1. Introduction
The most pressing need in cancer is to design effective treatments that minimize damage to normal tissue especially against metastastic lesions that become refractory to treatment. Molecular imaging in cancer can drive both discovery and optimization of treatment. It can be applied to identify targets specific to cancer with imaging, design agents against targets and visualize their delivery, monitor response to treatment, and minimize collateral damage to normal tissue. Theranostic imaging, where diagnosis is combined with therapy, is a rapidly growing application of cancer molecular imaging. It is particularly suitable for a disease that is as complex as cancer, especially since genomic and proteomic profiling can provide an extensive ‘fingerprint’ of each tumor. Using this information, theranostic agents can be designed to personalize treatment, and minimize damage to normal tissue. Some of the challenges in theranostic imaging are quantitative image analyses, cost of synthesizing theranostic agents, solving immunogenicity problems associated with these agents, challenges with cGMP synthesis, obtaining FDA/IRB approval, and the costs of clinical trials. Despite these challenges, the exciting opportunities in theranostic imaging that are occurring at the interface of chemistry, molecular biology, and imaging provide tangible advances in finding effective treatments against cancer.
2. Challenges in cancer and cancer-specific targets
While several effective therapies including surgery are available for primary cancers, there is a compelling need to find effective treatments for metastatic disease, as it typically becomes refractory to treatment. In the United States, for example, breast cancer alone claims approximately 40,000 lives annually. Approximately 3–6% of women presenting with breast cancer have metastatic disease and a further 50–70% of patients with localized breast cancer will suffer a systemic relapse [1]. Patients with untreated metastatic breast cancer (MBC) have a median survival of 9–12 months, and even with treatment, less than 2% of patients with MBC have a five year survival rate [2]. Similarly, in the US alone, prostate cancer (PCa) claims 30,000 lives annually [3]. Half of patients with advanced disease have local recurrence while the other half demonstrate metastases, with or without local recurrence. Metastatic lesions are not amenable to surgery since the development of metastases in distant secondary organs is widespread, preventing surgery with wide margins. In addition, metastatic lesions demonstrate increased resistance to conventional treatment, typifying the ability of cancer cells to adapt and survive.
Theranostic imaging requires the delivery of therapeutic cargo to cancer-specific targets that can be noninvasively imaged. Receptors and antigens expressed specifically by cancer cells provide the most straightforward targets and are being exploited for noninvasive imaging (Fig. 1). For example, approximately 20–30% of breast cancers express the human epidermal growth factor receptor (Her-2), and the use of Trastuzumab, a humanized monoclonal antibody directed against the extracellular domain of Her-2 has provided a breakthrough in the treatment of Her-2 positive MBC [4]. Patients with Her-2/neu overexpressing cancers respond well to antibody targeting of Her-2/neu with Trastuzumab or with the dual tyrosine kinase inhibitor lapatinib that inhibits EGFR/ErbB1 and Her-2/ErbB2, but a large percentage develop resistance to Her-2/neu targeted therapies [5]. Prostate-specific membrane antigen (PSMA) is a type II integral membrane protein that has abundant expression on the surface of prostate carcinomas, particularly in androgen-independent, advanced and metastatic disease [6,7]. PSMA possesses the criteria of an ideal target for immunotherapy, i.e., expression primarily restricted to the prostate, abundantly expressed as protein at all stages of the disease, presented at the cell surface but not shed into the circulation, and association with enzymatic or signaling activity [6,8].
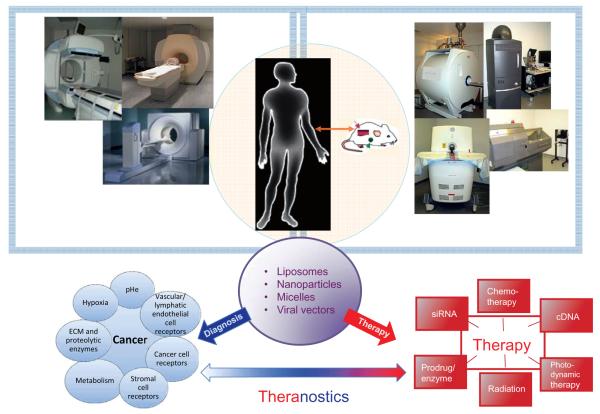
Fig. 1.
Schematic outlining the basis and the components of theranostic imaging in cancer. Several noninvasive imaging modalities such as MRI/S, PET, CT/SPECT and optical imaging available for preclinical imaging (right) are easily translated to the clinic (left) and can be incorporated for theranostic imaging. The delivery of carriers (purple circle) decorated with imaging reporters and therapeutic cargo (red) to cancer-specific targets (blue) can be visualized for theranostic imaging.
Most tumors, however, do not express cancer cell specific receptors, and there is an increasing need to mine other sources such as metabolism, angiogenesis, inflammation, the tumor microenvironment (TME), and stromal cell receptors for tumor specific delivery for image-guided targeted molecular medicine (Fig. 1). Indeed aberrant glucose and choline metabolism, as well as angiogenesis, are continuing to provide diagnostic tools for detecting cancer [9]. Physiological environments in tumors are characterized by hypoxia, acidic extracellular pH, and substrate deprivation. The infrastructure that surrounds and supports cancer cells is termed the TME and broadly covers the extracellular matrix (ECM), cancer associated fibroblasts (CAFs), adipocytes, pericytes, multiple immune cells such as tumor associated macrophages (TAMs), and vascular and lymphatic endothelial cells. The TME is being increasingly exploited for theranostic imaging [10].
3. Carriers and treatments
As shown in the schematic in Fig. 1, several imaging modalities with a bench to bedside span such as magnetic resonance imaging/spectroscopy (MRI/S), positron emission tomography (PET), single photon emission computerized tomography (SPECT), as well as optical imaging, that is increasingly being explored for intra-operative imaging, are available for theranostic imaging. Multiple innovative nanoplatforms are available to combine diagnostic imaging with therapy (Fig. 1). These are chosen based on the imaging modality, the therapeutic cargo and the target. Typically, these nanoplatforms are liposomes, nanoparticles, micelles and viral vectors that are decorated with imaging reporters and deliver conventional therapy or molecularly targeted medicine such as complementary DNA (cDNA) or small interfering RNA (siRNA). There is an increasing trend to combine imaging modalities and, as a result, the nanoplatforms are decorated with multimodal imaging reporters.
In our program we are developing targeted nanoplexes carrying multimodality imaging reporters together with siRNA and a prodrug enzyme for theranostic imaging of metastatic PCa. In prodrug enzyme therapy, a drug-activating enzyme is delivered to the tumor followed by the systemic administration of a non-toxic prodrug, to minimize side effects. The ability to image the delivery of the prodrug enzyme can be exploited to time prodrug administration to minimize damage to normal tissue. We previously synthesized a prototype agent consisting of the prodrug enzyme cytosine deaminase (CD) labeled with multimodal MR and optical imaging reporters [11]. CD converts a non-toxic prodrug 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU) that can be detected by 19F MRS. We extended this approach to develope a prototype targeted nanoplex that delivers a prodrug enzyme together with multiple siRNA for theranostic imaging of metastatic disease. We have incorporated a low molecular weight PSMA binding agent [12] in the nanoplex to target the nanoplex to PCa cells. Since choline kinase (Chk) is significantly upregulated in aggressive cancer cells [13] we have used siRNA against Chk in the nanoplex. The nanoplatform being developed will allow us to deliver siRNA together with a prodrug enzyme, under image guidance for developing theranostic imaging of metastatic disease. The nanoplexes have the ability to deliver multiple siRNA. These strategies can be extended, in the future, to down-regulate multidrug resistance pathways, or repair enzymes to increase the efficiency of chemo- or radiation therapy, or target other receptors and antigens.
4. Conclusion
The field of theranostic imaging has tremendous scope for personalized molecular medicine in cancer. While cancer cell receptors and antigens present the most facile targets for theranostics, future areas will include targeting specific microenvironments or stromal compartments, cancer stem cells, and imaging and targeting permissive or preventive microenvironmental niches for cancer stem cells. A major challenge for this exciting new field is to rapidly translate and implement the most promising of these agents to the clinic.
Acknowledgements
Support from P50 CA103175, P30 CA006973, R01 CA73850, R01 CA82337, R01 CA136576, R01 CA138515, R01 CA138264, R01 CA151838 and R01 CA134675 is gratefully acknowledged.
Footnotes
Competing interests: The authors have no conflict of interest to declare.
References
- 1.Sanchez-Munoz A, Perez-Ruiz E, Ribelles N, Marquez A, Alba E. Maintenance treatment in metastatic breast cancer. Expert Rev Anticancer Ther. 2008;8(12):1907–12. doi: 10.1586/14737140.8.12.1907. [DOI] [PubMed] [Google Scholar]
- 2.Dizdar O, Altundag K. Emerging drugs in metastatic breast cancer. Expert Opin Emerg Drugs. 2009;14(1):85–98. doi: 10.1517/14728210802625671. [DOI] [PubMed] [Google Scholar]
- 3.Tuncel M, Souvatzoglou M, Herrmann K, et al. [(11)C]Choline positron emission tomography/computed tomography for staging and restaging of patients with advanced prostate cancer. Nucl Med Biol. 2008;35(6):689–95. doi: 10.1016/j.nucmedbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Metro G, Mottolese M, Fabi A. HER-2-positive metastatic breast cancer: trastuzumab and beyond. Expert Opin Pharmacother. 2008;9(15):2583–601. doi: 10.1517/14656566.9.15.2583. [DOI] [PubMed] [Google Scholar]
- 5.Freudenberg JA, Wang Q, Katsumata M, Drebin J, Nagatomo I, Greene MI. The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp Mol Pathol. 2009;87(1):1–11. doi: 10.1016/j.yexmp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulke N, Varlamova OA, Donovan GP, et al. The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proc Natl Acad Sci U S A. 2003;100(22):12590–5. doi: 10.1073/pnas.1735443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X, Bennett M, Thorpe PE. Anti-tumor effects and lack of side effects in mice of an immunotoxin directed against human and mouse prostate-specific membrane antigen. Prostate. 2004;61(1):1–11. doi: 10.1002/pros.20074. [DOI] [PubMed] [Google Scholar]
- 8.Tasch J, Gong M, Sadelain M, Heston WD. A unique folate hydrolase, prostate-specific membrane antigen (PSMA): a target for immunotherapy? Crit Rev Immunol. 2001;21(1–3):249–61. [PubMed] [Google Scholar]
- 9.Penet MF, Mikhaylova M, Li C, et al. Applications of molecular MRI and optical imaging in cancer. Future Med Chem. 2010;2(6):975–88. doi: 10.4155/fmc.10.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stasinopoulos I, Penet MF, Chen Z, Kakkad S, Glunde K, Bhujwalla ZM. Exploiting the tumor microenvironment for theranostic imaging. NMR Biomed. 2011;24(6):636–47. doi: 10.1002/nbm.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Penet MF, Winnard P, Jr., Artemov D, Bhujwalla ZM. Image-guided enzyme/prodrug cancer therapy. Clin Cancer Res. 2008;14(2):515–22. doi: 10.1158/1078-0432.CCR-07-1837. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Byun Y, Barinka C, et al. Bioisosterism of urea-based GCPII inhibitors: Synthesis and structure-activity relationship studies. Bioorg Med Chem Lett. 2010;20(1):392–7. doi: 10.1016/j.bmcl.2009.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11(12):835–48. doi: 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]