqd function causes a defect in anchoring of transport particles at the dorsoanterior P bodies7. Instead of docking at the dorsoanterior corner, grk transport particles are in continuous flux along the anterior and grk is inappropriately translated7. We therefore favor a model in which sqd is required for translational repression of grk mRNA during its transport, and for anchoring the transcript at the P body edge. In this model, Orb is also present at the edge of the P body and is required for translational activation of grk mRNA. RNA, such as grk, that is excluded from the core of the P body is translationally activated at the edge of the bodies. In contrast, bcd and probably other transcripts, that enter the ribosome-depleted core of P bodies remain translationally repressed. Furthermore, the finding that excess grk RNA leads to an increase in the proportion of grk in the P body core, leads us to speculate that this is a mechanism for regulating Grk protein levels.
P bodies are one of several related electron dense and RNP-rich cellular structures26 that include stress granules27, the Xenopus mitochondrial cloud28, the zebrafish Balbiani body29, and nematode P-granules30. The dynamic nature of the protein and mRNA components of these structures has not often been studied in detail, except in C.elegans where P-granules have been shown to behave in a fluid like manner31. Our observation of highly dynamic independent movements of a P body protein and mRNA provides a general molecular basis for the fluid like behavior of P-granules and related granules. There has been considerable debate about whether P body formation is essential for mRNA decay and translational repression or a consequence of these events32-35. The consensus has been that P body formation is essential for mRNA decay and translational repression32-35 and our work highlights another function for P bodies in translational regulation of key transcripts through partitioning between the core and edge of the bodies.
Supplementary Material
References
- 1.St Johnston D. Moving messages: the intracellular localization of mRNAs. Nat. Rev. Mol. Cell Biol. 2005;6:363–375. doi: 10.1038/nrm1643. [DOI] [PubMed] [Google Scholar]
- 2.Weil TT, Forrest KM, Gavis ER. Localization of bicoid mRNA in late oocytes is maintained by continual active transport. Dev Cell. 2006;11:251–262. doi: 10.1016/j.devcel.2006.06.006. doi:S1534-5807(06)00262-0 [pii]10.1016/j.devcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Cha BJ, Koppetsch BS, Theurkauf WE. In vivo analysis of Drosophila bicoid mRNA localization reveals a novel microtubule-dependent axis specification pathway. Cell. 2001;106:35–46. doi: 10.1016/s0092-8674(01)00419-6. doi:S0092-8674(01)00419-6 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Clark A, Meignin C, Davis I. A Dynein-dependent shortcut rapidly delivers axis determination transcripts into the Drosophila oocyte. Development. 2007;134:1955–1965. doi: 10.1242/dev.02832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuman-Silberberg FS, Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGFa-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- 6.St Johnston D, Driever W, Berleth T, Richstein S, Nüsslein-Volhard C. Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development Suppl. 1989;107:13–19. doi: 10.1242/dev.107.Supplement.13. [DOI] [PubMed] [Google Scholar]
- 7.Delanoue R, Herpers B, Soetaert J, Davis I, Rabouille C. Drosophila Squid/hnRNP helps Dynein switch from a gurken mRNA transport motor to an ultrastructural static anchor in sponge bodies. Dev Cell. 2007;13:523–538. doi: 10.1016/j.devcel.2007.08.022. doi:10.1016/j.devcel.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Wilsch-Brauninger M, Schwarz H, Nusslein-Volhard C. A sponge-like structure involved in the association and transport of maternal products during Drosophila oogenesis. J Cell Biol. 1997;139:817–829. doi: 10.1083/jcb.139.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snee MJ, Macdonald PM. Dynamic organization and plasticity of sponge bodies. Dev Dyn. 2009;238:918–930. doi: 10.1002/dvdy.21914. doi:10.1002/dvdy.21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckham C, et al. The DEAD-box RNA helicase Ded1p affects and accumulates in Saccharomyces cerevisiae P-bodies. Mol Biol Cell. 2008;19:984–993. doi: 10.1091/mbc.E07-09-0954. doi:E07-09-0954 [pii]10.1091/mbc.E07-09-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minshall N, Kress M, Weil D, Standart N. Role of p54 RNA helicase activity and its C-terminal domain in translational repression, P-body localization and assembly. Mol Biol Cell. 2009;20:2464–2472. doi: 10.1091/mbc.E09-01-0035. doi:E09-01-0035 [pii]10.1091/mbc.E09-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herpers B, Rabouille C. mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulum-golgi units involved in gurken transport in Drosophila oocytes. Mol Biol Cell. 2004;15:5306–5317. doi: 10.1091/mbc.E04-05-0398. doi:10.1091/mbc.E04-05-0398E04-05-0398 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura A, Amikura R, Hanyu K, Kobayashi S. Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Development. 2001;128:3233–3242. doi: 10.1242/dev.128.17.3233. [DOI] [PubMed] [Google Scholar]
- 14.Dobbie I, et al. In: Live Cell Imaging: A Laboratory Manual. Second Edition Goldman Robert D., Swedlow Jason R., Spector David L., editors. Cold Spring Harbor Laboratory Press; 2010. pp. 203–214. [Google Scholar]
- 15.Jaramillo AM, Weil TT, Goodhouse J, Gavis ER, Schupbach T. The dynamics of fluorescently labeled endogenous gurken mRNA in Drosophila. J Cell Sci. 2008;121:887–894. doi: 10.1242/jcs.019091. doi:jcs.019091 [pii]10.1242/jcs.019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrest KM, Gavis ER. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol. 2003;13:1159–1168. doi: 10.1016/s0960-9822(03)00451-2. doi:S0960982203004512 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Bertrand E, et al. Localization of ASH1 mRNA particles in living yeast. Mol Cell. 1998;2:437–445. doi: 10.1016/s1097-2765(00)80143-4. doi:S1097-2765(00)80143-4 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, Parker R. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA. 2005;11:371–382. doi: 10.1261/rna.7258505. doi:rna.7258505 [pii]10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang JS, Tan L, Wolf MR, Schedl P. Functioning of the Drosophila orb gene in gurken mRNA localization and translation. Development. 2001;128:3169–3177. doi: 10.1242/dev.128.16.3169. [DOI] [PubMed] [Google Scholar]
- 20.Neuman-Silberberg FS, Schupbach T. Dorsoventral axis formation in Drosophila depends on the correct dosage of the gene gurken. Development. 1994;120:2457–2463. doi: 10.1242/dev.120.9.2457. [DOI] [PubMed] [Google Scholar]
- 21.Wilkie GS, Davis I. Drosophila wingless and pair-rule transcripts localize apically by dynein-mediated transport of RNA particles. Cell. 2001;105:209–219. doi: 10.1016/s0092-8674(01)00312-9. doi:S0092-8674(01)00312-9 [pii] [DOI] [PubMed] [Google Scholar]
- 22.MacDougall N, Clark A, MacDougall E, Davis I. Drosophila gurken (TGFalpha) mRNA localizes as particles that move within the oocyte in two dynein-dependent steps. Dev Cell. 2003;4:307–319. doi: 10.1016/s1534-5807(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 23.Bokel C, Dass S, Wilsch-Brauninger M, Roth S. Drosophila Cornichon acts as cargo receptor for ER export of the TGFalpha-like growth factor Gurken. Development. 2006;133:459–470. doi: 10.1242/dev.02219. doi:dev.02219 [pii]10.1242/dev.02219. [DOI] [PubMed] [Google Scholar]
- 24.Weil TT, Parton R, Davis I, Gavis ER. Changes in bicoid mRNA anchoring highlight conserved mechanisms during the oocyte-to-embryo transition. Curr Biol. 2008;18:1055–1061. doi: 10.1016/j.cub.2008.06.046. doi:S0960-9822(08)00803-8 [pii]10.1016/j.cub.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horner VL, et al. The Drosophila calcipressin sarah is required for several aspects of egg activation. Curr Biol. 2006;16:1441–1446. doi: 10.1016/j.cub.2006.06.024. doi:S0960-9822(06)01751-9 [pii]10.1016/j.cub.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Moser JJ, Fritzler MJ. Cytoplasmic ribonucleoprotein (RNP) bodies and their relationship to GW/P bodies. Int J Biochem Cell Biol. 2010;42:828–843. doi: 10.1016/j.biocel.2009.11.018. doi:10.1016/j.biocel.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. doi:10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King ML, Messitt TJ, Mowry KL. Putting RNAs in the right place at the right time: RNA localization in the frog oocyte. Biol Cell. 2005;97:19–33. doi: 10.1042/BC20040067. doi:10.1042/BC20040067. [DOI] [PubMed] [Google Scholar]
- 29.Kloc M, Bilinski S, Etkin LD. The Balbiani body and germ cell determinants: 150 years later. Curr Top Dev Biol. 2004;59:1–36. doi: 10.1016/S0070-2153(04)59001-4. doi:10.1016/S0070-2153(04)59001-4S0070215304590014 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Updike D, Strome S. P granule assembly and function in Caenorhabditis elegans germ cells. J Androl. 2010;31:53–60. doi: 10.2164/jandrol.109.008292. doi:10.2164/jandrol.109.008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brangwynne CP, et al. Germline P Granules Are Liquid Droplets That Localize by Controlled Dissolution/Condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. doi:10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 32.Eulalio A, Behm-Ansmant I, Schweizer D, Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol Cell Biol. 2007;27:3970–3981. doi: 10.1128/MCB.00128-07. doi:10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. doi:10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Balagopal V, Parker R. Polysomes, P bodies and stress granules: states and fates of eukaryotic mRNAs. Curr Opin Cell Biol. 2009;21:403–408. doi: 10.1016/j.ceb.2009.03.005. doi:10.1016/j.ceb.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoecklin G, Mayo T, Anderson P. ARE-mRNA degradation requires the 5′-3′ decay pathway. EMBO Rep. 2006;7:72–77. doi: 10.1038/sj.embor.7400572. doi:7400572 [pii]10.1038/sj.embor.7400572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.