Abstract
The p73 protein is a transcription factor and member of the p53 protein family that expresses as a complex variety of isoforms. ΔNp73α is an N-terminally truncated isoform of p73. We found that ΔNp73 protein is upregulated in human gastric carcinoma suggesting that ΔNp73 may play an oncogenic role in these tumors. Although it has been shown that ΔNp73α inhibits apoptosis and counteracts the effect of chemotherapeutic drugs, the underlying mechanism by which this p73 isoform contributes to chemotherapeutic drug response remains to be explored. We found that ΔNp73α upregulates MDR1 mRNA and p-glycoprotein (p-gp), which is involved in chemotherapeutic drug transport. This p-gp upregulation was accompanied by increased p-gp functional activity in gastric cancer cells. Our data suggest that upregulation of MDR1 by ΔNp73α is mediated by interaction with p53 at the MDR1 promoter.
Keywords: p73, p53, gastric tumor
Introduction
Gastric cancer is the second most common cause of death due to cancer worldwide (Parkin et al., 2005). Resistance to chemotherapy is a major problem in treatment of gastric carcinomas. Tumor cells often acquire drug resistance phenotype through the upregulation of the mdr1 gene. A product of the mdr1 gene, p-glycoprotein (p-gp), is a 170-kDa transmembrane protein that functions as an energy-dependent drug efflux pump, the substrates of which include a wide range of anticancer drugs. MDR1 is often overexpressed in primary gastric adenocarcinoma and its precursor lesions (Vollrath et al., 1991; Wallner et al., 1993; Lacueva et al., 1998; Choi et al., 2002). This increase in p-gp levels correlates with an increased resistance to the chemotherapeutic drug, doxorubicin, in primary gastric cancer cells (Hotta et al., 1999).
Tumor-suppressor protein p53 regulates mdr1 gene expression. Wild-type p53 (wtp53) suppresses MDR1 promoter constructs, as well as expression of the endogenous mdr1 gene, whereas several common p53 mutants are able to activate the MDR1 promoter (Chin et al., 1992; Dittmer et al., 1993; Thottassery et al., 1997).
Accumulating evidence suggests that p53 functions in concert with p73, another member of the p53 protein family. Extensive structural similarity exists between p53 and p73, but the highest is found in the DNA-binding domain, in which p73 shares approximately 60% amino-acid identity with p53. p73 is expressed as a complex variety of protein isoforms with and without N-terminal transactivation domain (TAD). Isoforms with TAD are termed TA, whereas isoforms without TAD are known as ΔN. TA isoforms of p73 transactivate p53 target genes and induce apoptosis (Kaghad et al., 1997). In contrast, ΔNp73α inhibits apoptosis and confers drug resistance (Fillippovich et al., 2001; Zaika et al., 2002). High expression levels of ΔNp73 significantly correlate with chemotherapeutic failure in ovarian cancer. Moreover, p53 is likely a main target of ΔNp73 in these tumors (Concin et al., 2005). In mice, ΔNp73 features proto-oncogenic properties; it facilitates cell immortalization and cooperates with oncogenic Ras in cellular transformation and in tumorigenesis (Stiewe and Putzer, 2002; Petrenko et al., 2003). Expression of ΔNp73 correlates with poor patient prognosis in neuroblastoma, lung, ovarian, prostate, colon and breast cancers (Casciano et al., 2002; Guan and Chen, 2005; Dominguez et al., 2006).
In this study, we examined the role of p73 isoforms in regulation of mdr1 gene expression in gastric cancer cells.
Results
Expression of ΔNp73α in gastric carcinoma
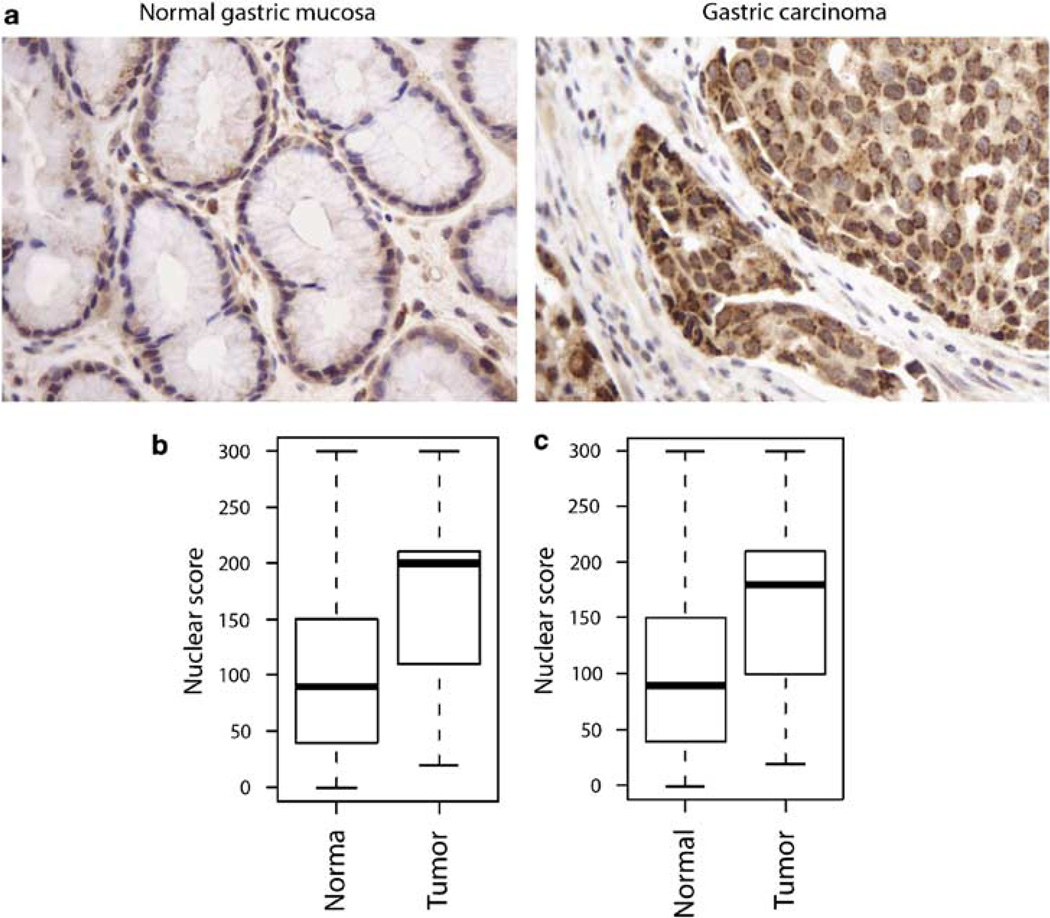
Protein expression of ΔNp73 was evaluated in 48 gastric carcinomas and 88 non-neoplastic gastric specimens. ΔNp73 immunoreactivity was found in both nuclei and cytoplasm of epithelial cells. However, intensity of ΔNp73 staining was weaker in non-neoplastic cells compared to that of tumor cells and was observed mostly in cytoplasm, whereas the tumor cells showed strong nuclear staining (Figure 1a). We found a statistically significant increase (P = 0.00014) in nuclear staining in the tumor samples compared to non-neoplastic mucosa (Figure 1b). Nuclear overexpression of ΔNp73 protein was detected in 59% (26 of 44 cases) analysed tumors.
Figure 1.
ΔNp73 protein is overexpressed in gastric adenocarcinoma. (a) Immunohistochemical staining of ΔNp73 protein in gastric cancer and normal gastric mucosa. A weak cytoplasmic staining was observed in non-neoplastic gastric mucosa; whereas, strong nuclear and cytoplasmic staining was found in tumor cells. (b) Comparison of ΔNp73 nuclear expression in gastric carcinoma and non-neoplastic tissue samples. (c) Comparison of ΔNp73 nuclear expression in gastric carcinoma and matched non-neoplastic mucosa samples.
Pairwise comparison of 44 informative carcinoma cases with matched non-neoplastic mucosa also demonstrated a statistically significant (P = 0.0011) increase in nuclear ΔNp73 staining in the tumors’ epithelia (Figure 1c). Interestingly, an increase of ΔNp73 expression was significantly higher in intestinal-type gastric carcinoma (P = 0.000015).
ΔNp73α upregulates mdr1 gene expression in gastric cancer cells
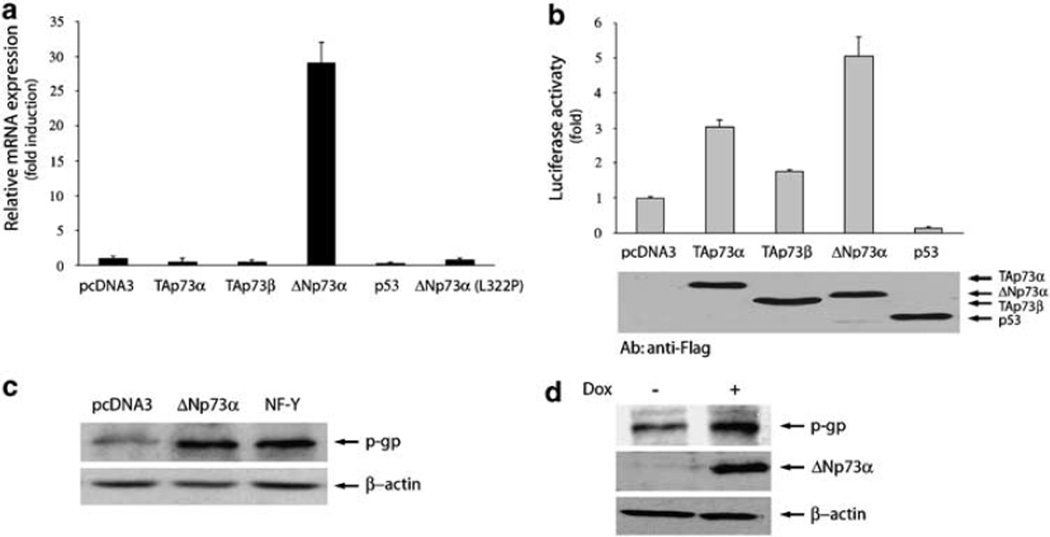
To assess whether p73 affects mdr1 gene expression, AGS cells were transfected with plasmids expressing various p73 isoforms, and MDR1 mRNA levels were determined by real-time PCR analysis (Figure 2a). MDR1 transcript level was increased more than 25-fold in ΔNp73α-transfected cells compared to vector control. In contrast to the ΔNp73α effect and in accord with previous reports (Chin et al., 1992; Thottassery et al., 1997), wtp53 inhibited MDR1. Interestingly, mutant ΔNp73α (L322P) was unable to increase the MDR1 mRNA level. Similarly, TAp73α or TAp73β did not affect MDR1 transcription. To confirm these findings, MDR1 promoter fragment (−1277 to +25) was cloned into pGL3 luciferase reporter vector, and luciferase assay was performed in AGS cells. As shown in Figure 2b, MDR1 promoter activity increased in cells expressing ΔNp73α compared to empty-vector transfected cells. Transfection of wtp53 led to a decrease in MDR1 promoter activity. Notably, TAp73α and TAp73β were able to activate the reporter, although at lower levels than ΔNp73α. Most likely, the observed difference in activation of mdr1 gene transcription by TAp73 isoforms (detected by real-time PCRan d luciferase assays, compare Figures 2a and b) is a reflection of differences in regulation of truncated (reporter construct) and endogenous MDR1 promoters. Equal expression of all transfected vectors was verified by western blotting with an antibody recognizing the Flag-tag (Figure 2b, lower panel).
Figure 2.
ΔNp73α activates MDR1 expression in gastric cancer cells. (a) Relative MDR1 mRNA levels in AGS cells transfected with indicated expression plasmids. (b) Activation of MDR1 luciferase reporter transfected with flag-tagged p73 isoforms and p53 in AGS cells (upper panel). Lower panel shows equal expression of p73 isoforms and p53 detected by western blotting. (c) Western blot analysis of p-glycoprotein (p-gp) expression in AGS cells transfected with indicated expression constructs. Transfection with NF-Y was used as a positive control. (d) Doxycycline-inducible upregulation of ΔNp73α increases p-gp level in stably-transfected AGS cells.
To determine whether ΔNp73α upregulates p-gp, we performed western blot analysis with a p-gp-specific antibody. As a positive control, cells transfected with NF-Y transcription factor, a known activator of mdr1 gene expression, were used (Hu et al., 2000). overexpression of ΔNp73α in AGS cells led to a significant increase in the endogenous p-gp level that was comparable to that induced by NF-Y (Figure 2c).
To further confirm these data, an AGS stable cell line expressing ΔNp73α in a doxycycline-inducible manner was generated. Using western blot analysis, we found that the induction of ΔNp73α increases the level of p-gp (Figure 2d). An exposure of a parental AGS cell line to the same concentration of doxycycline did not affect the p-gp expression (data not shown).
ΔNp73α causes the increase in p-gp transporter activity in gastric cancer cells
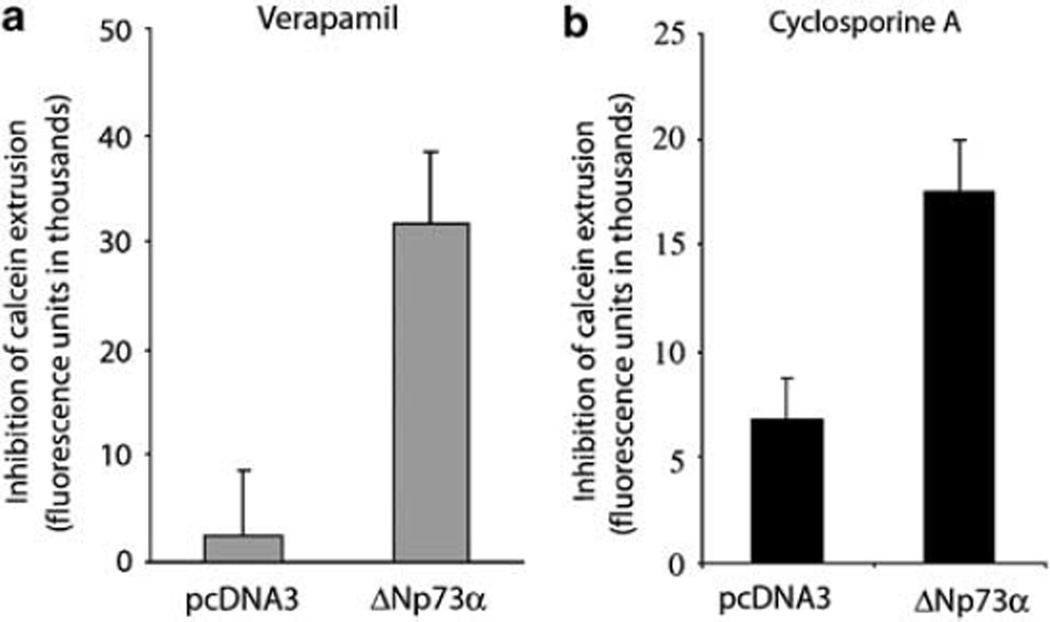
Next, changes in MDR1 functional activity were measured by the calcein uptake assay using two well-known MDR1 inhibitors verapamil and cyclosporine A. We observed a significant increase in the p-gp activity in AGS cells expressing ΔNp73α compared to control cells transfected with vector alone (Figures 3a and b). Interestingly, TAp73α was unable to elevate the p-gp transporter activity (data not shown). These results demonstrate that ΔNp73α increases calcein extrusion, thus suggesting that the observed upregulation of mdr1 gene expression correlates with the increase in p-gp transporter activity.
Figure 3.
ΔNp73α increases p-glycoprotein (p-gp) transporter activity in gastric cells. (a) Relative accumulation of fluorescent MDR1 substrate, calcein AM, in cells treated with p-gp inhibitor, varapamil, versus untreated control. AGS cells were transfected with indicated expression plasmids and the drug uptake analysis was performed as described in the Materials and Methods section. (b) Same as (a), except that cyclosporine A was used as a MDR1 inhibitor.
Activation of the mdr1 gene expression by ΔNp73α depends on p53 activity
Similar to the experiment with AGS cells described above, we evaluated the effect of ΔNp73α on MDR1 expression in additional cell lines having different p53 statuses. Surprisingly, endogenous MDR1 mRNA was increased only in cells expressing wtp53 (AGS and U2OS; Figures 2a and 4c); whereas, no upregulation was detected in p53-null (H1299) or p53-mutant (Caco-2) cells (Figures 4a and b). HeLa cells, which express very low levels of p53 due to the presence of HPV E6 protein (Hoppe-Seyler and Butz, 1993) were also non-responsive to ΔNp73α transfection (data not shown), indicating that p53 is involved in the regulation of MDR1 expression by ΔNp73α.
Figure 4.
Effect of ΔNp73α on MDR1 transcription is cell-line dependent. (a) Real-time PCR analysis of MDR1 mRNA in p53-null H1299 cells transfected with indicated expression vectors. (b) Same as (a), except that the mutant p53 cell line, Caco-2, was used. (c) Same as (a), except that U2OS cells, which harbor wtp53, were used.
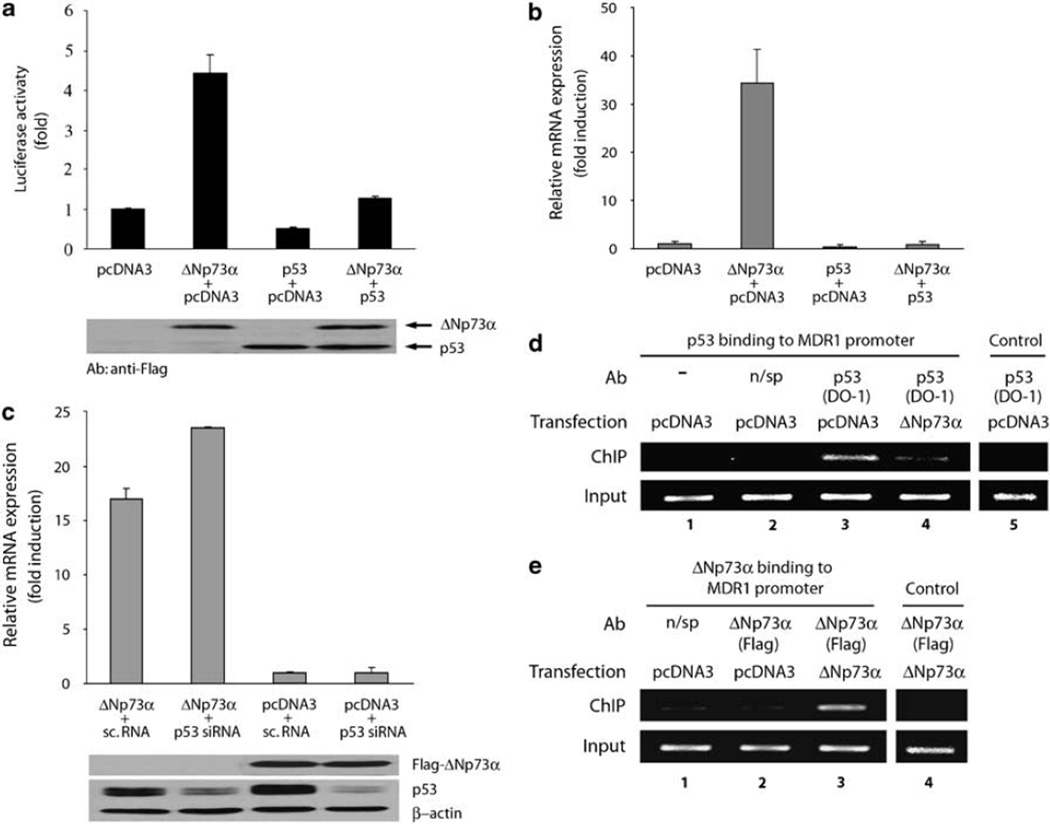
To assess the role of p53, MDR1 luciferase assay was performed in AGS cells transfected with p53 and ΔNp73α in combination or alone. ΔNp73α activity was abrogated by transfection of wtp53 (Figure 5a). Thus, p53 counteracts ΔNp73α function on MDR1 promoter. Equal expression of ΔNp73α and p53 in this experiment was verified by western blot analysis (Figure 5a, lower panel). To further confirm these data, we investigated whether wtp53 would have a similar effect on endogenous MDR1 mRNA levels. Likewise to the luciferase experiment described above, p53 completely suppressed upregulation of MDR1 mRNA induced by ΔNp73α (Figure 5b). Next, we co-transfected AGS cells with ΔNp73α or pcDNA3 and either small interfering RNA (siRNA) specific for p53 (p53 siRNA) or nonspecific dsRNA (sc.RNA). A decrease in the endogenous p53 protein level caused by p53 siRNA led to a further increase of ΔNp73α activity, and endogenous MDR1 mRNA compared to cells co-transfected with ΔNp73α and unspecific dsRNA (Figure 5c). The efficiency of p53 inhibition by siRNA and equal expression of ΔNp73α were confirmed by western blotting (Figure 5c, lower panel). Interestingly, downregulation of p53 alone did not result in a significant increase of mdr1 gene expression suggesting that ΔNp73α is essential for full activation of the MDR1 promoter (Figure 5c). Previously, it has been reported that p53 binds to the MDR1 promoter (Johnson et al., 2001). Therefore, we examined the effect of ΔNp73α on DNA-binding of p53 using chromatin immunoprecipitation (ChIP). Our ChIP analysis confirmed that endogenous p53 specifically binds to the MDR1 promoter in AGS cells (Figure 5d, lane 3). Transfection of ΔNp73α led to a significant decrease of p53 binding to the same promoter (Figure 5d, compare lanes 3 and 4). A similar effect was observed in AGS cells expressing ΔNp73α in a tet-inducible manner (data not shown). The ChIP assay also revealed that ΔNp73α binds to the MDR1 promoter (Figure 5e, lane 3).
Figure 5.
ΔNp73α regulates mdr1 gene expression in a p53-dependent manner. (a) Analysis of MDR1 luciferase reporter in AGS cells co-transfected with indicated plasmids. ΔNp73α and p53 were flag-tagged to facilitate their detection. Western blot analysis shows equal expression of ΔNp73α and p53 detected with Flag-specific antibody (lower panel). (b) Expression of MDR1 mRNA in the AGS cells transfected with indicated expression plasmids. (c) siRNA directed against p53 increases effect of ΔNp73α on MDR1 transcription. AGS cells were co-transfected with ΔNp73α or pcDNA3 and either small interfering RNA (siRNA) directed against p53 or nonspecific dsRNA. Western blot analysis of ΔNp73α and endogenous p53 in AGS cells (lower panel). (d) ΔNp73α inhibits specific binding of p53 to the MDR1 promoter. Chromatin extracted from AGS cells transfected with indicated plasmids was immunoprecipitated with p53 (DO-1) antibody (lanes 3, 4 and 5), unspecific mouse antibody (lane 2) or without antibody (lane 1) and then amplified by PCR using human MDR1 promoter-specific primers. As an additional control, primers that amplify unrelated DNA (human darpp32 gene) were used (lane 5). Bottom panels show PCR amplification of input DNA. (e) In vivo characterization of the ΔNp73α binding to the MDR1 promoter by chromatin immunoprecipitation (ChIP) assay. The same as (d), except ChIP was performed with anti-flag M2 antibody.
Discussion
In this study, we investigated expression of the p73 isoform, ΔNp73, in gastric cancer. Using an immunohistochemical approach, we found that the ΔNp73 protein expression is significantly increased in the gastric carcinoma as compared to non-neoplastic gastric mucosa. Elevated ΔNp73 staining was observed in the nuclei of cancer cells primarily in intestinal-type tumors. This increase is consistent with reports by us and others, which suggest that ΔNp73α has oncogenic and anti-apoptotic properties (Stiewe and Putzer, 2002; Zaika et al., 2002; Petrenko et al., 2003). Several studies have also shown that ΔNp73 may contribute to drug resistance in vitro and in vivo (Vossio et al., 2002; Zaika et al., 2002; Uramoto et al., 2004; Concin et al., 2005). These findings raise an important question about targets of ΔNp73. Here, we provide proof of a novel link between ΔNp73α and upregulation of one of the major markers of drug resistance, MDR1.
We found that ΔNp73α upregulates endogenous MDR1 mRNA and protein in gastric epithelial cells. Furthermore, upregulation of p-gp by ΔNp73α correlates with an increase of its transporter activity. A recent study has found upregulation of MDR1 mRNA by TAp73 isoforms in neuroblastoma cells (Johnson et al., 2005). We did not observe this phenomenon in this study, which suggests that the p73 effect might be cell-line dependent. Indeed, we found ΔNp73-induced upregulation only in cell lines with wtp53 (see Figure 4). Interestingly, ΔNp73α (L322P) mutant, which has an impaired ability to inhibit p53, was unable to activate mdr1 gene transcription (Zaika et al., 2002). This finding points to p53’s role in the regulation of MDR1 by ΔNp73α. We explored this hypothesis further and found that co-transfection of ΔNp73α together with wtp53 leads to suppression of the mdr1 gene expression induced ΔNp73α. Inversely, inhibition of endogenous p53 by siRNA increased MDR1 mRNA accumulation in response to ΔNp73α. Thus, ΔNp73-induced upregulation of MDR1 is controlled by p53.
Depending on the target gene, p53 usually acts either as a transcriptional activator or repressor (Vousden, 2006). In the contest of the mdr1 gene, p53 suppresses mdr1 gene transcription through a direct binding to its promoter (Johnson et al., 2001). The transcriptional inhibition of MDR1 expression by p53 was also confirmed in this study (see Figures 2a and b). We and others also found that ΔNp73 physically interacts with p53 (Pozniak et al., 2000; Nakagawa et al., 2002; Zaika et al., 2002). In addition, our ChIP analysis revealed that upregulation of ΔNp73α interferes with p53-specific binding to the MDR1 promoter (see Figure 5d). However, the data also showed that downregulation of p53 alone is not sufficient to upregulate MDR1, and ΔNp73α is required for full activation of the MDR1 transcription. Using ChIP, we found that ΔNp73α binds to the MDR1 promoter. These data combined with the recent characterization of a novel transactivation domain in ΔNp73 (Liu et al., 2004) suggests that ΔNp73 may function as a transcription factor.
In summary, the results demonstrate that ΔNp73α activates mdr1 gene expression and increases p-gp transporter activity in gastric cancer cells. This activation resulted from inhibition of p53 activity and ΔNp73 binding to the MDR1 promoter. These data provide the first evidence that ΔNp73α compromises p53 function as a repressor and reveal a novel mechanism that may underlie drug resistance in gastric tumors.
Materials and methods
Tissue sample, tissue array construction and immunohistochemistry
After the institutional review board approval, 48 carcinoma cases with matched non-neoplastic mucosa and 40 additional non-neoplastic gastric samples resected at Vanderbilt University Medical Center were histologically verified and representative regions were selected for inclusion in a tissue microarray.
For immunohistochemical staining, slides were pretreated in citrate buffer in a pressure cooker. Anti-ΔNp73 antibody (Imgenex, San Diego, CA, USA) was applied at room temperature and immunostaining was detected using EnVision + HRP kit (DakoCytomation, Ely, Cambridgeshire, UK). This antibody recognizes the unique epitope located on the N-terminus of ΔNp73 and does not crossreact with TAp73. Specificity of ΔNp73 staining was verified by omitting a primary antibody step in the protocol. The nuclei were counterstained with hematoxylin. Immunohistochemical results were evaluated for intensity and staining frequency in nuclear and cytoplasmic compartments. The intensity of staining was graded as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). The frequency was graded according to the percentage of positive cells. Total nuclear and cytoplasmic scores were calculated by multiplying the intensity score by the percentage of positive cells.
Cell culture and generation of ΔNp73α-Tet-on cells
AGS cells were maintained in Ham’s F-12 media (Invitrogen, Carlsbad, CA, USA). U2OS, Caco-2 and H1299 cell lines were grown in Dulbecco’s modified Eagle’s medium (Invitrogen). Culture media were supplemented with 10% fetal bovine serum.
To generate cells expressing ΔNp73α in a doxycycline-inducible manner, 1.8Kb NotI-ClaI fragment containing flag-ΔNp73α was subcloned into the pTRE2pur vector (Clontech, Palo Alto, CA, USA). AGS Tet-on cells (Clontech) were transfected with ΔNp73α-pTRE2pur and selected with puromycin according to the manufacturer’s protocol.
Vectors, antibodies, transfection and luciferase assay
pcDNA3-based vectors expressing human p53, p73α, p73β, ΔNp73α and tetramerization domain mutant, ΔNp73L322P, have been described previously (Zaika et al., 2002; Tomkova et al., 2006). pMDR1-luc reporter plasmid was constructed by insertion of MDR1 promoter fragment generated by PCR (−1277 to +25) into pGL3 luciferase vector (Promega, Madison, WI, USA). Cells were transfected with LipofectA-MINE 2000 (Invitrogen) or FuGENE 6 (Roche, Indianapolis, IN, USA) reagents using the manufacturers’ protocols. Target proteins were detected using specific antibodies: MDR1 (Sigma, St Louis, MO, USA), p53 (DO-1, Calbiochem, La Jolla, CA, USA), anti-Flag (M2, Sigma) and actin (Cell Signaling, Danvers, MA, USA). Luciferase activity was measured using a Dual-Luciferase Reporter Assay kit (Promega) as described earlier (Tomkova et al., 2004). All experiments were performed in triplicate. Data is presented as average ± s.d.
RNA isolation and real-time PCR analysis
Total RNA was extracted using Trizol (Invitrogen). Reverse transcription was performed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Quantitative real-time PCR was carried out using an iCycler (Bio-Rad). The PCR primers were as follows: MDR1 forward, 5′-GGG TGG TGT CAC AGG AAG AG-3′, and MDR1 reverse, 5′-AAC AAG GGC ACG AGC TAT GG-3′; HPRT forward, 5′-TTG GAA AGG GTG TTT ATT CCT CA-3′, and HPRT reverse, 5′-TCC AGC AGG TCA GCA AAG AA-3′. All reactions were performed in duplicate. The expression level of MDR1 mRNA was normalized to HPRT1 mRNA expression. Data is shown as fold expression ± s.d. compared to corresponding controls.
Small interfering RNA
AGS cells were co-transfected with ΔNp73α or pcDNA3 and either siRNA oligonucleotide specific for human p53 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or unspecific control dsRNA (Ambion, Austin, TX, USA).
p-gp functional activity
p-gp activity was assessed using Vybrant Multidrug Resistance Assay Kit (Molecular Probes, Invitrogen). p-gp activation was quantified by measuring an increase in intracellular calcein-AM fluorescence after exposure to MDR1 inhibitors. Briefly, AGS cells were grown in 96-well plates, transfected with indicated plasmids and allowed to recover. After 24 h, the medium was aspirated and cells were preincubated with either phosphate buffered saline or phosphate buffered saline supplemented with MDR1 inhibitor verapamil (100 µm) or cyclosporin A (10 µm) for 30 min at 37 °C. Calcein-AM was then added to the cells at final concentration 0.4 µm and the plates were incubated for 1 h at 37 °C. Calcein-AM containing solutions were replaced with ice-cold phosphate buffered saline and fluorescence was measured using a microplate reader. Experiments were conducted in six parallel wells and results were normalized for protein concentration. Data are presented as average ± s.e.
Chromatin immunoprecipitation
ChIP analysis was performed in AGS cells (either transfected with flag-tagged ΔNp73 or expressing ΔNp73 in a tet-inducible manner and corresponding control cells) using the ChIP assay kit (Upstate Biotechnology, Lake Placid, NY, USA) as described earlier (Tomkova et al., 2006). Bindings of p53 and ΔNp73 to the MDR1 promoter were analysed by PCR using following primers: 5′-ACCTGTTTCGCAGTTTCTCG-3′ and 5′-AAGAGCCGCTACTCGAATGA-3′. The control primers, 5′-AGAGAGGGGCACAGCACTAA-3′ and 5′-GGCAAGC TATCCAGGCAATA-3′, were used to amplify unspecific DNA (darpp32 gene). PCR conditions were as follows: 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min for 35 cycles and a final extension for 7 min at 72 °C. PCR products were separated on 1.5% agarose gel and visualized with ethidium bromide.
Statistical analysis
Wilcoxon rank sum test was used for non-pairwise comparison of gastric carcinoma and non-neoplastic mucosa samples. Wilcoxon signed rank test was used for pairwise comparison of matching tumor and normal cases. P ≤ 0.05 were considered statistically significant.
Acknowledgements
We thank Drs Belkhiri and Revetta for their assistance.
Grant support: This work was supported by the National Cancer Institute: grants NIH CA108956, NIH CA129655 and NIH 5PO CA095103.
References
- Casciano I, Mazzocco K, Boni L, Pagnan G, Banelli B, Allemanni G, et al. Expression of DeltaNp73 is a molecular marker for adverse outcome in neuroblastoma patients. Cell Death Differ. 2002;9:246–251. doi: 10.1038/sj.cdd.4400993. [DOI] [PubMed] [Google Scholar]
- Chin KV, Ueda K, Pastan I, Gottesman MM. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992;255:459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- Choi JH, Lim HY, Joo HJ, Kim HS, Yi JW, Kim B, et al. Expression of multidrug resistance-associated protein1, P-glycoprotein, and thymidylate synthase in gastric cancer patients treated with 5-fluorouracil and doxorubicin-based adjuvant chemotherapy after curative resection. Br J Cancer. 2002;86:1578–1585. doi: 10.1038/sj.bjc.6600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concin N, Hofstetter G, Berger A, Gehmacher A, Reimer D, Watrowski R, et al. Clinical relevance of dominant-negative p73 isoforms for responsiveness to chemotherapy and survival in ovarian cancer: evidence for a crucial p53–p73 cross-talk in vivo. Clin Cancer Res. 2005;11:8372–8383. doi: 10.1158/1078-0432.CCR-05-0899. [DOI] [PubMed] [Google Scholar]
- Dittmer D, Pati S, Zambetti G, Chu S, Teresky AK, Moore M, et al. Gain of function mutations in p53. Nat Genet. 1993;4:42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- Dominguez G, Garcia JM, Pena C, Silva J, Garcia V, Martinez L, et al. DeltaTAp73 upregulation correlates with poor prognosis in human tumors: putative in vivo network involving p73 isoforms, p53, and E2F-1. J Clin Oncol. 2006;24:805–815. doi: 10.1200/JCO.2005.02.2350. [DOI] [PubMed] [Google Scholar]
- Fillippovich I, Sorokina N, Gatei M, Haupt Y, Hobson K, Moallem E, et al. Transactivation-deficient p73alpha (p73Deltaexon2) inhibits apoptosis and competes with p53. Oncogene. 2001;20:514–522. doi: 10.1038/sj.onc.1204118. [DOI] [PubMed] [Google Scholar]
- Guan M, Chen Y. Aberrant expression of DeltaNp73 in benign and malignant tumours of the prostate: correlation with Gleason score. J Clin Pathol. 2005;58:1175–1179. doi: 10.1136/jcp.2005.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe-Seyler F, Butz K. Repression of endogenous p53 transactivation function in HeLa cervical carcinoma cells by human papillomavirus type 16 E6, human mdm-2, and mutant p53. J Virol. 1993;67:3111–3117. doi: 10.1128/jvi.67.6.3111-3117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta T, Tanimura H, Iwahashi M, Tani M, Tsunoda T, Noguchi K, et al. P-glycoprotein-expressing tumor cells are resistant to anticancer drugs in human gastrointestinal cancer. Surg Today. 1999;29:591–596. doi: 10.1007/BF02482982. [DOI] [PubMed] [Google Scholar]
- Hu Z, Jin S, Scotto KW. Transcriptional activation of the MDR1 gene by UV irradiation. Role of NF-Y and Sp1. J Biol Chem. 2000;275:2979–2985. doi: 10.1074/jbc.275.4.2979. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Ince TA, Scotto KW. Transcriptional repression by p53 through direct binding to a novel DNA element. J Biol Chem. 2001;276:27716–27720. doi: 10.1074/jbc.C100121200. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Shepard EM, Scotto KW. Differential regulation of MDR1 transcription by the p53 family members. Role of the DNA binding domain. J Biol Chem. 2005;280:13213–13219. doi: 10.1074/jbc.M414646200. [DOI] [PubMed] [Google Scholar]
- Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- Lacueva FJ, Teruel A, Calpena R, Medrano J, Mayol MJ, Perez-Vazquez MT, et al. Detection of P-glycoprotein in frozen and paraffin-embedded gastric adenocarcinoma tissues using a panel of monoclonal antibodies. Histopathology. 1998;32:328–334. doi: 10.1046/j.1365-2559.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Liu G, Nozell S, Xiao H, Chen X. DeltaNp73beta is active in transactivation and growth suppression. Mol Cell Biol. 2004;24:487–501. doi: 10.1128/MCB.24.2.487-501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Takahashi M, Ozaki T, Watanabe Ki K, Todo S, Mizuguchi H, et al. Autoinhibitory regulation of p73 by Delta Np73 to modulate cell survival and death through a p73-specific target element within the Delta Np73 promoter. Mol Cell Biol. 2002;22:2575–2585. doi: 10.1128/MCB.22.8.2575-2585.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Petrenko O, Zaika A, Moll UM. deltaNp73 facilitates cell immortalization and cooperates with oncogenic Ras in cellular transformation in vivo. Mol Cell Biol. 2003;23:5540–5555. doi: 10.1128/MCB.23.16.5540-5555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozniak CD, Radinovic S, Yang A, McKeon F, Kaplan DR, Miller FD. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science. 2000;289:304–306. doi: 10.1126/science.289.5477.304. [DOI] [PubMed] [Google Scholar]
- Stiewe T, Putzer BM. Role of p73 in malignancy: tumor suppressor or oncogene? Cell Death Differ. 2002;9:237–245. doi: 10.1038/sj.cdd.4400995. [DOI] [PubMed] [Google Scholar]
- Thottassery JV, Zambetti GP, Arimori K, Schuetz EG, Schuetz JD. p53-dependent regulation of MDR1 gene expression causes selective resistance to chemotherapeutic agents. Proc Natl Acad Sci USA. 1997;94:11037–11042. doi: 10.1073/pnas.94.20.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkova K, Belkhiri A, El-Rifai W, Zaika AI. p73 isoforms can induce T-cell factor-dependent transcription in gastrointestinal cells. Cancer Res. 2004;64:6390–6393. doi: 10.1158/0008-5472.CAN-04-2176. [DOI] [PubMed] [Google Scholar]
- Tomkova K, El-Rifai W, Vilgelm A, Kelly MC, Wang TC, Zaika AI. The gastrin gene promoter is regulated by p73 isoforms in tumor cells. Oncogene. 2006;25:6032–6036. doi: 10.1038/sj.onc.1209610. [DOI] [PubMed] [Google Scholar]
- Uramoto H, Sugio K, Oyama T, Nakata S, Ono K, Morita M, et al. Expression of deltaNp73 predicts poor prognosis in lung cancer. Clin Cancer Res. 2004;10:6905–6911. doi: 10.1158/1078-0432.CCR-04-0290. [DOI] [PubMed] [Google Scholar]
- Vollrath V, Chianale J, Gonzalez S, Duarte I, Andrade L, Ibanez L. Multidrug resistance gene and P-glycoprotein expression in gastric adenocarcinoma and precursor lesions. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60:133–138. doi: 10.1007/BF02899538. [DOI] [PubMed] [Google Scholar]
- Vossio S, Palescandolo E, Pediconi N, Moretti F, Balsano C, Levrero M, et al. DN-p73 is activated after DNA damage in a p53-dependent manner to regulate p53-induced cell cycle arrest. Oncogene. 2002;21:3796–3803. doi: 10.1038/sj.onc.1205465. [DOI] [PubMed] [Google Scholar]
- Vousden KH. Outcomes of p53 activation—spoilt for choice. J Cell Sci. 2006;119:5015–5020. doi: 10.1242/jcs.03293. [DOI] [PubMed] [Google Scholar]
- Wallner J, Depisch D, Gsur A, Gotzl M, Haider K, Pirker R. MDR1 gene expression and its clinical relevance in primary gastric carcinomas. Cancer. 1993;71:667–671. doi: 10.1002/1097-0142(19930201)71:3<667::aid-cncr2820710303>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Zaika AI, Slade N, Erster SH, Sansome C, Joseph TW, Pearl M, et al. DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J Exp Med. 2002;196:765–780. doi: 10.1084/jem.20020179. [DOI] [PMC free article] [PubMed] [Google Scholar]