Abstract
Mouse engineered cardiac tissue constructs (mECTs) are a new tool available to study human forms of genetic heart disease within the laboratory. The cultured strips of cardiac cells generate physiologic calcium transients and twitch force, and respond to electrical pacing and adrenergic stimulation. The mECT can be made using cells from existing mouse models of cardiac disease, providing a robust readout of contractile performance and allowing a rapid assessment of genotype–phenotype correlations and responses to therapies. mECT represents an efficient and economical extension to the existing tools for studying cardiac physiology. Human ECTs generated from iPSCMs represent the next logical step for this technology and offer significant promise of an integrated, fully human, cardiac tissue model.
Introduction
The study of cardiac physiology and the pathophysiology mechanisms underlying cardiac disease depends upon the availability of model systems that accurately reproduce functional parameters of the in situ heart. Developing robust cardiac models is complicated by the unique ability and requirement of cardiac muscle to rapidly and dynamically respond to a wide range of cues including neural and hormonal signals, ion currents, and changes in preload and afterload. The performance of the individual cardiomyocyte is not dependent simply on its own internal function, but also influenced by the complex extracellular environment. Given the wide range of existing engineered mouse models of cardiac disease, development of a robust tool to study cardiac physiology in these models is clearly desirable.
For many years, a technically accessible and cost-effective approach to study cardiac cells has been the 2D monolayer culture of isolated rat cardiomyocytes (Louch et al., 2011). The ability to isolate ventricular cardiomyocytes from fetal, neonatal, and adult rat hearts, and subsequently from mouse hearts, and grow them in 2D monolayer cultures fostered great advances in the understanding of cardiac cell function, particularly at the molecular level. The monolayer environment, however, lacks the rich cell–cell connections between both cardiomyocytes and non-myocytes found in vivo, as well as the interactions with the extracellular matrix and mechanical forces that are important to maintain morphology, maturity, and molecular composition (Akins et al., 2010; Kira et al., 1994). In the 2D environment, cardiomyocytes tend to regress to a less mature phenotype losing some of the ability to respond normally to physiologic stimuli (Akins et al., 2010). The 2D monolayer cultures also provide a limited window for study as the non-myocyte cell population typically overgrows the plates within 2 weeks.
Isolated perfused heart preparations developed over a 100 years ago by Oscar Langendorff overcome the limitations of the monolayer cultures mentioned earlier (Bell et al., 2011). The perfused heart preparations generate dynamic functional data on the level of the whole organ. These preparations have allowed significant advances in understanding ischemia-reperfusion injury, response to pharmacotherapy, and cardiac toxicity. However, the technique can be quite challenging to master, particularly the isolated perfused mouse heart, and the experimental set-up poses limits on experiment duration and throughput. Additionally, many of the existing genetic mouse models with a cardiac phenotype undergo significant secondary remodeling (e.g., hypertrophy or dilation) prior to reaching an appropriate size in which traditional physiologic testing by either isolation of papillary muscles, perfused isolated heart, or direct left ventricular catheterization can be performed.
Growing cardiomyocytes in three-dimensional culture
Over the past 15 years, several research groups developed and refined methods to grow neonatal rat or embryonic chick cardiomyocytes in 3D configurations (Eschenhagen et al., 1997; Tobita et al., 2006; Zimmermann et al., 2000). The isolated cells are mixed with biodegradable matrices, which so far have included rat collagen type I, Matrigel, or fibrin(o-gen), which then polymerize into sheets, rings, or cylinders. The cardiomyocytes and non-myocytes within the matrix align along the axis of stress forming a structure that closely resembles the intact myocardium with complex cell–cell connections. During the first few days in culture, the engineered cardiac tissue (ECT or alternatively, engineered heart tissue, EHT) condenses as the extracellular matrix is remodeled by the cellular components. Spontaneous and increasingly organized contractions are seen within 48 h. Similar to native myocardium, ECTs have an outer layer of non-myocytes, contain fibroblasts and macrophages, and demonstrate occasional evidence of endothelial-lined capillary-like structures. Application of either cyclic strain or electrical pacing during the culture period promotes further differentiation and tissue homogeneity with uniformly aligned cardiomyocytes forming gap junctions containing localized connexin-43 deposition. Depending on the ECT configuration and testing apparatus, twitch force, calcium transients, and action potentials can be measured. The constructs remain viable in culture for 4–6 weeks without overgrowth of non-myocytes or loss of contractile performance. An explanation for why CMs within ECT can remain in culture for extended periods without overgrowth of fibroblasts as occurs in monolayer cultures may be related to contact inhibition within the 3D tissue environment. Table 1 provides an overview of the strengths and weaknesses of the mECT approach compared to more traditional in vitro methods.
Table 1.
Comparison between conventional approaches and ECT.
| Monolayer culture |
Langendorff preparation |
Papillary muscle/ trabeculae |
ECT culture |
|
|---|---|---|---|---|
| Study immature CMs | +++ | ++++ | ||
| Study adult CMs | + | ++++ | +++ | |
| Mechano-electrical coupling | +++ | ++++ | ++++ | |
| Over-/under-express genes | +++ | ++ | ++++ | |
| Perform extended duration experiments | ++ | ++++ | ||
| Assess contractile function | + | ++++ | ++++ | +++ |
| Measure calcium transients | +++ | ++ | ++ | ++++ |
| Measure calcium sensitivity of force | ++++ | ++ | ||
| Assess impact of acute afterload | ++ | ++++ | ++ | ++++ |
| Electrophysiology of intact tissue | ++++ | +++ | ++ |
The potential applications of ECT as both a research tool and potential source of replacement tissue are driving its development forward. Sheets of matrix and cardiomyocytes can be stacked to build thicker composites that may be suitable for grafting into regions of infarcted myocardium to correct functional deficits (Fujimoto et al., 2011; Zimmermann et al., 2006), while cellularized tubes can be used as pulsatile vascular grafts (Patterson et al., 2012). ECT rings have been scaled down in size, and their functional measurements are automated to facilitate use as a high-throughput drug screening platform (Hansen et al., 2010). ECT may also provide insights into cardiac developmental changes as the cells recapitulate important steps in differentiation and maturation during reintegration into the 3D environment. The ability to grow ECT under mechanical load or with additional biomechanical stressors makes them well suited to study cardiac adaptive response to environmental stress.
Mouse ECT recapitulates cardiac physiology
In contrast to neonatal rat cardiomyocytes, neonatal mouse cardiomyocyte cultures have been utilized to a far lesser extent in cardiovascular research. Reasons for this may be based on the difficulty in obtaining enough starting cells given the size of the neonatal mouse heart, as well as difficulties in enzymatically dissociating cardiomyocytes from the myocardial extracellular matrix while preserving an acceptable viability. Over the past several years, an increasing number of researchers have overcome the initial hurdles and standardized the monolayer culture of both neonatal and adult mouse cardiomyocytes (Nistri et al., 2012; O’Connell et al., 2007).
Recent refinements of the ECT approach now enable the use of mouse cardiomyocytes. This advance creates an opportunity to evaluate cardiac contractility and correlate function with intracellular events using cells obtained from any of the existing genetically modified models of heart disease (de Lange et al., 2011). The technique remains based on gentle enzymatic dissociation of post-natal day 0 ventricles with a short-duration slow rotational culture to promote small aggregations of cells prior to mixing with the soluble matrix. After 7–14 days in culture, the cells have remodeled the extracellular matrix to form a condensed strip approximately 1mm in diameter and 1.5 cm long (Fig. 1). Spontaneous, organized contractions begin within 2–3 days during the culture period and exhibit an intrinsic rate that is faster than similar rat ECT. The mouse ECT (mECT) exhibits increased twitch force with incremental stretch (Frank–Starling response), readily accept extrinsic pacing to frequencies as high as 10 Hz, and generate physiologic calcium transients (Fig. 2). Adrenergic signaling pathways are intact as demonstrated by positive inotropic response to dobutamine and chronotropic response to isoprenaline.
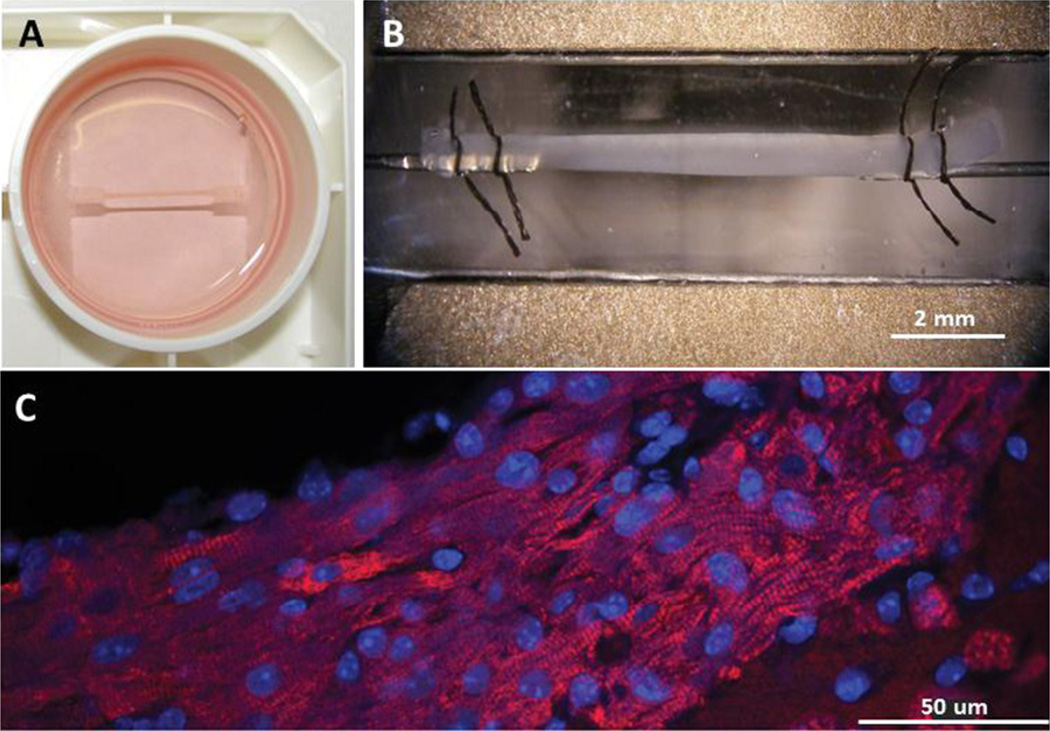
Fig. 1. Mouse ECT structure.
(A) mECT suspended from tabs and submerged in growth media within a 6-well FlexCell tissue culture plate. (B) mECT mounted by sutures to a force transducer in an open perfusion chamber. Electrodes seen along upper and lower margins of chamber. Length of mECT approximately 1 cm, width 0.8 mm. (C) mECT labeled with anti-myosin binding protein C antibody (red) and DAPI nuclear counter stain (blue) showing alignment of cells with long axis of ECT and striated cardiomyocytes.
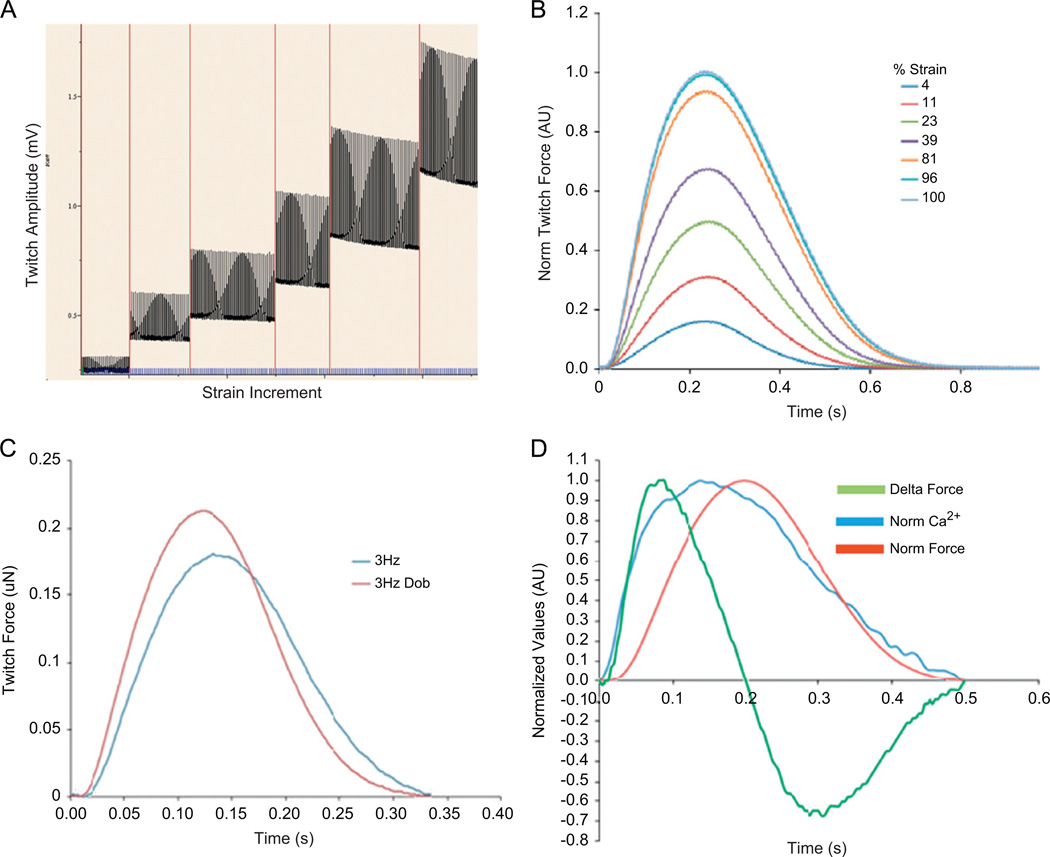
Fig. 2. Mouse ECT twitch force and calcium transients: representative contractile data from mECT.
(A and B) Frank–Starling relationship with increasing force magnitude with increasing strain. (C) mECT responds to dobutamine with increased force magnitude and accelerated twitch kinetics. (D) simultaneous Fura2AM measured calcium transient and twitch force with superimposed dF/dT tracing demonstrating the relationship between the calcium transient and twitch force.
One of the recognized challenges of mECT is the relatively large number of starting cells (8 × 105) required for each construct. To make 6 mECT therefore requires 4 × 106 cells, or up to 28 pups (4 litters). While some mouse knock-out lines have large, reliable litter sizes, other mutations result in smaller litters making this approach less feasible. Reducing the size of the mECT using an approach similar to the 24-well/silicone post-apparatus developed by the Eschenhagen group may reduce the number of cells required and extend the availability of this technology to a wider range of existing murine models (Hansen et al., 2010). Alternatively, using murine embryonic stem cells (ESCs) that retain some proliferative capacity to make mECT may also provide an alternative source of starting cells (Ou et al., 2011; Xi et al., 2010). Careful characterization of the mESC-derived CMs would be needed to verify that the expected cardiac phenotype is reproduced upon differentiation.
Developing an in vitro integrated myocardial tissue system that recapitulates the mature cardiac phenotype including not only the sarcomere but also the ion channel composition is of clear benefit. Several important questions remain about the maturity of the cardiomyocytes in mECT and the ability to experimentally control their maturation. The electrophysiology of the 3D cardiac constructs also remains poorly characterized. While the response to electrical pacing, intact calcium handling, and coordinated contraction is encouraging, the function of major conductance channels, the shape, and propagation of the action potential remain unexplored. Patch clamping cells from dissociated ECT, sharp electrode action potential measurement, and optical electrical mapping techniques should be feasible and would provide valuable information.
Modeling human genetic heart disease using mouse ECT
The mECT platform is particularly well suited to study genetic forms of heart disease in which an intrinsic contractile phenotype is suspected or when applied stressors are hypothesized to promote the development of a contractile abnormality. The mECT approach has been applied to study familial hypertrophic cardiomyopathy using a mouse model deficient in cardiac myosin binding protein C (de Lange et al., 2011). A potential advantage of the neonatal cardiomyocyte mECT is that pathological remodeling events may not yet be present. The unremodeled mECT can thus reveal details about the primary cellular abnormalities caused by a specific genetic mutation without the confounding effects of remodeling. The response of the mECT to environmental stress (e.g., mechanical loading, increased metabolic demand, or pharmacologic exposure) can then be determined acutely or over an extended period of weeks. After obtaining contractile, calcium transient, action potential, and metabolic data, mECT can be further processed to examine morphology and perform gene/protein expression studies.
Genetic manipulation in the mouse heart, while a time-honored standard, has required both significant patience and financial resources to develop either germ-line or inducible knock-out and knock-in models (Chu et al., 2002). The mECT provides an opportunity to save considerable time and expense depending on the suitability of the gene in question. Exogenous proteins are readily transduced into isolated murine cardiomyocytes using either adenovirus, adeno-associated, or lentivirus. The entire sequence from generating the virus to evaluating the contractile effects within the functional mECT can be accomplished in as little as 3 months. The sarcomere proteins may be particularly suited to this approach as they can be readily expressed and incorporated correctly within the sarcomere (de Lange et al., 2011). Proteins containing single amino acid mutations can be introduced into wild-type cells or cells derived from knockout model in which the wild-type protein has been genetically ablated. This novel use of existing mouse models expands their potential utility to study diseases with genetic heterogeneity, such as familial HCM. The mECT can thus function as an adjunct to traditional mouse models, permitting rapid and cost-effective identification of phenotype-positive mutations that would then merit generation of a unique mouse model.
Developing human ECT to study human heart disease
The development of an accessible human cardiac cell-based ECT (hECT) is highly desirable. Several groups are actively pursuing this goal with reports beginning to appear in the literature. In 2011, the successful formation of functional ECT from human embryonic stem cells in a fibrin-based matrix was reported (Schaaf et al., 2011). Another group simultaneously presented their initial report of hECT using cardiomyocytes derived from induced pluripotent stem cells (iPSCMs). The iPSCM hECTs develop into muscle strips with similar organization and mechanical function seen using rat and mouse cardiomyocytes (Tulloch et al., 2011). As iPS cell lines are established using fibroblasts isolated from patients with specific genetic diseases, we have the opportunity to study cardiac muscle function in an integrated mechanically active construct within the full genetic context of the individual from whom the cells were isolated. This will allow for the first-time assessment of genotype–environment–phenotype interactions at the level of an affected individual or family.
There are several issues pertaining to the human iPSCMs that will need to be resolved as the hECT technique is developed. The phenotype of the iPSCM falls somewhere between that of an immature and mature cardiomyocyte. The effect of the 3D environment on maturation status is likely profound and will need careful optimization. Cell composition, including fibroblasts and endothelial cells, will also need to be carefully considered as these non-myocytes influence the mechanical properties of the tissue and the development of microvasculature (Tulloch et al., 2011). The ability to intentionally modify the cardiac cell phenotype through manipulating the culture environment or genetic modification presents one of the real advantages of the ECT that can now be applied in studies of human cardiac tissue. Important human disease processes, such as heart failure, which are associated with perturbations in gene expression patterns with unclear impact on heart function, can now be studied using the controlled hECT system. hECT in combination with murine models of cardiac disease will provide a powerful tool to generate important insights into the pathophysiology of human heart disease.
Conclusions
The generation of 3D mouse-derived engineered cardiac tissue models enables the measurement of contractile function, as well as collection of calcium handling, and molecular data in a system that more closely mimics the intact heart than traditional 2D cultures. The ability to grow integrated cardiac tissue preparations in a sheet, ring, or strip conformations allows application of this technology to a wide range of experimental questions. This advance supports the evaluation of cardiac function and response to stress and pharmacologic treatment in the extensive number of currently existing mouse models of human disease. Additionally, new models of genetic heart disease can be efficiently and inexpensively created using the mouse ECT. Mouse ECT is thus an important addition to the available methods for the study of healthy and diseased myocardium. Further adaptation of this technology to utilize human iPS-derived cardiomyocytes will facilitate the study of human cardiac disease in a fully human context.
Acknowledgments
Preparation of this manuscript was supported by R01HL107367-01 (JCR) and Children’s Cardiomyopathy Foundation Grant 133-PRJ32ZU (JCR).
Footnotes
Disclosures: Neither Dr Ralphe nor Dr de Lange has any disclosures.
REFERENCES
- Akins RE, Jr, Rockwood D, Robinson KG, Sandusky D, Rabolt J, Pizarro C. Three-dimensional culture alters primary cardiac cell phenotype. Tissue Engineering Part A. 2010;16:629–641. doi: 10.1089/ten.tea.2009.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. Journal of Molecular and Cellular Cardiology. 2011;50:940–950. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Chu G, Haghighi K, Kranias EG. From mouse to man: understanding heart failure through genetically altered mouse models. Journal of Cardiac Failure. 2002;8:S432–S449. doi: 10.1054/jcaf.2002.129284. [DOI] [PubMed] [Google Scholar]
- de Lange WJ, Hegge LF, Grimes AC, et al. Neonatal mouse-derived engineered cardiac tissue: a novel model system for studying genetic heart disease. Circulation Research. 2011;109:8–19. doi: 10.1161/CIRCRESAHA.111.242354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenhagen T, Fink C, Remmers U, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB Journal. 1997;11:683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- Fujimoto KL, Clause KC, Liu LJ, et al. Engineered fetal cardiac graft preserves its cardiomyocyte proliferation within post-infarcted myocardium and sustains cardiac function. Tissue Engineering Part A. 2011;17:585–596. doi: 10.1089/ten.tea.2010.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Eder A, Bonstrup M, et al. Development of a drug screening platform based on engineered heart tissue. Circulation Research. 2010;107:35–44. doi: 10.1161/CIRCRESAHA.109.211458. [DOI] [PubMed] [Google Scholar]
- Kira Y, Nakaoka T, Hashimoto E, Okabe F, Asano S, Sekine I. Effect of long-term cyclic mechanical load on protein synthesis and morphological changes in cultured myocardial cells from neonatal rat. Cardiovascular Drugs and Therapy. 1994;8:251–262. doi: 10.1007/BF00877334. [DOI] [PubMed] [Google Scholar]
- Louch WE, Sheehan KA, Wolska BM. Methods in cardiomyocyte isolation, culture, and gene transfer. Journal of Molecular and Cellular Cardiology. 2011;51:288–298. doi: 10.1016/j.yjmcc.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistri S, Pini A, Sassoli C, et al. Relaxin promotes growth and maturation of mouse neonatal cardiomyocytes in vitro: clues for cardiac regeneration. Journal of Cellular and Molecular Medicine. 2012;16:507–519. doi: 10.1111/j.1582-4934.2011.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods in Molecular Biology. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- Ou D-B, He Y, Chen R, et al. Three-dimensional co-culture facilitates the differentiation of embryonic stem cells into mature cardiomyocytes. Journal of Cellular Biochemistry. 2011;112:3555–3562. doi: 10.1002/jcb.23283. [DOI] [PubMed] [Google Scholar]
- Patterson JT, Gilliland T, Maxfield MW, et al. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: from the bench to the clinic and back again. Regenerative Medicine. 2012;7:409–419. doi: 10.2217/rme.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf S, Shibamiya A, Mewe M, et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One. 2011;6:e26397. doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita K, Liu LJ, Janczewski AM, et al. Engineered early embryonic cardiac tissue retains proliferative and contractile properties of developing embryonic myocardium. American Journal of Physiology Heart and Circulatory Physiology. 2006;291:H1829–H1837. doi: 10.1152/ajpheart.00205.2006. [DOI] [PubMed] [Google Scholar]
- Tulloch NL, Muskheli V, Razumova MV, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circulation Research. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J, Khalil M, Shishechian N, et al. Comparison of contractile behavior of native murine ventricular tissue and cardiomyocytes derived from embryonic or induced pluripotent stem cells. FASEB Journal. 2010;24:2739–2751. doi: 10.1096/fj.09-145177. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnology and Bioengineering. 2000;68:106–114. [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nature Medicine. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]