Abstract
Sulfated non-anticoagulant heparins (S-NACHs) might be preferred for potential clinical use in cancer patients without affecting hemostasis as compared to low molecular weight heparins (LMWHs). We investigated anti-tumor effects, anti-angiogenesis effects, and mechanisms of S-NACH in a mouse model of pancreatic cancer as compared to the LMWH tinzaparin. S-NACH or tinzaparin with or without gemcitabine were administered, and tumor luminescent signal intensity, tumor weight, and histopathology were assessed at the termination of the study. S-NACH and LMWH efficiently inhibited tumor growth and metastasis, without any observed bleeding events with S-NACH as compared to tinzaparin. S-NACH distinctly increased tumor necrosis and enhanced gemcitabine response in the mouse pancreatic cancer models. These data suggest the potential implication of S-NACH as a neoadjuvant in pancreatic cancer.
Keywords: Gemcitabine, hemostasis, low molecular weight heparin, pancreatic cancer, non-anticoagulant heparin, anti-angiogenesis, anti-cancer, tumor suppressor, tumor survival, thrombospondin
1. Introduction
Pancreatic cancer, which is usually diagnosed at an advanced stage, carries the highest fatality rate among all human cancers [1,2]. Reasons for low survival include aggressive tumor, high metastatic potential, and late presentation at the time of diagnosis. Despite the introduction of gemcitabine and attempts at developing combination chemotherapy regimens, pancreatic cancer remains a highly aggressive and chemo-resistant tumor [1], and there is need for improved methods to treat this deadly disease. Heparin and low molecular weight heparins (LMWHs) are used in pancreatic and other cancer patients mainly to prevent or treat deep vein thrombosis [3]. In addition to antithrombotic effects, LMWHs release tissue factor pathway inhibitor proteins [4] and nitric oxide [5], attenuate TNF-alpha induced inflammation [6], and inhibit heparinases [7] and selectin [8], supporting their potential anti-cancer and anti-inflammatory role. However, a direct anti-cancer effect for heparins or LMWHs in cancer patients without thrombosis still remains to be demonstrated clinically.
LMWHs have been shown to illicit significant anti-tumor responses in a variety of cancers in both animal and some clinical studies [9-16], suggesting the potential for increasing patient survival. However, it has been difficult to draw definitive conclusions about survival benefits because the studies often involved populations that were heterogeneous in terms of histology type and stage of tumor. Major bleeding problems associated with systemic effects of LMWH on thrombin and factor Xa [17,18] are the limiting factor in continuation of treatments or dose escalation in clinical trials. The anti-thrombin-binding sequence accounts for most of the systemic anticoagulant activity of clinically used heparins and LMWH, and it is localized in only one third of the large molecule [19]. It has been shown that the anti-metastatic efficacy is not primarily based on its anticoagulant activity [20], and animal studies using non-anticoagulant species of heparin indicate that it is possible to separate the anti-metastatic and anticoagulant activities of heparin [21,22].
We have formulated LMWH that has no effect on the systemic coagulation factors yet releases tissue factor pathway inhibitor protein, a key endogenous inhibitor of the TF/VIIa complex from the endothelium [22]. We have also demonstrated the efficacy of a sulfated non-anticoagulant form of LMWH, S-NACH, as an anti-metastatic agent in a B16 melanoma mouse model, without any significant impact on coagulation [22,23]. The additional finding that S-NACH exhibits anti-angiogenesis activity suggests another mechanism that could contribute to its role in tumor suppression [22].
In this study, we investigated a possible role of S-NACH on tumor growth in an orthotopic pancreatic cancer mouse model, comparing the results with a standard LMWH, tinzaparin, which is used clinically. Our data show that S-NACH has direct anti-cancer effects, with comparable effects on the inhibition of pancreatic cancer proliferation and angiogenesis in the mouse pancreatic tumor model and the chick chorioallantoic membrane (CAM) model, but without any effects on hemostasis. We used in vivo and ex vivo bioluminescent imaging, correlating tumor signal intensity with viability, and histopathological data to identify a profile of activity and possible mechanism for S-NACH anti-tumor efficacy in this pancreatic cancer model.
2. Materials and methods
2.1 Cancer Cell Lines and Reagents
Human pancreatic cancer cell lines, MPanc96 and SUIT2 expressing firefly luciferase, were provided by Dr. Arumugam (MD Anderson Cancer Center, Houston, TX). Cell culture reagents and hemoglobin standard, Drabkin’s reagent, and other common reagents were purchased from Sigma (St. Louis, MO). D-Luciferin potassium salt was purchased from Caliper Life Sciences (Hopkinton, MA), and gemcitabine was purchased from Thermo Fisher Scientific (Waltham, MA). Matrigel was purchased from BD Bioscience (San Jose, CA). Tinzaparin was obtained from Leo Pharma Inc. (Ballerup, Denmark), and S-NACH was synthesized at Rensselaer Polytechnic Institute (Rensselaer, NY). Anti-pSer15-p53 (9286) was obtained from Cell Signaling (Danvers, MA), Anti-XIAP (sc-55550), Anti-THBS1 (sc-65612), and Anti-p21 (sc-6246) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX).
2.2 Cells and Cell Culture
Mpanc96-luc and SUIT2-luc cells were grown in DMEM supplemented with 5% fetal bovine serum, 1% penicillin, and 1% streptomycin. Cells were cultured at 37°C to sub-confluence and treated with 0.25% (w/v) trypsin/EDTA to affect cell release from culture flask. After washing cells with culture medium, cells were suspended in DMEM (free of phenol red and fetal bovine serum) and counted.
2.3 Tumor Growth in the CAM Cancer Implant Model
The CAM cancer implant model has been described previously [24] and was used here to study the effect of heparin derivatives (S-NACH versus tinzaparin) along with gemcitabine at 1.0 μg/CAM on tumor angiogenesis and tumor growth. Briefly, pancreatic cancer cells in exponential growth phase were harvested as described above. One × 106 cells in 30 μl of medium were mixed with an equal volume of Matrigel and implanted in the chorioallantoic membrane of 7-day old chick eggs. The effect of these treatments was determined after 8 days of implantation. Results are presented as a mean tumor weight (g) per treatment group and tumor hemoglobin (mg/dl) ± SEM, n = 10 per group.
2.4 Animal Studies
Immune-deficient female NCr nude homozygous mice aged 5-6 weeks and weighing 18-20 g were purchased from Harlan Laboratories (Indianapolis, IN). All animal studies were conducted at the animal facility of the Veteran Affairs Medical Center, Albany, NY, in accordance with the institutional guidelines for humane animal treatment and according to the current NIH guidelines. Mice were maintained under specific pathogen-free conditions and housed under controlled conditions of temperature (20-24°C), humidity (60-70%), and 12 h light/dark cycle with ad libitum access to water and food. Mice were allowed to acclimatize for 5 d prior to the start of study.
2.5 Pancreatic Tumor Orthotopic Implant and Treatments
MPanc96-luc and SUIT2-luc cells were harvested as described above and were orthotopically implanted (2 × 105 cells in 50 μl PBS per mouse) in the pancreas of anesthetized athymic nude mice. Just before treatment initiation, animals (n = 5-10 per group) were randomized by tumor mass detected by an in vivo imaging system (IVIS, described below). Initially heparin derivatives S-NACH and tinzaparin at 10 mg/kg were administered subcutaneously (s.c.) daily. In the subsequent studies S-NACH at 20 mg/kg and tinzaparin at 5 mg/kg were administered s.c. daily. Gemcitabine at 100 mg/kg was injected intraperitoneally twice a week alone or in combination with either S-NACH or tinzaparin. Treatment protocols for this study are summarized in Table I. All mice used for treatment response evaluations were euthanized after 28 days.
Table 1.
Treatment schedule of the immune-deficient, female NCr nude homozygous mice
| Treatment group | Dosage in mg/kg (administration) | Frequency |
|---|---|---|
| Control | saline (s.c.) | daily |
| S-NACH | 20 (s.c.) | daily |
| Tinzaparin | 5 (s.c.) | daily |
| Gemcitabine | 100 (i.p.) | twice a week |
| S-NACH + gemcitabine | 20 (s.c.) (S-NACH) | daily |
| 100 (i.p.) (gemcitabine) | twice a week | |
| Tinzaparin + gemcitabine | 5 (s.c.) (tinzaparin) | daily |
| 100 (i.p.) (gemcitabine) | twice a week |
s.c. = subcutaneously; i.p. = intraperitoneally
2.6 In vivo Imaging System (IVIS)
Imaging was performed once per week to monitor tumor growth. Mice bearing MPanc96-luc or SUIT2-luc tumors were anaesthetized using isoflurane, injected s.c. with 50 μl D-luciferin (30 mg/ml), then imaged. Photographic and luminescence images were taken at constant exposure time. Xenogen IVIS® Living Image software version 3.2 was used to quantify non-saturated bioluminescence in regions of interest. Light emission between 5.5 × 106-7.0 × 1010 photons was assumed to be indicative of viable luciferase-labeled tumor cells while emissions below this range were considered as background. Bioluminescence was quantified as photons/second for each region of interest. In vivo tumor kinetic growth and metastasis were monitored by signal intensity. Ex vivo imaging was performed to confirm the signal intensity in the tumors after the termination the study.
2.7 Histopathology
All specimens were analyzed by histology for routine analysis. Specimens were fixed in 10% buffered formalin, processed routinely, and embedded in paraffin. Then, after fixation, the specimens were transferred into the embedding chambers to hold the specimens in position until the paraffin became solid to prevent further rotation. Four μm serial sections were cut, and then stained using haematoxylin and eosin. Sections were evaluated for various pathologic parameters using a light microscope (Leica, Buffalo Grove, IL).
2.8 Studies of Cultured Pancreatic Cell Growth in vitro
Cells were cultured as previously described for other cell lines [25], except that the medium contained 10% fetal bovine serum throughout the course of each study. Media were replenished daily, including the addition of tinzaparin or S-NACH at 40 μg.
2.9 Immunoblotting
Extracts of cytosolic proteins were obtained from control and treated cells, after which the total protein content was quantitated and proteins resolved on discontinuous PAGE. Proteins were then electro-blotted to nitrocellulose membranes (Millipore, Bedford, MA) as previously described [26]. The membranes were treated with 5% milk in tris-buffered saline containing 0.1% Tween™ and incubated overnight with one of the following: monoclonal anti-pSer15-p53 (hyper-phosphorylated p53), anti-XIAP, anti-THBS1, and anti-p21. Primary antibody incubation was followed by treatment with the secondary rabbit anti-mouse IgG antibody. Immunoblots of β-actin were also prepared to control for equalization of proteins. Results presented reflect 3 western blot experiments and data are represented as mean ± SEM, n = 3, *P < 0.05, and **P < 0.01.
2.10 Statistical Analysis
Analysis of the in vivo study results was by one-way ANOVA using StatView software (Adept Scientific, Acton, MA). The mean ± SEM from each experimental group was compared with its respective control, and statistical significance was defined as P <0.05. For the in vitro studies, the unpaired t-test was used for analysis.
3. Results
3.1 Treatment Effects on Tumor Angiogenesis and Tumor Growth in the CAM Model
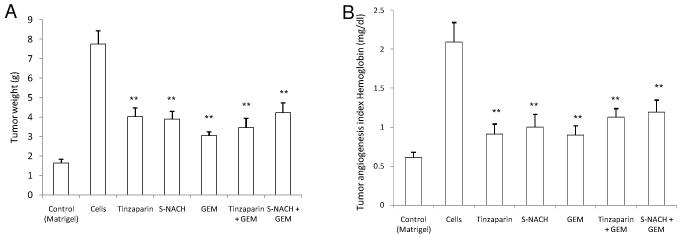
Either S-NACH or tinzaparin at 1 μg/CAM significantly (*P < 0.01) inhibited pancreatic cancer cell (MPanc96-luc) mediated tumor growth (Fig. 1A) and angiogenesis (Fig. 1B) in the CAM model. Like gemcitabine, treatment with either S-NACH or tinzaparin resulted in 60-80% inhibition of tumor growth (Fig. 1A) and tumor angiogenesis (Fig. 1B), without any further increase when combined with gemcitabine.
Fig. 1. Effect of S-NACH, tinzaparin, and gemcitabine (GEM) on human pancreatic carcinoma (MPanc96).
(A) Tumor growth and (B) tumor angiogenesis in the chick CAM cancer cell implant model. S-NACH, tinzaparin, and gemcitabine were added at 1 μg/CAM. Matrigel is a negative control, and all other groups have MPanc96 cancer cells at 1 × 106 cells/CAM with or without the test compounds. Data represent mean ± SEM, n = 10 per group. Significant reductions in tumor size and tumor angiogenesis with all agents are evident as compared to control (**P < 0.01).
3.2 Treatment Effect on Orthotopic Pancreatic Tumor Growth, Bioluminescent Signal, and Necrosis
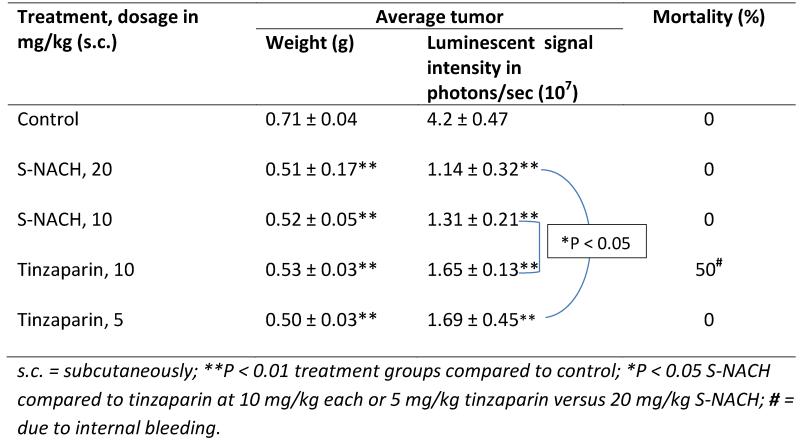
One hundred percent of mice orthotopically injected with MPanc96-luc or SUIT2-luc cells developed tumors within the pancreas. Tumors showed strong bioluminescent signal intensities with increasing size over time. Treatment with S-NACH (20 mg/kg body weight) and tinzaparin (5 mg/kg body weight) was started 2 days after MPanc96-luc or SUIT2-luc cell implantation in the pancreas (Table 1). In a preliminary study, we noted severe bleeding at the site of the injection with tinzaparin at 10 mg/kg, while S-NACH at doses 10 and 20 mg/kg did not have any adverse bleeding complications. In addition, the mortality rate was 50% in animals treated with tinzaparin at 10 mg/kg compared to saline or S-NACH treated groups (Table 2). Data in Table 2 illustrate greater efficacy for S-NACH at either daily 10 or 20 mg/kg, s.c., versus tinzaparin at daily 10 mg/kg, s.c., on viable cancer cell signal intensity. Since the maximal tolerated dose for tinzaparin was shown to be around daily 5 mg/kg, s.c., which was well tolerated, we continued the rest of the studies with that dose of tinzaparin instead of daily 20 mg/kg, s.c.
Table 2.
Effect of S-NACH or tinzaparin on the orthotopic tumor weight and mortality of female NCr nude homozygous mice
|
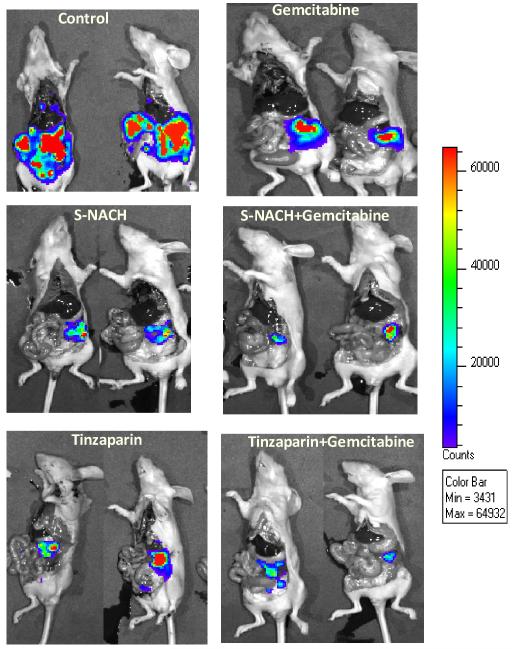
Daily treatment of orthotopic pancreatic tumors resulted in suppression of tumor signal intensity as determined by IVIS. Repeated administration resulted in sustained inhibition of luciferin signal strength (viable cells). However, in the control group, tumor luminescence increased and expanded, an indication of metastasis, shown in open-cavity imaging of representative mice (Fig. 2) or ex vivo imaging of excised tumors from mice for different pancreatic cancer cell implants MPanc96 (Fig. 3A) and SUIT2 (Fig. 4A). Common sites of distant metastasis in the control group were the liver, peritoneum, abdominal lymph nodes, bones, kidneys, and the small and large intestine.
Fig. 2. Effect of S-NACH, tinzaparin, and gemcitabine on MPanc96 tumor growth in vivo.
Representative open-cavity bioluminescence images of mice in each group taken at autopsy showing the effect of S-NACH, tinzaparin, and gemcitabine on tumor growth in vivo. Orthotopic pancreatic tumors developed from MPanc96 cells expressing the luciferase gene and were treated with S-NACH (20 mg/kg body weight daily) or tinzaparin (5 mg/kg body weight daily). Gemcitabine (100 mg/kg body weight) was injected intraperitoneally twice a week alone or in combination with either S-NACH or tinzaparin. Bioluminescence images showed reduction in tumor volume in treated groups at the end of the study (4 weeks).
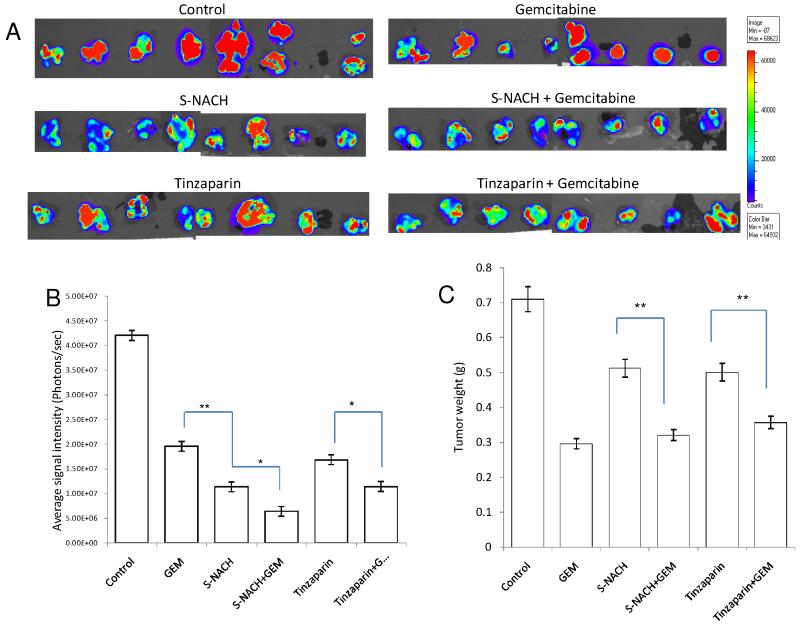
Fig. 3. Effect of S-NACH or tinzaparin and gemcitabine on MPanc96-luc tumor growth and tumor bioluminescence Intensity.
(A) Bioluminescence images of the excised orthotopic pancreatic tumors of MPanc96 cells bearing luciferase gene after 4 weeks of treatment with S-NACH, tinzaparin, gemcitabine, and combinations. (B) Average signal intensity of MPanc96-luc cells for each treatment showing significant reduction compared to control. (C) Tumor weight at the termination of study of MPanc96-luc cells. Data represent mean tumor weight (g) ± SEM, n = 8 per group (A), signal intensity (photons/sec) ± SEM, (*P < 0.05, **P < 0.01). GEM = gemcitabine.
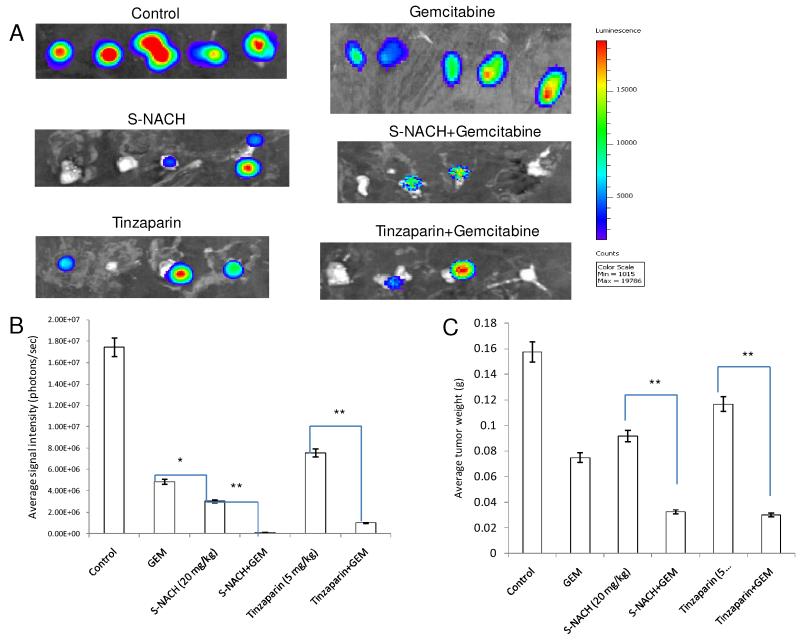
Fig. 4. Effect of S-NACH or tinzaparin and gemcitabine on SUIT2-luc tumor growth and tumor bioluminescence Intensity.
(A) Bioluminescence images of the excised orthotopic pancreatic tumors of SUIT2 cells bearing luciferase gene after 4 weeks of treatment with S-NACH, tinzaparin, gemcitabine, and combinations. (B) Average signal intensity of SUIT2-luc cells for each treatment showing reduction compared to control. (C) Tumor weight at the termination of study of SUIT2-luc cells. Data represent mean tumor weight (g) ± SEM, n = 5 per group (A), signal intensity (photons/sec) ± SEM, (*P < 0.05, **P < 0.01). GEM = gemcitabine. Study was repeated with n = 5 per group to confirm the data. (B) and (C) show the data averaged over both experiments.
Gemcitabine, alone or in combination with heparin derivatives, reduced luciferin signal intensity and tumor weights (P < 0.01), shown at 28 days following initiation of MPanc96-luc (Figs. 3B, 3C) or SUIT2-luc cell pancreatic tumors (Figs. 4B, 4C). However, divergent patterns emerged with respect to tumor signal intensity and tumor weights in certain treatment groups. Of particular interest, tumor luciferin intensity was significantly decreased in S-NACH and S-NACH + gemcitabine groups (P < 0.01), while tumor weights were only moderately decreased in comparison to gemcitabine alone at the end of the study (Figs. 3B, 3C and 4B, 4C). In comparison to control, either S-NACH or tinzaparin treatments resulted in 25-30% inhibition of tumor mass. However, signal intensity of tumors showed a highly significant decrease in the S-NACH treated groups when compared to other treatments and controls (P < 0.01). Gemcitabine-treated animals developed smaller tumors (tumor weights), but the tumor signal intensity was significantly higher than that in S-NACH (+/− gemcitabine) groups. These treatments caused no decreases in animal body weights (data not shown), an index of the lack of treatment toxicity.
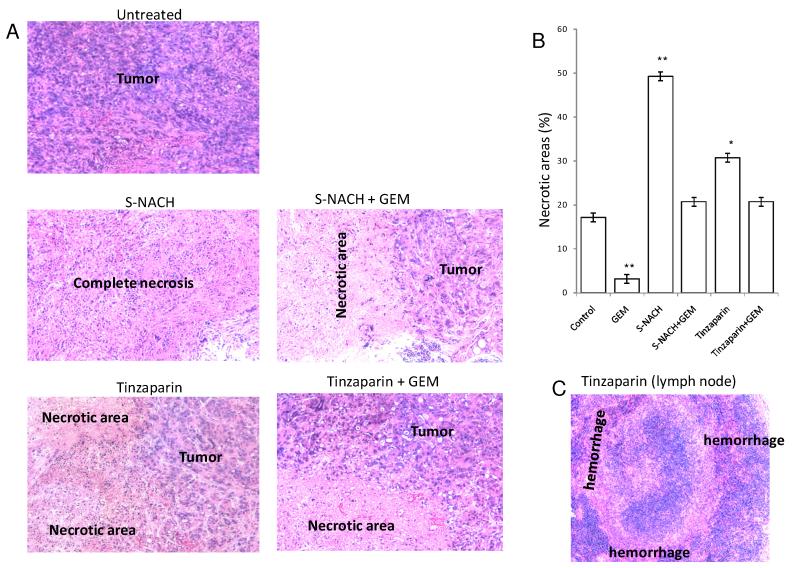
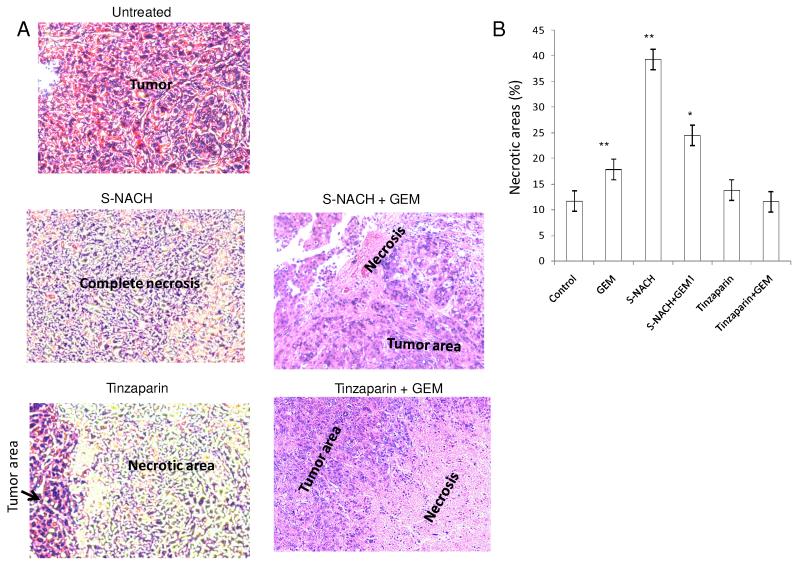
To address the discrepancies between tumor bioluminescence indices and tumor weights and to investigate possible mechanisms involved in the divergent patterns, we performed histological analysis of tumors from all treatment groups of MPanc96-luc (Fig. 5) and SUIT2-luc (Fig. 6). Histology showed that untreated animals have high-grade (anaplastic) features as common to advanced stage pancreatic cancer. S-NACH treated tumors showed large regions of necrosis (P < 0.01) when compared to other treated groups (Figs. 5B and 6B). In contrast, tinzaparin treatment resulted in modest increase in necrotic area as compared to S-NACH (Fig. 5B and 6B). Necrotic areas included both early stage (fragmented and small nucleus) and late stage (ghost cells without nucleus) areas indicating that S-NACH had effects on early and later aspects of cell death. Tumor necrosis induced by S-NACH was inversely proportional to the bioluminescent signal in the tumor, since only live cells show bioluminescent signal. Even at its maximal tolerated dose (daily 5 mg/kg body weight, s.c.) tinzaparin-treated animals showed a high percentage of hemorrhage and bleeding in the tumor and lymph nodes (Fig. 5C). Further studies as shown below were undertaken to address the molecular mechanisms involved in the histopathologically observed increased tumor necrosis mediated by tinzaparin or S-NACH.
Fig. 5. Effect of S-NACH or tinzaparin and gemcitabine on MPanc96 tumor necrosis.
(A) Representative micrographs of H and E stained histological sections of orthotopic pancreatic tumors showing increased necrotic areas after treatment with S-NACH and tinzaparin compared to untreated tumor with viable cells and large nuclei. (B) Histopathological analysis of the orthotopic pancreatic tumors of MPanc96 cells treated with S-NACH showed significant increase in necrotic areas compared to untreated tumors (*P < 0.05, **P < 0.01). GEM = gemcitabine. (C) Tinzaparin-treated animals show extensive bleeding in lymph node as represented by hemorrhage areas.
Fig. 6. Effect of S-NACH or tinzaparin and gemcitabine on SUIT2 tumor necrosis.
(A) Representative micrographs of H and E stained histological sections of orthotopic pancreatic tumors showing increased necrotic areas after treatment with S-NACH and tinzaparin compared to untreated tumor with viable cells and large nuclei. (B) Histopathological analysis of the orthotopic pancreatic tumors of SUIT2 cells treated with S-NACH showed significant increase in necrotic areas compared to control (*P < 0.05, **P < 0.01). GEM = gemcitabine.
3.3 Effects of Tinzaparin and S-NACH on Abundance of Apoptosis and Angiogenesis Markers
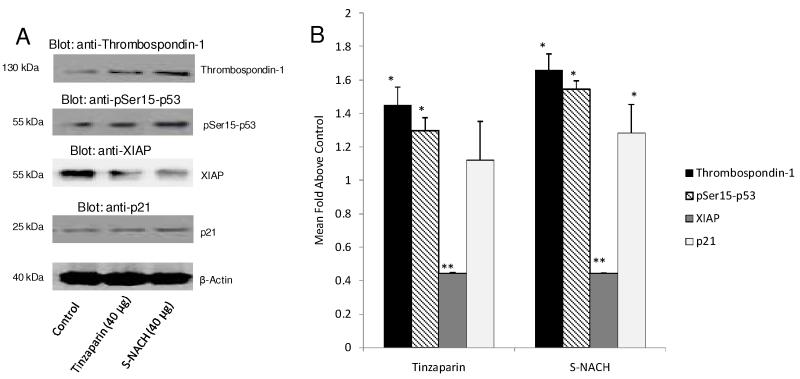
We examined the effects of tinzaparin and S-NACH on the abundance of proteins relevant to angiogenesis and apoptosis in MPanc96 pancreatic cancer cells treated with either tinzaparin or S-NACH at 40 μg using immunoblotting (Fig. 7). Thrombospondin-1 (THBS1) is an endogenous anti-angiogenesis protein that shows reduced gene expression in cancer cells. The overall abundance of THBS1 protein by immunoblotting was increased 1.5- to 2-fold by S-NACH over control (P < 0.05), with a similar trend for tinzaparin. Data also showed that cellular abundance of hyper-phosphorylated p53 (1.3- to 1.5-fold) and p21 (1.25- to 1.35-fold) increased overall above control levels, with statistical significance (P < 0.05) in hyper-phosphorylated p53 for the S-NACH and tinzaparin treatment groups. A significant increase in p21 protein above control was observed with S-NACH (P < 0.05) but not with tinzaparin. In further support for the promotion of apoptosis by S-NACH and tinzaparin, X-linked inhibitor of apoptosis protein (XIAP) was significantly suppressed by 50-60% with either tinzaparin or S-NACH as compared to control (P < 0.01).
Fig. 7. Effects of tinzaparin and S-NACH on abundance of apoptosis and angiogenesis-related proteins in MPanc96 cells in vitro.
(A) Immunoblots of total proteins harvested from the samples were prepared; β-actin was used as an internal control. Cells were cultured for 72 h in the presence of control (PBS), tinzaparin, or S-NACH (40 μg). (B) Increased thrombospondin-1 protein level was shown with either tinzaparin or S-NACH (*P < 0.05) as compared to control. Either tinzaparin or S-NACH increased expression of phosphorylated p53 protein (*P < 0.05), with relatively greater increase with S-NACH. P21 protein levels were significantly (*P < 0.05) increased by S-NACH but not by tinzaparin as compared to control. Either tinzaparin or S-NACH significantly decreased the protein level of XIAP (**P < 0.01) as compared to control. Data represent mean ± SEM, n = 3, (*P < 0.05 and **P < 0.01).
4. Discussion
Here we report data from the CAM pancreatic cancer implant model and an orthotopic pancreatic cancer model to support the potential therapeutic usefulness of S-NACH for the treatment of pancreatic cancer. The current study shows that S-NACH significantly inhibits tumor growth and tumor angiogenesis in this pancreatic cancer model. Data in Fig. 1 were derived from in vitro studies in the CAM model examining the effect of S-NACH versus tinzaparin on pancreatic cancer growth and tumor angiogenesis after local additions of either agent to the cancer cells implanted in matrigel. In contrast, data in Figs. 3C and 4C were derived from orthotopic implant of pancreatic cancer cell in the pancreas and after daily treatment with either agent for up to 4 weeks. These are two distinct systems. The limitation of the CAM model system is that it just gives an assessment of the impact of these agents on tumor growth and tumor angiogenesis where the interaction between heparin derivatives and the chemotherapeutic gemcitabine was not demonstrated as compared to results from the in vivo orthotopic model. Further studies at lower concentrations of the heparin derivatives (S-NACH or LMWH) with lower concentrations of gemcitabine might enable us to illustrate the level of interaction among the heparins and gemcitabine in the CAM model.
However, the use of orthotopic models of human pancreatic cancer in the nude mouse replicate human disease with high fidelity and allow for testing of novel treatment strategies as shown here and by others in different model systems [27,28]. Use of the stable and intensely bioluminescent MPanc96 and SUIT2 cell lines made the detection of tumors in live animals possible, allowed the tracking of their behavior from the time of implantation, and facilitated monitoring of growth, responses to treatment, and quantitation of tumor burdens [29]. Our results are consistent with a report that another modified non-anticoagulant heparin inhibits bioluminescent signals in subcutaneous myeloma tumors [30].
In the pancreatic cancer orthotopic model, gemcitabene alone demonstrated significant effect on tumor mass that is not different when combined with either S-NACH or tinzaparin (Fig. 3C). However, either S-NACH or tinzaparin alone resulted in moderate decrease in tumor mass (Fig. 3C). In contrast, an enhanced response on cancer cell viability (signal intensity, Fig. 3B) was demonstrated with either S-NACH or tinzaparin combined with gemcitabine. The data was more distinct with regard to the enhancement of the anti-cancer efficacy against the SUIT2 pancreatic cancer at the tumor mass and cell viability levels with either S-NACH or tinzaparin combined with gemcitabine (Figs. 4B and 4C).
This study demonstrated for the first time that S-NACH has a direct effect on the survival of pancreatic cancer tumor cells in vivo. S-NACH treatments resulted in effective inhibition of pancreatic tumor growth and angiogenesis in the CAM model, consistent with its effects on other tumors [31]. Our in vivo data showed that S-NACH inhibits metastasis of pancreatic cancer as demonstrated by the complete absence of bioluminescent signals when internal organs were exposed after the termination of study. In contrast, the untreated control group showed extensive metastasis into multiple organs. The absence of bioluminescence associated with live cells in the S-NACH treatment groups was confirmed by data showing the corresponding increase (~ 50%) of necrosis in histological studies. The anti-angiogenesis and anti-metastatic activity of S-NACH may be due, at least in part, to the tissue factor pathway inhibitor protein release from endothelial cells, similar to effects demonstrated for LMWH [7,22,32].
The discrepancies between effects of S-NACH or tinzaparin on tumor mass versus impact on tumor cell viability (bioluminescence) might be explained based on the histological data (increased tumor necrosis) along with increased tumor apoptosis proteins (phosphorylated p53 and p21) and decreased tumor survival protein (XIAP). Furthermore, either tinzaparin or S-NACH up-regulate the production of the anti-angiogenesis protein thrombospondin.
Gemcitabine showed less necrosis as compared to control MPanc96 pancreatic cancer cells implanted orthotopically but higher necrosis compared to control SUIT2 pancreatic cancer cells implanted orthotopically. This was shown despite an overall reduction in tumor mass or cancer cell viability by gemcitabine in either tumor type. The only apparent difference is that the growth rate of MPanc96 was 4-to 5-fold greater than the SUIT2 pancreatic cancer in terms of tumor mass and the signal intensity of viable cancer cells in the control arms.
Our data clearly demonstrate that S-NACH, without any systemic anticoagulant effects, possesses equivalent or improved anti-cancer efficacy in comparison to the LMWH tinzaparin. S-NACH at a high dose (20 mg/kg body weight) had no systemic anti-coagulation effects in mice; however, tinzaparin (5 mg/kg body weight) increased bleeding, evident in the histological sections of tumor and lymph. Previously, we showed that tinzaparin (10 mg/kg) doubled the bleeding time and mortality (Table 2) compared to the control group, and S-NACH (10 mg/kg) had no effect on bleeding time. When the dose of S-NACH was increased to 20 mg/kg, there was still no difference in mean bleeding time. Further, clinical studies have demonstrated that prolonged administration of heparin derivatives can result in improved survival in certain patient populations but with risk of bleeding [33,34].
Experiments using either S-NACH or tinzaparin + gemcitabine in animal models indicate significantly enhanced chemotherapeutic response of the pancreatic tumors as shown by reduced luminescent signal intensity with increased necrotic areas when compared to gemcitabine treatment alone. The results in this report are consistent with our previous data with S-NACH in a breast cancer model [35]. In that study S-NACH significantly increased the uptake of chemotherapeutic agents in addition to suppressing tumor growth and significantly prolonging survival [35]. S-NACH has been shown to inhibit platelet-associated P-selectin dependent processes in a manner similar to other glycosaminoglycans [36]. Our results indicate that S-NACH can be utilized in chemotherapy combinations to improve chemo responsiveness. We propose that the increased uptake of gemcitabine, if comparable to what was seen with paclitaxel and doxorubicin in our previous study [35], could allow for reduction of gemcitabine dose, with a decrease in concomitant toxicities. In addition, heparin compounds might impact on P-glycoprotein and associated pump activity [37], one of the main drug resistant mechanisms [38], suggesting that S-NACH may also potentially overcome gemcitabine chemo-resistance. Data also showed that S-NACH or tinzaparin significantly increased necrosis, and their combination with gemcitabine reduced necrosis compared to S-NACH alone. The mechanism for the latter effect is unclear. These data might suggest the potential of pre-treatment with either S-NACH or LMWH prior to gemcitabine or their uses at different cycles. Further studies are required to address these interactions.
Data also showed a direct effect of S-NACH and tinzaparin on the induction of anti-angiogenesis protein THBS1 and pro-apoptosis proteins hyper-phosphorylated p53, and p21, while suppressing the anti-apoptosis protein XIAP in MPanc96 cells. These data suggest direct effects of heparin on cancer cells that are independent of its anticoagulant activity.
S-NACH, the non-anticoagulant heparin, is preferable for potential clinical use because of the possibility that it could be administered at high doses, thereby fully exploiting the anti-cancer and anti-metastatic components of heparin, without bleeding complication. S-NACH could have a broader application because it could be utilized, either alone or in combination with chemotherapeutic agents, to treat pancreatic cancer or other cancers.
Acknowledgement
Financial Support: This work was supported by the National Institutes of Health (grant number 1R21CA124931-01 to S.A.M.). We appreciate the excellent editing by Dr. Kelly A. Keating, Pharmaceutical Research Institute (PRI) and the technical support by members of the PRI.
Abbreviations
- CAM
chick chorioallantoic membrane
- LMWH
low molecular weight heparin
- S-NACH
sulfated non-anticoagulant heparin
- THBS1
Thrombospondin-1
- XIAP
X-linked inhibitor of apoptosis protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- [1].Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- [2].Hidalgo M. Pancreatic cancer. N. Engl. J. Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- [3].Sgouros J, Maraveyas A. Excess premature (3-month) mortality in advanced pancreatic cancer could be related to fatal vascular thromboembolic events. A hypothesis based on a systematic review of phase III chemotherapy studies in advanced pancreatic cancer. Acta Oncol. 2008;47:337–346. doi: 10.1080/02841860701687267. [DOI] [PubMed] [Google Scholar]
- [4].Fareed J, Leong WL, Hoppensteadt DA, Jeske WP, Walenga J, Wahi R, Bick RL. Generic low-molecular-weight heparins: some practical considerations, Semin. Thromb. Hemost. 2004;30:703–713. doi: 10.1055/s-2004-861513. [DOI] [PubMed] [Google Scholar]
- [5].Oguchi T, Doursout MF, Kashimoto S, Liang YY, Hartley CJ, Chelly JE. Role of heparin and nitric oxide in the cardiac and regional hemodynamic properties of protamine in conscious chronically instrumented dogs. Anesthesiology. 2001;94:1016–1025. doi: 10.1097/00000542-200106000-00016. [DOI] [PubMed] [Google Scholar]
- [6].Salas A, Sans M, Soriano A, Reverter JC, Anderson DC, Pique JM, Panes J. Heparin attenuates TNF-alpha induced inflammatory response through a CD11b dependent mechanism. Gut. 2000;47:88–96. doi: 10.1136/gut.47.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vlodavsky I, Ilan N, Nadir Y, Brenner B, Katz BZ, Naggi A, Torri G, Casu B, Sasisekharan R. Heparanase, heparin and the coagulation system in cancer progression. Thromb. Res. 2007;120(Suppl 2):S112–120. doi: 10.1016/S0049-3848(07)70139-1. [DOI] [PubMed] [Google Scholar]
- [8].Ludwig RJ, Boehme B, Podda M, Henschler R, Jager E, Tandi C, Boehncke WH, Zollner TM, Kaufmann R, Gille J. Endothelial P-selectin as a target of heparin action in experimental melanoma lung metastasis. Cancer Res. 2004;64:2743–2750. doi: 10.1158/0008-5472.can-03-1054. [DOI] [PubMed] [Google Scholar]
- [9].Icli F, Akbulut H, Utkan G, Yalcin B, Dincol D, Isikdogan A, Demirkazik A, Onur H, Cay F, Buyukcelik A. Low molecular weight heparin (LMWH) increases the efficacy of cisplatinum plus gemcitabine combination in advanced pancreatic cancer. J. Surg. Oncol. 2007;95:507–512. doi: 10.1002/jso.20728. [DOI] [PubMed] [Google Scholar]
- [10].Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, Prins MH, Raskob G, Segers AE, Cariou R, Leeuwenkamp O, Lensing AW. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann. Intern. Med. 2004;140:867–873. doi: 10.7326/0003-4819-140-11-200406010-00007. [DOI] [PubMed] [Google Scholar]
- [11].Pross M, Lippert H, Misselwitz F, Nestler G, Kruger S, Langer H, Halangk W, Schulz HU. Low-molecular-weight heparin (reviparin) diminishes tumor cell adhesion and invasion in vitro, and decreases intraperitoneal growth of colonadeno-carcinoma cells in rats after laparoscopy. Thromb. Res. 2003;110:215–220. doi: 10.1016/s0049-3848(03)00296-2. [DOI] [PubMed] [Google Scholar]
- [12].Mousa SA, Petersen LJ. Anti-cancer properties of low-molecular-weight heparin: preclinical evidence. Thromb. Haemost. 2009;102:258–267. doi: 10.1160/TH08-12-0832. [DOI] [PubMed] [Google Scholar]
- [13].Gray E, Mulloy B, Barrowcliffe TW. Heparin and low-molecular-weight heparin. Thromb. Haemost. 2008;99:807–818. doi: 10.1160/TH08-01-0032. [DOI] [PubMed] [Google Scholar]
- [14].von Delius S, Ayvaz M, Wagenpfeil S, Eckel F, Schmid RM, Lersch C. Effect of low-molecular-weight heparin on survival in patients with advanced pancreatic adenocarcinoma. Thromb. Haemost. 2007;98:434–439. [PubMed] [Google Scholar]
- [15].Hejna M, Raderer M, Zielinski CC. Inhibition of metastases by anticoagulants. J. Natl. Cancer Inst. 1999;91:22–36. doi: 10.1093/jnci/91.1.22. [DOI] [PubMed] [Google Scholar]
- [16].Akl EA, van Doormaal FF, Barba M, Kamath G, Kim SY, Kuipers S, Middeldorp S, Yosuico V, Dickinson HO, Schunemann HJ. Parenteral anticoagulation for prolonging survival in patients with cancer who have no other indication for anticoagulation. Cochrane Database Syst. Rev. 2007:CD006652. doi: 10.1002/14651858.CD006652. [DOI] [PubMed] [Google Scholar]
- [17].Iorio A, Guercini F, Pini M. Low-molecular-weight heparin for the long-term treatment of symptomatic venous thromboembolism: meta-analysis of the randomized comparisons with oral anticoagulants. J. Thromb. Haemost. 2003;1:1906–1913. doi: 10.1046/j.1538-7836.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- [18].Mousa SA. Role of current and emerging antithrombotics in thrombosis and cancer. Drugs Today (Barc) 2006;42:331–350. doi: 10.1358/dot.2006.42.5.973580. [DOI] [PubMed] [Google Scholar]
- [19].Cox M, Nelson D. Lehninger, Principles of Biochemistry. W. H. Freeman; 2004. [Google Scholar]
- [20].Lee AY, Rickles FR, Julian JA, Gent M, Baker RI, Bowden C, Kakkar AK, Prins M, Levine MN. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J. Clin. Oncol. 2005;23:2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- [21].Vlodavsky I, Abboud-Jarrous G, Elkin M, Naggi A, Casu B, Sasisekharan R, Ilan N. The impact of heparanese and heparin on cancer metastasis and angiogenesis, Pathophysiol. Haemost. Thromb. 2006;35:116–127. doi: 10.1159/000093553. [DOI] [PubMed] [Google Scholar]
- [22].Mousa SA, Linhardt R, Francis JL, Amirkhosravi A. Anti-metastatic effect of a non-anticoagulant low-molecular-weight heparin versus the standard low-molecular-weight heparin, enoxaparin. Thromb. Haemost. 2006;96:816–821. doi: 10.1160/th06-05-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mousa SA. Inhibitory effect of C-reactive protein on the release of tissue factor pathway inhibitor from human endothelial cells: reversal by low molecular weight heparin. Int. Angiol. 2006;25:10–13. [PubMed] [Google Scholar]
- [24].Yalcin M, Bharali DJ, Lansing L, Dyskin E, Mousa SS, Hercbergs A, Davis FB, Davis PJ, Mousa SA. Tetraidothyroacetic acid (tetrac) and tetrac nanoparticles inhibit growth of human renal cell carcinoma xenografts. Anticancer Res. 2009;29:3825–3831. [PubMed] [Google Scholar]
- [25].Davis PJ, Shih A, Lin HY, Martino LJ, Davis FB. Thyroxine promotes association of mitogen-activated protein kinase and nuclear thyroid hormone receptor (TR) and causes serine phosphorylation of TR. J. Biol. Chem. 2000;275:38032–38039. doi: 10.1074/jbc.M002560200. [DOI] [PubMed] [Google Scholar]
- [26].Zhang S, Cao HJ, Davis FB, Tang HY, Davis PJ, Lin HY. Oestrogen inhibits resveratrol-induced post-translational modification of p53 and apoptosis in breast cancer cells. Br. J. Cancer. 2004;91:178–185. doi: 10.1038/sj.bjc.6601902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tsuzuki Y, Mouta Carreira C, Bockhorn M, Xu L, Jain RK, Fukumura D. Pancreas microenvironment promotes VEGF expression and tumor growth: novel window models for pancreatic tumor angiogenesis and microcirculation. Lab. Invest. 2001;81:1439–1451. doi: 10.1038/labinvest.3780357. [DOI] [PubMed] [Google Scholar]
- [28].Ramachandran V, Arumugam T, Hwang RF, Greenson JK, Simeone DM, Logsdon CD. Adrenomedullin is expressed in pancreatic cancer and stimulates cell proliferation and invasion in an autocrine manner via the adrenomedullin receptor, ADMR. Cancer Res. 2007;67:2666–2675. doi: 10.1158/0008-5472.CAN-06-3362. [DOI] [PubMed] [Google Scholar]
- [29].Caceres G, Zhu XY, Jiao JA, Zankina R, Aller A, Andreotti P. Imaging of luciferase and GFP-transfected human tumours in nude mice. Luminescence. 2003;18:218–223. doi: 10.1002/bio.729. [DOI] [PubMed] [Google Scholar]
- [30].Casu B, Vlodavsky I, Sanderson RD. Non-anticoagulant heparins and inhibition of cancer. Pathophysiol. Haemost. Thromb. 2008;36:195–203. doi: 10.1159/000175157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mousa SA, Feng X, Xie J, Du Y, Hua Y, He H, O’Connor L, Linhardt RJ. Synthetic oligosaccharide stimulates and stabilizes angiogenesis: structure-function relationships and potential mechanisms. J. Cardiovasc. Pharmacol. 2006;48:6–13. doi: 10.1097/01.fjc.0000238591.90062.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Amirkhosravi A, Meyer T, Amaya M, Davila M, Mousa SA, Robson T, Francis JL. The role of tissue factor pathway inhibitor in tumor growth and metastasis. Semin. Thromb. Hemost. 2007;33:643–652. doi: 10.1055/s-2007-991531. [DOI] [PubMed] [Google Scholar]
- [33].Riess H, Pelzer U, Hilbig A, Stieler J, Opitz B, Scholten T, Kauschat-Bruning D, Bramlage P, Dorken B, Oettle H. Rationale and design of PROSPECT-CONKO 004: a prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy) BMC Cancer. 2008;8:361. doi: 10.1186/1471-2407-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van Tienhoven G, Buller HR. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J. Clin. Oncol. 2005;23:2130–2135. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- [35].Phillips PG, Yalcin M, Cui H, Abdel-Nabi H, Sajjad M, Bernacki R, Veith J, Mousa SA. Increased tumor uptake of chemotherapeutics and improved chemoresponse by novel non-anticoagulant low molecular weight heparin. Anticancer Res. 2011;31:411–419. [PubMed] [Google Scholar]
- [36].Stevenson JL, Varki A, Borsig L. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb. Res. 2007;120(Suppl 2):S107–111. doi: 10.1016/S0049-3848(07)70138-X. [DOI] [PubMed] [Google Scholar]
- [37].Angelini A, Di Febbo C, Ciofani G, Di Nisio M, Baccante G, Di Ilio C, Cuccurullo F, Porreca E. Inhibition of P-glycoprotein-mediated multidrug resistance by unfractionated heparin: a new potential chemosensitizer for cancer therapy. Cancer Biol. Ther. 2005;4:313–317. doi: 10.4161/cbt.4.3.1503. [DOI] [PubMed] [Google Scholar]
- [38].Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 2005;14(Suppl 1):35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]