Abstract
Background and Purpose:
Neurosteroids exert their antiepileptic effects via GABAA and NMDA receptors. Another cell death mechanism is excessive Ca2+ influx into cells. Calbindin-D28k (CB) is a protein that modulates intracellular Ca2+ in the nervous system. We evaluated whether androsterone up-regulates the expression of CB and has a neuroprotective effect by controlling Ca2+ after pilocarpine-induced status epilepticus (SE) in mice.
Methods:
SE was induced in ICR mice by injection of pilocarpine. Two hours after SE, mice were treated intraperitoneally (i.p.) with androsterone (100–200 mg/kg) or vehicle, and compared with other control groups. Two days after injection, immunohistochemical staining for CB was performed using a hippocampal slice from each mice group. We also used cresyl violet staining to compare changes in hippocampal structures.
Results:
Two days after pilocarpine-induced SE, androsterone increased the expression of CB in the hippocampus compared with control SE mice. The number of CB-positive cells was 1±0.4 cells/mm3 in pilocarpine-only group, 14±1.1 cells/mm3 in pilocarpine plus androsterone 100 mg group and 29±2.5 cells/mm3 in pilocarpine plus androsterone 200 mg group (p<0.001).
Conclusions:
These results suggest that the neuroprotective effect of androsterone after pilocarpine- induced SE may be mediated by an increased expression of CB.
Keywords: Neurosteroid, Androsterone, Calbindin-D28k, Hippocampus, Seizure
Introduction
Neurosteroids are a type of steroid produced in the central nervous system rather than the adrenal gland. Neurosteroids rapidly alter neuronal excitability through interaction with neurotransmittergated ion channels of GABAA receptors.1,2 In addition, neurosteroids may also exert effects on gene expression via intracellular steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to epilepsy and traumatic brain injury treatment.3
Systemic administration of pilocarpine-induced status epilepticus (SE) consequentially generates death of pyramidal cells in the CA1 and CA3 fields of the hippocampus.4 This is due to excessive elevation of intracellular Ca2+ levels, resulting from overactivation of glutamate receptors.5,6 Therefore, regulation of intracellular Ca2+ levels is an important factor in neuronal survival after SE.
Calcium binding proteins, such as calbindin-D28k (CB), parvalbumin, and calretinin, are present in hippocampal non-pyramidal cells.7 It was reported that they have the capacity to buffer and transport intracellular Ca2+ in the nervous system.8,9 CB is ubiquitously expressed in the hippocampus, and particularly abundant in the granule cell-mossy fiber system.9,10 A number of studies have reported that CB has a neuroprotective function modulating intracellular Ca2+ levels against various central nervous system insults, including epilepsy.11,12
In previous studies, decreased CB expression was observed in epileptic conditions13, and some steroids, such as testosterone and estrogen, stimulated CB synthesis.14,15 However, the effect of neurosteroids on CB expression in epilepsy is unclear.
Therefore, we performed this study to examine whether neurosteroids, particularly androsterone, affect CB expression. We aimed to understand the relationship between the neuroprotective effects of androsterone and changes in CB expression with pilocarpine-induced SE.
Methods
The Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved all procedures. Male Institute for Cancer Research (ICR) mice (25 to 30 g, Orient Bio Co., Gyeonggi-do, Korea) were used in these experiments. Mice were housed under a 12 h light/dark cycle with food and water ad libitum. Lithium chloride (127 mg/kg, Sigma, St. Louis, MO, USA) was injected intraperitoneally (i.p) 24 h prior to pilocarpine administration. Next, mice were pretreated with methscopolamine-bromide (1 mg/kg, i.p., Sigma) 30 min prior to pilocarpine, and single dose of pilocarpine (30 mg/kg, i.p., Sigma) was administered. Seizures were scored using Racine’s scale, and the duration of status epilepticus (SE) was determined by behavioral assessment. The beginning of SE was defined as the onset of continuous generalized seizure activity (stage 4 or 5 based on Racine’s scale) without regaining normal behavior between the seizures. All mice received i.p. injections of 0.9% saline: 5 mL twice on the day with SE and 5 ml twice (in the morning and evening) of the day after SE. Two hours after SE, mice were treated with androsterone (100 and 200 mg/kg, i.p.). We compared with three other groups: the saline-only injection group (normal), the androsterone 200 mg-only injection group (androsterone-only), and the pilocarpine-only injection group (pilocarpine-only).
Mice were anesthetized and transcardially perfused with heparinized saline, followed by 3.7% formaldehyde in phosphate-buffered saline (PBS). Brains were isolated and postfixed in the same fixative overnight at 4°C. Fixed frozen brains were sectioned coronally at 20 μm using a cryostat. Sections collected from single animals were used for histological analyses, such as cresyl violet staining and immunohistochemistry (n =6 mice in each experimental group).
For the DAB substrate reaction, sections were incubated with 3% H2O2 in PBS with 0.3% Triton X-100. CB was stained using the monoclonal anti-calbindin D28K antibody (1:3000, Sigma). The primary antibody was reacted overnight at 4°C. The secondary antibody, biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA), was reacted at room temperature for 1 h. During the procedure, all sections were washed with PBS between each step. We followed the manufacturer’s protocols for the M.O.M kit (Vector Labs). Visualization was performed using the Vectastain ABC-DAB system for 1 h at room temperature (Vector Labs).16
Sections were stained in 0.2% cresyl fast violet acetate for 5 min and rinsed in water. They were then rinsed in 95% alcohol and differentiated in cresyl violet differentiator [95% alcohol (90 mL), chloroform (10 mL), acetic acid (three drops)]. Sections were rinsed in absolute alcohol, cleared, and mounted on slides.16
Data are expressed as the mean ± standard error of the mean. Statistical comparisons were made using one-way analysis of variance (ANOVA), followed by Bonferroni’s post hoc tests (SAS, Cary, NC, USA). The level of significance was set at p<0.05 and 0.001.
Results
In the cresyl violet staining, hippocampal CA1 and CA3 pyramidal neurons were markedly decreased and morphologically-damaged neurons were observed in the pyramidal layer of the pilocarpine-only group. Hippocampal structures were relatively preserved in all other groups (Fig. 1). In the pilocarpine plus androsterone (200 mg) group, the structures of the hippocampal neurons in the CA1 and CA3 regions were as well preserved as the normal group (Fig. 1D).
Figure 1.
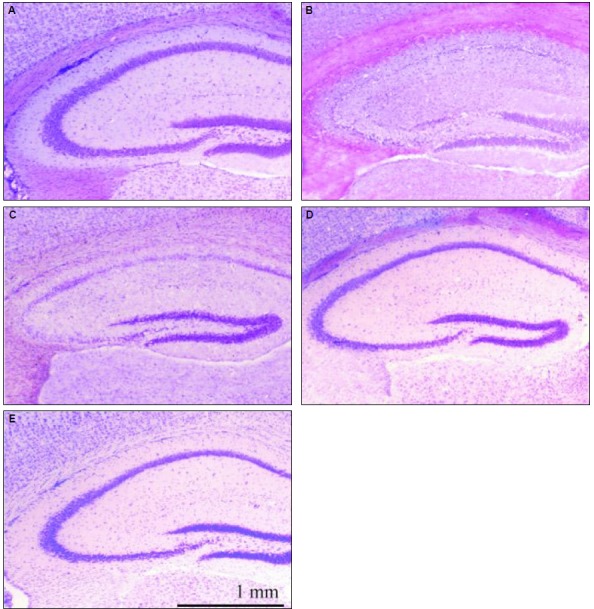
Cresyl violet staining of the mouse hippocampus. Severe neuronal degeneration in CA1 and CA3 pyramidal cells was detected in the pilocarpine-induced SE group (B) compared to the other groups. (A) normal control; (B) pilocarpine-only group; (C) pilocarpine plus androsterone 100 mg group; (D) pilocarpine plus androsterone 200 mg group; (E) androsterone-only group.
We evaluated the direct effect of androsterone on CB without SE and compared this with the normal and androsterone-only group (Fig. 2). CB-positive neurons were highly expressed in the stratum radiatum of the CA1 and CA3 region, stratum lucidum of the CA3 region, stratum molecular of the dentate gyrus (DG) and interneurons in the androsterone-only group (Fig. 2G and H).
Figure 2.
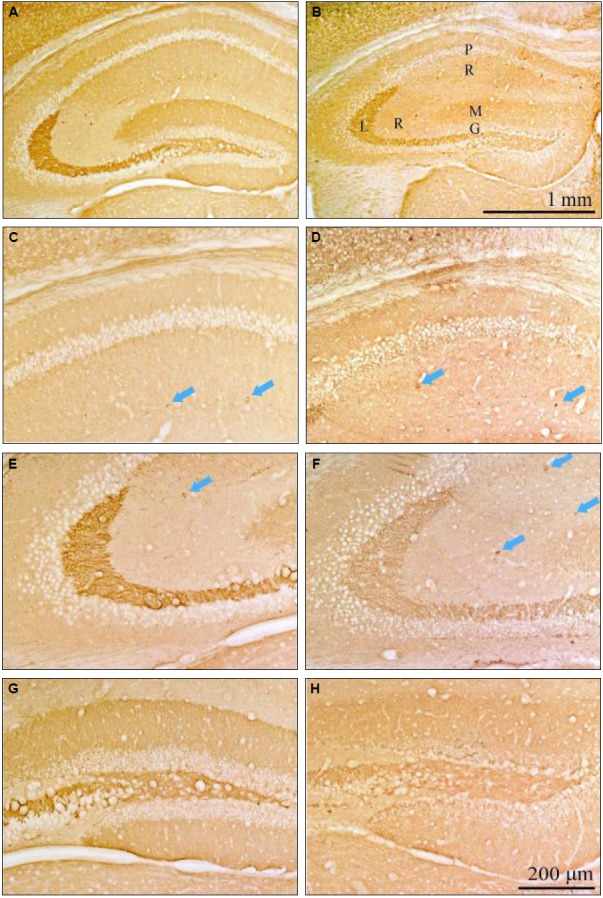
CB-immunostained hippocampi from the normal (A, C, E, and G) and androsterone-only groups (B, D, F, and H). CB-positive neurons were expressed in the stratum radiatum of the CA1 and CA3 regions, the stratum lucidum of the CA3 region, the stratum molecular of the DG, and interneurons (arrows). Expression was increased in the androsterone-only group (D and F) compared to the normal group (C and E). G, granular; L, lucidum; M, molecular; P, pyramidal; R, radiatum.
We also compared CB changes after pilocarpine-induced SE. CB-positive cells were increased in all subfields of hippocampus in the pilocarpine plus androsterone group compared to the pilocarpine-only group (Fig. 3). In particular, CB-positive cells increased dose-dependently in the pilocarpine plus androsterone group, the stratum radiatum of the CA1 and CA3 region, the stratum lucidum of the CA3 region (Fig. 3D to I), and the stratum molecular of the DG (Fig. 3J to L). In the pilocarpine-only group, CB-positive cells were dramatically reduced in the entire hippocampus. Especially in the stratum radiatum of the CA1 and CA3 regions, expression of CB-positive cells, such as interneurons, was reduced in pilocarpine-only group compared to the normal control group. Additionally, the CB staining intensity was decreased in the stratum lucidum of the CA3 region (Fig. 4).
Figure 3.
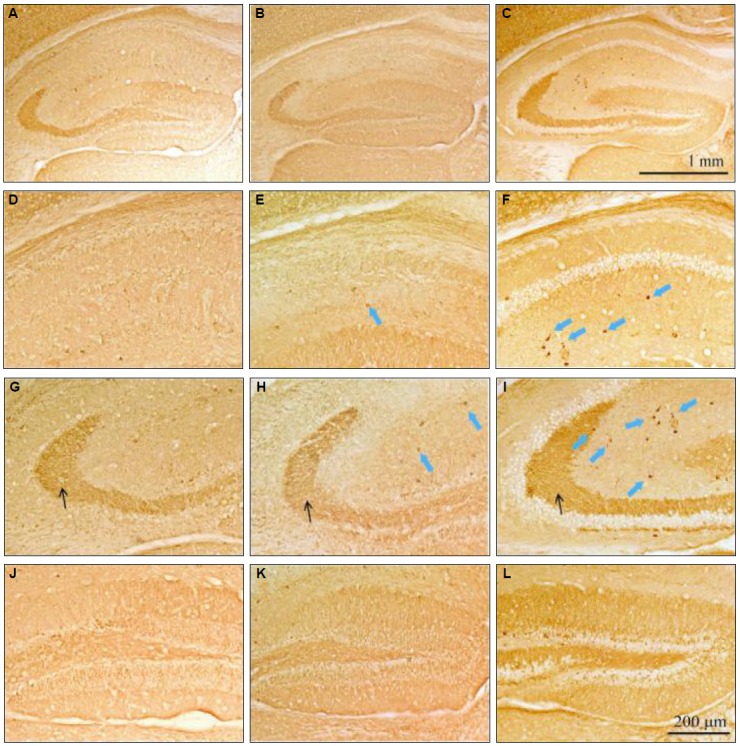
CB-immunostained hippocampi from the pilocarpine-only group (A, D, G, and J), pilocarpine plus androsterone 100 mg groups (B, E, H and K) and pilocarpine plus androsterone 200 mg groups (C, F, I and L). CB-positive neurons increased in the stratum radiatum of the CA1 and CA3 regions, the stratum lucidum of the CA3 region (blue arrows), and the stratum molecular of the DG (black arrow area) compared with the normal group.
Figure 4.
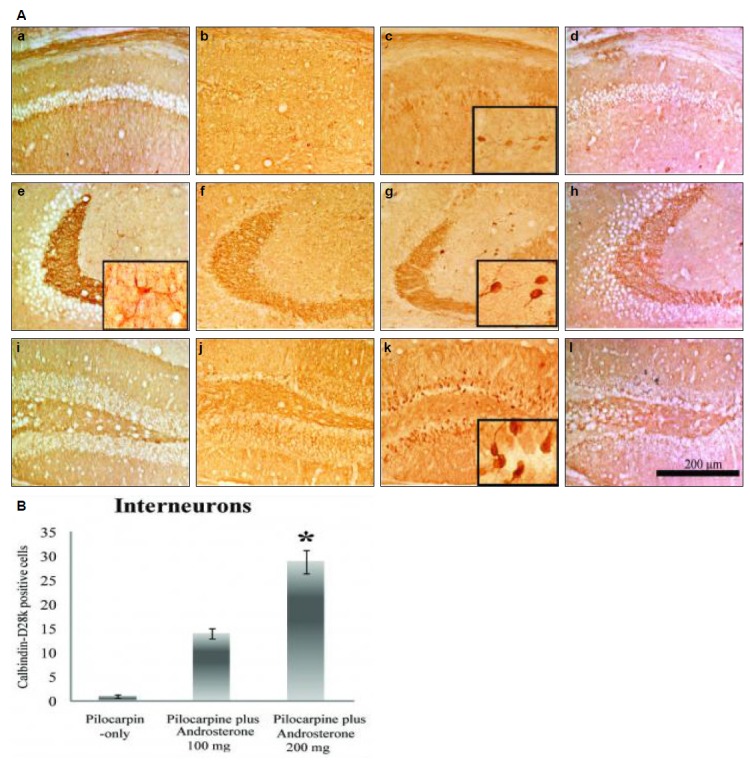
(A) Representative CB-immunostained hippocampi from the normal group (a, e, i), pilocarpine-only group (b, f, j), pilocarpine plus androsterone 200 mg group (c, g, k), and androsterone-only group (d, h, l). In the pilocarpine plus androsterone group, CB-positive neurons increased in the stratum radiatum of the CA1 and CA3 regions, the stratum lucidum of the CA3 region, and the stratum molecular of the DG compared to the other groups. Insets show the high-magnified images c, e, g and k. (B) Quantitation of CB-positive cells in the stratum radiatum of hippocampus. In the pilocarpine plus androsterone groups, CB-positive interneuron cells increased dose dependently. *p<0.001
As shown in Fig. 4, quantification of CB-positive interneurons in the stratum radiatum of the CA1 and CA3 regions was performed in the pilocarpine-only and pilocarpine plus androsterone groups. CB-positive cells in the pilocarpine-only group were not present; however, in the pilocarpine plus androsterone group, the number of CB-positive interneurons increased significantly compared to the pilocarpine-only group
The number of CB positive cells was 1±0.4 cells/mm3 in pilocarpine-only group, 14±1.1 cells/mm3 in pilocarpine plus androsterone 100 mg group and 29±2.5 cells/mm3 in pilocarpine plus androsterone 200 mg group (p<0.001) (Fig. 4B). In addition, the number of CB-positive interneurons increased dose-dependently. Finally, in the stratum lucidum of the CA3 region, the CB staining intensity in the pilocarpine plus androsterone group increased.
Discussion
Our results show a decrease in CB-positive cells in the stratum radiatum of the CA1 and CA3 regions of the hippocampus after pilocarpine induced-SE in the pilocarpine-only group. In contrast, androsterone treatment after pilocarpine-induced SE induced CB expression more than the pilocarpine-only group. CB-positive cells were detected more often in the stratum radiatum of the CA1 and CA3 regions in the androsterone plus pilocarpine group, and the morphology of neuronal cells was well preserved compared to the pilocarpine-only group. In the androsterone-only group, there tended to be more CB-positive compared to the normal control group.
Calcium influx and intracellular Ca2+ play role in several important functions, including production of action potentials, neurotransmitter release, cell-to-cell interactions, and neuronal plasticity in the central nervous systems. It has been reported that the failure to regulate intracellular Ca2+ concentration induces excessive Ca2+ influx into neuronal cells, resulting in a neuronal cell death cascade.17 In epilepsy, abnormal Ca2+ levels are found both in vitro and in vivo.18–20 Ca2+ levels were significantly and chronically elevated during bicuculline-induced epilepsy in vitro. Bicuculline, the GABAA receptor antagonist, also induced neuronal cell death.21,22 Therefore, Ca2+ is considered to play an important role in epileptogenesis.
CB is an intracellular calcium-binding protein, and functions in Ca2+ buffering systems. Therefore, CB may protect neuronal cells against in excessive Ca2+, including subpopulations of interneurons and the GABAergic character of a comparatively large group of non-pyramidal cells in the hippocampus.7,23,24 Hence, CB may regulate excitation of neuronal cells between excitatory and inhibitory neuronal cells. Previous studies found that decreased CB expression was observed in epileptic conditions.13 In addition, it was reported that some steroids, such as testosterone and estrogen, stimulated calbindin synthesis14,15 to regulate development of sexually dimorphic structures.18,19 Calbindin can modulate depolarization of dopaminergic cells,25 which is related to hippocampal cell death.26
Our finding of decreased numbers of CB-positive cells after pilocarpine treatment suggest that decreased CB is related to calcium homeostasis, and could be a major susceptibility to excite toxicity. The increase in CB after pilocarpine plus androsterone treatment suggests that androsterone modulates expression of calcium-binding proteins in neuronal cells during SE, which occurs in response to abnormal conditions such as excessive Ca2+. Additionally, the pyramidal cells in the hippocampus were preserved well in the pilocarpine plus androsterone group compared to the pilocarpine-only group. Taken together, our data suggest the increase in CB expression mediated by androsterone is related to neuronal cell protection in SE.
In conclusion, our observations suggest that the androsterone-mediated increase in CB-positive cells plays a role in buffering excess Ca2+ and protects neuronal cells. Androsterone may modulate Ca2+ homeostatic mechanisms in epilepsy and function in neuroprotection against excess Ca2+.
References
- 1.Kaminski RM, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–27. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mroz K, Mroz T, Wielosz M, Tutka P. Effects of androsterone on convulsions in various seizure models in mice. Pharmacol Rep. 2009;61:564–9. doi: 10.1016/s1734-1140(09)70100-8. [DOI] [PubMed] [Google Scholar]
- 3.Hill M, Zarubova J, Marusic P, et al. Effects of valproate and carbamazepine monotherapy on neuroactive steroids, their precursors and metabolites in adult men with epilepsy. J Steroid Biochem Mol Biol. 2010;122:239–52. doi: 10.1016/j.jsbmb.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Fujikawa DG. The temporal evolution of neuronal damage from pilocarpine-induced status epilepticus. Brain Res. 1996;725:11–22. doi: 10.1016/0006-8993(96)00203-x. [DOI] [PubMed] [Google Scholar]
- 5.Ferkany JW, Zaczek R, Coyle JT. Kainic acid stimulates excitatory amino acid neurotransmitter release at presynaptic receptors. Nature. 1982;298:757–9. doi: 10.1038/298757a0. [DOI] [PubMed] [Google Scholar]
- 6.Yi SS. Time-dependent changes of calbindin D-28K and parvalbumin immunoreactivity in the hippocampus of rats with streptozotocin-induced type 1 diabetes. J Vet Sci. 2013;14:373–80. doi: 10.4142/jvs.2013.14.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toth K, Freund TF. Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience. 1992;49:793–805. doi: 10.1016/0306-4522(92)90357-8. [DOI] [PubMed] [Google Scholar]
- 8.Heizmann CW, Braun K. Changes in Ca(2+)-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992;15:259–64. doi: 10.1016/0166-2236(92)90067-i. [DOI] [PubMed] [Google Scholar]
- 9.Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–8. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q, Wang S, Hamberger A, Celio MR, Haglid KG. Delayed decrease of calbindin immunoreactivity in the granule cell-mossy fibers after kainic acid-induced seizures. Brain Res Bull. 1997;43:551–9. doi: 10.1016/s0361-9230(97)00006-3. [DOI] [PubMed] [Google Scholar]
- 11.Bellido T, Huening M, Raval-Pandya M, Manolagas SC, Christakos S. Calbindin-D28k is expressed in osteoblastic cells and suppresses their apoptosis by inhibiting caspase-3 activity. J Biol Chem. 2000;275:26328–32. doi: 10.1074/jbc.M003600200. [DOI] [PubMed] [Google Scholar]
- 12.Guo Q, Christakos S, Robinson N, Mattson MP. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc Natl Acad Sci U S A. 1998;95:3227–32. doi: 10.1073/pnas.95.6.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter DS, Harrison AJ, Falenski KW, Blair RE, DeLorenzo RJ. Long-term decrease in calbindin-D28K expression in the hippocampus of epileptic rats following pilocarpine-induced status epilepticus. Epilepsy Res. 2008;79:213–23. doi: 10.1016/j.eplepsyres.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nys Y, Baker K, Lawson DE. Estrogen and a calcium flux dependent factor modulate the calbindin gene expression in the uterus of laying hens. Gen Comp Endocrinol. 1992;87:87–94. doi: 10.1016/0016-6480(92)90153-b. [DOI] [PubMed] [Google Scholar]
- 15.Stuart EB, Thompson JM, Rhees RW, Lephart ED. Steroid hormone influence on brain calbindin-D(28K) in male prepubertal and ovariectomized rats. Brain Res Dev Brain Res. 2001;129:125–33. doi: 10.1016/s0165-3806(01)00191-2. [DOI] [PubMed] [Google Scholar]
- 16.Kim GW, Kim HJ, Cho KJ, Kim HW, Cho YJ, Lee BI. The role of MMP-9 in integrin-mediated hippocampal cell death after pilocarpine-induced status epilepticus. Neurobiol Dis. 2009;36:169–80. doi: 10.1016/j.nbd.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez-Jimenez FJ, Garcia-Ruiz PJ, de Bustos F. [Calcium, neuronal death and neurological disease] Rev Neurol. 1996;24:1199–209. [PubMed] [Google Scholar]
- 18.Parsons JT, Churn SB, Kochan LD, DeLorenzo RJ. Pilocarpine-induced status epilepticus causes N-methyl-D-aspartate receptor-dependent inhibition of microsomal Mg(2+)/Ca(2+) ATPase-mediated Ca(2+) uptake. J Neurochem. 2000;75:1209–18. doi: 10.1046/j.1471-4159.2000.0751209.x. [DOI] [PubMed] [Google Scholar]
- 19.Parsons JT, Halkitis PN, Bimbi D, Borkowski T. Perceptions of the benefits and costs associated with condom use and unprotected sex among late adolescent college students. J Adolesc. 2000;23:377–91. doi: 10.1006/jado.2000.0326. [DOI] [PubMed] [Google Scholar]
- 20.Pal S, Sombati S, Limbrick DD, Jr, DeLorenzo RJ. In vitro status epilepticus causes sustained elevation of intracellular calcium levels in hippocampal neurons. Brain Res. 1999;851:20–31. doi: 10.1016/s0006-8993(99)02035-1. [DOI] [PubMed] [Google Scholar]
- 21.Pal S, Limbrick DD, Jr, Rafiq A, DeLorenzo RJ. Induction of spontaneous recurrent epileptiform discharges causes long-term changes in intracellular calcium homeostatic mechanisms. Cell Calcium. 2000;28:181–93. doi: 10.1054/ceca.2000.0146. [DOI] [PubMed] [Google Scholar]
- 22.Frantseva MV, Velazquez JL, Hwang PA, Carlen PL. Free radical production correlates with cell death in an in vitro model of epilepsy. Eur J Neurosci. 2000;12:1431–9. doi: 10.1046/j.1460-9568.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 23.Sloviter RS. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280:183–96. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- 24.Gulyas AI, Freund TF. Pyramidal cell dendrites are the primary targets of calbindin D28k-immunoreactive interneurons in the hippocampus. Hippocampus. 1996;6:525–34. doi: 10.1002/(SICI)1098-1063(1996)6:5<525::AID-HIPO5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 25.Frye CA, Paris JJ, Walf AA, Rusconi JC. Effects and Mechanisms of 3alpha, 5alpha,-THP on emotion, motivation, and reward functions involving pregnane xenobiotic receptor. Front Neurosci. 2011;5:136. doi: 10.3389/fnins.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozzi Y, Vallone D, Borrelli E. Neuroprotective role of dopamine against hippocampal cell death. J Neurosci. 2000;20:8643–9. doi: 10.1523/JNEUROSCI.20-22-08643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


