Abstract
Objective
Longitudinal neuropsychological assessments were performed to determine if adjuvant chemotherapy was associated with cognitive dysfunction in men with non-seminomatous germ cell tumors (NSGCT).
Methods
Patients with NSGCT status post orchiectomy that either received adjuvant chemotherapy (n=55) or did not (n=14) were recruited. Patients were tested before chemotherapy, one week post chemotherapy (or three months later in the surveillance group) and 12 months after the baseline evaluation.
Results
Compared to the surveillance group, patients treated with chemotherapy had higher rates of cognitive decline at 12 months (overall cognitive decline: 0%, 52% and 67% in the surveillance, LE and HE group, respectively), greater number of tests that declined (mean of 0.1, 1.4 and 2.0 in the surveillance, LE and HE group, respectively), and more frequent worsening in motor dexterity (0%, 48% and 46% in the surveillance, LE and HE group, respectively). Compared to the surveillance group, patients receiving more cycles of chemotherapy demonstrated worse psychomotor speed and learning and memory. Younger age was associated with greater incidence of overall cognitive decline at 12 month follow-up.
Conclusions
Men with NSGCT that received chemotherapy demonstrated greater rates of cognitive decline in a dose-response manner. Reductions in motor dexterity were most common. Decline in learning and memory also was evident particularly at later follow up time points and in men receiving more chemotherapy. Men that receive chemotherapy for NSGCT are at risk for cognitive decline and may benefit from monitoring and referral for psychosocial care.
Keywords: Chemotherapy, Cognitive Function, Neurotoxicity, Quality of Life, Testicular Cancer, Oncology
INTRODUCTION
Cognitive dysfunction in cancer patients is receiving increased attention as a survivorship issue due to its potential to interfere with occupational, scholastic, and social activities. We previously reported cognitive impairment in men with newly diagnosed non-seminomatous germ cell tumors (NSGCT) of the testis prior to receipt of chemotherapy [1]. Similar findings have been reported for patients with breast [2-5], prostate [6], and small cell lung cancer [7]. Recent preclinical studies have shed light on the neurobiologic mechanisms of neurotoxicities associated with some chemotherapies – the interested reader is referred to the following reviews [8,9].
Studies with longitudinal designs that include cognitive testing prior to chemotherapy are necessary to identify treatment-related cognitive decline [10,11]. Reports from longitudinal trials have documented treatment-related cognitive decline in 13-70% of patients with breast cancer who receive chemotherapy [12]. To date, three retrospective cross-sectional study [13-15] and one prospective study has that conducted longitudinal testing in a histologically mixed sample of testicular cancer patients [16] have been reported. Skaali et al. found no difference in rates of cognitive change 1-year after completion of chemotherapy between patients that received no chemotherapy, one cycle of chemotherapy or multiple cycles of chemotherapy. However, rates of decline on individual cognitive tests ranged from 0-7% in patients who did not receive chemotherapy versus 0-23% in patients who received chemotherapy. Additionally, the development of tinnitus (a well-known neurotoxicity of platinum-based chemotherapies) was 3.5 times more frequent (21% versus 6%) in patients with cognitive decline versus those without cognitive decline.
Given the excellent long term survival of many testicular cancer patients, it is of critical importance to understand the nature, extent and temporal course of disease-related symptoms and treatment-related toxicities to help direct survivorship research and clinical care [17].
METHODS
Study Site and Participants
Newly diagnosed patients with NSGCT from the genitourinary medical service of MD Anderson Cancer Center, Houston, Texas were recruited. Details about eligibility requirements and recruitment have been previously published [1]. Upon enrollment, the research staff obtained informed consent from all participants.
Procedures
A systematic, consecutive sampling procedure was used to identify participants. Patients with newly diagnosed NSGCT were identified from clinic schedules. Research staff reviewed medical records to evaluate the eligibility criteria. Eligible patients were recruited to the study after orchiectomy but prior to receiving adjuvant treatment. Two groups of patients were recruited: a surveillance group that did not receive adjuvant therapy and a group that received adjuvant chemotherapy. The chemotherapy group was further separated into a low exposure (2-3 cycles of chemotherapy) and a high exposure group (4-7 cycles of chemotherapy). At the time of study enrollment, participants completed a baseline assessment consisting of cognitive tests and self-report measures. A “post-treatment” assessment was completed one week after adjuvant chemotherapy, or 3 months after the baseline assessment for participants who did not receive chemotherapy. The final assessment was completed 12 months after baseline. Each assessment required 60 minutes. The study was approved by the institutional review board of MD Anderson Cancer Center.
Measures
A battery of 6 neuropsychological tests assessing multiple cognitive domains including attention, psychomotor speed, learning and memory, language, executive, and motor function was administered (see Wefel et al. [1]). Published normative data that adjust for age, education, handedness and gender where appropriate were used to convert raw test scores to standardized scores (z-scores; mean = 0, SD = 1) to facilitate comparisons among measures. Alternate forms were used when available to minimize practice effects.
The self-report assessment consisted of sociodemographic and psychosocial measures. Depressive symptoms were assessed with the Centers for Epidemiologic Studies – Depression (CES-D) scale [18] and anxiety was assessed using the State-Trait Anxiety Inventory (STAIS) [19]. Clinically significant symptoms of distress (i.e., depressive or anxious symptoms) were defined as ratings on the CES-D ≥ 27 (raw score) and STAIS ≤ 5th percentile. Evidence from medically ill populations suggests the cut off score of 27 on the CES-D provides better sensitivity and specificity compared to other commonly used cut offs [20,21].
Disease stage and tumor marker (i.e., AFP, hCG, and LDH) data were collected from the medical records. Staging was determined using the American Joint Committee on Cancer Staging for Testicular Germ Cell Tumors criteria [22] and risk categories were determined as defined by the International Germ Cell Cancer Collaborative Group (IGCCCG) [23].
Statistical Analysis
Change in cognitive function was determined using the standardized regression-based (SRB) approach used by Stewart et al.[24] and proposed by Mcsweeny et. al. [25] and Sawrie et. al.[26] SRB regression models were developed using the standardized scores for each cognitive test. Age and education were included as covariates in all regression models given their well-known relationship with cognitive function. Using the surveillance group, the 3-month post-treatment follow-up time point was regressed on the baseline time point for the first model (post-treatment model), and the 12-month follow-up time was regressed on the 3-month post-treatment follow-up for the second model (12-month model). An SRB score at the 3-month post-treatment time point was generated for each subject by subtracting the actual score from the post-treatment model predicted score, and then dividing by the standard error of the estimate derived from the surveillance group. The SRB score at the 12-month time point was similarly generated using the 12-month model. This score is expressed in standard deviation units and reflects either improvement or decline in cognitive function. Cognitive decline on a specific test was defined as an SRB score ≤ −2.0. Cognitive improvement on a specific test was defined as an SRB score of ≥+2.0. Overall cognitive decline was defined as declining on ≥2 tests and overall cognitive improvement was defined as improving on ≥2 tests.
SRB change scores were computed and percent declined or improved on each test was compared between treatment groups. To test for treatment group differences, Chi-square and Fisher's exact test were used to identify differences in the percentages of people declining on each neurocognitive test and in the incidence of overall cognitive decline at the post-treatment and 12-month follow-up time points. ANOVA and Kruskal-Wallis tests were utilized to test if the number of tests showing decline was different between groups. Fisher's exact test and independent sample tests were used to identify predictors of decline/improvement. Unadjusted results are presented due to the exploratory nature of this study. Results were summarized using frequencies, percentages, means, standard deviations and p-values. All analyses were conducted using SAS version 9.1 and SPSS 12.0.
RESULTS
Sixty-nine patients with NSGCT completed neuropsychological evaluation (Table 1). Mean age was 31.0 (± 7.5) years (range 18.5 – 50.7 years). On average, patients had completed 14.6 (± 2.7) years of education (range 8 – 20 years). Ethnically, 52 (75%) were Caucasian, 12 (17%) were Hispanic, 3 (4%) were African American and 2 (2%) were of other ethnicities.
Table 1.
Baseline Differences in Demographic and Clinical Factors, and Cognitive Test Results by Treatment Group
| DOMAIN | TEST | S (N=14) | LE (N=25) | HE (N=30) | P-Value |
|---|---|---|---|---|---|
|
Mean (SD)
| |||||
| Attention | DSpan1 | −0.2 (0.6) | −0.1 (0.8) | 0.03 (1.0) | .672 |
| Psychomotor Speed | DSymbol1 | 0.4 (0.6) | 0.3 (1.0) | 0.3 (1.0) | .944 |
| TMTA1 | 0.7 (0.6) | 0.3 (0.7) | 0.1 (1.3) | .787 | |
| Learning and Memory | HVLT1 | −0.4 (0.9) | −1.2 (1.5) | −1.1 (1.2) | .561 |
| Executive | TMTB1 | −0.2 (2.5) | −0.4 (1.8) | −0.8 (2.1) | .427 |
| Language | COWA1 | 0.5 (0.9) | 0.2 (0.9) | 0.2 (1.0) | .684 |
| Motor | GPD1 | −0.2 (0.8) | −0 .9 (1.0) | −1.1 (1.7) | .135 |
| GPND1 | −0.5 (0.9) | −0.6 (0.9) | −0.7 (1.1) | .429 | |
| Age (years) | 34.4 (5.7) | 32.4 (7.4) | 28.4 (7.5) | .065 | |
| Education (years) | 15.4 (1.9) | 14.5 (3.1) | 14.3 (2.6) | .385 | |
| Depression | CESD2 | 10.2 (9.7) | 15.6 (11.5) | 12.1 (10.3) | .470 |
| Anxiety | STAIS1 | 0.6 (1.0) | −0.3 (1.2) | 0.1 (1.2) | .103 |
|
% (N) | |||||
| Stage of Disease | |||||
| Stage 1 | 92.9 (13) | 76.0(19) | 60.0 (18) | ||
| Stage 2 | 7.1 (1) | 16.0(4) | 16.7 (5) | .174 | |
| Stage 3 | 0 (0) | 8.0(2) | 23.3 (7) | ||
Note: S=Surveillance; LE=Low Exposure Chemotherapy; HE=High Exposure Chemotherapy
Z-score
Raw score
DSpan: WAIS-R Digit Span30, DSymbol: WAIS-R Digit Symbol30, TMTA: Trail Making Test A31, HVLT: Hopkins Verbal Learning Test32, TMTB: Trail Making Test B31, COWA: Controlled Oral Word Association33, GPD/ND: Lafayette Grooved Pegboard Dominant/Non-Dominant Hand34, CESD: Center for Epidemiological Studies – Depression18, STAIS: State-Trait Anxiety Inventory – State19
Low exposure (LE) chemotherapy patients (n=25) received the following regimens: CEB × 2 (n=4), CEB × 3 (n=1), BEP × 2 (n=9), BEP × 3 (n=10), and BEP × 2 and ATP × 1 (n=1). High exposure (HE) chemotherapy patients (n=30) received the following regimens: CEB × 4 (n=3), BEP × 4 (n=11), EP × 4 (n= 1), BEP × 2 and EP × 2 (n=2); and one patient received each of the following regimens: CISCA/VB × 4, CISCA/VB × 1 and ACE × 3, CISCA/VB × 5, CISCA/VB × 6, CISCA/VB × 2 and ACE × 2 and BEP × 2, CISCA/VB × 2 and ACE × 3 and BOP × 1 and POMB × 1, BEP × 4 and ATP × 2, BEP × 4 and VIP × 2, and, BEP × 3 and CISCA × 2 and EP × 1. The median and Fisher's exact tests showed no significant differences between groups at baseline on age, education, stage of disease, depression, anxiety or any of the neurocognitive tests (Table 1).
Sixty-two patients completed the post-treatment assessment and 54 patients completed the 12-month assessment. Attrition was highest in the surveillance group, 21.4 % at the post-treatment follow-up and 35.7% at the 12-month follow-up. Attrition in the LE group was 8% and 16% at the post-treatment and 12-month follow-up assessments, respectively. The HE group's attrition rates were 6.7% and 20% at the post-treatment and 12-month follow-up assessments, respectively. Baseline comparisons between completers and dropouts at the post-treatment and 12-month follow-up showed no differences in cognitive test performance, age, education, stage of disease, CESD or STAIS.
SRB Change Scores Over Time – Baseline to Post-treatment Follow-Up
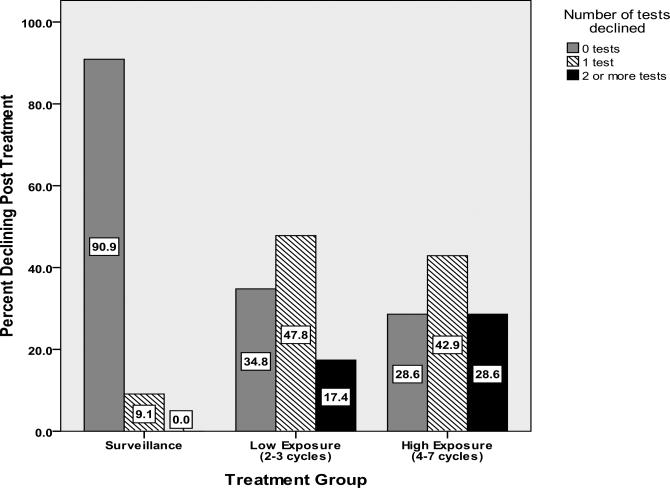
Both LE and HE groups (vs. surveillance group) demonstrated greater rates of decline in dominant hand fine motor dexterity (Table 2). The HE group additionally evidenced greater decline on a measure of psychomotor speed. There were no statistically significant differences in the frequency of overall cognitive decline, although there was a trend suggesting greater impairment that appeared to be related to the extent of exposure to treatment, with 0% showing overall cognitive decline in the surveillance group, 17%% in the LE group and 29% in the HE group. Using ANOVA, both the LE and HE groups declined on significantly more tests than the surveillance group (Table 2, Figure 1). These results were verified with the non-parametric Kruskal-Wallis test.
Table 2.
Cognitive Decline From Baseline to Post-treatment by Treatment Group
| DOMAIN | TEST | S (N=11) | LE (N=23) | HE (N=28) | FISHER'S EXACT P (LE vs S) | FISHER'S EXACT P (HE vs S) |
|---|---|---|---|---|---|---|
|
PERCENT DECLINED
| ||||||
| Attention | DSpan | 0 | 0 | 0 | * | * |
| Psychomotor Speed | DSymbol | 0 | 9 | 39 | 1.0 | 0.017 |
| TMTA | 0 | 4 | 14 | 1.0 | 0.309 | |
| Learning and Memory | HVLT | 0 | 4 | 0 | 1.0 | * |
| Executive | TMTB | 9 | 4 | 7 | 1.0 | 1.0 |
| Language | COWA | 0 | 0 | 0 | * | * |
| Motor | GPD | 0 | 35 | 39 | 0.034 | 0.017 |
| GPND | 0 | 22 | 14 | 0.150 | 0.309 | |
| Overall Decline# | 0 | 17 | 29 | 0.280 | 0.08 | |
|
MEAN (SD) | ||||||
| Number Tests Declined (ANOVA) | 0.1 (0.3) | 0.8 (0.9) | 1.1 (1.0) | 0.05 | 0.005 | |
Note: S=Surveillance; LE=Low Exposure Chemotherapy; HE=High Exposure Chemotherapy; DSpan: WAIS-R Digit Span, DSymbol: WAIS-R Digit Symbol, TMTA: Trail Making Test A, HVLT: Hopkins Verbal Learning Test, TMTB: Trail Making Test B, COWA: Controlled Oral Word Association, GPD/ND: Lafayette Grooved Pegboard Dominant/Non-Dominant Hand
Decline on ≥ 2 tests
No statistics computed due to zero declines in both groups
Figure 1.
Cognitive Decline From Baseline to Post-treatment on 0,1 and 2+ Tests by Group.
There were no between group differences in overall cognitive improvement. Compared to the surveillance group, the LE group demonstrated more frequent improvement on a measure of psychomotor speed (DSymbol: surveillance=0%, LE=39%, HE=7%; p<0.05). There were no other statistically significant differences on any individual cognitive test. However, the HE group improved on significantly more tests than the surveillance group (Kruskal Wallis p<0.05).
SRB Change Scores Over Time – Post-treatment to 12-Month Follow-Up
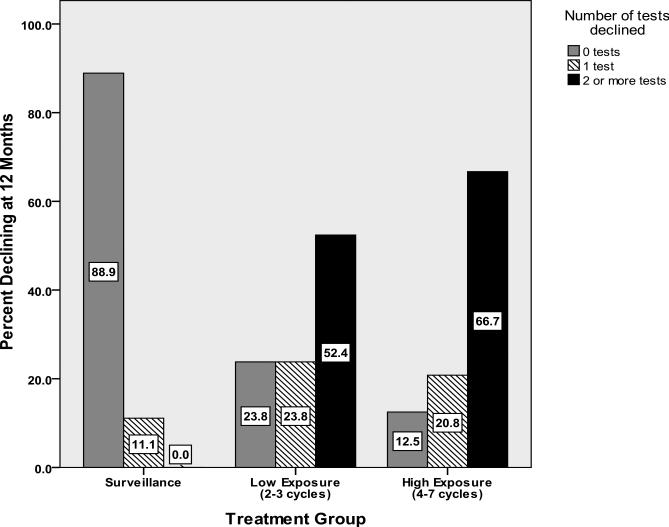
Both LE and HE groups demonstrated greater rates of decline (vs. surveillance group) in dominant hand fine motor dexterity (Table 3). The HE group additionally evidenced greater decline on a measure of psychomotor speed. Learning and memory showed a trend toward greater decline in the HE group. LE and HE groups evidenced significantly greater rates of overall cognitive decline and declined on more tests compared to the surveillance group (Table 3, Figure 2).
Table 3.
Cognitive Decline From Post-treatment to 12 Month Follow-Up by Treatment Group
| DOMAIN | TEST | S (N=9) | LE (N=21) | HE (N=24) | FISHER'S EXACT P (LE vs S) | FISHER'S EXACT P (HE vs S) |
|---|---|---|---|---|---|---|
|
PERCENT DECLINED
| ||||||
| Attention | DSpan | 0 | 10 | 0 | 1.0 | * |
| Psychomotor Speed | DSymbol | 0 | 0 | 0 | * | * |
| TMTA | 0 | 19 | 58 | 0.287 | <0.005 | |
| Learning and Memory | HVLT | 0 | 14 | 33 | 0.534 | 0.073 |
| Executive | TMTB | 0 | 14 | 21 | 0.534 | 0.290 |
| Language | COWA | 11 | 24 | 13 | 0.637 | 1.0 |
| Motor | GPD | 0 | 10 | 25 | 1.0 | 0.156 |
| GPND | 0 | 48 | 46 | 0.013 | 0.015 | |
| Overall Decline# | 0 | 52 | 67 | 0.006 | 0.001 | |
|
MEAN (SD) | ||||||
| Number Tests Declined (ANOVA) | 0.1 (0.3) | 1.4 (1.0) | 2.0 (1.2) | 0.001 | <0.0001 | |
Note: S=Surveillance; LE=Low Exposure Chemotherapy; HE=High Exposure Chemotherapy; DSpan: WAIS-R Digit Span, DSymbol: WAIS-R Digit Symbol, TMTA: Trail Making Test A, HVLT: Hopkins Verbal Learning Test, TMTB: Trail Making Test B, COWA: Controlled Oral Word Association, GPD/ND: Lafayette Grooved Pegboard Dominant/Non-Dominant Hand
Decline on ≥ 2 tests
No statistics computed due to zero declines in both groups
Figure 2.
Cognitive Decline From Post-treatment to 12 Months on 0,1 and 2+ Tests by Group.
The LE and HE groups did not demonstrate a statistically significant difference (vs. surveillance group) in rates of improvement on any test or in overall improvement. However, parametric and non-parametric analyses demonstrated that both the LE and HE groups improved on significantly more tests than the surveillance group (ANOVA p <0.05, Kruskall Wallis p <0.05).
Predictors of Cognitive Change at the Post-treatment and 12-Month Follow-Up
Bivariate exploratory analyses using Fisher's Exact tests and independent sample tests were used to examine if the following covariates exhibited potential association with overall cognitive decline or improvement at the post-treatment and 12-month time points: biomarkers (AFP, HCG, LDH), age, depression, state anxiety, years of education, risk status based on the IGCCCG, baseline cognitive impairment and stage of disease. Overall decline post-treatment was not associated with any of these predictors. At the 12-month follow-up, only age was related to overall decline, with those who declined being younger (mean age=28.1 years; SD=7.5) compared to non-decliners (mean age=33.6; SD=6.8). There was no relationship between any of the predictors and overall cognitive improvement at the post-treatment or 12-month follow-up.
DISCUSSION
Men with NSGCT who received chemotherapy evidenced more frequent overall cognitive decline and decrease in psychomotor speed. They also evidenced decline on a greater number of tests at both the post-treatment and 12-month follow-up time points when compared to men with NSGCT who did not receive chemotherapy. Additionally, decline in learning and memory at the 12-month follow-up was more common in men who received more cycles of chemotherapy. At both time points there appeared to be a “dose-response” relationship suggesting that greater chemotherapy exposure was associated with decline on more tests and greater overall cognitive decline. However, it is not possible to distinguish if the impact on cognition was due to the dose versus the regimen of chemotherapy given.
Our exploratory analysis indicated that younger age was potentially associated with a greater incidence of overall cognitive decline at the 12-month follow-up. However, after controlling for chemotherapy group due to trends toward statistically significant differences in mean age between groups, this effect was no longer significant. There was no association between stage of disease, risk status, clinical biomarkers, baseline cognitive impairment, education, or changes in mood and the development of cognitive decline or improvement at any time point. It is possible that younger patients were more at risk for androgen suppression and the associated adverse effects on cognition [27]. Unfortunately, testosterone levels were not serially monitored in the current study. It is also possible that this finding is an artifact of age being confounded with treatment exposure.
At the post-treatment time point, men treated with chemotherapy were more likely to demonstrate improvements in psychomotor speed. Similarly, men treated with chemotherapy demonstrated improvement on a greater number of tests at the 12-month follow-up time point. Although there were no mean group differences on cognitive tests at baseline, more men treated with chemotherapy were performing at lower levels on 6 out of 8 tests, which may have resulted in greater rates of regression to the mean and the appearance of more frequent improvement. Improvement may also reflect beneficial effects of treatment on unexamined disease factors. Importantly, there were not between group differences in distributions of patients performing at the upper limits of the tests that would confound interpretation of differential rates of decline.
Many of the patients received chemotherapy regimens with platinum-containing agents, which are known to be associated with peripheral neuropathy. It is therefore not surprising that they exhibited declines in upper extremity fine motor dexterity and tests of psychomotor function. Surprisingly, Skaali et al. [16] did not report adverse effects on motor function in their patients who were also treated with platinum-containing regimens.
Using a similar battery of tests that assessed similar cognitive domains, Skaali et al.[16] reported cognitive decline in approximately 15% of patients treated with chemotherapy. In our study, cognitive decline post-treatment was identified in 17% and 29% in the LE and HE exposure groups respectively. At 12-months, cognitive decline was identified in 52% and 67% of the LE and HE exposure groups, respectively. The difference in rates of cognitive impairment between the current study and that of Skaali et al. is quite substantial, particularly at the 12-month follow-up. This may be due in part to our sample being more heavily treated; however, even our LE chemotherapy group experienced much higher rates of cognitive decline.
The observation of increased rates of cognitive decline at the 12-month follow-up when patients were off treatment calls attention to potential late effects [11] and the survivorship issues these cancer patients must face [17]. Additional longitudinal studies with long term post-treatment follow-up assessments appear warranted to examine the course of cognitive decline after completion of chemotherapy.
There was evidence of adverse impact on learning and memory; however, this appears to have occurred less frequently than we have seen in women with breast cancer [11]. In the present study we employed an SRB approach to define cognitive change as it has been previously demonstrated to identify cognitive decline more frequently compared to a reliable change index (RCI) approach and can simultaneously control for the impact of covariates [28]. However, the surveillance sample used to generate the regression equations was small and may not be representative of the larger NSGCT population that does not receive chemotherapy. When we analyzed our data using the practice effect adjusted RCI methodology (RCI+PE, data not shown), as we have previously done in studies of chemotherapy in breast cancer survivors [11], there were no statistically significant between group effects on any test, lower rates of treatment associated decline in memory function (18%, 4%, 11%, respectively, for the surveillance, LE and HE groups) than that observed with the SRB methodology, similar rates of overall decline post-treatment ( 9%, 22% and 29%, respectively, for the surveillance, LE and HE groups) and lower rates of cognitive decline at the 12-month follow-up time point (0%, 14% and 8%, respectively, for the surveillance, LE and HE groups). However, we must caution about making direct comparisons between the SRB results in men with NSGCT and the RCI+PE results in women with BC. As demonstrated by Ouimet et al. [28], these approaches can yield different results due to the fact that SRB methodology allows for inclusion of covariates and predicts change scores using the same test-retest interval for all groups. When considering the RCI+PE analyses, compared to our previous studies demonstrating chemotherapy related cognitive dysfunction in women with BC that received largely FAC-based chemotherapies, it appears that men with NSGCT who receive platinum-containing regimens have less frequent decline in memory, which deserves further attention and may reflect differential effects of chemotherapy regimens and differences in other aspects of the patient populations (e.g., age).
Although our consent rate was good (76%) in this relatively rare tumor type, the small sample sizes in each group, high attrition especially in the surveillance group and heterogeneous chemotherapy regimens in the LE and HE groups are limitations that may impact the power of the analyses. Additionally, the current sample derived from a large tertiary cancer center may not be representative of all NSGCT patients.
We previously reported an unexpectedly high incidence of pretreatment cognitive dysfunction in men with NSGCT [1]. The results of the current study further demonstrate that men who receive chemotherapy experience greater declines in motor function that is likely consistent with the development of peripheral neuropathy in individuals treated with platinum-containing agents. Additionally, greater exposure to chemotherapy was associated with stronger effects on overall cognitive decline and the number of tests showing decline at post-treatment and the 12-month follow-up as well as more frequent decline in learning and memory at the 12-month follow-up. The observation of a “dose-response” relationship between chemotherapy exposure and cognitive dysfunction has been previously reported in women with breast cancer [29] and suggests that NSGCT patients with greater chemotherapy exposure are at increased risk for adverse cognitive outcomes and may benefit from closer monitoring. However, we are not able to rule out an alternative explanation that the regimens used in the HE group, and not the “dose”, are the primary cause of cognitive decline. While there was no evidence that any demographic, clinical, mood or biomarker variable was associated with cognitive dysfunction immediately after chemotherapy, younger age was associated with cognitive decline at the long-term follow up time point. Replication of this finding is necessary in order to establish if this is a reliable predictor of an at-risk subgroup that may benefit from closer monitoring or risk adapted therapy. Due to the absence of longitudinal data on hormonal function we were unable to determine if alterations in men's hormonal milieu contribute to cognitive dysfunction.
Given the epidemiology of NSGCT, most men are young at the time of diagnosis, in the midst of their careers and with numerous social demands that require optimal cognitive functioning. Identifying affected patients is important so that psychosocial support and compensatory interventions may be offered to patients experiencing these symptoms.
Acknowledgments
Sponsor: This work was supported by the Lance Armstrong Foundation [award to E.R.G.].
REFERENCES
- 1.Wefel JS, Vidrine DJ, Veramonti TL, et al. Cognitive impairment in men with testicular cancer prior to adjuvant therapy. Cancer. 2011;117:190–6. doi: 10.1002/cncr.25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wefel JS, Lenzi R, Theriault R, et al. ‘Chemobrain’ in breast carcinoma?: a prologue. Cancer. 2004;101:466–75. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 3.Cimprich B, So H, Ronis DL, et al. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14:70–8. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 4.Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–13. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- 5.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–52. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohile SG, Lacy M, Rodin M, et al. Cognitive effects of androgen deprivation therapy in an older cohort of men with prostate cancer. Crit Rev Oncol Hematol. 2010;75:152–59. doi: 10.1016/j.critrevonc.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyers CA, Byrne KS, Komaki R. Cognitive deficits in patients with small cell lung cancer before and after chemotherapy. Lung Cancer. 1995;12:231–35. doi: 10.1016/0169-5002(95)00446-8. [DOI] [PubMed] [Google Scholar]
- 8.Seigers R, Schagen SB, Van Tellingen O, Dietrich J. Chemotherapy-related cognitive dysfunction: current animal studies and future directions. Brain Imaging Behav. 2013 doi: 10.1007/s11682-013-9250-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35:729–41. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Wefel JS, Lenzi R, Theriault RL, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–99. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 11.Wefel JS, Saleeba AK, Buzdar AU, et al. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–56. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 12.Wefel JS, Vardy J, Ahles T, et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncology. 2011;12:660–68. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 13.Schagen SB, Boogerd W, Muller MJ, et al. Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncol. 2008;47:63–70. doi: 10.1080/02841860701518058. [DOI] [PubMed] [Google Scholar]
- 14.Gritz ER, Wellisch DK, Landsverk JA. Psychosocial sequelae in long-term survivors of testicular cancer. J Psychosoc Oncol. 1988;6:41–63. [Google Scholar]
- 15.Pedersen AD, Rossen P, Mehlsen MY, Pedersen CG, Zachariae R, von der Maase H. Long-term cognitive function following chemotherapy in patients with testicular cancer. J Int Neuropsychol Soc. 2009;15:296–301. doi: 10.1017/S1355617709090316. [DOI] [PubMed] [Google Scholar]
- 16.Skaali T, Fossa SD, Andersson S, et al. A prospective study of neuropsychological functioning in testicular cancer patients. Ann Oncol. 2011;22:1062–70. doi: 10.1093/annonc/mdq553. [DOI] [PubMed] [Google Scholar]
- 17.Travis LB, Beard C, Allan JM, et al. Testicular cancer survivorship: research strategies and recommendations. J Natl Cancer Inst. 2010;102:1114–30. doi: 10.1093/jnci/djq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 19.Spielberger CD, Gorsuch RL, Lushene R, et al. The State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- 20.Zich JM, Attkisson CC, Greenfield TK. Screening for depression in primary care clinics: the CES-D and the BDI. Int J Psychiatry Med. 1990;20:259–77. doi: 10.2190/LYKR-7VHP-YJEM-MKM2. [DOI] [PubMed] [Google Scholar]
- 21.Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain. 1979;13:163–70. doi: 10.1097/00002508-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Green FL, Fritz AG, Balch CM, et al. AJCC Cancer Staging Manual. 6th ed. Springer; New York: 2002. [Google Scholar]
- 23.Group IGCC International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. Journal of Clinical Oncology. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 24.Stewart A, Collins B, Mackenzie J, et al. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology. 2008;17:122–30. doi: 10.1002/pon.1210. [DOI] [PubMed] [Google Scholar]
- 25.McSweeny AJ, Chelune GJ, Naugle RI, et al. “T Scores for change”: an illustration of a regression approach to depicting change in clinical neuropsychology. The Clinical Neuropsychologist. 1993;7:300–12. [Google Scholar]
- 26.Sawrie SM, Marson DC, Boothe AL, et al. A method for assessing clinically relevant individual cognitive change in older adult populations. J Gerontol B Psychol Sci Soc Sci. 1999;54:116–24. doi: 10.1093/geronb/54b.2.p116. [DOI] [PubMed] [Google Scholar]
- 27.Green HJ, Pakenham KI, Headley BC, et al. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: a randomized controlled trial. BJU Int. 2002;90:427–32. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- 28.Ouimet LA, Stewart A, Collins B, et al. Measuring neuropsychological change following breast cancer treatment: an analysis of statistical models. J Clin Exp Neuropsychol. 2009;31:73–89. doi: 10.1080/13803390801992725. [DOI] [PubMed] [Google Scholar]
- 29.van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, Rodenhuis S. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–8. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; San Antonio: 1981. [Google Scholar]
- 31.Reitan RM. Trail Making Test Manual for Administration and Scoring. Reitan Neuropsychology Laboratory; Tucson: 1992. [Google Scholar]
- 32.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5:125–42. [Google Scholar]
- 33.Benton AL, Hamsher K. Multilingual Aphasia Examination. AJA Associates; Iowa City: 1983. [Google Scholar]
- 34.Trites R. L. Lafayette Grooved Pegboard Neuropsychological Test Manual. Royal Ottawa Hospital; Ottawa: 1977. [Google Scholar]