Abstract
The resistance rates of Helicobacter pylori to amoxicillin and metronidazole therapy are higher in eastern Taiwan as compared to national and worldwide rates. The high resistance rate in this territory justified a search for a better eradication regimen. We conducted an open-labeled, prospective, randomized, and controlled study in a tertiary referral hospital in eastern Taiwan. Between December 2007 and December 2009, a total of 153 Helicobacter pylori-positive, therapy-naïve patients with a positive rapid urease test were recruited for random assignment to two seven-day treatment groups: levofloxacin (500 mg), amoxicillin/clavulanate (875 mg/125 mg), and rabeprazole (20 mg) twice per day (LAcR) or clarithyromicin (500 mg), amoxicillin (1000 mg), and rabeprazole (20 mg) twice per day (CAR). Helicobacter pylori eradication was assessed using the 13C-urea breath test or rapid urease test performed at least 4 weeks after the end of treatment. After exclusion, 146 patients were enrolled and allocated in the study. The Helicobacter pylori eradication rates analyzed by both intention to treat (78.1% versus 57.5%, P = 0.008) and perprotocol (80.9% versus 61.8%, P = 0.014) were significantly higher for the LAcR group. In conclusion, the seven-day LAcR regimen provided improved Helicobacter pylori eradication efficacy when compared with the standard CAR triple therapy in eastern Taiwan.
1. Introduction
Helicobacter pylori (H. pylori) colonizes the human stomach chronically and is the causative agent of numerous benign and malignant gastric diseases [1]. A seroprevalence study in Taiwan showed that the seropositivity of H. pylori infection was 54.4% [2]. Eradication of H. pylori with a standard triple therapy using a proton pump inhibitor (PPI), amoxicillin, and clarithromycin or metronidazole was recommended to prevent gastric cancer and to avoid recurrence of peptic ulcer diseases [1, 3]. The recommended duration of the standard triple therapy from Asia-Pacific Consensus Guidelines is seven days [4]. Clarithromycin or metronidazole is suggested if the H. pylori local primary resistance rate is lower than 15~20% for the former or less than 40% for the latter [3]. Antimicrobial resistances in many countries have increased. As a consequence, the triple therapy eradication rate was less than 80% on an intention-to-treat (ITT) basis [5]. In eastern Taiwan, the primary resistance rates of metronidazole (51.9%), amoxicillin (36.1%), and clarithromycin (13.5%) in clinical isolates of H. pylori were higher [6] than those reported from other regions of Taiwan (Table 1) and worldwide [7–12]. However, the eradication rate of a seven-day triple therapy using clarithromycin and amoxicillin has never been reported for patients in eastern Taiwan.
Table 1.
Primary resistance rate of H. pylori reported in Taiwan (published since 2000 to 2010 D.C.)
| Author (study period) [reference] |
Location (region) |
Metronidazole | Clarithromycin | Amoxicillin | Levofloxacin | Tetracycline |
|---|---|---|---|---|---|---|
| Hu et al. (2004~2005) [6] | Hualien (east) | 51.9% | 13.5% | 36.1% | Nil | 0% |
| Yang et al. (1997~1999) [7] | Taipei (north) | 9% (6/67) | 18% (12/67) | Nil | Nil | Nil |
| Poon et al. (1998~2000) [8] | Taichung (west) | 41.7% (35/84) | 10.7% (9/84) | 0% (0/84) | Nil | Nil |
| Poon et al. (2001~2004) [8] | Taichung (west) | 25.4% (34/134) | 6.7% (9/134) | 0% (0/134) | Nil | Nil |
| Hung et al. (1998~2007) [9] | Tainan (south) | 27.6% (58/210) | 9.5% (20/210) | 1.0% (2/210) | 5.7% (12/210) | 0.5% (1/210) |
| Wu et al. (2007~2008) [10] | Kaohsiung (south) | 33.5% (56/167) | 6.6% (11/167) | 0.6% (1/167) | 10.2% (17/167) | 0.6% (1/167) |
| Liou et al. (2007~2009) [11] | Taipei (north) | Nil | 7.5% (20/266) | 2.5% (7/279) | 5.7% (16/280) | Nil |
Regimens containing levofloxacin (500 mg b.i.d.) plus amoxicillin (1 g b.i.d.) (LA) and a PPI have been evaluated recently as an alternative to the standard antibiotics [1, 13]. The use of LA-based regimens as a first-line treatment for H. pylori is encouraging but still controversial [11]. Beta-lactamase production in H. pylori is the principal mechanism of amoxicillin resistance [14]. In vitro studies [15, 16] and clinical trials [17–19] showed promising results by using amoxicillin plus a beta-lactamase inhibitor like clavulanic acid to attenuate H. pylori resistance to amoxicillin. Thus, our aim was to evaluate the efficacy and tolerability of a seven-day levofloxacin (500 mg b.i.d.), amoxicillin/clavulanate (1 g b.i.d.) plus rabeprazole (20 mg b.i.d) (LAcR) regimen versus the guideline-recommended seven-day triple therapy for treatment of H. pylori infection [4].
2. Methods
2.1. Study Population
In this single center prospective study, we included H. pylori-positive adult patients assessed by the rapid urease test between December 2007 and December 2009. We excluded patients under the age of 20, those who had received anti-H. pylori therapy previously, those with concomitant illness or conditions (i.e., cardiopulmonary, hepatic, renal, or neoplastic diseases), those who were pregnant or breast-feeding women, and those allergic to any of the drugs used. The protocol was approved by the Institutional Review Board (IRB) of Buddhist Tzu-Chi General Hospital (IRB 096-28) and registered in ClinicalTrials.gov (NCT01575899). Informed consents were obtained from all participants.
2.2. Study Design
Eligible patients were assigned into two groups by a computer generated random table with blocks based on gender. Sealed envelopes which were opened in the outpatient clinic without blinding were used for allocation concealment. The LAcR group received levofloxacin, 500 mg (Cravit, Daiichi-Sankyo, Japan) b.i.d., amoxicillin/clavulanate, 875 mg/125 mg (Augmentin, GlaxoSmithKline, UK) b.i.d., and rabeprazole, 20 mg (Pariet, Eisai, Japan) b.i.d., for 7 days. The standard triple therapy group served as the control group and was treated with clarithyromicin, 500 mg (Klaricid, Abbott, USA) b.i.d., amoxicillin, 1000 mg (Amoxicillin capsule 250 mg, Yung-Shin, Taiwan) b.i.d., and rabeprazole, 20 mg b.i.d. (CAR), for seven days.
2.3. Drug Compliance and Adverse Events
Drug compliance was defined as intake of more than 80% of each prescribed medication. Compliance and incidence of adverse events were collected by phone calls and in outpatient clinics. Each symptom was graded as either absent or present. H. pylori eradication was established based on a negative 13C-urea breath test or a negative rapid urease (CLO) test. The confirmation tests were carried out at least 4 weeks after completion of eradication therapy by operators unaware of the patients' medication and H. pylori status.
2.4. Statistical Analysis
The primary end point of this study was to evaluate the eradication rate of the LAcR regimen. The evaluation of H. pylori eradication efficacy was performed on both an intention-to-treat (ITT) and a perprotocol (PP) analyses. The sample size was predetermined for this paired cohort study, taking the following parameters into consideration: initial estimate of the difference in efficacy = 15% (i.e., 87% versus 72%) [5, 13]; alpha = 0.05; power = 80%, and gamma = 0.2. The number of patients thus calculated in each group was 88. The final sample size was 100 patients when assuming that 15% of the patients were lost to follow-up. Interim analyses with periodic reports every 4 months were requested by our IRB, which allowed investigators to adjust study course according to the interim results.
Categorical data were compared using the chi-square test employing Yates correction for continuity or Fisher's exact test as appropriate. Continuous data were compared with Student's t-test and expressed as mean ± SD. The stratified chi-square test was used for subgroup analysis between the two groups. Multiple logistic regression analyses were used for determination of major factors that affected treatment efficacy. Statistical analyses were performed using the SPSS 12.0 statistical software for Windows.
3. Results
3.1. Interim Analysis Result
The fifth interim analysis performed on December 2009 revealed that the difference in the eradication rates between the LAcR and CAR regimens was statistically significant (P = 0.014). According to the stopping boundaries corresponding to the fifth interim analysis for the Pocock (0.0158) or O'Brien-Fleming approach (0.0417) [20], this study was terminated early for ethical reasons. A total of 153 cases were screened, 7 cases were excluded, and 146 cases were enrolled and treated.
3.2. Patient Characteristics
The participant flow for this study is shown in Figure 1. As shown in Table 2, the baseline demographic data including age, gender, area of residence, endoscopic diagnosis, and follow-up methods were not significantly different between the two groups. The area of residence was defined by the domicile addresses registered by the participants. An address was considered “urban” if located in a city, while it was considered “rural” if in a township. Old population in this study, according to the current definition of the United Nations, was defined as ≥60 years old.
Figure 1.
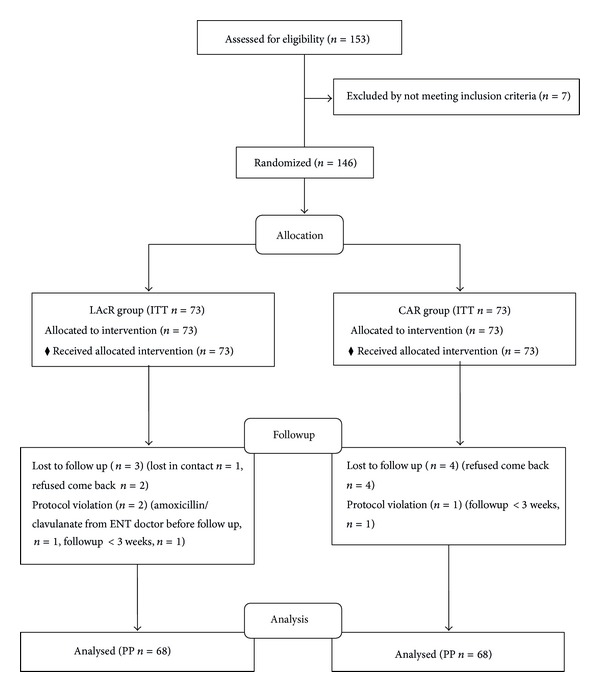
The participant flow chart.
Table 2.
Demographic characteristics of participants.
| All (n = 146) | LAcR (n = 73) | CAR (n = 73) | P value | |
|---|---|---|---|---|
| Mean age (years) | 53.73 ± 13.29 | 52.82 ± 12.08 | 54.63 ± 14.42 | 0.413 |
| Age < 60 years old, n (%) | 98 (67.1) | 51 (69.9) | 47 (64.4) | |
| Age ≧ 60 years old, n (%) | 48 (32.9) | 22 (30.1) | 26 (35.6) | 0.481 |
| Gender | ||||
| Male, n (%) | 71 (48.6) | 38 (52.1) | 33 (45.2) | |
| Female, n (%) | 75 (51.4) | 35 (47.9) | 40 (54.8) | 0.408 |
| Resident area | ||||
| Urban, n (%) | 54 (37.0) | 30 (41.1) | 24 (32.9) | |
| Rural, n (%) | 92 (63.0) | 43 (58.9) | 49 (67.1) | 0.304 |
| Endoscopic finding | ||||
| With peptic ulcer, n (%) | 65 (44.5) | 30 (41.1) | 35 (47.9) | |
| Without peptic ulcer, n (%) | 81 (55.5) | 43 (58.9) | 38 (52.1) | 0.405 |
| Follow-up method | ||||
| C13 urea breath test | 130 (89.0) | 65 (89.0) | 65 (89.0) | 0.881 |
| CLO test | 9 (6.2) | 5 (6.9) | 4 (5.5) | |
| Lost to follow up | 7 (4.8) | 3 (4.1) | 4 (5.5) |
3.3. Effects of Therapy on H. pylori Eradication
The PP eradication rates (PP-ER) for the LAcR and the CAR groups were 55/68 (80.9%) and 42/68 (61.8%), respectively (P = 0.014). The ITT eradication rates (ITT-ER) for the LAcR and the CAR groups were 57/73 (78.1%) and 42/73 (57.5%), respectively (P = 0.008) (Table 3). In the subgroup analysis, compared with the CAR regimen, the LAcR therapy showed significantly higher PP-ER (52.6% versus 83.9%, P = 0.006) and ITT-ER (51.3% versus 77.1%, P = 0.021) in patients ≧54 years old, higher PP-ER (55.3% versus 82.9%, P = 0.006) and ITT-ER (53.1% versus 81.4%, P = 0.004) in those living in rural areas, and higher PP-ER (57.1% versus 84.6%, P = 0.009) and ITT-ER (52.6% versus 81.4%, P = 0.006) in those without endoscopic diagnosis of peptic ulcer diseases (Table 3). A multivariate logistic regression analysis confirmed that the unique factor that led to successful H. pylori eradication was the modality of treatment (Table 4).
Table 3.
Comparison of eradication rate and subgroup analysis.
| Perprotocol analysis | Intention-to-treat analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| LAcR, n (%) | CAR, n (%) | P value | Odd ratio (95% CI) | LAcR, n (%) | CAR, n (%) | P value | Odd ratio (95% CI) | |
| All | 55 (80.9) | 42 (61.8) | 0.014* | 2.619 (1.20~5.70) |
57 (78.1) | 42 (57.5) | 0.008* | 2.629 (1.28~5.42) |
| Age | ||||||||
| <60 years old | 37 (75.5) | 31 (72.1) | 0.710 | 1.194 (0.47~3.03) | 37 (72.5) | 31 (66.0) | 0.479 | 1.364 (0.58~3.23) |
| ≧60 years old | 20 (95.2) | 11 (42.3) | 0.000* | 27.273 (3.17~235) | 20 (90.9) | 11 (42.3) | 0.000* | 13.636 (2.62~70.91) |
| Gender | ||||||||
| Male | 30 (81.1) | 20 (64.5) | 0.123 | 2.357 (0.78~7.11) | 30 (78.9) | 20 (60.6) | 0.091 | 2.438 (0.86~6.94) |
| Female | 25 (80.6) | 22 (59.5) | 0.060 | 2.841 (0.94~8.59) | 27 (77.1) | 22 (55.0) | 0.044* | 2.761 (1.01~7.55) |
| Resident area | ||||||||
| Urban | 21 (77.8) | 16 (76.2) | 0.748 | 1.094 (0.28~4.23) | 22 (73.3) | 16 (66.7) | 0.594 | 1.375 (0.43~4.44) |
| Rural | 34 (82.9) | 26 (55.3) | 0.006* | 3.923 (1.45~10.62) | 35 (81.4) | 26 (53.1) | 0.004* | 3.870 (1.50~10.02) |
| Endoscopic finding | ||||||||
| With peptic ulcer | 22 (75.9) | 22 (66.7) | 0.426 | 1.571 (0.51~4.80) | 22 (73.3) | 22 (62.9) | 0.368 | 1.625 (0.56~4.69) |
| Without peptic ulcer | 33 (84.6) | 20 (57.1) | 0.009* | 4.125 (1.38~12.36) | 35 (81.4) | 20 (52.6) | 0.006* | 3.938 (1.45~10.68) |
*P < 0.05.
Table 4.
Multiple logistic regression analysis.
| Perprotocol | Intention to treat | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age | ||||
| <60 years old | 1.0 (referent) | 1.0 (referent) | ||
| ≥60 years old | 1.33 (0.61–2.92) | 0.478 | 1.16 (0.55–2.47) | 0.696 |
| Gender | ||||
| Male | 1.0 (referent) | 1.0 (referent) | ||
| Female | 1.14 (0.53–2.46) | 0.737 | 1.18 (0.57–2.44) | 0.648 |
| Resident area | ||||
| Urban | 1.0 (referent) | 1.0 (referent) | ||
| Rural | 1.49 (0.65–3.42) | 0.342 | 1.12 (0.53–2.37) | 0.775 |
| Endoscopic finding | ||||
| With peptic ulcer | 1.0 (referent) | 1.0 (referent) | ||
| Without peptic ulcer | 1.05 (0.48–2.26) | 0.909 | 1.05 (0.51–2.16) | 0.900 |
| Treatment | ||||
| CAR | 1.0 (referent) | 1.0 (referent) | ||
| LAcR | 2.67 (1.22–5.84) | 0.014* | 2.57 (1.24–5.33) | 0.011* |
*P < 0.05.
3.4. Tolerance to H. pylori Eradication Therapy
One patient from the LAcR group developed severe vomiting during the therapy. This patient received supportive treatment at the emergency room. After recovery, she stopped the LAcR regimen and was lost to follow-up. The association between this event and the LAcR therapy could not be confirmed. Mild adverse events were reported by a few patients in both groups without significant differences (Table 5).
Table 5.
Comparison of side effects.
| LAcRa | CAR | P value | |
|---|---|---|---|
| All, n (%) | 10 (13.7) | 11 (15.1) | 0.814 |
| Abdominal pain | 2 | 2 | |
| Flatus/abdominal fullness | 1 | 3 | |
| Loose stool/diarrhea | 3 | 1 | |
| Nausea/hiccough | 4 | 4 | |
| Vomiting | 2a | 0 | |
| Change in appetite | 2 | 4 | |
| Insomnia | 1 | 0 |
aOne of the two cases stopped the LAcR therapy due to severe vomiting.
4. Discussion
Our study results suggest that the standard seven-day triple therapy is not suitable for patients in Eastern Taiwan because the H. pylori ITT-ER was only 57.5%. The LAcR regimen achieved a significantly higher H. pylori eradication rate, suggesting that the use of amoxicillin/clavulanate can increase the overall response rate. In addition, the LAcR regimen may have attenuated the influence of some socioeconomic factors contributing to a high failure rate by the standard triple therapy among rural residents. In fact, we found that the differences on primary resistance rates of metronidazole (52.8% versus 47.7%, P = 0.95), amoxicillin (22.2% versus 25.0%, P = 0.98), and clarithromycin (13.9% versus 22.7%, P = 0.58) in clinical isolates of H. pylori from patient living in urban and rural areas of eastern Taiwan were not statistically significant [21].
The efficacy of this seven-day levofloxacin plus amoxicillin/clavulanate (LAc) based regimen has never been reported previously. However, we found a similar study in northern Taiwan that evaluated a seven-day regimen using LA as a first-line therapy. As expected, this “LA” combination revealed an ITT-ER of 74.2%, which is inferior to our “LAc” combination with an ITT-ER of 78.1% [11].
Two studies evaluated the antiresistance effect of amoxicillin/clavulanate to its counterpart, amoxicillin, in a seven-day PPI, clarithromycin plus amoxicillin/clavulanate or amoxicillin regimen. One (with omeprazole) showed a significant improvement in the ITT-ER (86.6% versus 66.6%, P < 0.05) [17]. The second study (with esomeprazole) showed a positive trend but did not achieve statistical significance (ITT-ER 72% versus 62%, P = 0.2188) [18]. Similarly, a trial comparing omeprazole, azithromycin plus amoxicillin/clavulanate, or amoxicillin also showed a beneficial trend (ITT-ER 86% versus 82%, P = 0.801; PP-ER 91.5% versus 85.4%, P = 0.543) but did not attain statistical significance [19]. In two studies, the omeprazole, metronidazole plus amoxicillin/clavulanate regimen for 2 weeks provided an 80.5% PP-ER in children from Iran [22] and a 76.4% PP-ER in adults from China [23].
Other strategies to increase the eradication rate when confronted with antibiotic resistance are to increase dosage and to extend treatment duration. Better H. pylori eradication rates have also been achieved elsewhere by 10- to 14-day versus seven-day LA regimens [24–28] with only two studies having less than 80% ITT-ER [24, 29]. We hypothesize that if we extend the duration of the LAcR regimen to 10–14 days, we will anticipate an ITT-ER beyond 80%. The optimal dosage and duration of a LAc-based regimen for an area with high H. pylori resistance to amoxicillin and/or clarithromycin require more investigations.
In this study, we chose rabeprazole as the PPI because its metabolism is less affected by CYP2C19 [30, 31] and CYP3A4 genotypes [31]. As a result, we anticipated less interpersonal variation in drug response, but this strategy may lead to a possible reduction in the synergistic effect of a PPI with antibiotics like clarithromycin [32, 33], metronidazole [33], or fluoroquinolone [34]. A study in China reported an ITT-ER of 75.4% for a seven-day LA-10 mg rabeprazole regimen, which was inferior to those of a seven-day LA-20 mg esomeprazole (85.2%) and a seven-day LA-40 mg esomeprazole (87.1%) regimen, respectively [35]. Thus, the susceptibility variation of CYP2C19/CYP3A4 genotypes and types/dosage of various PPIs used in this LAc-based regimen are also important considerations to improve H. pylori eradication rate. We have been engaged in an investigation of the influence of CYP2C19 genotypes on the efficacy of H. pylori eradication by the LAcR regimen as a second-line treatment.
We found that the H. pylori eradication rate of this LAcR regimen was not affected by common host factors (i.e., old age, rural residence, and ulcer/nonulcer status) that could affect the standard triple therapy. The poorer H. pylori eradication rate by the standard triple therapy in rural area may not be true in different countries. For example, rural areas in northern Wales had a higher ITT-ER (92%) by the standard triple therapy [36] when compared to rural China (PP-ER 69.59%, ITT-ER 65.6%) [37]. Our study results first point out that H. pylori eradication rate could be affected by problems in rural areas such as poor sanitary conditions and personal hygiene, antibiotic abuse in agriculture, limited education resources, and inadequate access to clean water. Since the majority of previous clinical trials on H. pylori treatment were performed in large city hospitals, the influence of rural residence on H. pylori eradication rate needs more therapeutic trials to confirm. Above all, one of the advantages of the LAcR regimen for first-line use is that clinicians need not be concerned about patients' socioeconomic status before prescribing this regimen in an area with high H. pylori resistance rate to conventional antibiotics. Their concerns can focus on penicillin allergy, compliance, and possible side effects. The limitation of this study is that the local H. pylori resistance rates to levofloxacin and amoxicillin/clavulanate are unknown.
5. Conclusion
The seven-day LAcR regimen for H. pylori eradication is a viable alternative to the standard seven-day clarithromycin-based triple therapy for eastern Taiwan. It is a rescue regimen before the discovery of an optimal first-line therapy for a region with high ampicillin and/or clarithromycin resistance rates. The efficacy of the standard eradication therapy is probably reduced by the high H. pylori resistance rate to amoxicillin and/or clarithromycin.
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this paper.
References
- 1.Chey WD, Wong BCY. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. The American Journal of Gastroenterology. 2007;102(8):1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 2.Lin J-T, Wang J-T, Wang T-H, Wu M-S, Lee T-K, Chen C-J. Helicobacter pylori infection in a randomly selected population, healthy volunteers, and patients with gastric ulcer and gastric adenocarcinoma. A seroprevalence study in Taiwan. Scandinavian Journal of Gastroenterology. 1993;28(12):1067–1072. doi: 10.3109/00365529309098311. [DOI] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56(6):772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. Journal of Gastroenterology and Hepatology. 2009;24(10):1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 5.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nature Clinical Practice Gastroenterology and Hepatology. 2008;5(6):321–331. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu CT, Wu CC, Lin CY, et al. Resistance rate to antibiotics of Helicobacter pylori isolates in eastern Taiwan. Journal of Gastroenterology and Hepatology. 2007;22(5):720–723. doi: 10.1111/j.1440-1746.2006.04743.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y-J, Yang J-C, Jeng Y-M, Chang M-H, Ni Y-H. Prevalence and rapid identification of clarithromycin-resistant Helicobacter pylori isolates in children. Pediatric Infectious Disease Journal. 2001;20(7):662–666. doi: 10.1097/00006454-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Poon S-K, Lai C-H, Chang C-S, et al. Prevalence of antimicrobial resistance in Helicobacter pylori isolates in Taiwan in relation to consumption of antimicrobial agents. International Journal of Antimicrobial Agents. 2009;34(2):162–165. doi: 10.1016/j.ijantimicag.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Hung K-H, Sheu B-S, Chang W-L, Wu H-M, Liu C-C, Wu J-J. Prevalence of primary fluoroquinolone resistance among clinical isolates of Helicobacter pylori at a University Hospital in Southern Taiwan. Helicobacter. 2009;14(1):61–65. doi: 10.1111/j.1523-5378.2009.00655.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clinical Gastroenterology and Hepatology. 2010;8(1):36.e1–41.e1. doi: 10.1016/j.cgh.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liou J-M, Lin J-T, Chang C-Y, et al. Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomised comparative trial with crossover design. Gut. 2010;59(5):572–578. doi: 10.1136/gut.2009.198309. [DOI] [PubMed] [Google Scholar]
- 12.de Francesco V, Giorgio F, Hassan C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. Journal of Gastrointestinal and Liver Diseases. 2010;19(4):409–414. [PubMed] [Google Scholar]
- 13.Saad RJ, Schoenfeld P, Hyungjin MK, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. The American Journal of Gastroenterology. 2006;101(3):488–496. doi: 10.1111/j.1572-0241.2006.00637.x. [DOI] [PubMed] [Google Scholar]
- 14.Tseng Y-S, Wu D-C, Chang C-Y, et al. Amoxicillin resistance with β-lactamase production in Helicobacter pylori . European Journal of Clinical Investigation. 2009;39(9):807–812. doi: 10.1111/j.1365-2362.2009.02166.x. [DOI] [PubMed] [Google Scholar]
- 15.Dore MP, Graham DY, Sepulveda AR, Realdi G, Osato MS. Sensitivity of amoxicillin-resistant Helicobacter pylori to other penicillins. Antimicrobial Agents and Chemotherapy. 1999;43(7):1803–1804. doi: 10.1128/aac.43.7.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horii T, Kimura T, Sato-Kawamura K, Nada T, Shibayama K, Ohta M. β-Lactamase inhibitors have antibacterial activities against Helicobacter pylori . Journal of Infection and Chemotherapy. 1999;5(4):206–207. doi: 10.1007/s101560050036. [DOI] [PubMed] [Google Scholar]
- 17.Ojetti V, Migneco A, Zocco MA, Nista EC, Gasbarrini G, Gasbarrini A. Beta-lactamase inhibitor enhances Helicobacter pylori eradication rate. Journal of Internal Medicine. 2004;255(1):125–129. doi: 10.1046/j.0954-6820.2003.01239.x. [DOI] [PubMed] [Google Scholar]
- 18.Crispino P, Iacopini F, Pica R, et al. β-Lactamase inhibition with clavulanic acid supplementing standard amoxycillin-based triple therapy does not increase Helicobacter pylori eradication rate. Digestive and Liver Disease. 2005;37(11):826–831. doi: 10.1016/j.dld.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Vcev A, Vceva A, Takac B, et al. Omeprazole, azithromycin and amoxicillin or amoxicillin plus clavulanic acid in eradication of Helicobacter pylori in duodenal ulcer disease. Acta Medica Croatica. 1998;52(4-5):209–214. [PubMed] [Google Scholar]
- 20.O'Brien PC. Data and safety monitoring. In: Redmond CK, Cotton T, editors. Biostatistics in Clincal Trials. West Sussex, UK: John Wiley & Sons; 2001. pp. 146–148. [Google Scholar]
- 21.Liu TT. The retrospective study of Helicobacter pylori infection and antibiotic resistant [M.S. thesis] Hualien, Taiwan: Buddhist Tzu Chi General Hospital and Tzu Chi University; 2006. http://fedetd.mis.nsysu.edu.tw/FED-db/cgi-bin/FED-search/view_etd?identifier=oai:www.etd.library.tcu.edu.tw:etd-0816106-101130&index_word= [Google Scholar]
- 22.Dehghani SM, Erjaee A, Imanieh MH, Haghighat M. Efficacy of the standard quadruple therapy versus triple therapies containing proton pump inhibitor plus amoxicillin and clarithromycin or amoxicillin-clavulanic acid and metronidazole for Helicobacter pylori eradication in children. Digestive Diseases and Sciences. 2009;54(8):1720–1724. doi: 10.1007/s10620-008-0547-9. [DOI] [PubMed] [Google Scholar]
- 23.Wong BC-Y, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. Journal of the American Medical Association. 2004;291(2):187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 24.Erçin CN, Uygun A, Toros AB, et al. Comparison of 7- and 14-day first-line therapies including levofloxacin in patients with Helicobacter pylori positive non-ulcer dyspepsia. Turkish Journal of Gastroenterology. 2010;21(1):12–16. doi: 10.4318/tjg.2010.0041. [DOI] [PubMed] [Google Scholar]
- 25.Gisbert JP, Bermejo MF, Infante JM, et al. Levofloxacin, amoxicillin, and omeprazole as first-line triple therapy for Helicobacter pylori eradication. Journal of Clinical Gastroenterology. 2009;43(4):384–385. doi: 10.1097/MCG.0b013e31816d921c. [DOI] [PubMed] [Google Scholar]
- 26.Marzio L, Coraggio D, Capodicasa S, Grossi L, Cappello G. Role of the preliminary susceptibility testing for initial and after failed therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and esomeprazole. Helicobacter. 2006;11(4):237–242. doi: 10.1111/j.1523-5378.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 27.Molina-Infante J, Perez-Gallardo B, Fernandez-Bermejo M, et al. Clinical trial: clarithromycin vs. levofloxacin in first-line triple and sequential regimens for Helicobacter pylori eradication. Alimentary Pharmacology and Therapeutics. 2010;31(10):1077–1084. doi: 10.1111/j.1365-2036.2010.04274.x. [DOI] [PubMed] [Google Scholar]
- 28.Gisbert JP, Fernández-Bermejo M, Molina-Infante J, et al. First-line triple therapy with levofloxacin for Helicobacter pylori eradication. Alimentary Pharmacology and Therapeutics. 2007;26(3):495–500. doi: 10.1111/j.1365-2036.2007.03384.x. [DOI] [PubMed] [Google Scholar]
- 29.Castro-Fernández M, Lamas E, Pérez-Pastor A, et al. Efficacy of triple therapy with a proton pump inhibitor, levofloxacin, and amoxicillin as first-line treatment to eradicate Helicobacter pylori . Revista Espanola de Enfermedades Digestivas. 2009;101(6):395–402. doi: 10.4321/s1130-01082009000600004. [DOI] [PubMed] [Google Scholar]
- 30.Padol S, Yuan Y, Thabane M, Padol IT, Hunt RH. The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a meta-analysis. The American Journal of Gastroenterology. 2006;101(7):1467–1475. doi: 10.1111/j.1572-0241.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 31.Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors—emphasis on rabeprazole. Alimentary Pharmacology and Therapeutics, Supplement. 1999;13(supplement 3):27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 32.Ushiama H, Echizen H, Nachi S, Ohnishi A. Dose-dependent inhibition of CYP3A activity by clarithromycin during Helicobacter pylori eradication therapy assessed by changes in plasma lansoprazole levels and partial cortisol clearance to 6β-hydroxycortisol. Clinical Pharmacology and Therapeutics. 2002;72(1):33–43. doi: 10.1067/mcp.2002.125559. [DOI] [PubMed] [Google Scholar]
- 33.Sapone A, Vaira D, Trespidi S, et al. The clinical role of cytochrome P450 genotypes in Helicobacter pylori management. The American Journal of Gastroenterology. 2003;98(5):1010–1015. doi: 10.1111/j.1572-0241.2003.07427.x. [DOI] [PubMed] [Google Scholar]
- 34.Allen A, Vousden M, Lewis A. Effect of omeprazole on the pharmacokinetics of oral gemifloxacin in healthy volunteers. Chemotherapy. 1999;45(6):496–503. doi: 10.1159/000007244. [DOI] [PubMed] [Google Scholar]
- 35.Pan X, Li Y, Qiu Y, et al. Efficacy and tolerability of first-line triple therapy with levofloxacin and amoxicillin plus esomeprazole or rabeprazole for the eradication of Helicobacter pylori infection and the effect of CYP2C19 genotype: a 1-week, randomized, open-label study in chinese adults. Clinical Therapeutics. 2010;32(12):2003–2011. doi: 10.1016/j.clinthera.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Ching SS, Sabanathan S, Jenkinson LR. Treatment of Helicobacter pylori in surgical practice: a randomised trial of triple versus quadruple therapy in a rural district general hospital. World Journal of Gastroenterology. 2008;14(24):3855–3860. doi: 10.3748/wjg.14.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Shen L, Ma J-L, et al. Eradication of H pylori infection in a rural population: one-day quadruple therapy versus 7-day triple therapy. World Journal of Gastroenterology. 2006;12(24):3915–3918. doi: 10.3748/wjg.v12.i24.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]


