Abstract
A recent research of cancer has indicated that the mutant of isocitrate dehydrogenase 1 and 2 (IDH1 and 2) genes will induce various cancers, including chondrosarcoma, cholangiocarcinomas, and acute myelogenous leukemia due to the effect of point mutations in the active-site arginine residues of isocitrate dehydrogenase (IDH), such as IDH1/R132, IDH2/R140, and IDH2/R172. As the inhibition for those tumor-associated mutant IDH proteins may induce differentiation of those cancer cells, these tumor-associated mutant IDH proteins can be treated as a drug target proteins for a differentiation therapy against cancers. In this study, we aim to identify the potent TCM compounds from the TCM Database@Taiwan as lead compounds of IDH2 R140Q mutant inhibitor. Comparing to the IDH2 R140Q mutant protein inhibitor, AGI-6780, the top two TCM compounds, precatorine and abrine, have higher binding affinities with target protein in docking simulation. After MD simulation, the top two TCM compounds remain as the same docking poses under dynamic conditions. In addition, precatorine is extracted from Abrus precatorius L., which represents the cytotoxic and proapoptotic effects for breast cancer and several tumor lines. Hence, we propose the TCM compounds, precatorine and abrine, as potential candidates as lead compounds for further study in drug development process with the IDH2 R140Q mutant protein against cancer.
1. Introduction
Nowadays, in accordance with more and more mechanisms of diseases being identified [1–6], there are increasing numbers of potential target proteins against each disease, which are useful for drug design [7–11]. The recent research of cancer has indicated that the mutant of isocitrate dehydrogenase 1 and 2 (IDH1 and 2) genes will induce various cancers [12, 13]. Somatic mutations in the isocitrate dehydrogenase 1 and 2 genes affecting point mutations in the active-site arginine residues of isocitrate dehydrogenase (IDH), such as IDH1/R132, IDH2/R140, and IDH2/R172, occur frequently in many cancers, including chondrosarcoma, cholangiocarcinomas, and acute myelogenous leukemia [14–22]. The inhibition for those tumor-associated mutant IDH proteins may induce differentiation of those cancer cells. The tumor-associated mutant IDH proteins can be treated as a drug target proteins for a differentiation therapy against cancers [23].
Nowadays, the computer-aided drug design has been widely used in drug designing [24, 25]. Increasing numbers of compounds extracted from traditional Chinese medicine (TCM) have been indicated as potential lead compounds against cancers [26–28], inflammation [29], influenza [30], viral infection [31], metabolic syndrome [32], diabetes [33], stroke [34–36], and many other diseases [37–41]. A recent research of mutant IDH2 protein shows a compound, AGI-6780, which can inhibit the tumor-associated mutant IDH2/R140Q [42]. For drug development of TCM compounds, we aim to identify the potent TCM compounds from the TCM Database@Taiwan [43] as lead compounds of IDH2 R140Q mutant inhibitor. As structural disordered disposition in the protein may induce the side effect and reduce the occupancy for ligand to bind with target protein [44, 45], PONDR-Fit protocol was performed to predict the disordered disposition in IDH2 protein before virtual screening. After virtual screening, the MD simulation was performed to validate the stability of interactions between IDH2 R140Q mutant proteins and each ligand.
2. Materials and Methods
2.1. Data Collection
The X-ray crystallography structure of the human mitochondrial isocitrate dehydrogenase (IDH2) R140Q mutant was downloaded from RCSB Protein Data Bank with PDB ID: 4JA8 [42]. To predict the disordered amino acids, PONDR-Fit [46] protocol was employed with the sequence of IDH2 protein from Swiss-Prot (UniProtKB: P48735). In preparation section, X-ray crystallography structure of IDH2 R140Q mutant protein was protonated with Chemistry at HARvard Macromolecular Mechanics (CHARMM) force field [47] and removed crystal water by Prepare Protein module in Discovery Studio 2.5 (DS2.5). The final structure of TCM compounds from TCM Database@Taiwan [43] was protonated and filtered by Lipinski's Rule of Five [48] using Prepare Ligand module in DS2.5. The binding site for virtual screening was defined by the volume of the cocrystallized IDH2 R140Q mutant inhibitor, AGI-6780.
2.2. Docking Simulation
The TCM compounds were docking into the binding site using a shape filter and Monte-Carlo ligand conformation generation by LigandFit protocol [49] in DS 2.5. The docking poses were optionally minimized with CHARMM force field [47] and filtered the similar poses by the clustering algorithm. Each docking pose was evaluated by the following Dock Score energy function:
-
Dock Score = − (ligand/receptor interaction energy + ligand internal energy).
2.3. Molecular Dynamics (MD) Simulation
The molecular dynamics (MD) simulation utilizing Gromacs 4.5.5 [50] was employed using classical molecular dynamics theory to simulate each protein-ligand complex under dynamic conditions. In preparation section, the IDH2 R140Q mutant proteins were prepared by pdb2gmx protocol of Gromacs to provide topology and parameters with charmm27 force field, and each ligand was prepared by SwissParam program [51]. A cubic box solvated using TIP3P water model was defined based upon the edge approx. 1.2 nm from the protein complexes periphery. In the minimization section, the steepest descent [52] minimization was employed with a maximum of 5,000 steps to remove bad van der Waals contacts. Gromacs program creates a neutral system using 0.145 M NaCl model, followed by another steepest descent minimization with a maximum of 5,000 steps to remove bad van der Waals contacts. In the equilibration section, Gromacs program performs a position-restrained molecular dynamics with the linear constraint algorithm for all bonds, NVT equilibration, Berendsen weak thermal coupling method, and particle mesh Ewald method. In the production section, Gromacs program performs a total of 5000 ps production simulation with time step in unit of 2 fs under NPT ensembles and particle mesh Ewald (PME) option. A series of protocols in Gromacs program was utilized to analyze the 5000 ps MD trajectories. The CAVER 3.0 [53] was employed to analyze the presumably pathways for small molecule under dynamics conditions.
3. Results and Discussion
3.1. Disordered Protein Prediction
The sequence of IDH2 protein from Swiss-Prot (UniProtKB: P48735) was employed to predict the disordered disposition by PONDR-Fit protocol. As illustrated in Figure 1, the key residues in the binding domain have no disordered disposition, which express a stable binding domain in protein folding. It indicates that the binding domain in the crystallography structure of target protein will be suitable for docking simulation as the residues in the binding domain have no significant variation.
Figure 1.
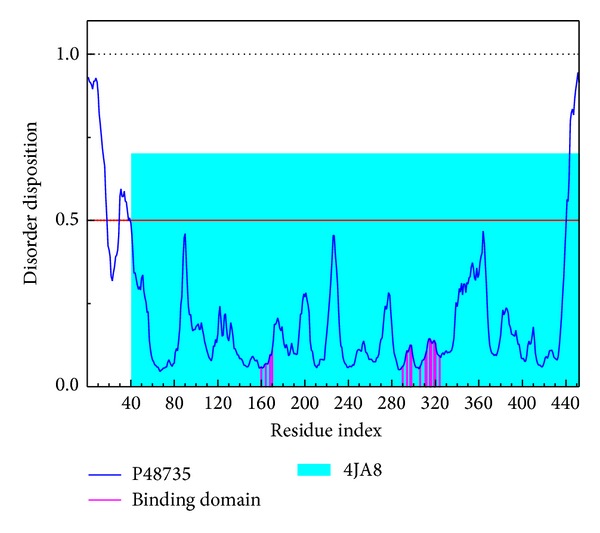
Disordered disposition predicted by PONDR-Fit.
3.2. Docking Simulation
To validate the accuracy of LigandFit protocol, we redock the cocrystallized IDH2 R140Q mutant inhibitor, AGI-6780, into the binding site of IDH2 R140Q mutant proteins. Root-mean-square deviation (RMSD) value between crystallized structure and docking pose of AGI-6780 is 0.3683 Å (Figure 2), which indicates that the docking simulation by LigandFit protocol is suitable for virtual screening with IDH2 R140Q mutant proteins. After virtual screening, the chemical scaffolds of AGI-6780 and top two TCM compounds are displayed in Figure 3 with Dock Score and sources. Precatorine is extracted from Abrus precatorius L., and abrine is extracted from Abrus fruticuIosus Wall. ex Wight et Arn. The compounds extracted from Abrus precatorius L. had been indicated to have the antimicrobial activity [54], antibacterial activity [55], cytotoxic and proapoptotic effects for breast cancer [56], and several tumor lines [57]. Figure 4 illustrated the docking poses of IDH2 R140Q mutant protein complexes with AGI-6780 and top two TCM compounds, respectively. The IDH2 R140Q mutant protein inhibitor, AGI-6780, has hydrogen bonds (H-bonds) with residues Gln316 in both chains of IDH2 R140Q mutant protein and a π interaction with residue Ile319 in chain B of IDH2 R140Q mutant protein. For the top TCM candidates, they also have H-bonds with residues Gln316 in both chains of IDH2 R140Q mutant protein as AGI-6780. For abrine, it has a π interaction with residue Ile319 in chain A of IDH2 R140Q mutant protein.
Figure 2.
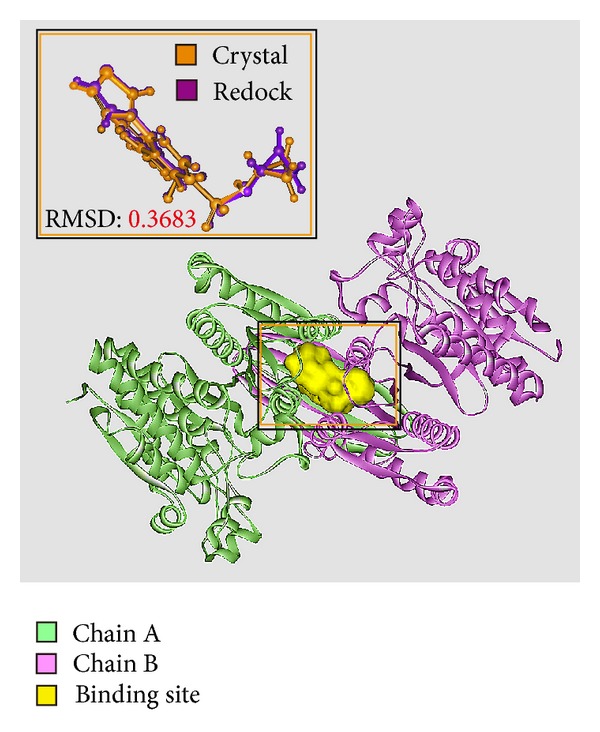
Binding site of IDH2 R140Q mutant protein defined as the volume of AGI-6780 and root-mean-square deviation value between crystallized structure (orange) and docking pose (violet) of AGI-6780.
Figure 3.
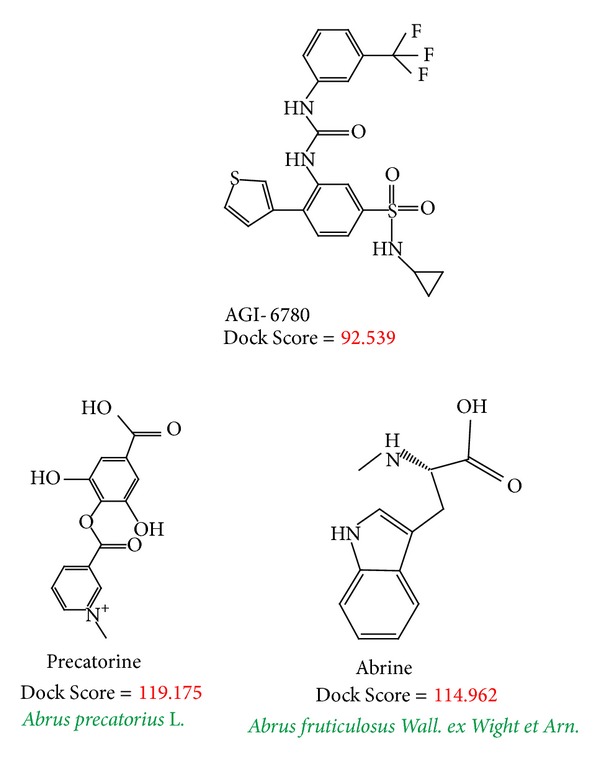
Chemical scaffold of controls and top two TCM candidates with their scoring function and sources.
Figure 4.
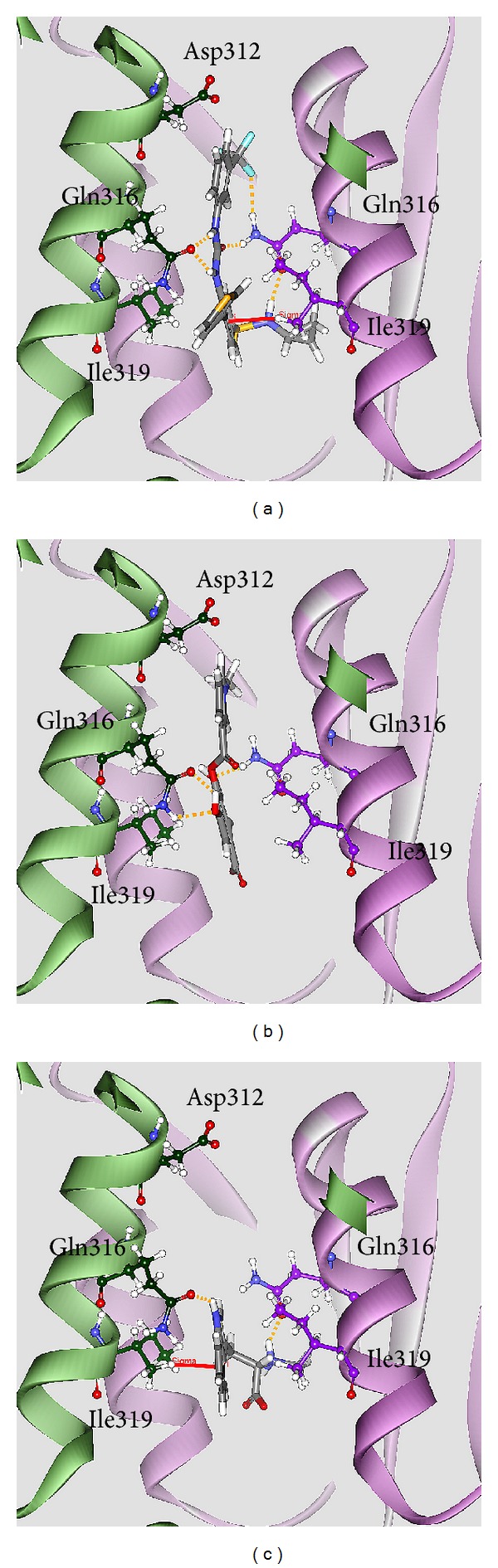
Docking pose of IDH2 R140Q mutant protein complexes with (a) AGI-6780, (b) precatorine, and (c) abrine.
3.3. Molecular Dynamics Simulation
For the docking simulation performed by LigandFit protocol, the receptor is a rigid body of IDH2 R140Q mutant proteins. The conformation of the IDH2 R140Q mutant protein may modify under dynamic conditions. We employed the MD simulation to validate the stability of interactions between IDH2 R140Q mutant proteins and each ligand. RMSDs illustrated the atomic fluctuations during MD simulation. Figure 5 displays the atomic fluctuations of IDH2 R140Q mutant proteins in apo form and complexes with AGI-6780, precatorine, and abrine and the atomic fluctuations of each compound during 5000 ps MD simulation. It shows that IDH2 R140Q mutant proteins tend to be stable after first 100 ps MD simulation, but the ligands except precatorine are fluctuate during MD simulation. To consider the variation radii of gyration for protein and total energy over 5000 ps MD simulation in Figure 6, it indicates that the radii of gyration for IDH2 R140Q mutant proteins in apo form were decreased after 4500 ps MD simulation, but the radii of gyration for complexes of IDH2 R140Q mutant proteins with AGI-6780, precatorine, and abrine were more stabilized. In addition, there is no significant change for the total energies of each IDH2 R140Q mutant protein complex during MD simulation in Figure 7. The variation of solvent accessible surface area over 5000 ps MD simulation in Figure 8 indicates that docking the ligands, AGI-6780, precatorine, and abrine, would not affect the solvent accessible surface of IDH2 R140Q mutant protein under dynamic conditions. The mean square displacement (MSD) for each protein and ligand in IDH2 R140Q mutant proteins and protein complexes with AGI-6780, precatorine, and abrine over 5000 ps of MD simulation is displayed in Figure 9. Root-mean-square fluctuation (RMSF) for each residue over 5000 ps MD simulation is displayed in Figure 10. They indicate that IDH2 R140Q mutant protein docking with precatorine and abrine causes similar diffusion constant and flexibility for IDH2 R140Q mutant proteins as AGI-6780.
Figure 5.
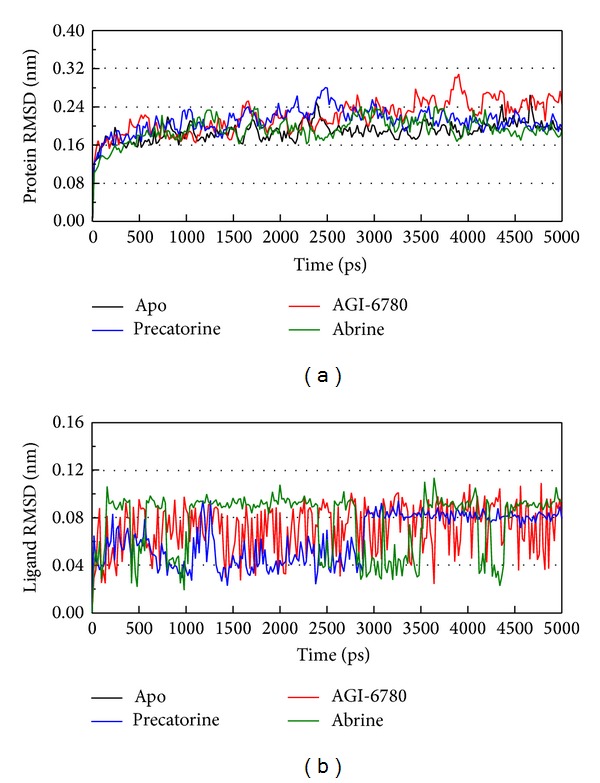
Root-mean-square deviations in units of nm for protein and ligand over 5000 ps of MD simulation for IDH2 R140Q mutant proteins and protein complexes with AGI-6780, precatorine, and abrine.
Figure 6.
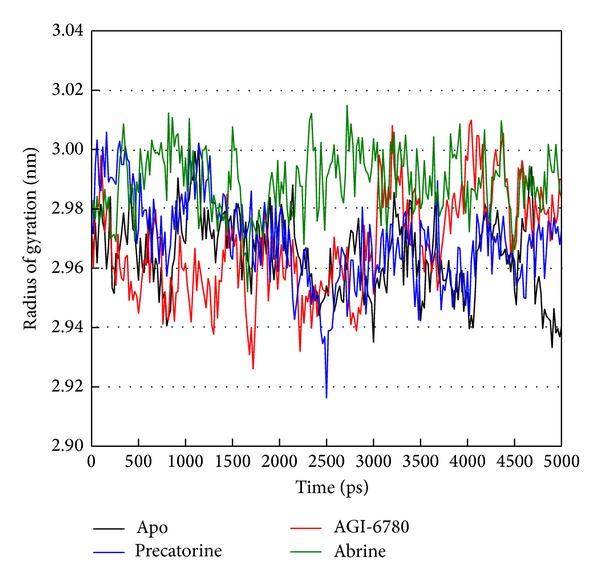
Radii of gyration for protein over 5000 ps of MD simulation for IDH2 R140Q mutant proteins and protein complexes with AGI-6780, precatorine, and abrine.
Figure 7.
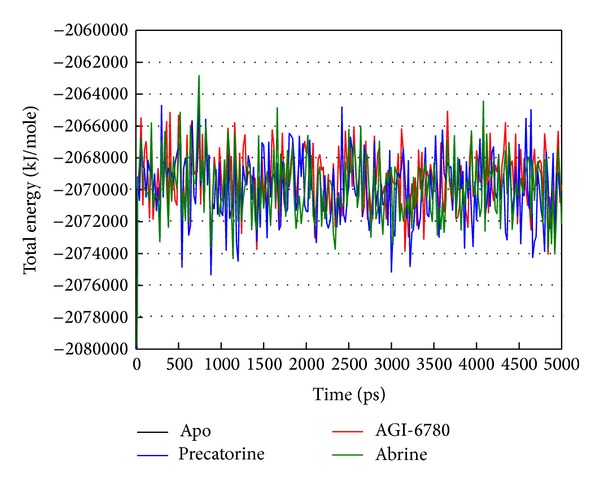
Variation of total energy for complex over 5000 ps of MD simulation for IDH2 R140Q mutant proteins and protein complexes with AGI-6780, precatorine, and abrine.
Figure 8.
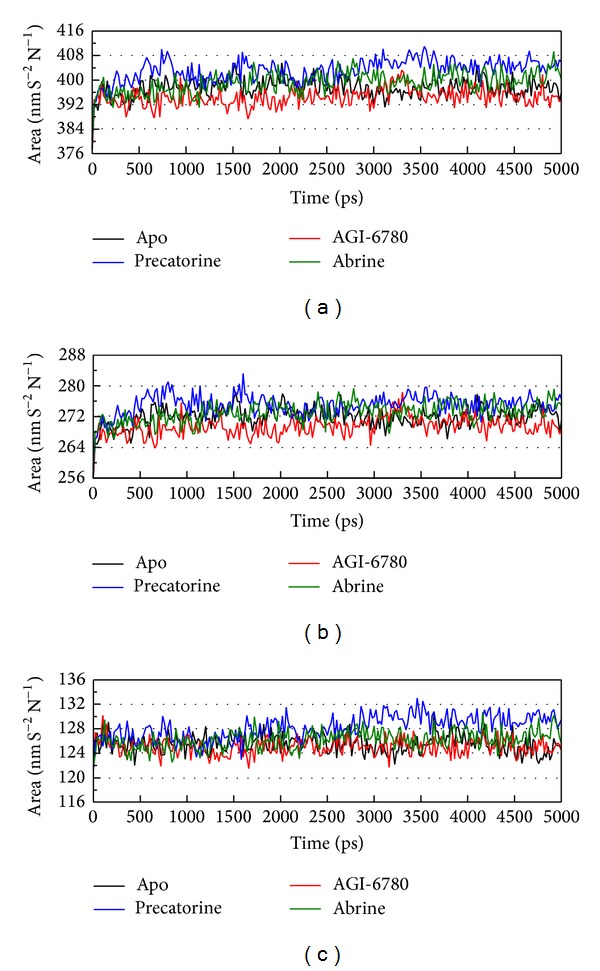
Variation of (a) total solvent accessible surface area, (b) hydrophobic surface area, and (c) hydrophilic surface area over 5000 ps of MD simulation for IDH2 R140Q mutant proteins and protein complexes with AGI-6780, precatorine, and abrine.
Figure 9.
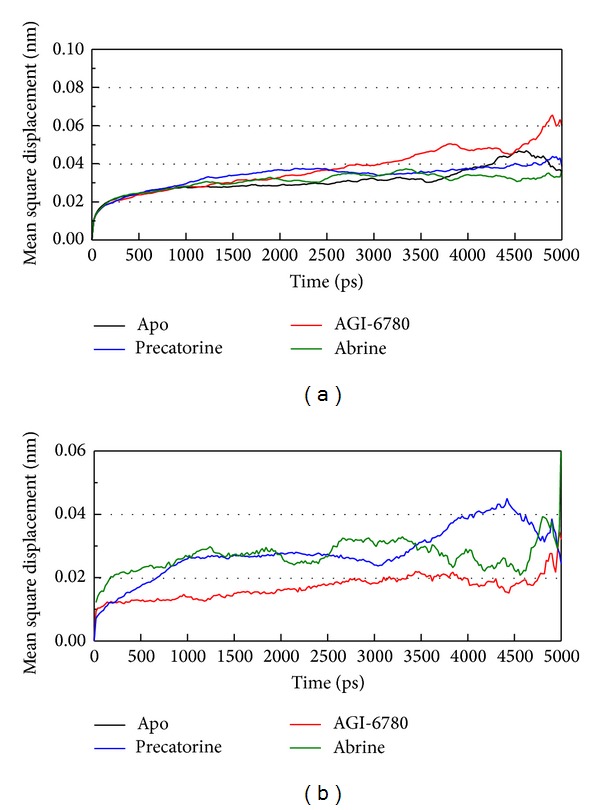
Mean square displacement (MSD) for (a) protein and (b) ligand over 5000 ps of MD simulation for IDH2 R140Q mutant proteins and protein complexes with AGI-6780, precatorine, and abrine.
Figure 10.
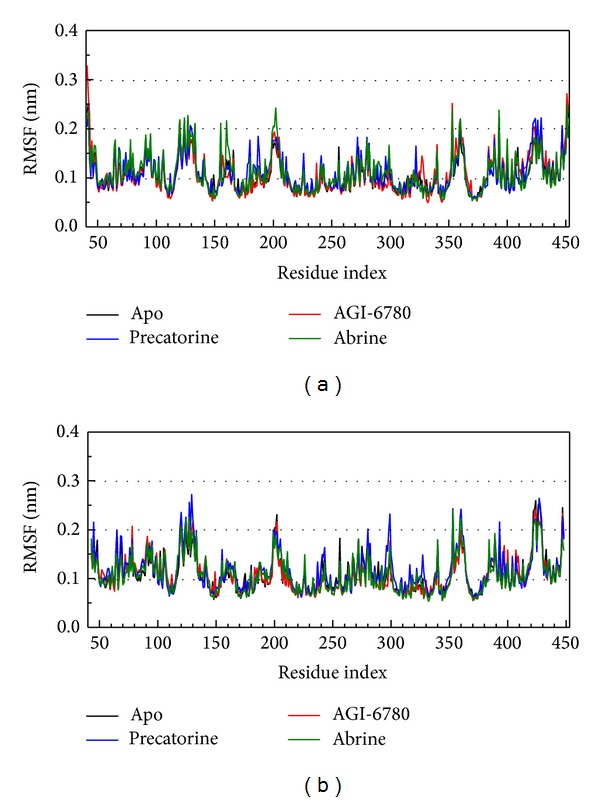
Root-mean-square fluctuation (RMSF) for residues in (a) chain A and (b) chain B of IDH2 R140Q mutant proteins and protein complexes with AGI-6780, precatorine, and abrine.
After MD simulation, we identify the representative structures of IDH2 R140Q mutant proteins in apo form and in each complex using the RMSD values and graphical depiction of the clusters analysis with a RMSD cutoff of 0.105 nm in Figure 11. The docking poses of the representative structures for complexes of IDH2 R140Q mutant proteins with AGI-6780, precatorine, and abrine are illustrated in Figure 12. To compare with the result in docking simulation, the IDH2 R140Q mutant protein inhibitor, AGI-6780, has stable hydrogen bonds (H-bonds) with residues Gln316 in both chains of IDH2 R140Q mutant protein and forms a π interaction with residue Val315 in chain B of IDH2 R140Q mutant protein. For TCM candidates, they have similar docking poses as docking simulation, which has stable H-bonds with residues Gln316. The H-bond occupancy for key residues in complexes of IDH2 R140Q mutant protein with AGI-6780 and top TCM compounds overall 5000 ps of molecular dynamics simulation in Table 1 displayed the stability of H-bonds. Analysis of transport pathways for each IDH2 R140Q mutant protein complex illustrated in Figure 13 shows the presumably pathways for small molecule. They indicate that IDH2 R140Q mutant protein docking with precatorine and abrine has similar effects of protein conformation as AGI-6780.
Figure 11.
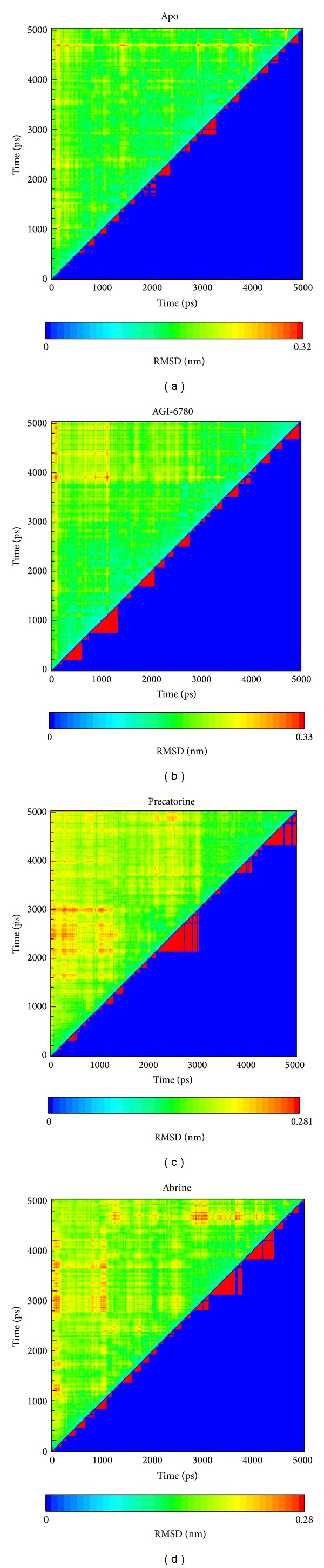
Root-mean-square deviation value (upper left half) and graphical depiction of the clusters with cutoff 0.105 nm (lower right half) for IDH2 R140Q mutant proteins and protein complexes with AGI-6780, precatorine, and abrine.
Figure 12.
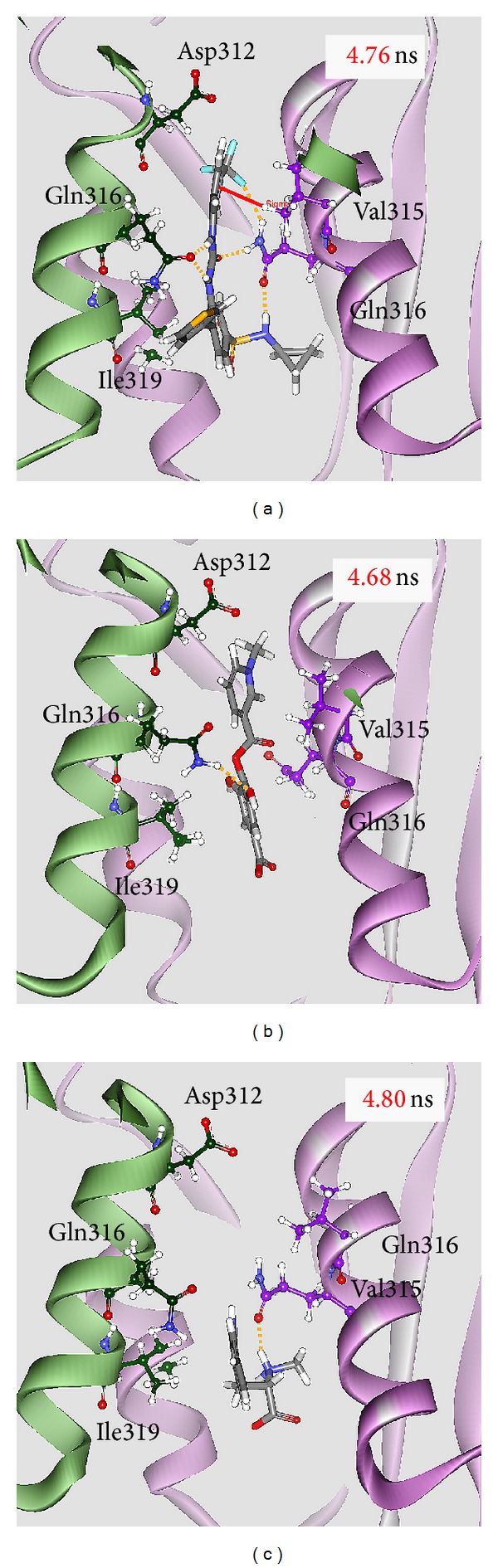
Docking poses of middle RMSD structure in the major cluster for IDH2 R140Q mutant protein complexes with AGI-6780, precatorine, and abrine.
Table 1.
H-bond occupancy for key residues of IDH2 R140Q mutant protein with AGI-6780 and top TCM compounds overall 5000 ps of molecular dynamics simulation.
| Name | H-bond interaction | Occupancy |
|---|---|---|
| AGI-6780 | A:Gln316:OE1/H37 | 100% |
| A:Gln316:OE1/H38 | 100% | |
| B:Gln316:HE22/O13 | 100% | |
| B:Gln316:OE1/H40 | 100% | |
|
| ||
| Precatorine | A:Gln316:HE22/O20 | 67% |
| A:Gln316:OE1/H29 | 14% | |
| B:Gln316:HE22/O9 | 4% | |
| B:Gln316:HE22/O16 | 12% | |
| B:Gln316:OE1/H28 | 4% | |
|
| ||
| Abrine | B:Gln316:HE22/N7 | 22% |
| B:Gln316:OE1/H30 | 95% | |
| B:Gln316:O/H30 | 2% | |
H-bond occupancy cutoff: 0.3 nm.
Figure 13.
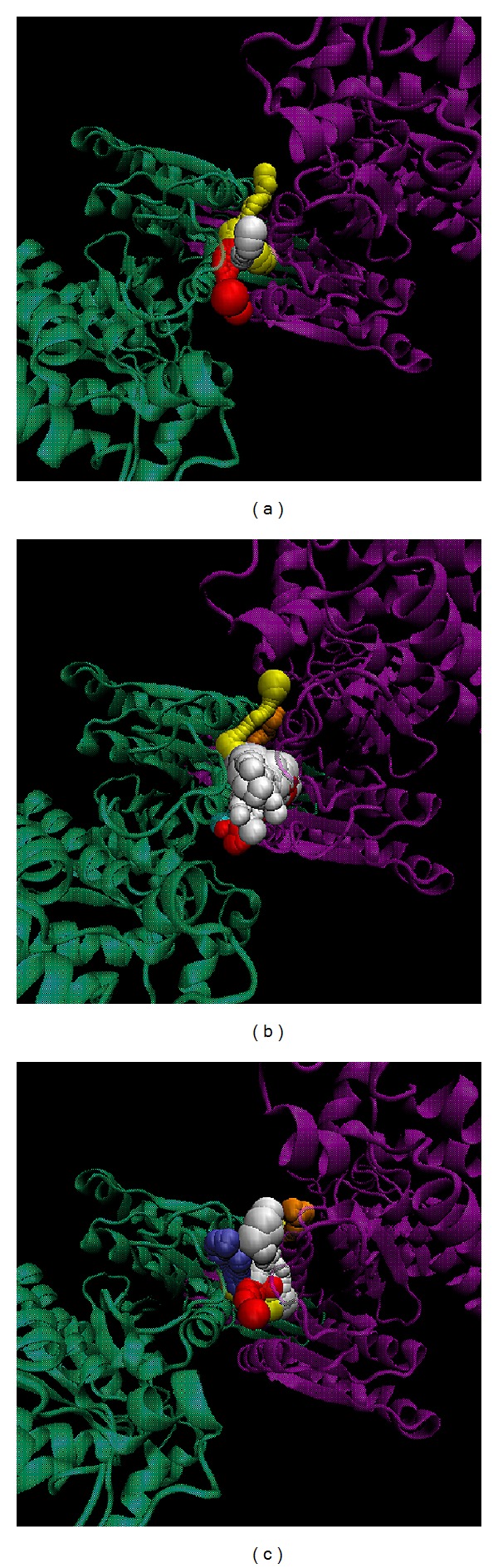
Analysis of transport pathways for IDH2 R140Q mutant protein complexes with (a) AGI-6780, (b) precatorine, and (c) abrine.
4. Conclusion
This study aims to investigate the potent lead TCM candidates for IDH2 R140Q mutant protein inhibitors against cancers. Compared to the IDH2 R140Q mutant protein inhibitor, AGI-6780, the top two TCM compounds, precatorine and abrine, have higher binding affinities with target protein in docking simulation. Both of them has H-bonds with residues Gln316 in both chains of IDH2 R140Q mutant protein as AGI-6780. After MD simulation, the top two TCM compounds remain as the same docking poses under dynamic conditions. In addition, precatorine is extracted from Abrus precatorius L., which has been indicated to have the cytotoxic and proapoptotic effects for breast cancer and several tumor lines. Hence, we propose the TCM compounds, precatorine and abrine, as potential candidates as lead compounds for further study in drug development process with the IDH2 R140Q mutant protein against cancer.
Acknowledgments
The research was supported by Grants from the National Science Council of Taiwan (NSC102-2325-B039-001, and NSC102-2221-E-468-027-), Asia University (ASIA100-CMU-2, ASIA101-CMU-2, and 102-ASIA-07), and China Medical University Hospital (DMR-103-058, DMR-103-001, and DMR-103-096). This study is also supported in part by Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH102-TD-B-111-004) and Taiwan Department of Health Cancer Research Center of Excellence (MOHW103-TD-B-111-03), and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Wen-Yuan Lee, Kuan-Chung Chen, and Hsin-Yi Chen contributed equally to this paper.
References
- 1.Chou I-C, Lin W-D, Wang C-H, et al. Association analysis between Tourette’s syndrome and two dopamine genes (DAT1, DBH) in Taiwanese children. BioMedicine. 2013;3(2):88–91. [Google Scholar]
- 2.Yamamoto T, Hung W-C, Takano T, Nishiyama A. Genetic nature and virulence of community-associated methicillin-resistant Staphylococcus aureus. BioMedicine. 2013;3(1):2–18. [Google Scholar]
- 3.Jiang Y, Li X, Yang W, et al. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Molecular Cell. 2014;53(1):75–87. doi: 10.1016/j.molcel.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y-M, Velmurugan BK, Kuo W-W, et al. Inhibitory effect of alpinate Oxyphyllae fructus extracts on Ang II-induced cardiac pathological remodeling-related pathways in H9c2 cardiomyoblast cells. BioMedicine. 2013;3(4):148–152. [Google Scholar]
- 5.Leung YM, Wong KL, Chen SW, et al. Down-regulation of voltage-gated Ca2+ channels in Ca2+ store-depleted rat insulinoma RINm5F cells. BioMedicine. 2013;3(3):130–139. [Google Scholar]
- 6.Mahamuni SP, Khose RD, Menaa F, Badole SL. Therapeutic approaches to drug targets in hyperlipidemia. BioMedicine. 2012;2(4):137–146. [Google Scholar]
- 7.Leissring MA, Malito E, Hedouin S, et al. Designed inhibitors of insulin-degrading enzyme regulate the catabolism and activity of insulin. PLoS ONE. 2010;5(5) doi: 10.1371/journal.pone.0010504.e10504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin M-C, Tsai S-Y, Wang F-Y, Liu F-H, Syu J-N, Tang F-Y. Leptin induces cell invasion and the upregulation of matrilysin in human colon cancer cells. BioMedicine. 2013;3(4):174–180. [Google Scholar]
- 9.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochemical Journal. 2001;353(3):417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su K-P. Inflammation in psychopathology of depression: clinical, biological, and therapeutic implications. BioMedicine. 2012;2(2):68–74. [Google Scholar]
- 11.Jao C-L, Huang S-L, Hsu K-C. Angiotensin I-converting enzyme inhibitory peptides: inhibition mode, bioavailability, and antihypertensive effects. BioMedicine. 2012;2(4):130–136. [Google Scholar]
- 12.Lokody I. Metabolism: IDH2 drives cancer in vivo. Nature Reviews Cancer. 2013;13(11):756–757. doi: 10.1038/nrc3619. [DOI] [PubMed] [Google Scholar]
- 13.Das BR, Tangri R, Ahmad F, Roy A, Patole K. Molecular investigation of isocitrate dehydrogenase gene (IDH) mutations in gliomas: first report of IDH2 mutations in Indian patients. Asian Pacific Journal of Cancer Prevention. 2013;14(12):7261–7264. doi: 10.7314/apjcp.2013.14.12.7261. [DOI] [PubMed] [Google Scholar]
- 14.Yen KE, Bittinger MA, Su SM, Fantin VR. Cancer-associated IDH mutations: biomarker and therapeutic opportunities. Oncogene. 2010;29(49):6409–6417. doi: 10.1038/onc.2010.444. [DOI] [PubMed] [Google Scholar]
- 15.Lu C, Venneti S, Akalin A, et al. Induction of sarcomas by mutant IDH2. Genes & Development. 2013;27(18):1986–1998. doi: 10.1101/gad.226753.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Liu Y, Lu C, et al. Cancer-associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibition. Genes & Development. 2013;27(18):1974–1985. doi: 10.1101/gad.226613.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grassian AR, Pagliarini R, Chiang DY. Mutations of isocitrate dehydrogenase 1 and 2 in intrahepatic cholangiocarcinoma. Current Opinion in Gastroenterology. 2014;30(3):295–302. doi: 10.1097/MOG.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 18.Wang JB, Dong DF, Wang MD, Gao K. IDH1 overexpression induced chemotherapy resistance and IDH1 mutation enhanced chemotherapy sensitivity in Glioma cells in vitro and in vivo. Asian Pacific Journal of Cancer Prevention. 2014;15(1):427–432. doi: 10.7314/apjcp.2014.15.1.427. [DOI] [PubMed] [Google Scholar]
- 19.Wang JB, Dong DF, Gao K, Wang MD. Mechanisms underlying the biological changes induced by isocitrate dehydrogenase-1 mutation in glioma cells. Oncology Letters. 2014;7(3):651–657. doi: 10.3892/ol.2014.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kats LM, Reschke M, Taulli R, et al. Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance. Cell Stem Cell. 2014;14(3):329–341. doi: 10.1016/j.stem.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mylonas E, Janin M, Bawa O, et al. Isocitrate dehydrogenase (IDH)2 R140Q mutation induces myeloid and lymphoid neoplasms in mice. Leukemia. 2014 doi: 10.1038/leu.2014.18. [DOI] [PubMed] [Google Scholar]
- 22.Sabit H, Nakada M, Furuta T, et al. Characterizing invading glioma cells based on IDH1-R132H and Ki-67 immunofluorescence. Brain Tumor Pathology. 2014 doi: 10.1007/s10014-013-0172-y. [DOI] [PubMed] [Google Scholar]
- 23.Bobrovnikova-Marjon E, Hurov JB. Targeting metabolic changes in cancer: novel therapeutic approaches. Annual Review of Medicine. 2014;65:157–170. doi: 10.1146/annurev-med-092012-112344. [DOI] [PubMed] [Google Scholar]
- 24.Chen CY-C. A novel integrated framework and improved methodology of computer-aided drug design. Current Topics in Medicinal Chemistry. 2013;13(9):965–988. doi: 10.2174/1568026611313090002. [DOI] [PubMed] [Google Scholar]
- 25.Huang H-J, Yu HW, Chen C-Y, et al. Current developments of computer-aided drug design. Journal of the Taiwan Institute of Chemical Engineers. 2010;41(6):623–635. [Google Scholar]
- 26.Chen C-Y, Chen CY-C. Insights into designing the dual-targeted HER2/HSP90 inhibitors. Journal of Molecular Graphics and Modelling. 2010;29(1):21–31. doi: 10.1016/j.jmgm.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Yang S-C, Chang S-S, Chen H-Y, Chen CY-C. Identification of potent EGFR inhibitors from TCM Database@Taiwan. PLoS Computational Biology. 2011;7(10) doi: 10.1371/journal.pcbi.1002189.e1002189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsou Y-A, Chen K-C, Lin H-C, Chang S-S, Chen CY-C. Uroporphyrinogen decarboxylase as a potential target for specific components of traditional Chinese medicine: a virtual screening and molecular dynamics study. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0050087.e50087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen K-C, Sun M-F, Yang S-C, et al. Investigation into potent inflammation inhibitors from traditional chinese medicine. Chemical Biology and Drug Design. 2011;78(4):679–688. doi: 10.1111/j.1747-0285.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- 30.Chang S-S, Huang H-J, Chen CY-C. Two birds with one stone? Possible dual-targeting H1N1 inhibitors from traditional Chinese medicine. PLoS Computational Biology. 2011;7(12) doi: 10.1371/journal.pcbi.1002315.e1002315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang HJ, Jian YR, Chen CYC. Traditional Chinese medicine application in HIV: an in silico study. Journal of Biomolecular Structure & Dynamics. 2014;32(1):1–12. doi: 10.1080/07391102.2012.745168. [DOI] [PubMed] [Google Scholar]
- 32.Chen K-C, Chang S-S, Huang H-J, Lin T-L, Wu Y-J, Chen CY-C. Three-in-one agonists for PPAR-a, PPAR-γ, and PPAR-d from traditional Chinese medicine. Journal of Biomolecular Structure and Dynamics. 2012;30(6):662–683. doi: 10.1080/07391102.2012.689699. [DOI] [PubMed] [Google Scholar]
- 33.Chen KC, Chang SS, Tsai FJ, Chen CY. Han ethnicity-specific type 2 diabetic treatment from traditional Chinese medicine? Journal of Biomolecular Structure & Dynamics. 2013;31(11):1219–1235. doi: 10.1080/07391102.2012.732340. [DOI] [PubMed] [Google Scholar]
- 34.Chen K-C, Yu-Chian Chen C. Stroke prevention by traditional Chinese medicine? A genetic algorithm, support vector machine and molecular dynamics approach. Soft Matter. 2011;7(8):4001–4008. [Google Scholar]
- 35.Chen K-C, Chang K-W, Chen H-Y, Chen CY-C. Traditional Chinese medicine, a solution for reducing dual stroke risk factors at once? Molecular BioSystems. 2011;7(9):2711–2719. doi: 10.1039/c1mb05164d. [DOI] [PubMed] [Google Scholar]
- 36.Chang T-T, Chen K-C, Chang K-W, et al. In silico pharmacology suggests ginger extracts may reduce stroke risks. Molecular BioSystems. 2011;7(9):2702–2710. doi: 10.1039/c1mb05228d. [DOI] [PubMed] [Google Scholar]
- 37.Tou WI, Chang S-S, Lee C-C, Chen CY-C. Drug design for neuropathic pain regulation from traditional Chinese medicine. Scientific Reports. 2013;3(article 844) doi: 10.1038/srep00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen KC, Jian YR, Sun MF, Chang TT, Lee CC, Chen CY. Investigation of silent information regulator 1 (Sirt1) agonists from traditional Chinese medicine. Journal of Biomolecular Structure & Dynamics. 2013;31(11):1207–1218. doi: 10.1080/07391102.2012.726191. [DOI] [PubMed] [Google Scholar]
- 39.Tang H-C, Chen CY-C. Investigation of the novel lead of melanocortin 1 receptor for pigmentary disorders. Evidence-Based Complementary and Alternative Medicine. 2014;2014:13 pages. doi: 10.1155/2014/254678.254678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H-J, Lee C-C, Chen CY-C. Pharmacological chaperone design for reducing risk factor of Parkinson's disease from traditional chinese medicin. Evidence-Based Complementary and Alternative Medicine. 2014;2014:12 pages. doi: 10.1155/2014/830490.830490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen HY, Chang SS, Chan YC, Chen CY. Discovery of novel insomnia leads from screening traditional Chinese medicine database. Journal of Biomolecular Structure & Dynamics. 2014;32(5):776–791. doi: 10.1080/07391102.2013.790849. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Travins J, DeLaBarre B, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340(6132):622–626. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 43.Chen CY-C. TCM Database@Taiwan: the world’s largest traditional Chinese medicine database for drug screening In Silico. PLoS ONE. 2011;6(1) doi: 10.1371/journal.pone.0015939.e15939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tou WI, Chen CY. May disordered protein cause serious drug side effect? Drug Discovery Today. 2014;19(4):367–372. doi: 10.1016/j.drudis.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 45.Chen CY-C, Tou WI. How to design a drug for the disordered proteins? Drug Discovery Today. 2013;18(19-20):910–915. doi: 10.1016/j.drudis.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Xue B, Dunbrack RL, Williams RW, Dunker AK, Uversky VN. PONDR-FIT: a meta-predictor of intrinsically disordered amino acids. Biochimica et Biophysica Acta: Proteins and Proteomics. 2010;1804(4):996–1010. doi: 10.1016/j.bbapap.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. CHARMM: a program for macromolecular energy minimization and dynamics calculations. Journal of Computational Chemistry. 1983;4(2):187–217. [Google Scholar]
- 48.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 49.Venkatachalam CM, Jiang X, Oldfield T, Waldman M. LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites. Journal of Molecular Graphics and Modelling. 2003;21(4):289–307. doi: 10.1016/s1093-3263(02)00164-x. [DOI] [PubMed] [Google Scholar]
- 50.Hess B, Kutzner C, Van Der Spoel D, Lindahl E. GRGMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. Journal of Chemical Theory and Computation. 2008;4(3):435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 51.Zoete V, Cuendet MA, Grosdidier A, Michielin O. SwissParam: a fast force field generation tool for small organic molecules. Journal of Computational Chemistry. 2011;32(11):2359–2368. doi: 10.1002/jcc.21816. [DOI] [PubMed] [Google Scholar]
- 52.Fletcher R. Optimization. London, UK: Academic Press; 1969. [Google Scholar]
- 53.Chovancova E, Pavelka A, Benes P, et al. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLoS Computational Biology. 2012;8(10) doi: 10.1371/journal.pcbi.1002708.e1002708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adelowotan O, Aibinu I, Adenipekun E, Odugbemi T. The in-vitro antimicrobial activity of Abrus precatorius (L) fabaceae extract on some clinical pathogens. The Nigerian Postgraduate Medical Journal. 2008;15(1):32–37. [PubMed] [Google Scholar]
- 55.Zore GB, Awad V, Thakre AD, et al. Activity-directed-fractionation and isolation of four antibacterial compounds from Abrus precatorius L., roots. Natural Product Research. 2007;21(9):838–845. doi: 10.1080/14786410701474928. [DOI] [PubMed] [Google Scholar]
- 56.Shafi Sofi M, Sateesh MK, Bashir M, et al. Cytotoxic and pro-apoptotic effects of Abrus precatorius L. on human metastatic breast cancer cell line, MDA-MB-231. Cytotechnology. 2013;65(3):407–417. doi: 10.1007/s10616-012-9494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reddy VV, Sirsi M. Effect of Abrus precatorius L. on experimental tumors. Cancer Research. 1969;29(7):1447–1451. [PubMed] [Google Scholar]


