Abstract
Williams-Campbell syndrome is a rare disorder characterized by deficiency of subsegmental bronchial cartilage and development of airway collapse and bronchiectasis that may subsequently progress to respiratory failure and death. There are only 2 published reports suggesting a familial association, and only one report of lung transplantation being used as a therapeutic modality. Due to postoperative airway complications, transplantation has not been recommended for this disease. We report the first lung transplant with prolonged survival, approaching 10 years, in a patient with Williams-Campbell syndrome, and provide further evidence to support a familial association.
Keywords: Williams-Campbell syndrome, bronchiectasis, lung transplantation, bronchial cartilage deficiency
Introduction
Williams-Campbell syndrome (WCS) is a rare disorder characterized by deficiency of cartilage in subsegmental bronchi (4th to 6th order bronchi) with subsequent development of airway collapse and bronchiectasis.1 Though many patients progress to respiratory failure in childhood, others have a more benign course and present with severe obstructive lung disease and bronchiectasis in adulthood. Since this disease may involve the cartilage of the large airways, the role of lung transplantation for this disorder is controversial. We report a case of successful lung transplantation in a patient with end-stage lung disease secondary to presumed familial WCS. Furthermore, this is only the third case in the English literature suggesting a familial association.2,3
Case Report
A 31-year-old man was admitted for respiratory failure and cor pulmonale in July 1999 to the University Hospital in San Antonio, Texas. Although he had no history of smoking, his chest radiograph revealed evidence of severe obstructive lung disease with extensive bullous disease. His electrocardiogram suggested pulmonary hypertension, and an echocardiogram showed severe right-ventricular dilatation with elevated pulmonary pressures. A ventilation-perfusion scan showed no evidence of pulmonary emboli. Pulmonary function tests revealed an FEV1 of 0.81 L (20% of predicted), FVC of 1.88 L (37% of predicted), and a diffusing capacity for carbon monoxide of 47% of predicted.
The patient’s childhood and family history were remarkable in that he and both of his siblings (one brother and one sister) had recurrent episodes of bronchitis and several episodes of pneumonia resulting in bronchiectasis. However, the patient did not recall substantial activity limitations. In 1979, at National Jewish Health System, all 3 siblings underwent an extensive evaluation, without a definitive diagnosis. The patient, his sister, and both parents carry the ΔF508 mutation; however, sweat chloride testing was negative in all family members on multiple occasions. The patient and his siblings were felt to have dysfunctional alpha-1 antitrypsin at that time. His parents remain healthy, while his sister developed moderate obstructive lung disease. The patient and his brother progressed to end-stage lung disease, and both of his brother’s 2 young children have recurrent pulmonary disease.
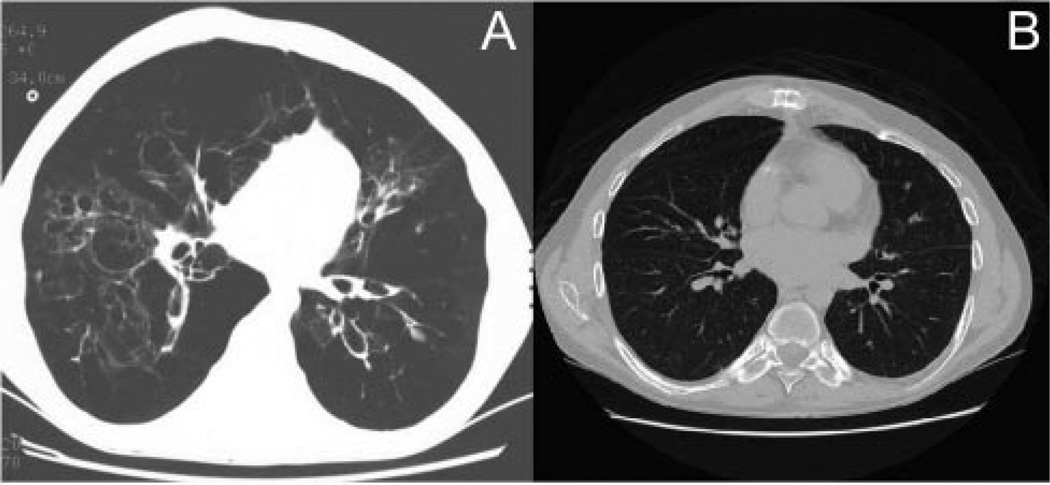
The patient received aggressive out-patient therapy with corticosteroids, oxygen, antibiotics, and bronchodilators. A functional assay for alpha-1 antitrypsin was normal, and his genotype was MM. A computed tomography (CT) scan of the chest showed extensive, thin-walled, cystic bronchiectasis, severe emphysema, and lower lobe bullous disease (Fig. 1). The chest radiologist felt the examination was consistent with alpha-1 antitrypsin deficiency, due to the marked basilar predominance of the bullous disease.
Fig. 1.
A: Pre-transplant chest computed tomogram (CT) showing thin-walled, cystic bronchiectasis and lower lobe severe bullous disease. B: Post-transplant chest CT showing no evidence of disease.
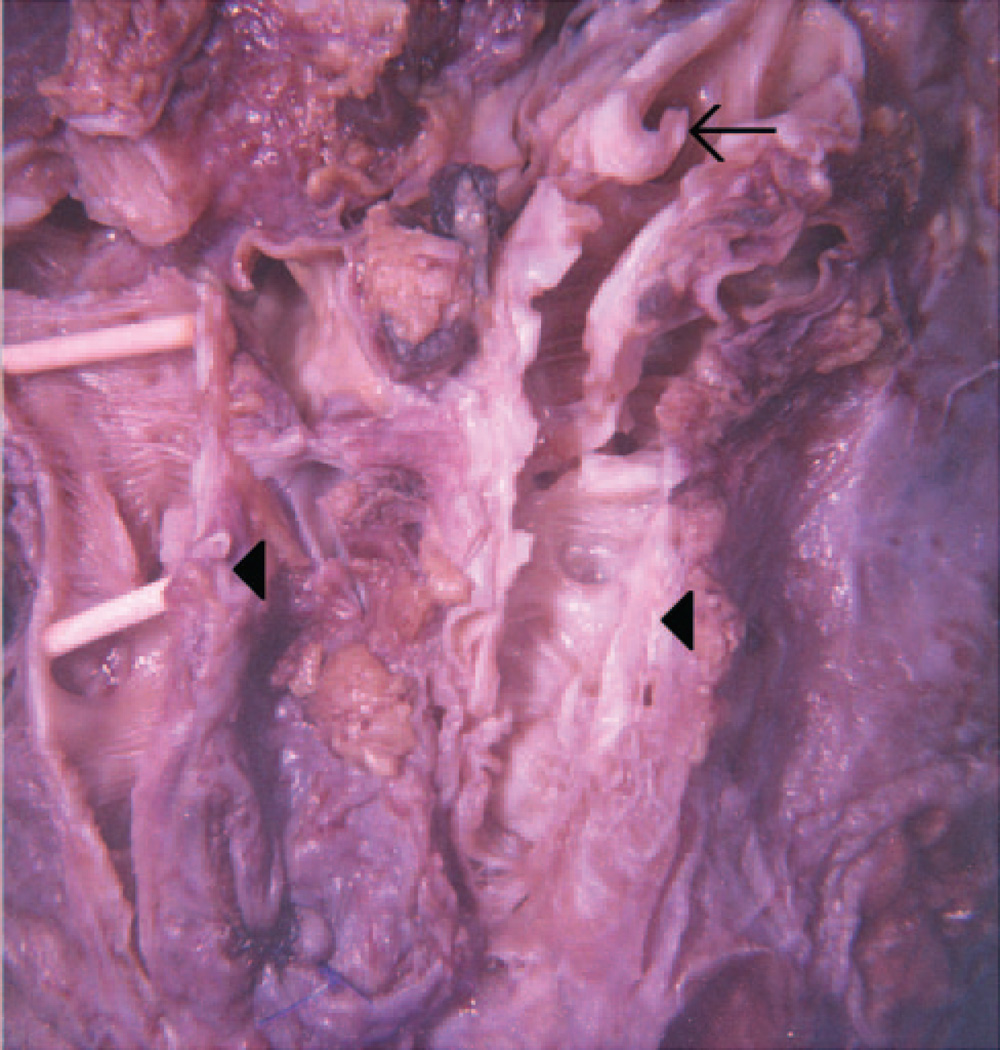
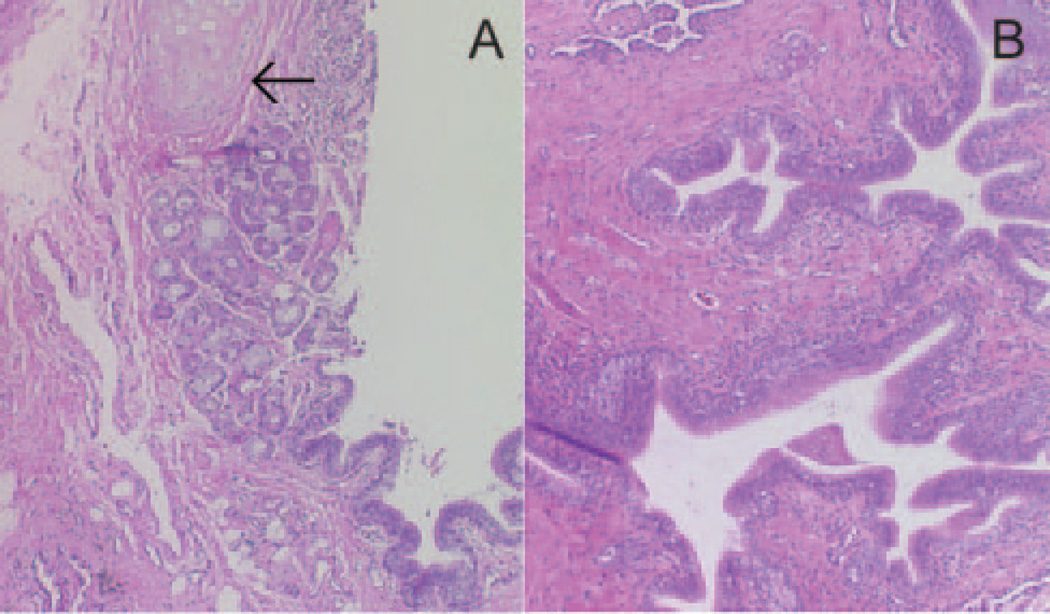
In 2001, the patient underwent bilateral lung transplantation. The explanted right lung was cut sagitally, and the right lower lobe bronchus was identified. Dissection was extended distally along the anterior- and medial-basal segment bronchi. Cartilage could be seen for 2 to 3 cm and then stopped abruptly, with a sudden change in the mucosal surface. At the end of the firm, cartilage-containing bronchial wall, the airway became soft, thin-walled, dilated and irregularly-shaped (Fig. 2). Histopathology revealed the sudden cessation of cartilage as well as enfolding of the airway epithelium, with a relative paucity of inflammation (Fig. 3).
Fig. 2.
Airway dissection of explanted right lung revealing abrupt loss of cartilage in segmental bronchi (arrow) with thin-walled, dilated, and tortuous airways devoid of cartilage (arrowheads).
Fig. 3.
Histopathology showing (A) normal airway with cessation of cartilage (arrow) beginning abruptly at the third-order bronchial division, and (B) dilated, enfolded airway without cartilage and lacking inflammation.
After the transplant the patient had recurrent Pseudomonas aeruginosa tracheobronchitis and received several courses of antibiotics. A flexible bronchoscopy showed granulation tissue at both anastomotic sites and mild narrowing of the bronchus intermedius. He slowly improved and all pulmonary symptoms resolved. His most recent pulmonary function tests revealed an FEV1 of 2.01 L (52% of predicted), and forced vital capacity of 3.55 L (76% of predicted). The patient is now nearly 10 years post-lung transplantation and doing well. He performs all activities of daily living and plays golf without difficulty. He does not require oxygen and shows no evidence of disease recurrence on CT scans of the chest. His brother underwent successful lung transplantation 4 years later at another institution and remains alive and well. His younger sister also carries a diagnosis of WCS, suffers from recurrent pulmonary infections, has an FEV1 of 27% of predicted, and is currently being evaluated for possible lung transplantation.
Discussion
In 1819, Laënnec described the abnormal dilation and distortion of bronchi caused by destruction of the elastic and muscular components of the bronchial wall, and introduced the concept of bronchiectasis.4 Though bronchiectasis was initially felt to be a congenital disease,5 most cases of non-cystic-fibrosis (CF) bronchiectasis are now felt to be associated with either an episode of necrotizing infection, a series of infections, or chronic infection by organisms including atypical mycobacterium. Non-CF congenital bronchiectasis is quite rare and usually characterized by structural alterations in the respiratory tract.
In 1960, Williams and Campbell reported 5 cases of childhood bronchiectasis showing bronchiectatic changes distal to the first division of the segmental bronchi, with deficiency in the bronchial cartilage plates.1 By 1972, another 11 cases were reported and the condition was named Williams-Campbell syndrome.6 Autopsy specimens in Williams and Campbell’s study demonstrated that this syndrome is confined to the pulmonary system. Pathologic features of the WCS include deficiency of bronchial cartilage extending to the first and second segmental divisions, but intact cartilaginous plates at bronchial bifurcations. One of the most striking features from the initial description is that, despite formalin fixation, the bronchial walls balloon out and appear “flabby as blood vessels.”1
In 1976, the first report of the occurrence of familial bronchiectasis in siblings was published and supported the theory that WCS was congenital, based on the uniformity of the cartilaginous defect.2 Characteristics of WCS included persistence of intact plates of cartilage at axial bifurcations (as far as the seventh generation), lack of destruction of other bronchial wall structures by inflammation, and the proximal extension of the cartilage defect to the level of the first and second segmental divisions.1
The definitive diagnosis of WCS can be made only by examination of resected or autopsy specimens7 but should be suspected, as stated by Williams, by clinical symptoms such as recurrent infection and diffuse bronchiectasis associated with cough, wheezing, and progressive loss of pulmonary function. One must rule out other causes of bronchiectasis, including allergic, metabolic, and genetic causes, or immunologic defects. As with other forms of bronchiectasis, bronchoscopy may reveal mucosal erythema, edema with purulent exudates, and evidence of segmental bronchomalacia; however, these features are not diagnostic.7 Several authors have demonstrated the ability of spiral multidetector dynamic CT to image bronchiectasis and, more specifically, to aid in the diagnosis ofWCS.8–12 Watanabe et al used CT imaging to aid in the diagnosis of WCS, with the finding of ballooning and collapsing of segmental airways during inspiration and expiration, respectively.8 George et al have described absence of cartilage ring impressions on the bronchial wall bilaterally from the main to the subsegmental bronchi, suggesting cartilage deficiency, on reconstructed images of the tracheobronchial tree.12
Since its first description, minimal impact has been made on the natural history of the disease or in the medical therapy. In the initial report 2 of the 5 patients died, and in Williams’s next report 4 of the patients died, all before 5 years of age.1,6 As there are no specific treatment options, out-patient and bedside management consists of supportive care and general measures for bronchiectasis, which include controlling secondary infections with the use of antibiotics (including chronic macrolide therapy), treating reactive airways with bronchodilators, and mobilizing secretions with postural drainage or devices that improve clearance of airway secretions.6,7,13 Noninvasive ventilation has also been used effectively for the respiratory failure of WCS.14 Some children exhibit a rapid downhill course, while others may have a more benign process compatible with prolonged survival.15 Jones et al reported 2 family members in whom respiratory symptoms developed within the first year of life and who were found to have pathologic changes consistent with WCS.3 This was the first report where surgery was used as a treatment option. One patient underwent triple lobectomy, while the other had a right upper lobectomy, both eventually resulting in severe restrictive and obstructive lung disease.
In 1998, Palmer et al described a 28-year-old man who was referred to Duke University with deteriorating pulmonary function tests.16 CT of the chest revealed extensive, thin-walled, cystic bronchiectasis, and bronchoscopy confirmed expiratory collapse of the distal airways. He was diagnosed with WCS and underwent bilateral, sequential lung transplantation. The native lungs showed typical findings of WCS, with characteristic absence of cartilage in the walls of the medium to small airways. The patient did well after surgery and was discharged on the 19th postoperative day. His pulmonary function substantially improved, and several surveillance bronchoscopies and biopsies did not show signs of infection or rejection. Several months later the patient developed recurrent, bacterial, pulmonary infections, and a Palmaz stent was placed in the belief that proximal airway collapse was contributing to the recurrent infections. Thirteen months after transplant, the patient died of P. aeruginosa pneumonia. Autopsy revealed a decreased amount of cartilage in the patient’s native right and left main bronchi. This finding was contrary to all previous reports, which described distal bronchial involvement with sparing of the proximal bronchial cartilage in patients with WCS. This was the first reported case of lung transplantation for WCS, which the authors recommended avoiding because of the potential high risk of complications in the proximal airways.16 Until our report, this was the only reported case of lung transplantation for WCS in the English literature. Contrary to the first report, our patient with WCS is nearly 10 years out from lung transplantation and continues to have excellent quality of life and adequate pulmonary function.
Given the rarity of the disease, guidelines from the International Society for Heart and Lung Transplantation give no specific recommendation on selection of patients for lung transplantation, but state that with non-CF bronchiectasis the lung transplant community has generally followed the guidelines used for CF patients. Per their recommendations, the transplant window can be established when FEV1 reaches <30% of predicted or with rapid deterioration of FEV1, with increasing frequency of exacerbations or an ICU stay, and with development of oxygen-dependent respiratory failure, hypercapnia, or pulmonary hypertension.17 As compared to other lung transplant patients, there was no difference in postoperative bedside management in our patient.
The presence of basilar emphysema and the ΔF508 mutation in the patient and his family members raises questions about the association with other diseases. Typically, basilar emphysema is seen most commonly with alpha-1 antitrypsin deficiency,18 but testing in this patient was negative. No specific mention of a basilar predominance was noted in the other reports of WCS, though emphysema has been commonly noted.3,12,19 Although they did carry the ΔF508 mutation, none of the family members was found to be homozygous, and the negative sweat chloride tests argue against the presence of CF. This finding suggests the possibility of an undiscovered mutation in the other copy of the CF transmembrane regulator (CFTR) gene that was responsible for the phenotype; however, given that both parents were asymptomatic while having had a copy of this gene, this is also unlikely. It is also noteworthy that loss of CFTR function in a porcine animal model has been associated with abnormalities in airway, and specifically cartilaginous, development.20 More research will be needed to determine if these factors play a role in WCS.
As in previously reported cases of WCS, our patient presented with classic clinical symptoms as well as the characteristic pathologic findings on resected specimens; however, what is unique about our report is the compelling evidence for a familial association, as well as the novel therapeutic approach, with sustained favorable results. Disease in the patient’s siblings and their children argues for autosomal dominant inheritance, but the absence of disease in his parents appears contradictory, suggesting possible non-Mendelian genetics, anticipation, or variable penetrance. In addition to supporting the concept of a congenital and familial basis of disease in WCS, as proposed by earlier papers, we suggest that lung transplantation may be a viable therapeutic option in carefully selected patients with this syndrome. The report by Palmer and colleagues16 does not mention whether any proximal airway evaluation was done for their patient prior to transplantation, but, given their findings, we strongly recommend a radiologic and/or bronchoscopic assessment of the mainstem bronchial cartilage in any patient being evaluated for transplant for WCS.
Acknowledgments
Dr Restrepo was partly supported by grant K23HL096054 from the National Heart, Lung, and Blood Institute.
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the University of Texas Health Science Center at San Antonio, the National Heart, Lung, and Blood Institute, or the National Institutes of Health.
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.Williams H, Campbell P. Generalized bronchiectasis associated with deficiency of cartilage in the bronchial tree. Arch Dis Child. 1960;35:182–191. doi: 10.1136/adc.35.180.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wayne KS, Taussig LM. Probable familial congenital bronchiectasis due to cartilage deficiency (Williams-Campbell syndrome) Am Rev Respir Dis. 1976;114(1):15–22. doi: 10.1164/arrd.1976.114.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Jones VF, Eid NS, Franco SM, Badgett JT, Buchino JJ. Familial congenital bronchiectasis: Williams-Campbell syndrome. Pediatr Pulmonol. 1993;16(4):263–267. doi: 10.1002/ppul.1950160410. [DOI] [PubMed] [Google Scholar]
- 4.Laënnec RTH, Forbes J. A treatise on the diseases of the chest, tr. by J. Forbes. London: S Wood; 1834. p. 736. [Google Scholar]
- 5.Grawitz P. Über angeborene bronchiektasie. Virchows Arch F Path Anat. 1880;(82):216. Article in German. [Google Scholar]
- 6.Williams HE, Landau LI, Phelan PD. Generalized bronchiectasis due to extensive deficiency of bronchial cartilage. Arch Dis Child. 1972;47(253):423–428. doi: 10.1136/adc.47.253.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis PB, Hubbard VS, McCoy K, Taussig LM. Familial bronchiectasis. J Pediatr. 1983;102(2):177–185. doi: 10.1016/s0022-3476(83)80515-0. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe Y, Nishiyama Y, Kanayama H, Enomoto K, Kato K, Takeichi M. Congenital bronchiectasis due to cartilage deficiency: CT demonstration. J Comput Assist Tomogr. 1987;11(4):701–703. [PubMed] [Google Scholar]
- 9.Naidich DP, McCauley DI, Khouri NF, Stitik FP, Siegelman SS. Computed tomography of bronchiectasis. J Comput Assist Tomogr. 1982;6(3):437–444. doi: 10.1097/00004728-198206000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Muller NL, Bergin CJ, Ostrow DN, Nichols DM. Role of computed tomography in the recognition of bronchiectasis. AJR Am J Roentgenol. 1984;143(5):971–976. doi: 10.2214/ajr.143.5.971. [DOI] [PubMed] [Google Scholar]
- 11.Di Scioscio V, Zompatori M, Mistura I, Montanari P, Santilli L, Luccaroni R, et al. The role of spiral multidetector dynamic CT in the study of Williams-Campbell syndrome. Acta Radiol. 2006;47(8):798–800. doi: 10.1080/02841850600849084. [DOI] [PubMed] [Google Scholar]
- 12.George J, Jain R, Tariq SM. CT bronchoscopy in the diagnosis of Williams-Campbell syndrome. Respirology. 2006;11(1):117–119. doi: 10.1111/j.1440-1843.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 13.Barker AF. Bronchiectasis. N Engl J Med. 2002;346(18):1383–1393. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 14.Wada H, Seto R, Yamada H, Nagao T, Hajiro T, Nakano Y. Respiratory failure of Williams-Campbell syndrome is effectively treated by noninvasive positive pressure ventilation. Intern Med. 2011;50(16):1729–1732. doi: 10.2169/internalmedicine.50.4971. [DOI] [PubMed] [Google Scholar]
- 15.Fraser RG, Macklem PT, Brown WG. Airway dynamics in bronchiectasis; a combined cinefluorographic-manometric study. Am J Roentgenol Radium Ther Nucl Med. 1965;93:821–835. [PubMed] [Google Scholar]
- 16.Palmer SM, Jr, Layish DT, Kussin PS, Oury T, Davis RD, Tapson VF. Lung transplantation for Williams-Campbell syndrome. Chest. 1998;113(2):534–537. doi: 10.1378/chest.113.2.534. [DOI] [PubMed] [Google Scholar]
- 17.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, et al. International guidelines for the selection of lung transplant candidates: 2006 update: a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(7):745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Gishen P, Saunders AJ, Tobin MJ, Hutchison DC. Alpha 1-antitrypsin deficiency: the radiological features of pulmonary emphysema in subjects of Pi type Z and Pi type SZ: a survey by the British Thoracic Association. Clin Radiol. 1982;33(4):371–377. doi: 10.1016/s0009-9260(82)80297-3. [DOI] [PubMed] [Google Scholar]
- 19.Hartman TE, Primack SL, Lee KS, Swensen SJ, Muller NL. CT of bronchial and bronchiolar diseases. Radiographics. 1994;14(5):991–1003. doi: 10.1148/radiographics.14.5.7991828. [DOI] [PubMed] [Google Scholar]
- 20.Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA, Smith AR, et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010;182(10):1251–1261. doi: 10.1164/rccm.201004-0643OC. [DOI] [PMC free article] [PubMed] [Google Scholar]