Abstract
In many parts of the world particularly sub-Saharan Africa, congenital syphilis is a significant public health problem. Though it is rare in most affluent countries there has been a slight resurgence recently in several European countries. The diagnosis of suspected cases and management of congenital syphilis may be confusing and the potential for severe disability is high when cases are missed. The cornerstone of congenital syphilis control is antenatal screening and treatment of mothers with penicillin and in affluent countries it should be strengthened among those at high risk. In developing countries antenatal care screening needs to be strengthened by implementing point-of-care decentralised screening and treatment, also alternative novel approaches to control congenital syphilis should be looked at and utilized. International health agencies and political parties should take steps urgently to support focused approaches to tackling the problem of continuing congenital syphilis.
Keywords: Antenatal care, congenital syphilis, sexually transmitted infections, penicillin
INTRODUCTION
Congenital syphilis (CS) should have become a medical curiosity long ago. However, its ongoing occurrence symbolizes failure of existing antenatal care system and control of sexually transmitted infections (STIs). Antenatal screening for syphilis is cost beneficial and cost effective and penicillin (main drug) is effective, cheap, and easily available. Yet CS remains an important cause of neurological, developmental and musculoskeletal disability and death in infants, especially in resource poor settings.[1] Furthermore, infected infants may experience severe sequelae which can be prevented by timely treatment of CS during pregnancy.[2]
However, diagnosis and management of CS may be confusing and when cases are missed potential for severe disability is high. Furthermore, diagnosis may be problematic as more than half of infants are asymptomatic and signs in symptomatic infants may be subtle and nonspecific. Newer diagnostic tests like enzyme immunoassays, polymerase chain reaction, and immunoblotting are more sensitive and specific but are mostly unavailable.[2]
Syphilis screening is implemented only sporadically in many countries, despite national policies on antenatal testing and widespread use of antenatal services, leaving disease undetected and untreated among pregnant women. Weak organization of services and costs of screening are major hurdles faced by programs. Guidelines developed for better-resourced settings are conservative, err on the side of overtreatment, difficult to implement in, or inappropriate for, poorly-resourced settings because of lack of investigative ability and pressure on health facilities to discharge infants early. Hence, decentralization of antenatal syphilis screening programs, on-site testing and immediate treatment can reduce CS cases.[1]
INCIDENCE OF CONGENITAL SYPHILIS
Adverse pregnancy outcomes are 12 times more likely in women with syphilis than seronegative women, with 2.5-fold higher risk even after treatment as compared to uninfected women. Adverse pregnancy outcomes occur in 80% of women with active syphilis, including stillbirth (40%), perinatal death (20%) and serious neonatal infection (20%).[3]
CS is estimated to occur in 25-75% of exposed infants. Approximately, 10-12% of infants born to mothers with positive serology for syphilis would die if untreated (mortality rate 1-3%).[4]
Incidence of CS in Eastern Europe and central Asia has increased. High rates of syphilis seropositivity (3-18%) have been reported form Africa with CS, accounting for 1% of pediatric admissions.[1] From Delhi and Andhra Pradesh, 82 (1965-1978) and 7 cases (1980-1990) were reported, respectively.[4] Case fatality rates of 6.4% in US, 15% in Africa and 38% in South Africa have been reported.[1]
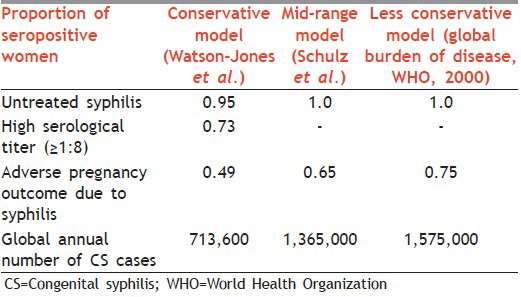
Table 1 gives estimates of annual incidence of CS.[5]
Table 1.
Estimates of annual incidence of CS
TRANSMISSION OF INFECTION
Transmission occurs transplacentally and may occur any time during gestation; however, risk of transmission to fetus depends on stage of maternal infection. Longer time that primary infection occurs before pregnancy, more benign the outcome with respect to rate and severity of infection. Untreated primary or secondary syphilis in pregnancy results in 25% risk of stillbirth, 14% risk of neonatal death, 41% risk of giving birth to a live but infected infant and 20% chance of giving birth to uninfected infant. Untreated late syphilis results in 12% risk of stillbirth, 9% risk of neonatal death, 2% risk of giving birth to infected infant, and 77% chance of giving birth to an uninfected infant. Women whose infection manifests itself during 1st year after delivery may also infect their infants.[2]
CLINICAL FEATURES
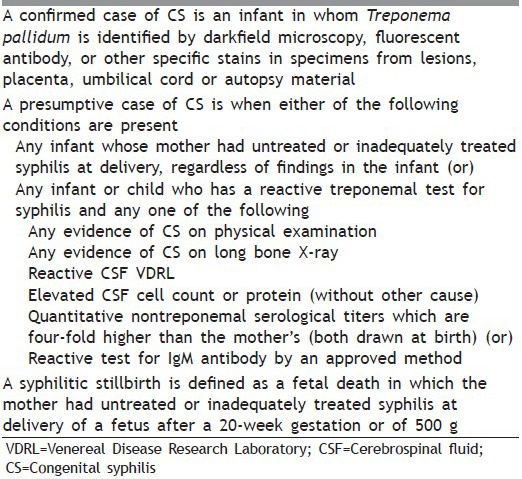
Tables 2 and 3 mention clinical findings related to CS in children younger than 1 year and older than 1 year, respectively. Table 4 gives surveillance case definition for CS.
Table 2.
Clinical findings related to CS in children younger than 1 year
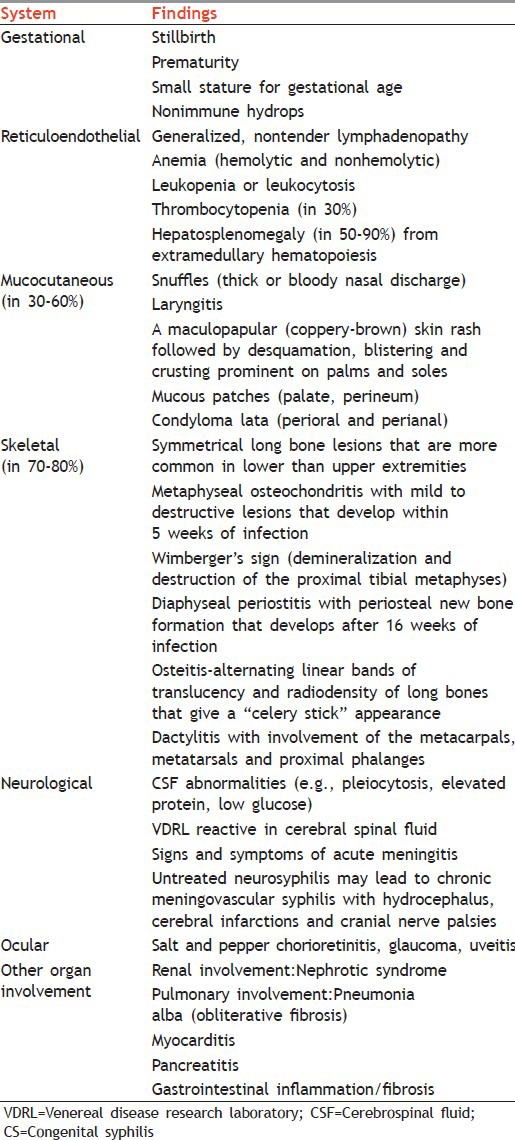
Table 3.
Clinical findings related to CS in children older than 1 year
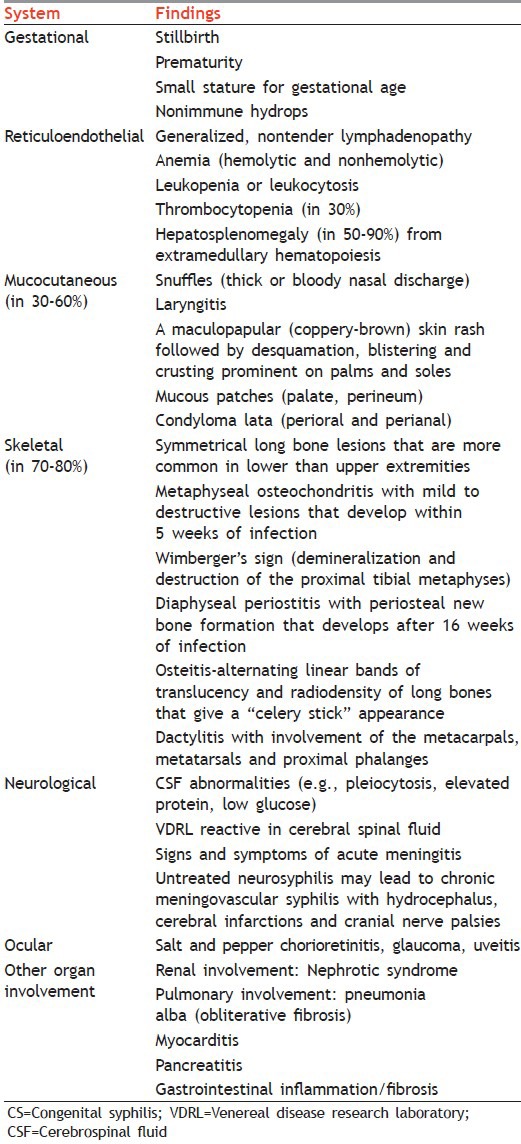
Table 4.
Surveillance case definition for CS
DIAGNOSIS OF CONGENITAL SYPHILIS
Suspicion can be first raised by positive maternal serology results. When diagnosis is suspected before or during delivery, histological examination of the placenta and cord should be performed for typical pathological changes and spirochetes. Ulcers or nasal discharge if present should be examined for spirochetes by dark field microscopy/fluorescent antibody techniques.
Definitive diagnosis can be established by demonstrating organism in tissue or body fluid by dark field microscopy, immunofluorescence or histopathology. Because maternal immunoglobulin M (IgM) does not cross placenta, detection of IgM by IgM assay (western blot) in infant indicates active infection. The sensitivity and specificity of the test is 83% and 90% respectively. Measurement of fetal IgM by fluorescent treponemal antibody absorption tests (FTA-ABS) or microhemagglutination assay-Treponema pallidum (MHA-TP) test is widely used, but it has high positive rate and the patient continues to have positive tests even after treatment. Newer tests include immunoassay, polymerase chain reaction and immunoblotting. Ideally all children should have both nontreponemal (venereal disease research laboratory [VDRL] or rapid plasma reagin [RPR]) and treponemal (FTA-ABS and/or MHA-TP) tests performed.
A presumptive serological diagnosis may also be made when
Infant's nontreponemal titer is four-fold higher than that of mother when both blood samples are drawn at the time of delivery
Infant's nontreponemal titer persists or increases after birth
Infant's treponemal antibody titer remains positive at 12-18 months of age.
In resource poor settings tests are restricted to use of VDRL or RPR. These tests detect IgG which is transferred transplacentally from mother to fetus making interpretation of a positive result difficult. Therefore, it is necessary to compare infant's titers with maternal serological titers using the same tests. Where testing during pregnancy is inadequate, testing women at delivery would identify maternal cases not detected during pregnancy.
ROLE OF LUMBAR PUNCTURE AND RADIOLOGY
Table 5 mentions the situations where radiology and lumbar puncture is warranted.
Table 5.
Situations where radiology and lumbar puncture is warranted

MANAGEMENT OF CONGENITAL Syphilis
WHO recommends that treatment of CS in developing countries should be based on the following:
Identifying maternal syphilis (by RPR) during pregnancy and/or at time of delivery
Identifying whether an infant is clinically symptomatic.
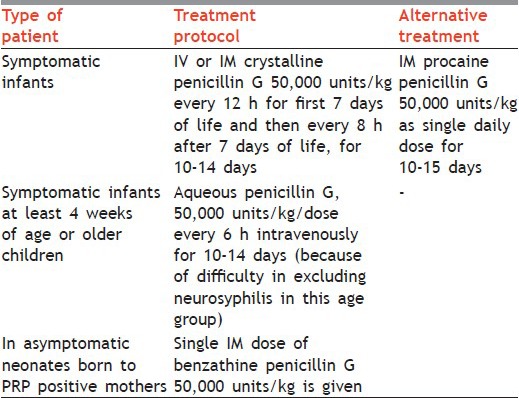
Treatment protocol is mentioned in Table 6.
Table 6.
Treatment protocol
Full course of penicillin to be given to infants in the situations is mentioned in Table 7.
Table 7.
Situations where full course of penicillin to is given to infants

Algorithm for treatment and diagnosis of CS is mentioned in Figure 1.
Figure 1.
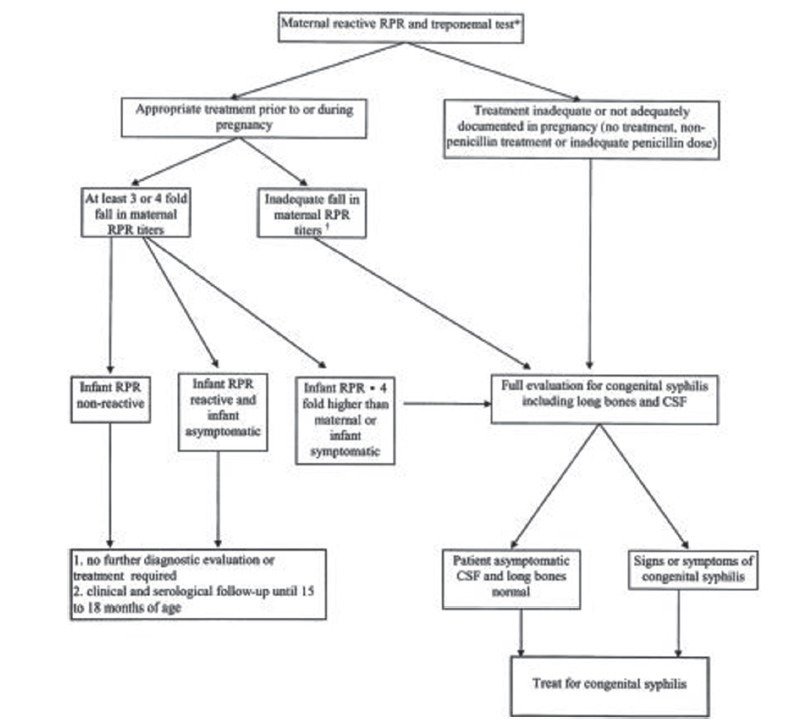
Algorithm for treatment and diagnosis of congenital syphilis
Alternative to immediate treatment is close follow-up with initiation of therapy when signs/symptoms of CS develop or when nontreponemal titers increase or fail to decline. Single dose benzathine penicillin G is recommended especially when follow-up cannot be assured, but failures occur with this regimen. Therefore, if adequate follow-up of an exposed, asymptomatic infant cannot be guaranteed, full course of therapy should be considered. Infants born to HIV coinfected mothers and in whom follow-up cannot be assured, should be treated for 10-14 days, regardless of maternal treatment history and symptomatology because the response to maternal treatment is unpredictable. Children born to mothers who develop signs or symptoms of secondary or early latent syphilis within 1 year after delivery should be tested and treated if serology is reactive.
SEROLOGICAL FOLLOW-UP
In children with CS, serial nontreponemal serology should be repeated at 1, 2, 4, 6, and 12 months. Transplacentally acquired nontreponemal antibody disappears in uninfected infants by 6 months of age and in treated children it becomes nonreactive by 12 months of age. Children with persistently positive titers, even at a low level, should be considered for the second course of treatment. Infants with treated neurosyphilis should have repeat cerebrospinal fluid (CSF) examination at 6-month intervals until results are normal. A positive CSF VDRL 6 months after treatment requires second course of treatment. Uninfected infants should also have serial nontreponemal titers, which should become nonreactive by 6 months.
WHY CONGENITAL SYPHILIS STILL CONTINUES TO BE A PROBLEM?
Poor antenatal care is a major factor for continued high incidence of CS, other factors being mentioned in Table 8.
Table 8.
Factor responsible for continued high incidence of CS

REDUCING SEVERITY AND BURDEN OF CONGENITAL SYPHILIS
Provider efforts to reduce CS in high-risk populations should focus on factors mentioned in Table 9. Since incidence of CS is related to prevalence of syphilis in population, strategies should be complemented by programs to prevent and control STIs and by syndromic management of STIs. Adjuvant strategies include establishing clear indicators and targets to ensure the adequacy of screening and monitoring and linking STIs services with mother and child health (MCH) services.
Table 9.
Important factors to be focused on to reduce CS in high-risk populations

Following factors are important in reducing the severity and burden of CS:
Preventing infection in women
CS can be prevented either by prevention or detection of infection in pregnant women. Programs promoting safe sex or control of STIs will prevent maternal infection, but if women become infected only screening programs can prevent effects of maternal infection on fetus. Hence these programs must be implemented during ANC.[8]
Access to ANC with emphasis on early access
In developing countries ANC is not available or not accessed; reasons ranging from fear of medical care to nonexistence of ANC. In industrialized countries problems like drug abuse interfere with access to ANC, while in developing countries women with syphilis are less likely to have risk factors which distinguish them from women without syphilis. Furthermore, women must access ANC early. It was earlier thought that fetus was protected from infection until 20th week of gestation due to placenta barrier which is not true. In developing countries first ANC visit occurs at 5th-6th week of gestation which may be late to prevent CS.[8]
Screening/testing of women
Ideal approach would be to screen women during the first trimester with nontreponemal tests and again early in the third trimester even in low prevalence population. Optimal approach would be to retest women who are at high risk or from high prevalence areas at 28 weeks gestation and closer to delivery as primary infection may occur after initial screening and there is a possibility of reinfection, especially if the partner is untreated. Minimal requirement would be that all women should undergo one screening test in their early pregnancy and if this does not happen they should be tested at delivery.
Provision of syphilis testing in ANC programs
Many ANC programs do not provide testing for syphilis and there is no national policy for this. Inability to maintain syphilis testing service that requires transportation of blood to centralized laboratory for testing is a major obstacle.[8]
On site testing
Solution to the problem of disassociation between testing and administrating treatment is use of on-site testing in antenatal clinics, allowing early receipt of reports leading to earlier treatment of mothers attending clinics late in their pregnancy. Furthermore, it would allow immediate identification of mothers who test positive, eliminating the need for costly and unreliable tracing. Onsite testing may have low sensitivity and may vary with site. Reduced sensitivity at lower titers is a minor concern since infants born to these mothers have a low risk of being infected. Furthermore, because the test has low positive predictive value about 9% women will be treated unnecessarily, which though not ideal, is unlikely to expose them to serious risks. However, there are difficulties like availability of trained staff and material. Furthermore, results obtained at clinics differ from those obtained from centralized laboratory. But decentralized testing leads to more women being detected and treated than centralized testing. Difficulties of conducting RPR at clinics has led to the development of technologically simple immunochromatographic strip tests, which is cost effective than centralized testing.[8]
Prompt receipt of results
For prompt receipt of results, tested women must return to clinic, or there must be notification system in place; frequently neither occur. Confidential system to notify women of their results at home would be a good alternative.[8]
Notification resulting in treatment
Treatment must be available at clinic itself as referral for therapy results in women being treated.
Interventions targeting vulnerable groups
Eliminating CS will only become possible if interventions targeting vulnerable groups are also implemented. Pan American Health Organization's role in eliminating CS being a good example.[6]
ECONOMIC PERSPECTIVE
Various analysis conclude that syphilis screening is cost effective and cost saving at threshold prevalence's <1% and therefore should continue because treating CS is expensive. Preventing an occasional case is economically worthwhile; also screening, detecting and treating infection in women can prevent consequences in women and their partners.[8]
PROGRAM PERSPECTIVE
In industrialized countries despite recommendations of screening for syphilis early in pregnancy many stillbirths and infections occur in infants (in US between 1992 and 1998 a mean of 135 infants annually died of CS). Biggest contributor to the occurrence of CS being failure to attend ANC. Programs focus on ensuring that pregnant women are tested at least once by the time of delivery, while some mandate that women at high-risk be tested again in the third trimester and yet again at the time of delivery.[8]
RECOMMENDED INTERVENTIONS
The WHO global strategy to eliminate congenital syphilis
Goal of WHO Strategy is an elimination of CS as public health problem, prevention of mother-to-child transmission (PMTCT) of syphilis through early antenatal care, treatment of sexual partners of infected women, and prophylactic treatment with a single dose of penicillin of all neonates born to RPR-positive mothers.[3]
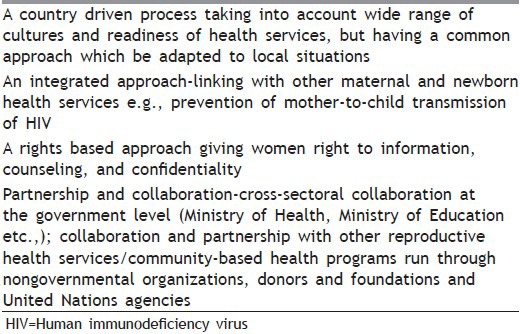
Table 10 mentions four pillars of WHO strategy (country level).[3] Table 11 gives four guiding principles for implementation.[3]
Table 10.
Four pillars of the WHO strategy (country level)

Table 11.
The four guiding principles for implementation
For success, work has to be done at following levels.
At international level
At international level prevention of CS should be made health system priority and following should be provided
Estimates of the global burden of CS
Active advocacy for CS prevention
Assessment of global CS prevention program policies
Program guidance for nation prevention programs
Links with international groups resulting in focused, coordinated international level effort.[8]
At national level
At national level, policy for testing and treating all pregnant women for syphilis should be developed giving it political and health care priority. At National level antenatal screening should be linked to programs for PMTCT of HIV and other programs to improve ANC. Evaluation of pilot programs to look for combined efficiencies would be useful. Community syphilis prevention and control efforts should be reinforced. Improvement of STIs services outside ANC, with efforts to notify infected male partners, would help achieve the goal of preventing women from getting infected.[8]
At local level
At the local level attendance early in pregnancy at ANC clinic should be encouraged. Laboratory testing should be decentralized.[8]
Challenges for congenital syphilis prevention at different levels
Successful implementation of syphilis screening programs depends on identifying barriers and then improving the healthcare system's capacity to provide services required. Challenges faced include
Lack of policy adherence
Lack of clarity regarding roles, responsibilities and accountability
Importance of integration of programs and services
Adequate access to maternal and newborn health services
Availability of onsite screening and prompt treatment
Lack of surveillance data
Lack of monitoring and evaluation.
Recommendations from review of evaluations of various programs are mentioned in Table 12.
Table 12.
Recommendations from the review of evaluations of programs

CONCLUSION
The elimination of CS can be achieved through implementation of various proven measures but requires technical support, funding and commitment among political forces, health officials, and public to prevent and treat CS cases and help countries reach their Millennium Development Goals. Stronger partnerships with clearly defined responsibilities should be developed among agencies responsible for national STI control, HIV/AIDS control, and making pregnancy safer initiatives to ensure universal coverage of CS control interventions.[10] Clinicians should adhere to standardized protocols in evaluation and management of at-risk newborns. Vigilant screening prenatally and at delivery and adequate follow-up are critical to reduce CS. Improved surveillance data and resources are needed for identification and follow-up of newborns at risk for CS.[11]
MULTIPLE CHOICE QUESTIONS
-
Q1.
What is the most common adverse pregnancy outcome in primigravida female having active syphilis?
- Neonatal Syphilis
- Stillbirth
- Prenatal Death
- Small for Date
-
Q2.
By what time does the transplacentally acquired nontreponemal antibody disappear in infected infants treated with penicillin?
- 12 months of age.
- 8 months of age
- 14 months of age
- 10 months
-
Q3.
Following are situations where radiology and lumbar puncture is warranted. Which is false?
- If an infant or child has signs or symptoms of CS
- If there is no documented maternal treatment in pregnancy
- If the mother was treated within 4 weeks of delivery
- If maternal treatment was inadequate or inadequately documented
- An eight-fold decline in titer following therapy was not documented
-
Q4.
Infants with neurosyphilis should have repeat CSF examination at six month intervals until results are normal. A second course of treatment is recommended if CSF VDRL is positive after __________ months of the first dose.
- 4months
- 12months
- 8months
- 6 months
-
Q5.
In which condition a presumptive serological diagnosis of neonatal syphilis may be made?
- Infant's nontreponemal titer is eight-fold higher than that of mother when both blood samples are drawn at the time of delivery.
- Infant's nontreponemal titer is four-fold higher than that of mother when both blood samples are drawn at the time of delivery.
- Infant's nontreponemal titer persists or decreases after birth.
- Infant's treponemal antibody titer remains positive at 20 months of age.
-
Q6.
A syphilitic stillbirth is defined as ________ ___________________________
- A fetal death in which the mother had untreated or inadequately treated syphilis at delivery of a fetus after a 22-week gestation or of 500 g.
- A fetal death in which the mother had untreated or inadequately treated syphilis at delivery of a fetus after a 24-week gestation or of 1500 g.
- A fetal death in which the mother had untreated or inadequately treated syphilis at delivery of a fetus after a 20-week gestation or of 500 g.
-
Q7.
What is Wimberger's sign?
- demineralization and destruction of the proximal tibial metaphyses.
- demineralization and destruction of the distal tibial metaphyses.
- demineralization and destruction of the proximal femoral metaphyses.
- demineralization and destruction of the distal femoral metaphyses.
-
Q8.
Cutaneous manifestation seen in congenital syphilis but not in acquired syphilis.
- Maculopapular rash
- Vesiculo-Bullous rash
- Mucous patch
- Condyloma lata
Answers: 1. B, 2. A, 3. E, 4. D, 5. B, 6. D, 7. A, 8. B
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Saloojee H, Velaphi S, Goga Y, Afadapa N, Steen R, Lincetto O. The prevention and management of congenital syphilis: An overview and recommendations. Bull World Health Organ. 2004;82:424–30. [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold SR, Ford-Jones EL. Congenital syphilis: A guide to diagnosis and management. Paediatr Child Health. 2000;5:463–9. doi: 10.1093/pch/5.8.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid GP, Stoner BP, Hawkes S, Broutet N. The need and plan for global elimination of congenital syphilis. Sex Transm Dis. 2007;34:S5–10. doi: 10.1097/01.olq.0000261456.09797.1b. [DOI] [PubMed] [Google Scholar]
- 4.Sharma VK, Khandpur S. Changing patterns of sexually transmitted infections in India. Natl Med J India. 2004;17:310–9. [PubMed] [Google Scholar]
- 5.Syphilis during pregnancy. The Global Elimination of Congenital Syphilis: Rationale and Strategy for Action. WHO. 2000:3–5. [Google Scholar]
- 6.Valderrama J, Zacarías F, Mazin R. Maternal syphilis and congenital syphilis in Latin America: Big problem, simple solution. Rev Panam Salud Publica. 2004;16:211–7. doi: 10.1590/s1020-49892004000900012. [DOI] [PubMed] [Google Scholar]
- 7.Warner L, Rochat RW, Fichtner RR, Stoll BJ, Nathan L, Toomey KE. Missed opportunities for congenital syphilis prevention in an urban southeastern hospital. Sex Transm Dis. 2001;28:92–8. doi: 10.1097/00007435-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Schmid G. Economic and programmatic aspects of congenital syphilis prevention. Bull World Health Organ. 2004;82:402–9. [PMC free article] [PubMed] [Google Scholar]
- 9.WHO; 2007. Challenges for congenital syphilis prevention at different levels of the health system. The Global Elimination of Congenital Syphilis: Rationale and Strategy for Action; pp. 10–3. [Google Scholar]
- 10.Hossain M, Broutet N, Hawkes S. The elimination of congenital syphilis: A comparison of the proposed World Health Organization action plan for the elimination of congenital syphilis with existing national maternal and congenital syphilis policies. Sex Transm Dis. 2007;34:S22–30. doi: 10.1097/01.olq.0000261049.84824.40. [DOI] [PubMed] [Google Scholar]
- 11.Martin D, Bertrand J, McKegney C, Thompson L, Belongia E, Mills W. Congenital syphilis surveillance and newborn evaluation in a low-incidence state. Arch Pediatr Adolesc Med. 2001;155:140–4. doi: 10.1001/archpedi.155.2.140. [DOI] [PubMed] [Google Scholar]


