Abstract
The central role of mitochondria as mediators of cell survival is indisputable and gathering increasing attention as a focal point for interventional strategies to mitigate apoptotic cell death in the wake of cardiomyopathic injury. A legacy of signal transduction studies has proven that mitochondrial integrity can be enhanced by kinases involved in cell survival. Among the many survival signaling cascades under investigation, the wide-ranging impact of Akt upon mitochondrial biology is well known. However, despite years of investigation, emerging research continues to reveal new mechanisms governing the protective effects of Akt signaling in the context of cardiomyocyte mitochondria. This review focuses on two emerging pathways that mediate preservation of mitochondrial function downstream of Akt: hexokinase and Pim-1 kinase.
Keywords: Akt, cardiomyocyte, heart, hexokinase, kinase, mitochondria, Pim-1
Mitochondrial integrity & survival kinases
The critical role of mitochondria as arbiters of cell survival is widely recognized and well documented in the myocardial context [1–11]. Since mitochondria act as integrators of multiple cellular conditions reflecting physiological and genomic stresses, it is reasonable to expect that kinase signaling mechanisms influencing cell survival impinge either directly or indirectly upon mitochondrial integrity. Indeed, a multitude of studies have documented the influence of each major kinase signaling pathway upon mitochondrial activity, including PKA, PKC, extracellular signal-regulated protein kinase, c-Jun N-terminal kinase, p38 kinase, glycogen synthase kinase-3β, and Akt (also known as PKB) [8,12–18]. In addition to the potent effect of pre-/post-conditioning upon signaling and mitochondrial protection [19–24], several upstream receptor-mediated hormonal and cytokine signaling pathways trigger the cascade of intracellular mitochondrial protective signaling, such as IL-6, leptin, estrogen and IGF [25–29]. The connection between IGF signaling, Akt and mitochondrial protection points to the interplay of Akt with mitochondria [27,30]. Moreover, the cardioprotective effects of Akt have been suggested to depend upon translocation from the cytosol to mitochondria [31] where it influences mitochondrial ATP-sensitive potassium channels and subsequently inhibits opening of the permeability transition pore to maintain mitochondrial integrity [2,15,32–35], as well as influencing redox state by blunting oxidative stress [36–40]. Although the kinetics of mitochondrial Akt activity have yet to be clarified in the cardiomyocyte, studies from other cell types show accumulation of mitochondrial Akt in active form occurs within minutes after stimulation and persists for 45 min [41,42]. Interestingly, agonist treatments that induce Akt have differential effects on kinase activity within mitochondria, implying distinct mechanisms for directing Akt kinase action depending upon the inductive stimulus [42]. Activation of Akt signaling has also been implicated in the process of mitochondrial biogenesis [43]. As the role of Akt in protection of mitochondrial integrity has been reviewed in detail recently [44], this section will summarize basic principles of intrinsic cell death and focus upon recent advances regarding hexokinases and Pim-1 downstream of Akt in the context of the myocardium.
Intrinsic apoptotic cascades
Mitochondrial-dependent apoptosis, also referred to as programmed cell death, is activated in response to extracellular or intracellular insults. Upon undergoing activation of programmed cell death, mitochondria act as cellular ‘executioners’ by releasing pro-apoptotic molecules normally held within the intermembrane space, such as cytochrome-C, apoptosis-inducing factor, Smac/Diablo, HtrA2/Omi and endonuclease G. These activators of apoptotic cascades lead to cell death via multiple independent mechanisms once mitochondrial membrane integrity is breached, so a critical facet of inhibiting apoptosis is prevention of mitochondrial membrane permeabilization. The stability of mitochondrial membranes is dictated by cytosolic Bcl-2 proteins, which comprise a large family of both pro- and anti-apoptotic members that exist in a dynamic balance, and it is at this level of regulation that Akt acts to preserve mitochondrial integrity.
Two pro-apoptotic members of the Bcl-2 protein family are Bad and Bax. Bad promotes apoptosis by forming heterodimers with anti-apoptotic Bcl-2 or Bcl-XL proteins, thereby inhibiting their protective actions. In comparison, Bax undergoes a conformational shift that allows for insertion into mitochondrial membranes and oligomerization with cytochrome-C to promote membrane permeabilization. Akt antagonizes pro-apoptotic actions of these Bcl-2 family members by kinase activity, phosphorylating both Bad (Ser 136) [45] and Bax (Ser 184). Phosphorylation dissociates Bad from complexes with Bcl-2/Bcl-XL proteins and promotes association with 14-3-3 to sequester Bad in the cytosol, thereby negating interference of Bad with protective signaling. The consequence of Bax phosphorylation by Akt is to promote heterodimerization with Bcl-XL or Mcl-1 (a Bcl-2-related protein), thereby sequestering Bax away from mitochondrial membranes. Alternatively, Akt may directly interfere with molecules that promote conformational change of Bax, such as Bid or Bif-1. Through modulation of cytosolic Bcl-2 family members via phosphorylation, Akt directly affects the initiation of mitochondrial membrane permeabilization that leads to apoptosis, and multiple studies have attempted to elucidate the relationship between Akt activation, actions on Bcl-2 family members and inhibition of cardiomyopathic damage [23,46–50]. Akt has also been suggested to suppress activities of pro-apoptotic molecules released from compromised mitochondria, such as apoptosis-inducing factor and HtrA2/Omi, but these observations in nonmyocardial contexts will need further studies to validate their role in the heart. Additionally, mitochondrial integrity is impacted by effects of Akt activity via altered gene transcription of forkhead family members, Mdm2, NFkB, CREB and YAP. Thus, Akt controls a multifaceted array of downstream mediators that are directly or indirectly responsible for regulation mitochondrial integrity (Figure 1) [51].
Figure 1. Akt preserves mitochondrial integrity against stress at multiple levels.
Depicts targets of activated Akt that reside at the cell membrane, cytoplasm and mitochondria that mediate anti-apoptotic actions of the kinase. Actions of Akt can either be inhibitory, as evidenced by Bad, Bax or GSK-3β phosphorylation, or stimulatory, in the case of mitochondrial HK-II. Collectively, the actions of Akt serve to maintain mitochondrial integrity and antagonize the apoptotic cascade.
Reproduced from with permission from [103].
ER: Endoplasmic reticulum; GSK: Glycogen synthase kinase; HK: Hexokinase; IAP: Inhibitor of apoptosis; NCX: Sodium–calcium exchanger; NHE: Sodium–hydrogen exchanger; P: Phosphate group; PT: Permeability transition; SR: Sarcoplasmic reticulum.
Relationship of Akt to hexokinase
Hexokinase: targets of Akt at the mitochondria
The intertwined relationship linking Akt to preservation of mitochondria creates the mechanistic basis for a sensing mechanism to regulate cellular energy metabolism and survival. Consider that most stimuli for cellular growth and proliferation operate via Akt-associated signaling to promote energy utilization derived from mitochondrial function. As such, preservation of mitochondrial integrity is a synergistic consequence of Akt function that enhances growth and survival processes via kinase activity that consumes ATP to phosphorylate target molecules. Availability of energy substrates is critical for growth and survival, and Akt also has a dependence upon glucose in order to antagonize apoptotic signaling. Current speculation posits that metabolic functions of Akt preceded and eventually evolved into additional roles in preservation of cell survival as well, with glucose-dependent antiapoptotic signaling of Akt interfacing through phosphorylation of hexokinases to protect mitochondria [52–54]. Fueling this model of Akt/mitochondrial symbiosis, a strong correlation also exists for preservation of mitochondrial integrity by Akt action to promote localization and stabilization of mitochondrial hexokinase on the outer membrane [55,56]. Hexokinase plays a central role in glucose metabolism and has been shown to antagonize intrinsic cell death by interfering with pro-apoptotic Bax function and/or stabilizing the voltage-dependent anion channel [57]. Several lines of evidence support hexokinase as the facilitator of Akt-mediated protective signaling at the mitochondrial site:
Ectopic expression of hexokinase mimics the effects of Akt activation to inhibit apoptosis
Hexokinase also requires glucose for antiapoptotic actitivty
Association of hexokinase with mitochondria correlates with the ability of Akt to inhibit apoptosis
Association of hexokinase with mitochondria is impaired in Akt-deficient cells following growth factor stimulation
Glucose deprivation impairs both Akt anti-apoptotic activity as well as reducing hexokinase association with mitochondria
Targeted disruption of hexokinase–mitochondria interaction impairs the protective actions of Akt
In cardiomyocytes, Akt acts directly on mitochondria to phosphorylate hexokinase-II, resulting in protection from oxidant or calcium-stimulated permeability-transition pore opening [58]. In conclusion, although we are at the tip of the proverbial iceberg with regard to assessing this new facet of Akt-mediated signaling in the myocardium, the increasingly apparent codependence of Akt and mitochondria for mutual functional activity points to an inexorably linked partnership through hexokinase that may be the evolutionary interface designed to balance energy conditions, cell metabolism, growth and survival under stress.
Relationship of Akt to Pim-1 kinase
Pim-1 biology
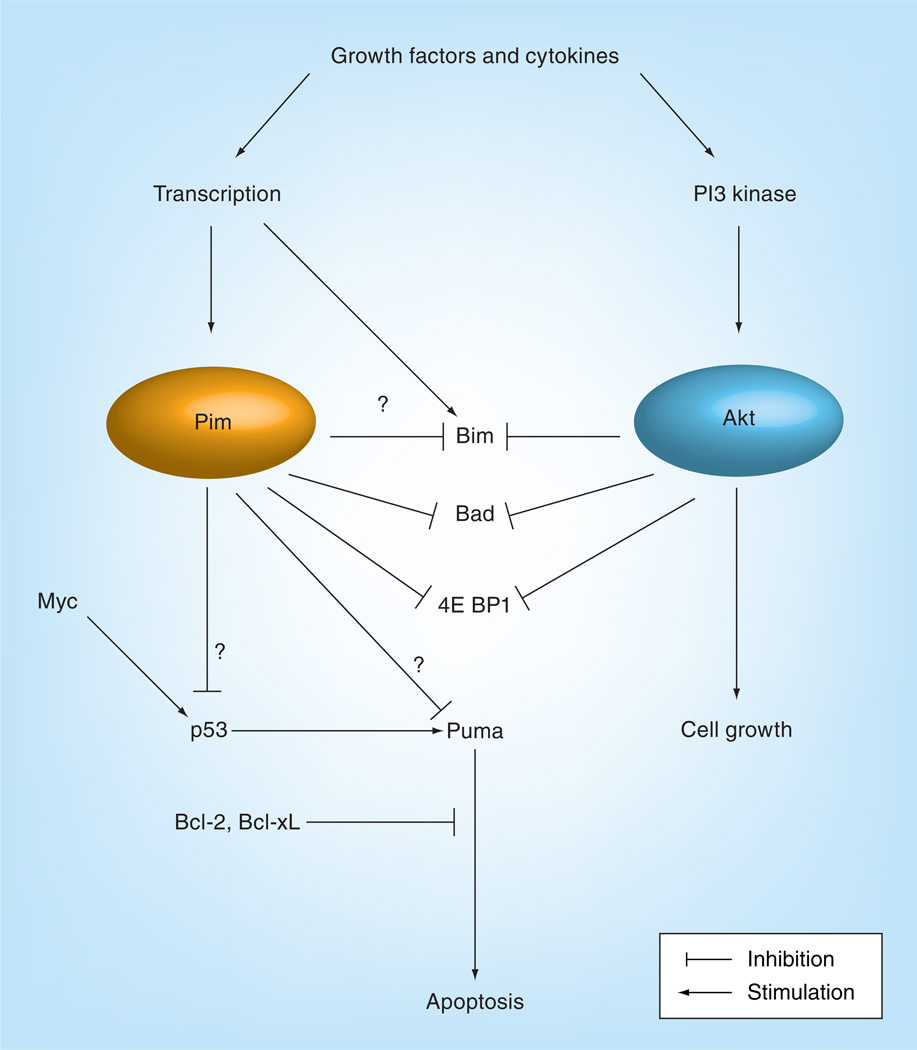
Prosurvival and proliferative effects of Akt activity in the myocardium are well documented [59–61]. However, recent evidence indicates that these actions previously ascribed to Akt are actually mediated by a downstream kinase called Pim-1, one of a three member family of serine/threonine kinases belonging to the calmodulin-dependent protein kinase-related group [62,63]. Similar to Akt in several respects, Pim-1 is also a serine/threonine kinase originally identified as a cellular oncogene that inhibits apoptosis and promotes proliferation (Figure 2) [64,65]. Pim-1 is expressed in various hematopoetic sites, including thymus, spleen, bone marrow and fetal liver, but can also be found in oral epithelia, prostate, hippocampus (in response to seizures), vascular smooth muscle (in response to injury) and many tumorigenic cell types [66–73]. In comparison, adult myocardium exhibits relatively low Pim-1 expression under normal conditions. Induction of Pim-1 is mediated through a variety of growth factors that can involve JAK/STAT pathway signaling with rapid accumulation of protein reminiscent of an early response gene [74–76]. Akt signaling has also been linked to induction of Pim-1 expression resulting from prolactin treatment [77].
Figure 2.
Although regulated by different intracellular cascades, the downstream substrate targets for Pim and Akt kinases intersect at regulators of apoptosis, influencing mitochondrial integrity.
Adapted with permission from [104].
Crosstalk between Akt & Pim-1
Similar to Akt, Pim-1 has many substrate targets that participate in gene transcription, cell cycle regulation, signal transduction and antagonizing apoptosis. For example, both Akt and Pim-1 both directly phosphorylate and inactivate Bad, a pro-apoptotic Bcl-2 family member [45,78,79]. Additional intersections exist at targets controlling the IκB/NF-κB transcription factor complex, regulation of protein synthesis via mTOR, and glycogen synthase kinase-3β phosphorylation [80]. Overlapping roles for Akt and Pim-1 as regulators of cellular proliferation and survival were found in studies of nontransformed hematopoetic stem cells [81]. Furthermore, the pharmacologic compound, LY294002, previously thought to specifically inhibit PI3K and subsequent Akt activation also directly inhibits Pim-1, suggesting that effects previously ascribed to block PI3K/Akt need to be reinterpreted for potential consequences of concurrent Pim-1 inhibition [82]. Collectively, these observations point to a close inter-relationship between Akt and Pim-1 in cellular signaling. However, mechanistically, there is a pivotal distinction between the two kinases: whereas Akt is activated by post-translational phosphorylation, Pim-1 is constitutively active and, therefore, must be controlled by protein turnover involving regulation at transcriptional, post-transcriptional, translational and post-translational levels. Thus, while Akt may be present but inactive, the only way to decrease Pim-1 activity is rapid turnover through proteosomal degradation [64].
Implications for myocardial biology
The link between Akt and Pim-1 in the myocardial context stems from observations that Akt accumulates in the nucleus following activation [83,84]. Last year, our group extended observations of Pim-1 expression to include the myocardium, where Pim-1 expression is found in cardiomyocytes of the postnatal heart and is downregulated within a few weeks after birth [85]. Induction of Pim-1 expression depends predominantly upon nuclear accumulation rather than cytoplasmic expression of Akt, indicating a specific subcellular role for Akt in modulation of Pim-1 activity. Induction of Pim-1 occurs after pathologic challenge to the adult heart, with accumulation and persistence of Pim-1 in surviving myocytes that border areas of infarction. Cardiacspecific expression of Pim-1 was highly protective in response to infarction challenge, whereas genetic deletion of Pim-1 rendered mice more susceptible to infarction damage despite significant compensatory increases in Akt expression and phosphorylation. These findings point to Pim-1 as a critical downstream participant in Akt-mediated cardioprotection, with implications for Pim-1 as a participant in survival, proliferative and reparative processes previously associated with Akt activity. Specifically with regard to mitochondrial protection, Pim-1 overexpression in cardiomyocytes elevates levels of Bcl-2 and Bcl-XL as well as increasing Bad phosphorylation under normal conditions [85] and after pathologic challenge in the heart [86], consistent with findings from noncardiac systems [64,81,87–89]. Recent studies in our laboratory have extended these findings in the myocardium of transgenic mice created with cardiac-specific overexpression of Pim-1 kinase that preserves mitochondrial integrity. Mitochondria isolated from hearts of Pim-1-overexpressing transgenic mice show protection from calcium-mediated mitochondrial swelling, t-bid-induced cytochrome-C release and peroxide-induced membrane depolarization [Sussman M, Unpublished Data]. Future studies expanding on the role of myocardial Pim-1 may lead to more focused avenues for intervening in cellular processes rather than Akt, since Pim-1 activity can be directly regulated by expression level and may not have the widespread and often deleterious impact of altered Akt signaling previously observed in the heart [59,90–95].
Expert commentary
Preservation of mitochondrial integrity and function remains a focal point of ongoing research, with the widely held belief that apoptosis can be mitigated by molecular interventional strategies. Cumulative evidence unquestionably points to the mitochondria as arbiters of the intrinsic cell death pathway influenced by a multitude of signaling cascades associated with cardioprotection. Therefore, it makes perfect sense to search for interventional approaches that serve to enhance mitochondrial resistance to pathological stress. Assuming we can identify a specific molecular target, signaling can be manipulated to focus on the target and, in so doing, preserve mitochondrial integrity. There is a litany of evidence to support a pivotal role for Akt kinase in preservation of cell survival from both cardiovascular and non-cardiovascular systems. Akt signaling has garnered so much attention because it is inherently complex and multifaceted, but this pervasive aspect of Akt influence makes it a difficult molecule to work with for therapeutic intervention. The consequences of Akt activation involve not just aspects of survival, but also proliferation, metabolism, protein synthesis and transcriptional regulation. Earlier efforts to exploit the prosurvival properties of Akt to enhance resistance to pathologic challenge using constitutively activated forms of the protein resulted in maladaptive remodeling, leading to hypertrophic or dilated cardiomyopathy and heart failure. Earlier this decade, my group embarked upon a series of studies based on the premise that location of Akt activity was a critical aspect of appropriate functional effects. Since Akt shows a regulated temporal and spatial distribution pursuant to activation, we postulated that nuclear accumulation of Akt would provide protective aspects of functional activity without the maladaptive consequences previously observed with membrane-targeted or phosphomimetic versions of the protein. Subsequent to those studies, we found that the cardioprotective effects of Akt depended on induction of another kinase: Pim-1 that is induced by nuclear Akt accumulation. This was an important finding because, unlike Akt, Pim-1 is constitutively active and appears to be regulated primarily by expression level. Moreover, although Pim-1 shares substrate specificity with Akt, Pim-1 effects appear to be directed primarily toward regulation of cell survival and proliferation. Since adult cardiomyocytes are notoriously difficult to provoke into cell cycle re-entry, Pim-1 is an attractive candidate for regulation of cell survival. This is especially true with respect to enhancement of mitochondrial integrity as Pim-1 antagonizes pro-apoptotic signaling mediated by the intrinsic cell death cascade. Furthermore, recent studies in our laboratory have found that Pim-1 enhances mitochondrial resistance to calciuminduced swelling and inhibits cytochrome-C release in response to apoptotic stimuli [Sussman M, Unpublished Data].
Along with understanding the kinase signaling responsible for cellular survival, we must tease out molecular targets of these kinases and how they influence mitochondrial integrity. The relationship of survival kinases to the Bcl-2 family members and the associated release of mitochondrial death mediators has been investigated extensively, but relatively little is known regarding the actions of kinase signaling at the surface or within the mitochondria within cardiomyocytes. In this context, although the role of hexokinase signaling and Akt-mediated regulation has been described in noncardiac systems, this relationship is only now being examined in cardiomyocytes. Hexokinase performs a critical role in the initial step of metabolizing glucose by phosphorylation, which leads to energy production. Although association of hexokinase with mitochondria is influenced by multiple factors in the cytoplasm, it appears that the protective effect of Akt depends, at least in part, on promoting interaction between hexokinase and mitochondria [57]. Interestingly, Akt is positioned at the crossroads of cell metabolism and survival under stress, with downstream effector molecules such as hexokinase serving to regulate mitochondrial function and integrity (Figure 3). Presumably, additional regulatory interactions remain to be discovered between Akt and intermediate mediators of mitochondrial activity, ultimately leading to consequences for mitochondrial structure and function extending beyond our current horizons of energy production, mitochondrial integrity and sensitivity to apoptotic signaling.
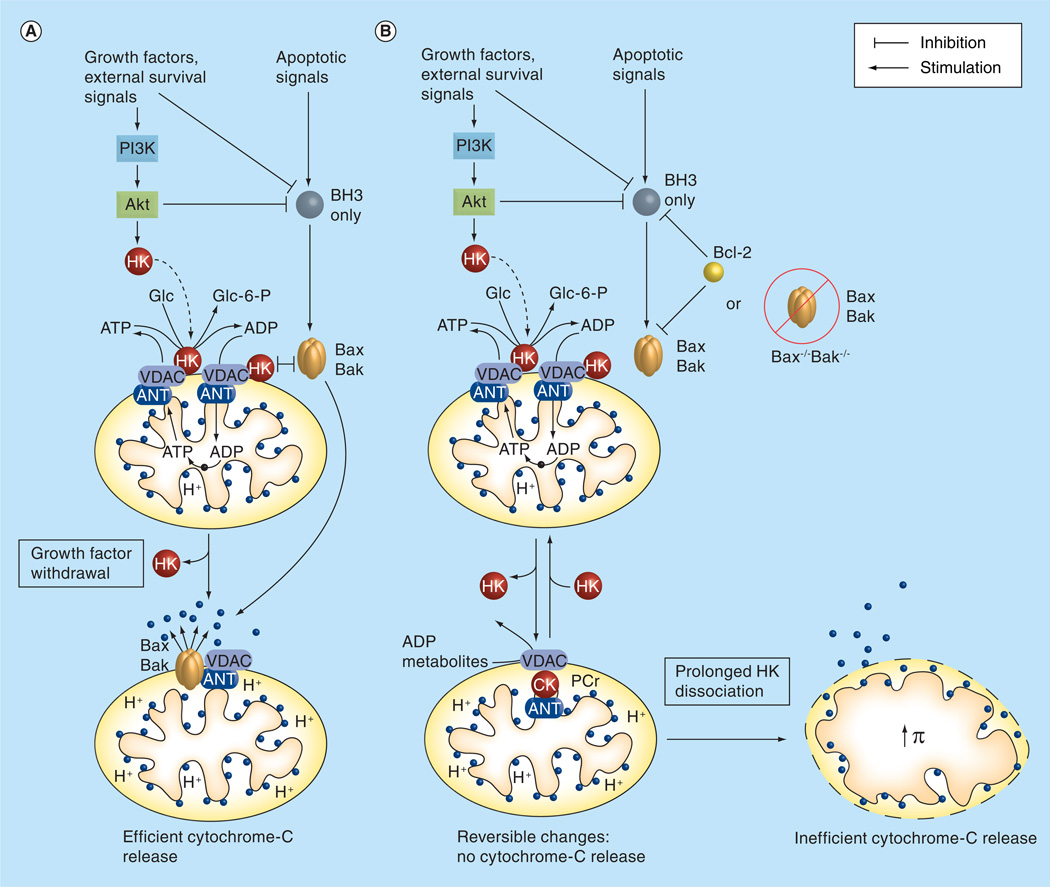
Figure 3. Mitochondrial HKs act at the nexus between survival and apoptotic signals competing to modulate mitochondrial integrity and cytochrome-C release.
(A) Activation of Akt by growth factors and PI3K leads to increased HK association with mitochondria. Activation of Bcl-2 homology 3-only members of the Bcl-2 protein family directly or indirectly activate Bax and/or Bak at the level of the mitochondria. HK and Bax/Bak may compete for mitochondrial association, with Bax/Bak displacement of HK leading to disrupted mitochondrial function and facilitated cytochrome-C release. Reduced Akt activity following growth factor withdrawal probably facilitates Bax/Bak-mediated cytochrome-C release by decreasing HK association with the mitochondria. (B) In the absence of functional Bax and Bak, as in cells nullizygous for Bax and Bax (Bax−/−, Bak−/−) or in cells overexpressing Bcl-2, prolonged HK dissociation by targeted disruption or a superimposed apoptotic stimulus leads to swelling of the mitochondrial matrix, outer mitochondrial membrane rupture, and ultimately cytochrome-C release, albeit with less efficiency and with delayed kinetics.
ANT: Adenine nucleotide translocase; CK: Creatine kinase; HK: Hexokinase; PCr: Phosphocreatine; VDAC: Voltage-dependent anion channel.
Reproduced with permission from [53].
Numerous studies document the targeting of certain components of signaling cascades specifically to mitochondria with subsequent effects on mitochondrial function that ultimately impact upon cellular biology. For example, components of the respiratory chain are subject to regulation by phosphorylation. The dynamic interplay of communication is probably bidirectional as well, since reactive oxygen species generated by mitochondria have profound influence on cellular signaling cascades and kinase activity. The use of mitochondrial targeting sequences is revealing a more refined perspective of kinase signaling than generic cytoplasmically expressed constructs. Presumably, these studies, together with loss-of-function experiments mediated by siRNA or genetic engineering approaches, represent the next wave of advances in dissection of mitochondrial signaling biology.
All of the sophisticated molecular biology and elegant experimental design will be fascinating for revealing the mysteries of mitochondrial biology, but from a therapeutic perspective it will all be for nothing if appropriate interventional targets are not identified and exploited by artificial manipulation. This can either be accomplished by pharmacologic or genetic engineering mechanisms but in either event the regulation of expression and cardiac-specific delivery will continue to challenge researchers as they strive for ever more precise tools to interfere with pathological processes occurring within cardiomyocytes. The tools to deliver the intracellular ‘silver bullet’ providing resistance to pathologic injury continues to be an area of intense and active investigation.
Five-year view
Assuming the past as a prolog, our understanding of mitochondrial survival signaling will only continue to expand the layers of complexity. By drilling down further into the downstream targets of signaling, we pinpoint specific molecules interacting directly on the surface or within the mitoplasm. The issue of where and when these kinases act upon mitochondria will become increasingly important. The field of signal transduction must begin to incorporate aspects of subcellular compartmentalization and temporal localization into assessments of kinase activities. Physiological stimuli often act at low levels and exhibit rapid-pulsed responses with subsequent decay. Such phenomena are challenging to study, let alone publish, as the community has become accustomed to profound changes and dramatic inductions resulting from overexpression or knockout approaches. However, it is important to be mindful that the cell operates within a balance of maintained homeostasis and perturbations of the system with nonphysiologic approaches may provide insights that are important but not necessarily relevant for understanding mitochondrial regulation. That being said, a targeted and focused approach to manipulating mitochondrial biology to enhance integrity in the face of pathological challenge for a regulated and sustained burst during times of stress is an attractive conceptual model around which to design therapeutic interventional strategies. Inducible promoters or those regulated by external stimuli, such as hypoxia, could be employed to gain more control over how intensely and how long the exogenously introduced gene is switched on.
Beyond the direct phosphorylation status of target proteins in the mitochondrion, we must become more adept at understanding the dynamics of mitochondrial behavior. An evolving aspect of this story are the issues of biogenesis, fission and fusion, which are gaining increasing attention from multiple aspects of mitochondrial function as well as disease pathogenesis [96–99]. There is evidence to support that regulation of fission and fusion plays an important role in maintaining mitochondrial function and regulation of cell survival. Therefore, kinase-mediated signaling is likely to emerge as a critical facet of the cellular machinery that controls the process of fission and fusion. Similar questions of location, timing and regulation by kinase signaling are also likely to apply in another emerging field of study: mitophagy [100–102]. This cannibalization aspect of autophagic processes is likely to be a normal homeostatic process for ensuring quality control in the mitochondrial population and removing malfunctioning performers from the cytosol before they become problematic. As might be anticipated, mitophagy is being implicated in regulation of cell survival and disease processes. In both mitochondrial dynamics as well as mitophagy, the issue is not simply the process per se but the rate and flux of the mitochondria as they move through the system. Current approaches too often rely on ‘snapshots’ of cell behavior that may provide a glimpse into regulatory mechanisms but may miss critical issues of rate and timing. Owing to the dynamic nature of mitochondrial regulation, we will be challenged to come up with intracellular reporter constructs that can be assessed and tracked in real-time imaging of live cells. As imaging and biological manipulation come together, we are likely to uncover a whole new world of insights and questions regarding the multilayered complexity characteristic of mitochondria, their behavior and how kinase signaling influences the preservation of mitochondrial integrity.
Key issues.
Mitochondria are central regulators of cell survival.
Kinase signaling has important consequences for preserving mitochondrial function.
Mitochondrial integrity can be enhanced by both direct and indirect effects of survival kinase signaling.
Akt kinase is a nodal regulator of cell survival that exerts multiple effects to preserve mitochondrial integrity, including enhancing anti-apoptotic proteins in the cytoplasm as well as acting directly in the mitochondrial subcellular compartment.
Hexokinase is tied to Akt signaling by multiple lines of evidence and plays an essential role in Akt-mediated protection.
Nuclear accumulation of Akt in cardiomyocytes induces expression of Pim-1 kinase that antagonizes several facets of apoptotic signaling, leading to enhanced preservation of mitochondrial function in the wake of pathologic challenge.
Manipulation of survival-signaling targets downstream of Akt may provide an entry point for therapeutic intervention to preserve mitochondrial integrity without the maladaptive consequences that have plagued efforts to use Akt as a cardioprotective strategy.
Future studies will need to factor in subcellular localization, timing and the dynamics of mitochondrial biology related to fission, fusion and mitophagy.
Acknowledgment
Mark A Sussman is the recipient of research funding from the National Heart, Lung, and Blood Institute and the National Aging Institute divisions of the National Institutes of Health, award numbers HL067245, HL085577, HL091102 and AG023071.
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
•• of considerable interest
- 1. Nakayama H, Chen X, Baines CP, et al. Ca2+ - and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J. Clin. Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. Papers of special note have been highlighted as: of considerable interest.
- 2.Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol. Biochem. 2007;20:1–22. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- 3.Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J. Biol. Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- 4.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu. Rev. Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 5.Das DK, Maulik N. Mitochondrial function in cardiomyocytes: target for cardioprotection. Curr. Opin. Anaesthesiol. 2005;18:77–82. doi: 10.1097/00001503-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Mott JL, Chang SW, Stevens M, Mikolajczak P, Zassenhaus HP. Mitochondrial DNA mutations activate programmed cell survival in the mouse heart. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2476–H2483. doi: 10.1152/ajpheart.00670.2004. [DOI] [PubMed] [Google Scholar]
- 7.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ. Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 8.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia–reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc. Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 9.McFalls EO, Liem D, Schoonderwoerd K, Lamers J, Sluiter W, Duncker D. Mitochondrial function: the heart of myocardial preservation. J. Lab. Clin. Med. 2003;142:141–148. doi: 10.1016/S0022-2143(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 10.Adams JW, Pagel AL, Means CK, Oksenberg D, Armstrong RC, Brown JH. Cardiomyocyte apoptosis induced by Gαq signaling is mediated by permeability transition pore formation and activation of the mitochondrial death pathway. Circ. Res. 2000;87:1180–1187. doi: 10.1161/01.res.87.12.1180. [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc. Res. 2008;77:334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 12.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic. Biol. Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc. Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Kowalczyk JE, Zablocka B. [Protein kinases in mitochondria] Postepy Biochem. 2008;54:209–216. [PubMed] [Google Scholar]
- 15.Li Y, Sato T. Dual signaling via protein kinase C and phosphatidylinositol 3′-kinase/Akt contributes to bradykinin B2 receptorinduced cardioprotection in guinea pig hearts. J. Mol. Cell. Cardiol. 2001;33:2047–2053. doi: 10.1006/jmcc.2001.1455. [DOI] [PubMed] [Google Scholar]
- 16.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cδ activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J. Biol. Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 17.Ohori K, Miura T, Tanno M, et al. Ser9 phosphorylation of mitochondrial GSK-3β is a primary mechanism of cardiomyocyte protection by erythropoietin against oxidant-induced apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2079–H2086. doi: 10.1152/ajpheart.00092.2008. [DOI] [PubMed] [Google Scholar]
- 18.Park SS, Zhao H, Mueller RA, Xu Z. Bradykinin prevents reperfusion injury by targeting mitochondrial permeability transition pore through glycogen synthase kinase 3β. J. Mol. Cell. Cardiol. 2006;40:708–716. doi: 10.1016/j.yjmcc.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MV, Philipp S, Krieg T, et al. Preconditioning-mimetics bradykinin and DADLE activate PI3-kinase through divergent pathways. J. Mol. Cell. Cardiol. 2007;42:842–851. doi: 10.1016/j.yjmcc.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das M, Gherghiceanu M, Lekli I, Mukherjee S, Popescu LM, Das DK. Essential role of lipid raft in ischemic preconditioning. Cell Physiol. Biochem. 2008;21:325–334. doi: 10.1159/000129391. [DOI] [PubMed] [Google Scholar]
- 21.Downey JM, Krieg T, Cohen MV. Mapping preconditioning’s signaling pathways: an engineering approach. Ann. NY Acad. Sci. 2008;1123:187–196. doi: 10.1196/annals.1420.022. [DOI] [PubMed] [Google Scholar]
- 22.Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim. Biophys. Acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchiyama T, Engelman RM, Maulik N, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109:3042–3049. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Park TS, Gidday JM. Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2573–H2581. doi: 10.1152/ajpheart.01098.2006. [DOI] [PubMed] [Google Scholar]
- 25.Smart N, Mojet MH, Latchman DS, Marber MS, Duchen MR, Heads RJ. IL-6 induces PI 3-kinase and nitric oxide-dependent protection and preserves mitochondrial function in cardiomyocytes. Cardiovasc. Res. 2006;69:164–177. doi: 10.1016/j.cardiores.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br. J. Pharmacol. 2006;149:5–13. doi: 10.1038/sj.bjp.0706834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai HC, Liu TJ, Ting CT, Sharma PM, Wang PH. Insulin-like growth factor-1 prevents loss of electrochemical gradient in cardiac muscle mitochondria via activation of PI 3 kinase/Akt pathway. Mol. Cell. Endocrinol. 2003;205:99–106. doi: 10.1016/s0303-7207(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 28.Satoh M, Matter CM, Ogita H, et al. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation. 2007;115:3197–3204. doi: 10.1161/CIRCULATIONAHA.106.657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tissier R, Waintraub X, Couvreur N, et al. Pharmacological postconditioning with the phytoestrogen genistein. J. Mol. Cell. Cardiol. 2007;42:79–87. doi: 10.1016/j.yjmcc.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Lai HC, Liu TJ, Ting CT, et al. Regulation of IGF-I receptor signaling in diabetic cardiac muscle: dysregulation of cytosolic and mitochondria HSP60. Am. J. Physiol. Endocrinol. Metab. 2007;292:E292–E297. doi: 10.1152/ajpendo.00189.2006. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad N, Wang Y, Haider KH, et al. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2402–H2408. doi: 10.1152/ajpheart.00737.2005. [DOI] [PubMed] [Google Scholar]
- 32.Davidson SM, Hausenloy D, Duchen MR, Yellon DM. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int. J. Biochem. Cell Biol. 2006;38:414–419. doi: 10.1016/j.biocel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Juhaszova M, Zorov DB, Kim SH, et al. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhamra GS, Hausenloy DJ, Davidson SM, et al. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res. Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi H, Miura T, Ishida H, et al. Limitation of infarct size by erythropoietin is associated with translocation of Akt to the mitochondria after reperfusion. Clin. Exp. Pharmacol. Physiol. 2008;35:812–819. doi: 10.1111/j.1440-1681.2008.04925.x. [DOI] [PubMed] [Google Scholar]
- 36.Nagy N, Malik G, Tosaki A, Ho YS, Maulik N, Das DK. Overexpression of glutaredoxin-2 reduces myocardial cell death by preventing both apoptosis and necrosis. J. Mol. Cell. Cardiol. 2008;44:252–260. doi: 10.1016/j.yjmcc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Hui ST, Andres AM, Miller AK, et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc. Natl Acad. Sci. USA. 2008;105:3921–3926. doi: 10.1073/pnas.0800293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philipp S, Critz SD, Cui L, Solodushko V, Cohen MV, Downey JM. Localizing extracellular signal-regulated kinase (ERK) in pharmacological preconditioning’s trigger pathway. Basic Res. Cardiol. 2006;101:159–167. doi: 10.1007/s00395-005-0566-z. [DOI] [PubMed] [Google Scholar]
- 39.Sahach VF, Korkach Iu P, Kotsiuruba AV, Rudyk OV, Vavilova HL. [Mitochondrial permeability transition pore opening inhibition by ecdysterone in heart mitochondria of aging rats] Fiziol. Zh. 2008;54:3–10. [PubMed] [Google Scholar]
- 40.Xu Z, Park SS, Mueller RA, Bagnell RC, Patterson C, Boysen PG. Adenosine produces nitric oxide and prevents mitochondrial oxidant damage in rat cardiomyocytes. Cardiovasc. Res. 2005;65:803–812. doi: 10.1016/j.cardiores.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasaki K, Sato M, Umezawa Y. Fluorescent indicators for Akt/protein kinase B and dynamics of Akt activity visualized in living cells. J. Biol. Chem. 2003;278:30945–30951. doi: 10.1074/jbc.M212167200. [DOI] [PubMed] [Google Scholar]
- 43.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ. Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parcellier A, Tintignac LA, Zhuravleva E, Hemmings BA. PKB and the mitochondria: AKTing on apoptosis. Cell Signal. 2008;20:21–30. doi: 10.1016/j.cellsig.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 46.Pastukh V, Ricci C, Solodushko V, Mozaffari M, Schaffer SW. Contribution of the PI 3-kinase/Akt survival pathway toward osmotic preconditioning. Mol. Cell. Biochem. 2005;269:59–67. doi: 10.1007/s11010-005-2536-z. [DOI] [PubMed] [Google Scholar]
- 47.Kato K, Yin H, Agata J, Yoshida H, Chao L, Chao J. Adrenomedullin gene delivery attenuates myocardial infarction and apoptosis after ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1506–H1514. doi: 10.1152/ajpheart.00270.2003. [DOI] [PubMed] [Google Scholar]
- 48.Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ. Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 49.Negoro S, Oh H, Tone E, et al. Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/caspase-3 interaction. Circulation. 2001;103:555–561. doi: 10.1161/01.cir.103.4.555. [DOI] [PubMed] [Google Scholar]
- 50.Kuwahara K, Saito Y, Kishimoto I, et al. Cardiotrophin-1 phosphorylates akt and BAD prolongs cell survival via a PI3K-dependent pathway in cardiac myocytes. J. Mol. Cell. Cardiol. 2000;32:1385–1394. doi: 10.1006/jmcc.2000.1177. [DOI] [PubMed] [Google Scholar]
- 51.Le Bras M, Rouy I, Brenner C. The modulation of inter-organelle cross-talk to control apoptosis. Med. Chem. 2006;2:1–12. doi: 10.2174/157340606775197787. [DOI] [PubMed] [Google Scholar]
- 52.Robey RB, Hay N. Mitochondrial hexokinases: guardians of the mitochondria. Cell Cycle. 2005;4:654–658. doi: 10.4161/cc.4.5.1678. [DOI] [PubMed] [Google Scholar]
- 53. Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. Excellent review of the interaction between Akt and hexokinase.
- 54.Birnbaum MJ. On the InterAktion between hexokinase and the mitochondrion. Dev. Cell. 2004;7:781–782. doi: 10.1016/j.devcel.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 55.Majewski N, Nogueira V, Bhaskar P, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Majewski N, Nogueira V, Robey RB, Hay N. Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol. Cell. Biol. 2004;24:730–740. doi: 10.1128/MCB.24.2.730-740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15(3):521–529. doi: 10.1038/sj.cdd.4402285. Demonstrates the role of hexokinase in cardiomyocyte protective signaling downstream of Akt.
- 59.Walsh K. Akt signaling and growth of the heart. Circulation. 2006;113:2032–2034. doi: 10.1161/CIRCULATIONAHA.106.615138. [DOI] [PubMed] [Google Scholar]
- 60.Shiraishi I, Melendez J, Ahn Y, et al. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ. Res. 2004;94:884–891. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- 61.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia–reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bullock AN, Debreczeni J, Amos AL, Knapp S, Turk BE. Structure and substrate specificity of the Pim-1 kinase. J. Biol. Chem. 2005;280:41675–41682. doi: 10.1074/jbc.M510711200. [DOI] [PubMed] [Google Scholar]
- 63.Hoover D, Friedmann M, Reeves R, Magnuson NS. Recombinant human Pim-1 protein exhibits serine/threonine kinase activity. J. Biol. Chem. 1991;266:14018–14023. [PubMed] [Google Scholar]
- 64.Wang Z, Bhattacharya N, Weaver M, et al. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. J. Vet. Sci. 2001;2:167–179. [PubMed] [Google Scholar]
- 65.Bachmann M, Moroy T. The serine/threonine kinase Pim-1. Int. J. Biochem. Cell Biol. 2005;37:726–730. doi: 10.1016/j.biocel.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 66.Mally MI, Vogt M, Swift SE, Haas M. Oncogene expression in murine splenic T cells and in murine T-cell neoplasms. Virology. 1985;144:115–126. doi: 10.1016/0042-6822(85)90310-1. [DOI] [PubMed] [Google Scholar]
- 67.Nagarajan L, Louie E, Tsujimoto Y, ar-Rushdi A, Huebner K, Croce CM. Localization of the human Pim oncogene (PIM) to a region of chromosome 6 involved in translocations in acute leukemias. Proc. Natl Acad. Sci. USA. 1986;83:2556–2560. doi: 10.1073/pnas.83.8.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meeker TC, Nagarajan L, ar-Rushdi A, Rovera G, Huebner K, Croce CM. Characterization of the human PIM-1 gene: a putative proto-oncogene coding for a tissue specific member of the protein kinase family. Oncogene Res. 1987;1:87–101. [PubMed] [Google Scholar]
- 69.Stewart BE, Rice RH. Differentiation-associated expression of the proto-oncogene Pim-1 in cultured human keratinocytes. J. Invest. Dermatol. 1995;105:699–703. doi: 10.1111/1523-1747.ep12324482. [DOI] [PubMed] [Google Scholar]
- 70.Liang H, Hittelman W, Nagarajan L. Ubiquitous expression and cell cycle regulation of the protein kinase PIM-1. Arch. Biochem. Biophys. 1996;330:259–265. doi: 10.1006/abbi.1996.0251. [DOI] [PubMed] [Google Scholar]
- 71.Leduc I, Karsunky H, Mathieu N, et al. The Pim-1 kinase stimulates maturation of TCRβ-deficient T cell progenitors: implications for the mechanism of Pim-1 action. Int. Immunol. 2000;12:1389–1396. doi: 10.1093/intimm/12.10.1389. [DOI] [PubMed] [Google Scholar]
- 72.Ellwood-Yen K, Graeber TG, Wongvipat J, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 73.Katakami N, Kaneto H, Hao H, et al. Role of Pim-1 in smooth muscle cell proliferation. J. Biol. Chem. 2004;279:54742–54749. doi: 10.1074/jbc.M409140200. [DOI] [PubMed] [Google Scholar]
- 74.Matikainen S, Sareneva T, Ronni T, Lehtonen A, Koskinen PJ, Julkunen I. Interferon-α activates multiple STAT proteins and upregulates proliferation-associated IL-2Rα, c-myc, and Pim-1 genes in human T cells. Blood. 1999;93:1980–1991. [PubMed] [Google Scholar]
- 75.Paukku K, Silvennoinen O. STATs as critical mediators of signal transduction and transcription: lessons learned from STAT5. Cytokine Growth Factor Rev. 2004;15:435–455. doi: 10.1016/j.cytogfr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Stout BA, Bates ME, Liu LY, Farrington NN, Bertics PJ. IL-5 and granulocyte-macrophage colony-stimulating factor activate STAT3 and STAT5 and promote Pim-1 and cyclin D3 protein expression in human eosinophils. J. Immunol. 2004;173:6409–6417. doi: 10.4049/jimmunol.173.10.6409. [DOI] [PubMed] [Google Scholar]
- 77.Krishnan N, Pan H, Buckley DJ, Buckley A. Prolactin-regulated Pim-1 transcription: identification of critical promoter elements and Akt signaling. Endocrine. 2003;20:123–130. doi: 10.1385/endo:20:1-2:123. [DOI] [PubMed] [Google Scholar]
- 78.Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 79.Yan B, Zemskova M, Holder S, et al. The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J. Biol. Chem. 2003;278:45358–45367. doi: 10.1074/jbc.M307933200. [DOI] [PubMed] [Google Scholar]
- 80.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J. Clin. Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hammerman PS, Fox CJ, Birnbaum MJ, Thompson CB. Pim and Akt oncogenes are independent regulators of hematopoietic cell growth and survival. Blood. 2005;105:4477–4483. doi: 10.1182/blood-2004-09-3706. Defines the synergistic relationship between Akt and Pim-1 signaling in the hematopoetic context.
- 82.Jacobs MD, Black J, Futer O, et al. Pim-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002. J. Biol. Chem. 2005;280:13728–13734. doi: 10.1074/jbc.M413155200. [DOI] [PubMed] [Google Scholar]
- 83.Kunkel MT, Ni Q, Tsien RY, Zhang J, Newton AC. Spatio–temporal dynamics of protein kinase B/Akt signaling revealed by a genetically encoded fluorescent reporter. J. Biol. Chem. 2005;280:5581–5587. doi: 10.1074/jbc.M411534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camper-Kirby D, Welch S, Walker A, et al. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ. Res. 2001;88:1020–1027. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 85. Muraski JA, Rota M, Misao Y, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat. Med. 2007;13:1467–1475. doi: 10.1038/nm1671. Demonstrates that the cardioprotective effect of Akt kinase depends upon the induction of Pim-1.
- 86.Muraski JA, Fischer KM, Wu W, et al. Pim-1 kinase antagonizes aspects of myocardial hypertrophy and compensation to pathological pressure overload. Proc. Natl Acad. Sci. USA. 2008;105:13889–13894. doi: 10.1073/pnas.0709135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841–1854. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim KT, Levis M, Small D. Constitutively activated FLT3 phosphorylates BAD partially through Pim-1. Br. J. Haematol. 2006;134:500–509. doi: 10.1111/j.1365-2141.2006.06225.x. [DOI] [PubMed] [Google Scholar]
- 89. Lilly M, Sandholm J, Cooper JJ, Koskinen PJ, Kraft A. The PIM-1 serine kinase prolongs survival and inhibits apoptosis-related mitochondrial dysfunction in part through a bcl-2-dependent pathway. Oncogene. 1999;18:4022–4031. doi: 10.1038/sj.onc.1202741. Details the anti-apoptotic effect of Pim-1 kinase via antagonizing intrinsic apoptotic cascade signaling.
- 90.Condorelli G, Drusco A, Stassi G, et al. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc. Natl Acad. Sci. USA. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsui T, Li L, Wu JC, Cook SA, et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J. Biol. Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 92.Matsui T, Nagoshi T, Hong EG, et al. Effects of chronic Akt activation on glucose uptake in the heart. Am. J. Physiol. Endocrinol. Metab. 2006;290:E789–E797. doi: 10.1152/ajpendo.00564.2004. [DOI] [PubMed] [Google Scholar]
- 93.Nagoshi T, Matsui T, Aoyama T, et al. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J. Clin. Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shiojima I, Sato K, Izumiya Y, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shioi T, McMullen JR, Kang PM, et al. Akt/protein kinase B promotes organ growth in transgenic mice. Mol. Cell. Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grandemange S, Herzig S, Martinou JC. Mitochondrial dynamics and cancer. Semin. Cancer Biol. 2009;19(1):50–56. doi: 10.1016/j.semcancer.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 97.Herzig S, Martinou JC. Mitochondrial dynamics: to be in good shape to survive. Curr. Mol. Med. 2008;8:131–137. doi: 10.2174/156652408783769625. [DOI] [PubMed] [Google Scholar]
- 98.Westermann B. Molecular machinery of mitochondrial fusion and fission. J. Biol. Chem. 2008;283:13501–13505. doi: 10.1074/jbc.R800011200. [DOI] [PubMed] [Google Scholar]
- 99.Berman SB, Pineda FJ, Hardwick JM. Mitochondrial fission and fusion dynamics: the long and short of it. Cell Death Differ. 2008;15:1147–1152. doi: 10.1038/cdd.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Devenish RJ. Mitophagy: growing in intricacy. Autophagy. 2007;3:293–294. doi: 10.4161/auto.4273. [DOI] [PubMed] [Google Scholar]
- 101.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 102.Mijaljica D, Prescott M, Devenish RJ. Different fates of mitochondria: alternative ways for degradation? Autophagy. 2007;3:4–9. doi: 10.4161/auto.3011. [DOI] [PubMed] [Google Scholar]
- 103.Miyamoto S, Rubio M, Sussman MA. Nuclear and mitochondrial signalling Akts in cardiomyocytes. Cardiovasc. Res. 2009;82(2):272–285. doi: 10.1093/cvr/cvp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.White E. The Pims and outs of survival signaling: role for the Pim-2 protein kinase in the suppression of apoptosis by cytokines. Genes Dev. 2003;17:1813–1816. doi: 10.1101/gad.1123103. [DOI] [PubMed] [Google Scholar]