Abstract
Varicella zoster virus (VZV) is the causative agent of varicella (chickenpox) and zoster (shingles). Investigating VZV pathogenesis is challenging as VZV is a human-specific virus and infection does not occur, or is highly restricted, in other species. However, the use of human tissue xenografts in mice with severe combined immunodeficiency (SCID) enables the analysis of VZV infection in differentiated human cells in their typical tissue microenvironment. Xenografts of human skin, dorsal root ganglia or foetal thymus that contains T cells can be infected with mutant viruses or in the presence of inhibitors of viral or cellular functions to assess the molecular mechanisms of VZV–host interactions. In this Review, we discuss how these models have improved our understanding of VZV pathogenesis.
Varicella zoster virus (VZV), which is a human alphaherpesvirus of the genus Varicellovirus, causes varicella (also known as chickenpox) and zoster (also known as shingles)1. Epidemiological evidence suggests that primary VZV infection begins with replication in epithelial cells of the upper respiratory mucosa, which is followed by the widely distributed vesicular rash that is typical of varicella after an incubation period of 10–21 days. This pattern probably reflects viral spread to the tonsils and other local lymphoid tissues, from where infected T cells can transport the virus via the bloodstream to the skin2 (FIG. 1a). During primary infection, virions presumably gain access to the sensory nerve cell bodies in ganglia by retrograde axonal transport from skin sites of replication or by T cell viraemia, and latent infection is established3. When viral replication is reactivated, VZV reaches the skin via anterograde axonal transport to cause the symptoms of zoster, which is characterized by a vesicular rash in the dermatome that is innervated by the affected ganglion. Both varicella and zoster skin lesions contain high concentrations of infectious virus and are thus responsible for transmission to susceptible individuals. Varicella epidemics occurred annually in the United States until a varicella vaccine (which is a live attenuated form of the VZV Oka strain) was introduced in 1995, but epidemics continue among children in countries that do not have immunization programs4–6.
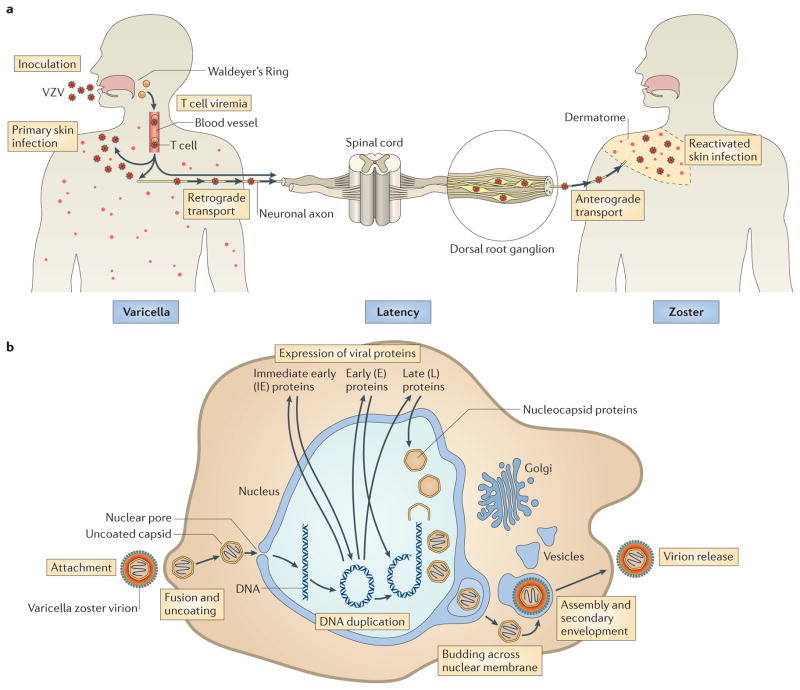
Figure 1. VZV life cycle and replication.
a | Model of the varicella zoster virus (VZV) life cycle. VZV infects the human host when virus particles reach mucosal epithelial sites of entry. Local replication is followed by spread to tonsils and other regional lymphoid tissues, where VZV gains access to T cells. Infected T cells then deliver the virus to cutaneous sites of replication. VZV establishes latency in sensory ganglia after transport to neuronal nuclei along neuronal axons or by viraemia. Reactivation from latency enables a second phase of replication to occur in skin, which typically causes lesions in the dermatome that is innervated by the affected sensory ganglion. b | Model of VZV replication. Enveloped VZV particles attach to cell membranes, fuse and release tegument proteins. Uncoated capsids dock at nuclear pores, where genomic DNA is injected into the nucleus and circularizes. On the basis of events that have been documented in herpes simplex virus 1 (HSV-1) replication, immediate-early genes are expressed, followed by early and late genes. Nucleocapsids are assembled and package newly synthesized genomic DNA, move to the inner nuclear membrane and bud across the nuclear membrane. Capsids enter the cytoplasm, and virion glycoproteins mature in the trans-Golgi region and tegument proteins assemble in vesicles; capsids undergo secondary envelopment and are transported to cell surfaces, where newly assembled virus particles are released.
The VZV genome has at least 71 known or predicted ORFs1,7 (BOX 1). Similar to all herpesviruses, VZV has a lipid-rich envelope, which is acquired from cellular membranes and into which viral glycoproteins are inserted. Within the envelope, a tegument layer that is predominantly composed of viral regulatory proteins surrounds an icosahedral nucleocapsid core that contains the linear double-stranded DNA genome1. The viral life cycle begins with VZV entry, which is a poorly understood process that is presumed to involve either direct fusion of viral particles with the plasma membrane or endocytosis (FIG. 1b). Viral envelope proteins are predicted to interact with cell surface molecules, such as mannose-6-phosphate receptor8 or myelin-associated glycoprotein9. VZV glycoprotein B (gB), gH and gL function as the core fusion complex9, but other envelope glycoproteins probably contribute as accessory proteins. After entry, the virions undergo uncoating, and tegument proteins, including the immediate-early protein 62 (IE62) — which is the major viral protein that functions as a transcription factor (that is, as a viral transactivator)1,10 — are released and might be transported to the nucleus before de novo protein synthesis occurs. Nucleocapsids anchor at nuclear pores, where viral genomes are injected into the nucleus. VZV genome replication and viral gene expression depend on virus-encoded and host cell transcription factors and cellular translation systems11. Furthermore, the tegument proteins ORF47 and ORF66 are important serine/threonine kinases that autophosphorylate and phosphorylate viral transcription factors and other VZV proteins12–18. IE62 forms regulatory complexes with cellular factors, such as transcription factor specificity protein 1 (Sp1), which has binding sites in many viral promoters11, to transactivate VZV genes. Similarly to other herpesviruses, nucleocapsids undergo primary envelopment, fusion with nuclear membranes and de-envelopment during transfer to the cytoplasm (FIG. 1b). Secondary envelopment occurs in the cisternae of the trans-Golgi network (TGN), where the capsids acquire tegument proteins and glycoprotein-containing membranes. Nascent virus particles then move to the cell surface in post-Golgi compartment vesicles; the first enveloped progeny virions are detected 9 hours after infection and many are present on cell surfaces within 12 hours of infection19. VZV differs from other herpesviruses in that assembled virions typically remain highly cell-associated. The same viral glycoproteins that are predicted to mediate entry are expressed on cell membranes and induce fusion of infected and uninfected cells, producing syncytia and multinucleated polykaryocytes, which further contributes to virus spread20–25.
Box 1. VZV genome and virion structure.
Genome structure
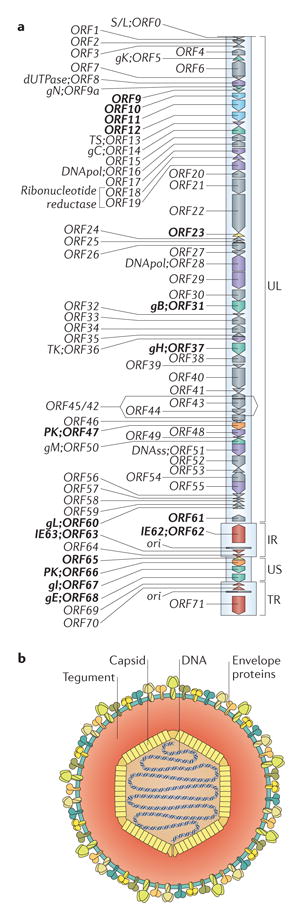
The VZV genome is a linear double-stranded DNA molecule of ~125,000 bp that encodes at least 71 unique ORFs and related promoter sequences1,7. Five phylogenetic VZV clades have been identified, but the most disparate still have 99.8% sequence conservation69. The genome consists of a unique long region (UL) of ~105,000 bp, a unique short region (US) of ~5,232 bp, and internal repeat (IR) and terminal repeat (TR) regions (see the figure, part a). The genes that encode ORF62 and ORF70, ORF63 and ORF71, and ORF64 and ORF69 are duplicated. The origin of replication (ori) is located in the repeat region. The VZV genome can circularize via unpaired bases at each end. About two-thirds of VZV ORFs are necessary for replication in vitro77, most of which are among the ~40 genes that are conserved in all herpesviruses, including eight glycoproteins (gB, gC, gE, gH, gI, gK, gL, gN; green), proteins that are involved in DNA replication (purple) and other functions, such as DNA cleavage and packaging, nucleic acid metabolism and capsid assembly. Replication proteins include the small and large subunits of the viral ribonucleotide reductase (known as ORF18 and ORF19), the two subunits of the viral DNA polymerase (known as ORF16 and ORF28), the single-stranded DNA-binding protein (known as ORF29), the origin of DNA replication binding protein (known as ORF51), two viral protein kinases (known as ORF47 and ORF66) and other enzymes that are involved in DNA replication, including dUTPase (known as ORF 8), thymidylate synthetase (known as ORF13), DNase (known as ORF48) and uracil DNA glycosylase (known as ORF59). Some VZV gene products have functional subdomains that are dispensable in cultured cells; others are dispensable for replication in vitro but are necessary for pathogenesis. The ORF9–ORF12 cluster of tegument proteins (blue) is conserved in the alphaherpesviruses. The products of the dispensable genes are of interest for their potential differential functions in tropism. Cloning the VZV genome into bacterial artificial chromosome vectors or as four or five overlapping fragments in cosmids enables the deletion of ORFs or targeted mutations of coding and non-coding sequences to define functions in vitro and in vivo (ORTs evaluated for pathogenesis indicated in bold, part a)40,53,107,108.
Virion formation and structure
VZV particles are ~80–120 nm in diameter (see the figure, part b). Linear VZV genomes are packaged into an icosahedral nucleocapsid core that is formed from proteins encoded by orf20, orf21, orf23, orf33, orf40 and orf41 (REF. 1) Capsids are surrounded by a tegument layer, which is a less well-defined structure that is made up of proteins with known or predicted regulatory functions, including the immediate-early (IE) viral transactivating factors that are encoded by orf4, orf62 and orf63, those that are encoded by the orf9–orf12 gene cluster, the two viral kinases ORF47 and ORF66, and others. The outer virion component is a lipid membrane envelope that is derived from cellular membranes with incorporated viral glycoproteins, including gB/gH–gL, which form the minimal fusion complex.
Investigating VZV pathogenesis is challenging as VZV is a highly human-specific virus that has little or no capacity to infect other species. This obstacle can be overcome by using human tissue xenografts in mice with severe combined immunodeficiency (SCID) (BOX 2). Infecting foetal thymus-liver T cell, skin and dorsal root ganglia (DRG) xenografts enables studies of the three major tissue tropisms of VZV: T cell-, skin- and neuro-tropism13,26,27. In these models, innate responses that modulate infectious processes can be assessed independently of adaptive immunity, which is absent in SCID mice. VZV-specific T cells are necessary to clear primary infection and prevent symptomatic reactivation from latency, but the xenograft models show the importance of intrinsic responses of differentiated cells in the absence of an adaptive immune response. Such studies can be done in knockout mouse models that have defects in adaptive immunity, but VZV does not infect mice. Furthermore, the xenograft models have the advantage of investigating infection in the various human tissue microenvironments that are targeted by VZV. Inoculating human tissue xenografts with mutant VZV can show functions of viral genes that are dispensable in tissue culture but necessary under the more stringent conditions that are present in intact tissues and fully differentiated human cells in vivo. To investigate which host cell factors are required during infection, small-molecule inhibitors or antibodies that block cell functions can be administered. In this Review, we summarize the characteristics of the infectious process in T cells, skin and dorsal root ganglia, the contributions of VZV proteins and functional motifs within these proteins to the capacity of VZV to infect differentiated human cells (TABLE 1) and the modulation of VZV infection by host cell factors within the tissue microenvironment. Investigating pathogenesis using these tools offers insights into how VZV causes the clinical manifestations of varicella and zoster and how this ubiquitous virus has so successfully survived in the human population.
Box 2. Modelling the pathogenesis of varicella zoster virus infection.
The pathogenesis of VZV infection can be modelled by infecting xenografts of human foetal tissues in mice with severe combined immunodeficiency (SCID)26,27,33. The xenografts are maintained for prolonged periods, as SCID mice lack T cell responses that mediate rejection of these foreign tissues. Skin xenografts are implanted beneath the mouse skin, and thymus-liver or dorsal root ganglia (DRG) xenografts are placed under the kidney capsule (see the figure). Then the human tissues become vascularized by anastomosis of capillaries in the xenografts with those in the surrounding murine tissues; endothelial cells of the microvasculature express human platelet endothelial cell adhesion molecule 1 (PECAM-1). Skin engraftment is established within 3–4 weeks; T cell and DRG xenografts require >8–12 weeks. Skin xenografts differentiate to have typical layers of dermal and epidermal cells, and epidermal keratinocytes differentiate into the anuclear corneocytes that comprise the stratum corneum, as in postnatal skin in humans. Thymus-liver xenografts contain immature dual-positive CD4+ CD8+ T cells but predominantly contain mature CD4+ T cell and CD8+ T cell populations. Neurons in DRG xenografts are surrounded by satellite cells, express neuronal subtype-specific proteins (such as TRKA and RT97), neural cell adhesion molecule (NCAM), synaptophysin and other markers such as herpes viral entry mediator (HVEM). After the period of engraftment, functional signalling pathways and effector proteins, including those that are involved in blocking apoptosis and innate antiviral responses (for example, signal transducer and activator of transcription (STAT) proteins, interferons and promyelocytic leukaemia protein (PML)) are present, as expected, in postnatal tissues. Effects of targeted mutations in the VZV genome are assessed by inoculating surgically exposed xenografts with fibroblasts that are infected with the parent wild-type or mutant virus, or with uninfected fibroblasts. The infectious process is similar when infected fibroblasts or when infected T cells — which are introduced into the mouse circulation — are used to deliver and release virions. To study the consequences of interfering with the functions of viral or cellular proteins, the mice are treated with antibodies or small-molecule inhibitors. Xenografts are recovered at different intervals after inoculation to assess viral titres, genome copies and viral transcripts; quantitative microscopy of tissue sections shows viral and cellular protein expression, genome localization, virion assembly and ultrastructural changes in cells. Furthermore, progression of infection can be monitored in vivo using recombinant VZV that expresses firefly luciferase. The SCID mouse model also provides a system for translational research to assess live attenuated VZV vaccines and antiviral drugs26,96,109.
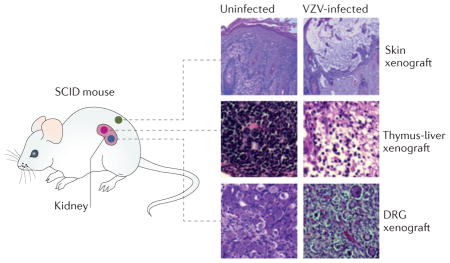
Table 1.
VZV protein functions in the pathogenesis of T cell, skin and DRG infection
| Protein | Characteristics | Cell culture* | Xenografts* |
|---|---|---|---|
| Glycoproteins | |||
| gB (ORF31) | Mature form generated by furin cleavage; ITIM motif in cytoplasmic domain; required for cell fusion together with gH–gL | Essential | Mutation of furin-cleavage site: impaired replication in skin |
| ITIM mutation; severely impaired replication and enhanced fusion in skin | |||
| gH (ORF37) | Ectodomain comprising domains I–III; forms heterodimer with gL; required for cell fusion together with gB and gL | Essential | Mutation of domain I amino terminus: impaired replication in skin |
| Mutation of domain III fusion loop; impaired replication in skin | |||
| gL (ORF60) | Forms heterodimer with gH | Presumed essential | Not tested |
| gE (ORF68) | Large unique N terminus (amino acids 1–187); forms heterodimer with gI (amino acids 208–236); binds to IDE; TGN-targeting and endocytosis motifs in cytoplasmic domain; MSP−gE is a natural variant | Essential; deletion of amino acids 1–187 blocks endocytosis | Deletion of amino acid residues 51–187: no replication in T cells and skin |
| Mutation of gI-binding domain: no effect in T cells, severely impaired replication in skin, prolonged replication and severe tissue damage in DRG | |||
| Mutation of IDE-binding domain: no effect in T cells, impaired replication in skin, no effect in DRG | |||
| Mutation of TGN-targeting motif: impaired replication in T cells and skin, no effect in DRG | |||
| MSP-gE: enhanced replication in skin | |||
| gI (ORF67) | Forms heterodimer with gE (via amino acids 105–125 and conserved cysteine residues); required for incorporation of gE into virions | Dispensable, decreased titres and plaque size | Deletion of gI: no replication in T cells and skin, prolonged replication in DRG |
| Mutation of gE-binding domain: prolonged replication in DRG | |||
| Deletion of amino acids 105–125: no replication in skin | |||
| Mutation of Sp1–USF-binding motif: no replication in T cells, impaired replication in skin, no effect in DRG | |||
| Mutation of ORF29-binding motif: impaired replication in T cells | |||
| Regulatory proteins | |||
| ORF9 | Tegument protein; binds to IE62 and ORF11; functions unknown | Essential | Not tested |
| ORF10 | Tegument protein; ORF62 and ORF71 transactivator; required for efficent virion assembly | Dispensable | Deletion of ORF10: no effect in T cells, impaired replication in skin |
| ORF11 | Tegument protein; required for normal levels of IE4, IE62, IE63 and gE; RNA-binding domain (amino acids 1–22); binding to ORF9 required for efficient virion assembly | Dispensable | Deletion of ORF11: impaired replication in skin |
| Mutation of RNA-binding motif: no effect in skin | |||
| Mutation of ORF9-binding motif: no replication in skin | |||
| ORF12 | Tegument protein; activates cell signalling pathways (such as ERK, p38, JNK and PI3K–AKT) that inhibit apoptosis and support viral replication | Dispensable | Deletion of ORF12: no effect in skin |
| ORF47 | Serine/threonine kinase (conserved); phosphorylates regulatory proteins and glycoproteins; binds to IE62; required for virion assembly | Dispensable | Deletion of ORF47: no replication in T cells and skin |
| Mutation of kinase motif: no replication in T cells, impaired replication in skin | |||
| ORF61 | Transactivator or repressor; dimerization required for regulatory functions; E3 ligase; PML dispersal by SIMs | Essential‡ | Deletion of SIMs: impaired replication in skin |
| Deletion of dimerization domain (amino acids 250–320): impaired replication in skin | |||
| ORF62 and ORF71 | IE62 is major viral transactivator; IFN inhibition | Essential | Deletion of ORF62 and ORF71 with ectopic ORF62: no replication in skin |
| ORF63 and RF70 | IE63 phosphoprotein; represses IE62; transactivates EF−1α | Essential‡ | Deletion of ORF63 and ORF70 with ectopic ORF63: no effect in T cells and skin |
| Mutation of phosphorylation sites: no effect in T cells, impaired replication in skin | |||
| ORF66 | Serine/threonine kinase (alphaherpesviruses); phosphorylates IE62; inhibits apoptosis | Dispensable | Deletion of ORF66: impaired replication in T cells, slightly impaired replication in skin |
| Mutation of kinase motif: impaired replication in T cells, slightly impaired replication in skin | |||
| Other VZV proteins | |||
| ORF23 | Small capsid surface protein; required for nuclear transport of other capsid proteins and capsid assembly | Dispensable | Deletion of ORF23: no replication in skin |
| ORF35 | Cell fusion | Dispensable, decreased cell fusion | Deletion of ORF35: slightly impaired replication in T cells, impaired replication in skin |
| ORF64 and ORF69 | Cell fusion | Dispensable, increased cell fusion | Deletion of ORF64 and ORF69: no effect in T cells and skin |
| ORF65 | Virion protein | Dispensable | Deletion of ORF65: no effect in T cells and skin |
DRG, dorsal root ganglia; EF-1α, elongation factor 1α; ERK, extracellular signal-regulated kinase; IDE, insulin-degrading enzyme; IE, immediate-early; IFN, interferon; ITIM, immunoreceptor tyrosine-based inhibition; JNK, c-JUN N-terminal kinase; PML, promyelocytic leukaemia protein; SIMs, SUMO-binding motifs; TGN, trans-Golgi network; VZV, varicella zoster virus.
Function in cells and tissues was assessed by mutagenesis of the VZV genome using cosmids or bacterial artificial chromosomes (BACs) to delete or insert stop codons or introduce targeted changes in the coding sequence
Indicates differences among published observations about whether the gene is essential or dispensable, including variations in the mutations tested and/or experimental conditions used to assess growth requirement.
T cell tropism
Discovering VZV tropism for T cells
VZV was initially classified as a neurotropic herpesvirus, but experiments using T cell xenografts in SCID mice in vivo and tonsil T cells in vitro have revealed that VZV also shows T cell tropism13,26,28 (FIG. 2). CD3+ T cells, including CD4+, CD8+ and dual CD4+CD8+ T cell subpopulations, are fully permissive for the replication and release of infectious virions. VZV infects tonsil T cells with high efficiency, which suggests that the virus is transferred from respiratory epithelial cells to T cells, presumably in the tonsils and other lymphoid tissues that comprise the Waldeyer’s ring (FIG. 1), similarly to the transfer of Epstein–Barr virus to tonsil B cells29. VZV can also infect dendritic cells, which might facilitate spread to lymph nodes30,31. VZV-infected CD4+ T cells predominantly show a memory T cell phenotype and express activation markers and skin-homing proteins, such as cutaneous leukocyte antigen (CLA) and CC-chemokine receptor 4 (CCR4), and are thus more likely to circulate through skin and other tissues28. In addition, VZV induces activation and skin homing proteins on naive T cells.
Figure 2. VZV T cell tropism.
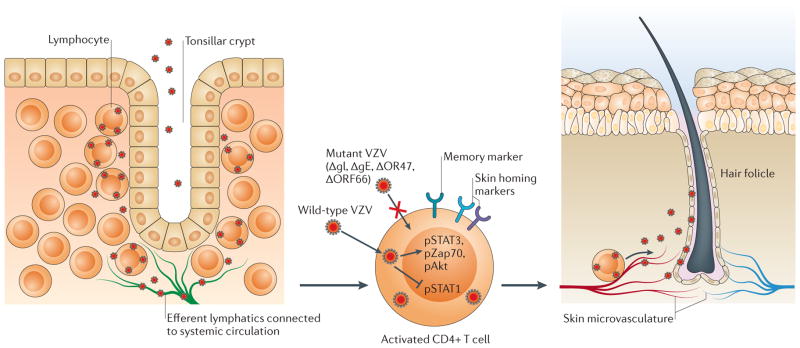
According to the model of varicella zoster virus (VZV) cell-associated viraemia, tonsil T cells are infected following VZV inoculation and replication in respiratory mucosal epithelial cells. T cells traffic into and out of tonsils across the squamous epithelial cells that line the tonsilar crypts (left panel). VZV has increased tropism for activated memory T cells that have skin-homing markers, which are common in tonsils (centre panel). These T cells are programmed for immune surveillance and can transport the virus across capillary endothelial cells into skin. VZV glycoprotein E (gE) (through its unique amino terminus), gI and the viral kinases ORF47 and ORF66 are important for T cell infection. Proteins that regulate cellular gene expression are activated (in the case of signal transducer and activator of transcription 3 (STAT3)) or inhibited (in the case of STAT1) in infected T cells. The microvasculature is extensive at the base of hair follicles, where T cells transit into the surrounding skin and initial VZV replication is observed (right panel).
In SCID mice with skin xenografts, infected human tonsil T cells can transport VZV and initiate skin infection2. At 24 hours after injection of T cells into the circulation, infected T cells appear in the skin that surrounds hair follicles, where the capillary microvasculature is extensive, which indicates that these cells retain the ability to transit across capillary endothelial walls by diapedesis. Infected T cells also deliver VZV to DRG xenografts in vivo, which suggests that viraemia facilitates the establishment of latency27. VZV promotes survival of infected T cells by inducing signal transducer and activator of transcription 3 (STAT3), which gives these cells time to reach tissues32. Notably, VZV does not trigger fusion of infected T cells, which indicates that virions must enter each T cell separately13,26,33. This characteristic differs from the multinucleated syncytia that are formed in VZV-infected skin34 and suggests that VZV has a cell type-specific capacity to suppress fusion, probably to retain the capacity of T cells to enter and exit tissues. The ability of VZV to infect memory T cells without disrupting their trafficking explains the development of the scattered varicella lesions and is consistent with the cell-associated viraemia that is observed in clinical cases35–37.
Viral determinants of T cell infection
The only glyco-proteins that are encoded by the short unique region of the VZV genome (BOX 1), gE and gI, have been investigated for their functions in T cells33,38–40 (TABLE 1). In contrast to its homologues in other alphaherpesviruses, the VZV membrane glycoprotein gE has a large unique amino-terminal region (consisting of amino acids 1–188), which is essential for replication39. Within this region, gE amino acid residues 51–187 are important for secondary virion envelopment and T cell infection. Although gE typically forms a heterodimer with gI via a cysteine-rich ectodomain region at residues 208–236, this interaction is not needed to infect T cells38,39. In addition, amino acid residues 27–90 are required for binding to insulin-degrading enzyme (IDE), which is a cellular protein that contributes to VZV replication in melanoma cells in vitro and is a proposed entry receptor41. However, disrupting these interactions by mutating gE does not affect T cell-xenograft infection, which indicates that IDE is not needed for T cell entry. The gE cytoplasmic domain contains an endocytosis motif that is required for replication, whereas a TGN-targeting motif in this domain is dispensable for replication in vitro but is important in T cells in vivo33. VZV gI is required for T cell infection42, and reducing gI synthesis by disrupting gI promoter sites for interactions with cellular transcription factors or ORF29 DNA-binding protein impairs replication in T cell xenografts43.
Five of the tegument proteins that surround the viral capsid have been evaluated for their contributions to T cell infection, including ORF10 protein, IE63, ORF65 protein and the two viral kinases, ORF47 protein and ORF66 protein13,16,17,44,45. ORF10 protein increases IE62 expression but is not required for T cell infection44. IE63 also regulates IE62 expression and transactivates cellular elongation factor 1α (EF-1α)49–52: IE63 functions that are needed to infect T cell xenografts are preserved even when the extensive serine/threonine phosphorylation domain is disrupted45. The ORF65 protein is also dispensable53. In contrast, the VZV kinases have a major role in T cells: the kinase activity of ORF47 directs the intracellular localization of IE62, gE and ORF47 and is needed for virion assembly. Although these functions are dispensable in vitro12,14,34, blocking ORF47 expression or disabling its kinase activity prevents VZV replication in T cell xenografts, which shows their importance in vivo13. Whereas ORF47 is conserved in all herpesviruses, only alphaherpesviruses have ORF66 kinase homologues1,7. ORF66 is important for virion assembly in differentiated T cells in vivo17,18 and in human corneal fibroblasts but not in other cells cultured in vitro15,18,46 (FIG. 1b). Furthermore, the ORF66 kinase inhibits apoptosis and counteracts the induction of interferon (IFN) signalling in infected T cells17,18. ORF66 also contributes to the downregulation of the major histocompatibility complex I and thereby interferes with CD8+ T cell-mediated elimination of infected cells47,48. Thus, the ORF66 kinase, which is dispensable in vitro, has multiple functions in T cells in vivo.
Cellular transcription factors as determinants of T cell tropism
Investigating VZV mutants that have disrupted binding sites for cellular transcription factors shows the importance of the synergistic regulation of viral genes by IE62 and cellular cofactors. For example, the transcription factor specificity protein 1 (Sp1) increases the recruitment of IE62 to viral promoters11,54, and mutation of the two Sp1-binding motifs in the gE promoter prevents VZV replication55. The gE promoter also has a site for upstream stimulatory factor (USF) binding, but it is not needed for the infection of tonsil T cells. Inhibiting the binding of Sp1 and USF to the gI promoter impairs VZV replication in T cell xenografts43. Thus, cellular cofactors that interact with IE62 have differential effects on particular viral gene promoters, which influence VZV replication in T cells in vivo.
Skin tropism
Innate cellular responses regulate skin pathogenesis. Lesion formation in infected skin xenografts is a highly regulated process that is determined by robust innate responses of epidermal and dermal cells (FIG. 3). Delivery of VZV to skin xenografts by infected T cells leads to the gradual formation of skin lesions over 10–21 days, which is consistent with the varicella incubation period2. Infected cells initially appear around hair follicles and viral proteins are then detected in clusters of adjacent cells, some of which fuse to create multinucleated polykaryocytes37. The uninfected cells that surround infectious foci show upregulation of interferon-α (IFNα) and IFNβ and the cellular transcription factors STAT1 and nuclear factor-κB (NF-κB), which orchestrate innate immune responses2,56.
Figure 3. VZV skin tropism.
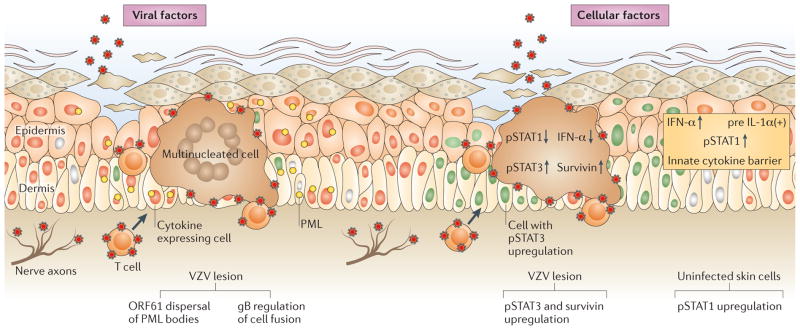
The schematic illustrates viral factors that ensure spread to the skin surface after varicella zoster virus (VZV) is delivered to cutaneous sites of replication by infected T cells or by retrograde axonal transport from neurons (left-hand side). Two examples of VZV proteins that are important for pathogenesis are shown: ORF61 protein has SUMO-interacting motifs that are important for dispersal of promyelocytic leukaemia nuclear bodies (PML-NBs)67 and the cytoplasmic domain of glycoprotein B (gB) has an immunoreceptor tyrosine-based inhibition motif that regulates cell–cell fusion and polykaryocyte formation25. VZV replication in skin triggers cellular responses, including changes that are induced in infected cells and changes in the uninfected cells adjacent to infected cells. Examples of VZV effects within infected cells are illustrated (right-hand side). VZV induces signal transducer and activator of transcription 3 (STAT3) activation, which triggers the expression of the anti-apoptotic protein survivin and inhibits the expression of interferon-α (IFNα) and STAT1 (REF. 32). In contrast to infected cells, surrounding uninfected cells exhibit upregulation of IFNs, STAT1, which activates IFN-stimulated factors such as PML, and other cell transacivators and innate cytokines2.
The importance of the IFNα and IFNβ response is evident from the enlarged lesions in skin xenografts that form if it is blocked with an antibody that targets the IFNα and IFNβ receptor2. During varicella in the human host, T cell-mediated immunity only occurs late in infection and is rarely detected until skin lesions have developed1. Adaptive responses are important to stop the infection57, but the initial control of viral replication by innate responses probably contributes to the persistence of VZV in the population, as a severe infection that overwhelms the host would limit opportunities for VZV transmission to other susceptible individuals.
Notably, replication of the vaccine virus Oka is reduced in skin xenografts, which is consistent with the situation in humans, who rarely develop lesions after subcutaneous inoculation of the vaccine4,5,26. Thus, the mutations that have accumulated in this attenuated strain (via passage in fibroblasts) have reduced its capacity to overcome intrinsic cutaneous barriers, even though the mutations have no effect on T cell tropism26 or neurotropism5. The Oka vaccine consists of a mixture of polymorphic viral clones, and some polymorphisms seem to be common, but none were identified as the molecular basis for attenuation. The evaluation of chimeric viruses constructed from segments of the pathogenic parental Oka strain and the attenuated vaccine in skin xenografts indicated that several genome regions contribute to viral attenuation in the skin58, which is consistent with the presence of a mixture of VZV clones with varying sequence changes in Oka vaccine preparations59.
VZV manipulation of cellular responses
VZV suppresses innate responses to produce a virus-filled lesion at the skin surface, where infected keratinocytes release newly-assembled virions60 (FIG. 3). Several viral proteins interfere with IFN-mediated responses in infected cells: IE62 inhibits the phosphorylation of IFN regulatory factor 3 (IRF3) by TANK-binding kinase 1 (TBK-1), thus blocking IFNβ production61, ORF47 kinase reduces IRF3 phosphorylation62 and IE63 inhibits the phosphorylation of the eukaryotic initiation factor 2 and its downstream IFNα effects63. The ORF66 kinase inhibits the activation of STAT1, which is induced in response to IFNα and IFNβ signaling and upregulates IFN-stimulated genes (ISGs)17. VZV also sequesters the p50–p65 heterodimer, which is the most abundant form of NFκB, in the cytoplasm of epidermal cells, by preventing degradation of the NF-κB inhibitor-α (IκBα)56. The latter effect is cell type-specific, as VZV induces NF-κB in monocytes48.
In addition to its inhibitory effects, VZV reprogrammes cell signalling to activate STAT3 in epidermal cells as well as T cells in vivo and fibroblasts in vitro32. Skin infection was severely impaired by treating SCID mice with a small-molecule inhibitor of STAT3, S31-201 (FIG. 3). STAT3-mediated upregulation of survivin, which is a cell protein that inhibits apoptosis, was necessary to support VZV infection. Although oncogenic herpesviruses manipulate this pathway to cause tumours, these experiments showed that VZV, which is a lytic herpesvirus, must also induce survivin. Interestingly, survivin expression is constitutive in the epithelial cells that line the hair follicles, which is the initial site of VZV replication in skin. VZV triggers autophagosome formation in skin cells, which might also reinforce the delay of apoptosis, thereby favouring infection64.
Promyelocytic leukemia protein (PML) in the regulation of skin infection
PML is a multifunctional protein that has antiviral effects against many viruses and is upregulated by IFNs. In VZV-infected cells, PML can form intranuclear cages that trap nascent virions and restrict their egress from the nucleus to the cytoplasm65,66. This antiviral effect is mediated by PML isoform IV, which binds to the ORF23 outer capsid protein via a unique carboxy-terminal domain and sequesters virions.
PML is a predominant component of nuclear structures known as PML nuclear bodies (PML-NBs; also known as nuclear domain 10). PML-NBs are more abundant in skin than in cultured cells, and VZV infection and the concomitant IFN secretion cause further accumulation in uninfected, adjacent epidermal and dermal cells, thus potentially limiting viral replication in vivo67 (FIG. 3). The ORF61 protein, which is expressed very early after infection19, functions to counteract this antiviral effect by binding to PML via SUMO-interacting motifs (SIMs) and disrupting the architecture of PML-NBs (FIG. 3). Mutation of the ORF61 SIMs has no consequences in vitro, but, in skin, it enables PML-NBs to remain abundant, severely impairs VZV replication and prevents viral penetration of the cutaneous basement membrane67. Again, IE62 synergism with Sp1 is necessary, as interfering with the binding of Sp1 to the ORF61 promoter limits ORF61 expression and reduces the extent of skin lesions68.
Cell–cell fusion and skin tropism
Cell–cell fusion is not strictly required for VZV spread, as virions that are released from infected cells can enter adjacent cells19. However, polykaryocyte formation is the classic pathological change that is induced by VZV in the skin. This pattern suggests that VZV reprogrammes infected cells to overcome the normal preservation of plasma membrane boundaries between differentiated cells and to mediate fusion of human skin cells in vivo, leading to facilitated virus spread, which overcomes innate barriers.
Glycoprotein gB and the heterodimer that is formed by gH and gL constitute the minimal VZV fusion complex and are candidates for mediating virus-induced cell fusion as well as virion entry9,20,23,24 (FIG. 1b; TABLE 1). The gB ectodomain has a highly conserved primary fusion loop that is essential for replication, and a furin protease recognition motif, which is not found in most alphaherpesvirus homologues24. Disrupting furin cleavage attenuates viral infection of skin xenografts, which indicates that this post-translational modification of gB is important for pathogenesis. According to the current model, herpesvirus gH (together with gL) activates gB to trigger its fusogenic function. For VZV, this function seems to depend, in part, on gH ectodomain residues and is required for skin pathogenesis, as administering a gH-specific monoclonal antibody blocks replication in skin xenografts22. Residues in the extreme amino-terminus of gH (which are dispensable in vitro) also contribute to skin infection in vivo23.
Although cell–cell fusion is a prominent characteristic of VZV pathogenesis, it must be tightly controlled during skin infection. Appropriately regulated cell–cell fusion depends on the cytoplasmic domain of gB and is mediated by a previously unrecognized immunoreceptor tyrosine-based inhibition motif (ITIM) in the gB cytoplasmic domain25 (FIG. 3). Phosphorylation of tyrosine 881 (Y881) in the ITIM modulates VZV-induced fusion, and blocking ITIM phosphorylation leads to a hyperfusogenic phenoptye in vitro and reduces the production of infectious virions. Inhibition of ITIM phosphorylation in vivo causes the aberrant fusion of skin cells in the outermost layer and markedly impairs lesion formation and infectious virus yields, which suggests that the gB cytoplasmic domain is essential for the control of polykaryocyte formation and optimal skin infection.
Glycoproteins gE and gI as determinants of skin infection
Although VZV isolates can be classified into several distinct clades that reflect their geographical origin, VZV is genetically stable, and unrelated isolates exhibit little variability in virulence69. However, VZV-MSP, which is a naturally occurring variant with a single amino acid change (D150N) in the gE ectodomain, has increased cell–cell spread in vitro and accelerated growth in skin xenografts compared with other isolates70. Highlighting the potential effect of changing a single gE residue, a serine to alanine substitution at gE position 31 markedly impairs skin replication39.
Residues 51–187 in the non-conserved ectodomain of gE are essential for both skin tropism and T cell tropism39, which reinforces the importance of this unique gE region for the VZV life cycle (TABLE 1). Blocking gE–gI heterodimerization interferes with gE maturation and surface expression and inhibits the incorporation of gI into virions. These functions that involve gE–gI-binding are important for skin tropism but are dispensable in T cells38. Binding of gE to IDE also contributes to skin tropism, but blocking the interaction of gE with this cellular protein has much less effect than eliminating gE–gI binding. Overall, gE domains, including the TGN-targeting motif in the C-terminus of gE33, are more important for the infection of skin than of T cells, which suggests that gE functions determine cell–cell spread rather than initial virion entry. Binding sites for cellular factors in the gE promoter, other than Sp1, are not necessary for replication or skin tropism55.
Deleting gI interferes with gE trafficking and secondary envelopment of virions in vitro and completely blocks infection of skin and T cells in vivo, which can be attributed to defective virion assembly41,71. Ensuring that gI expression levels are sufficient for normal skin pathogenesis depends on the enhancing effect of the ORF29 DNA-binding protein for IE62 transactivation43. Co-regulation of the gI promoter by Sp1 and USF with IE62 is essential in skin, whereas it enables a low level of replication in T cell xenografts43, which indicates tissue-specific differences in the requirement for cell transactivators that influence levels of gI expression.
Tegument and capsid proteins in skin infection
Like other herpesviruses, the VZV genome has duplicate copies of genes in the repeat regions, including those that encode the tegument proteins, IE62 (encoded by orf62 and orf71), IE63 (encoded by orf63 and orf70) and ORF64 protein (encoded by orf64 and orf69)1,7 (BOX 1). The importance of this genomic organization for pathogenesis is not known. Deleting the genes for IE62 or IE63 is lethal for VZV replication but the orf64–orf69 gene pair is dispensable, which indicates that gene duplication is not conserved to protect against the loss of an essential gene50. However, when skin xenografts are infected with VZV mutants that have single copies of the genes for IE62 and IE63 genes, the second copy is restored by recombination during replication, which suggests that gene duplication is important in vivo45,72. IE62 production in skin is necessary, as is evident from impaired infection when the binding site for the ORF29 viral cofactor is mutated in the orf62–orf70 promoter. A single copy of orf63 at a non-native site in the genome provides sufficient IE63 for replication in skin; however, lesion formation is substantially impaired if IE63 phosphorylation is disrupted, which indicates that there are differential requirements for the IE63 phosphoprotein in skin and in T cells45.
The VZV tegument contains proteins that are encoded by a cluster of genes (known as orf9–orf12) that is conserved in alphaherpesvirus genomes7. Of these genes, only orf9 is essential in vitro. The ORF10 protein increases expression of IE62 and is important for skin infection, which shows that interactions of IE62 with viral cofactors — which are dispensable in vitro — are necessary for pathogenesis in vivo44,73. The ORF11 protein contributes essential functions in skin xenografts via its binding to the ORF9 protein during virion tegument assembly; however, the capacity of the ORF11 protein to bind to mRNA is not necessary, which shows that distinct functions of the same VZV protein differ in their effects on skin tropism74. The ORF12 protein — which activates ERK1/2, inhibits apoptosis75 and manipulates cell cycle progression by activating the PI3K/AKT pathway76 — is dispensable in skin73. Thus, although functions of some gene products are essential, conservation of the orf9–12 cluster does not signify a uniform requirement of these genes for pathogenesis in differentiated human tissues.
Disrupting the ORF47 protein kinase function, which causes the nuclear retention of ORF47 and IE62 proteins in vitro, severely impairs skin pathogenesis14,34. Deleting orf47 also reduces replication in skin organ cultures77. However, despite reduced virion formation, the ORF47 kinase mutant elicits some polykaryocyte formation in skin xenografts as long as binding of the ORF47 protein to IE62 is preserved34. By contrast, replication of the ORF47 kinase mutant in T cell xenografts is completely blocked, which suggests that even minimal cell fusion can support VZV spread in the skin, whereas T cell infection depends on the complete assembly and release of infectious virions13,14. Notably, disabling ORF66 kinase activity has very little effect on virulence in the skin, despite its importance for T cell tropism13. Thus, the ORF47 and ORF66 viral kinases make different and tissue-specific contributions to VZV pathogenesis.
The small capsid protein that is encoded by orf23 is dispensable for replication in vitro, owing to redundant ORF33.5-mediated transport of the major capsid protein into the nucleus78. However, this mechanism is not sufficient in skin, which indicates that there are strict requirements for VZV capsid assembly in vivo.
Neurotropism
Before the DRG-xenograft model was developed, VZV neurotropism in human tissues could only be examined in sensory ganglia that were obtained at autopsy. These studies showed that VZV genomes (~2–9 copies per cell) are present in about 4% of neurons during latency79,80. Transcripts of ten VZV genes have been reported in autopsy ganglia, and orf63 transcripts are the most abundant81,82, but the extent to which detection represents post-mortem release of gene silencing is not known. In some studies, VZV proteins were rare or absent, whereas others reported frequent expression in the neuronal cytoplasm83,84; however, eliminating an artefact, which results from antibody reactivity against blood group A determinants, confirms that VZV protein expression is rare in latency85. By contrast, ganglia from individuals who had zoster a few months before death harbour VZV proteins and inflammatory proteins in up to 25% of neurons86,87. Prolonged replication in ganglia might be a factor in some cases of postherpetic neuralgia.
VZV infection and latency in DRG xenografts
In DRG xenografts, viral proteins, genomes and infectious virus are detectable for 3–4 weeks after VZV inoculation27 (FIG. 4). Productive infection is followed by a transition to VZV latency after 4–8 weeks. During this phase, infectious virus is no longer detectable, viral genome copies decline, levels of orf62 and orf63 transcripts are reduced and gB transcription ceases. This pattern of DRG infection is identical after direct inoculation or VZV transfer from infected T cells. The transition to persistence in neurons occurs despite the absence of adaptive immunity and markedly contrasts with the progressive lytic infection that is observed in skin and T cell xenografts. Thus, VZV gene silencing in neurons is a cell type-specific characteristic of the VZV–host interaction. Infection of explanted foetal DRG neurons in vitro showed that IE63 has anti-apoptotic functions that might facilitate this transition88,89.
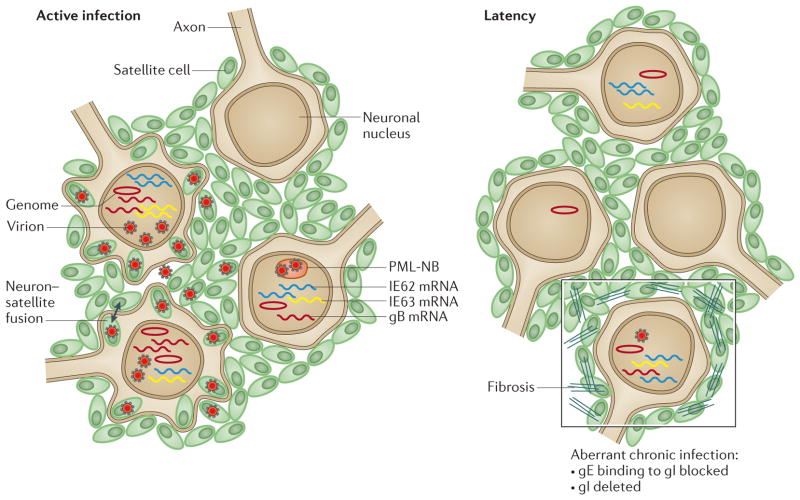
Figure 4. VZV neurotropism in DRG xenografts.
This schematic illustrates active infection of dorsal root ganglia (DRG) which is characterized by the transcription of genes (for example, genes encoding glycoprotein B (gB), immediate early protein 62 (IE62) and IE63) that produce proteins that are required for lytic infection, varicella zoster virus (VZV) genome synthesis, virus assembly in neurons and satellite cells, release of VZV into intracellular spaces and fusion of some neurons and satellite cells27 (left panel). Virions are captured in cages that are formed by promyelocytic leukaemia nuclear bodies (PML-NBs) in some neurons and satellite cells66. By contrast, latency (right panel) is associated with the persistence of VZV genomes and immediate-early (IE) transcripts, whereas late gene transcription, such as transcription of gB, ceases and virion formation ceases. When DRG are infected with VZV mutants in which binding of gE to gI is blocked or in which gI is deleted, the transition to latency is disrupted (right panel; outlined box), infectious virions continue to be produced at low levels and in the case of disrupted binding of gE to gI, tissue destruction is extensive, which is associated with disruption of the cell matrix, elimination of many neurons and the proliferation of satellite cells.
As observed in skin xenografts, VZV infection of DRG is regulated by the capacity of PML-NBs to sequester nucleocapsids in neurons and satellite cells65. Cages that consist of PML fibres surround both nascent and fully formed virions in infected cell nuclei. Notably, neurodegenerative disorders, such as Huntington’s disease, are also associated with large PML-NBs that retain aberrant polyglutamine proteins. PML isoform IV cages have the capacity to sequester both viral capsids and the aberrant Huntington’s disease protein, HttQ72 (REF. 90), which suggests that neurons have a conserved mechanism to sense and entrap protein aggregates.
How VZV reactivation is triggered is not known, but intrinsic cellular mechanisms might also restore gene silencing, resulting in abortive replication. If replication continues, VZV is transported to skin, causing zoster (FIG. 1). VZV-specific T cells then function together with innate cutaneous responses to control the infection. VZV cell-mediated immunity is deficient in elderly and immunocompromised patients, which accounts for more frequent and severe zoster in these populations92. Whether VZV-specific T cells also limit reactivation in ganglia is not known.
Neuron–satellite-cell fusion
When VZV replicates in DRG xenografts, VZV genomic DNA, viral proteins and virion production are detectable in both neurons and satellite cells27,92. Importantly, VZV can induce fusion and polykaryocyte formation between differentiated neurons and surrounding satellite cells (FIG. 4), whereas herpes simplex virus 1 (HSV-1) does not induce cell fusion in DRG92,93. Neuron–satellite cell fusion is reported in ganglia from patients with zoster at the time of death94, which indicates that DRG xenograft infections are a model of this consequence of VZV reactivation. This formation of neuron–satellite-cell polykaryons amplifies the spread of VZV to neuronal cell bodies in the ganglion. As VZV is transported to the skin by neuronal axons that extend from neuronal cell bodies, spread within ganglia would increase the extent of infection of the dermatome during zoster. These pathological changes are not readily reversible and help to explain why zoster can be associated with prolonged neuropathic pain1.
Glycoproteins gE and gI as determinants of neurotropism
Both gE and gI have functions that influence VZV pathogenesis in DRG xenografts (TABLE 1). Infection is not altered when binding of gE to IDE is disrupted, although neural cells express IDE, and interference with the TGN-trafficking motif of gE also has no effect on neurotropism95. By contrast, blocking gE–gI heterodimer formation impairs cell–cell spread in DRG and reduces replication during acute infection, with no infectious virus detected until four weeks after inoculation. However, instead of transitioning to persistence, the gE mutant causes widespread cytopathic changes in neurons, satellite cells and the surrounding tissue, for at least two months after inoculation96,97. Thus, the gE–gI interaction is crucial for preventing a chronic, highly destructive process in sensory ganglia.
Deleting gI, similarly to blocking heterodimer formation, results in the prolonged production of infectious virus particles despite reduced virion assembly and gE mislocalization97. Impaired virion assembly is associated with the accumulation of aberrant membrane stacks in the TGN region and altered Golgi structures both in DRG neurons and in vitro98. These effects, like those that are observed when gE cannot bind to gI, might be due to the absence of cell fusion, such that the virus can only spread slowly from cell to cell and host innate responses that faciliate the transition to persistence are less effectively triggered. Of interest, gI promoter regulation by Sp1 and USF is not required for neuropathogenesis97, in contrast to T cell and skin infection, which, again, indicates that cellular factors contribute in a cell type-specific manner to viral gene expression and therefore to tissue tropism. Surprisingly, the ability of VZV to replicate in DRG in the absence of gI suggests that the requirements for VZV infection of neural cells are less stringent than those that are required for infection of T cells or the skin. Finally, the unexpected finding that disrupting the formation of gE–gI heterodimers, or deleting gI, causes chronic infection highlights the need to evaluate effects on neurovirulence in vivo and shows that the consequences of VZV mutations cannot be predicted from their effects in other tissue microenvironments.
Perspective
These studies in the SCID mouse model show that virus–host cell interactions result in a well-regulated infectious process in each of the tissue microenvironments that is important for VZV pathogenesis. In infected cells, the virus reprogrammes cell signalling pathways by inducing or downregulating cellular factors, such as the STATs, to support replication. VZV also has tissue-specific effects that are important for pathogenesis, such as suppressing the fusion of infected T cells. Although many viral proteins are incidental for VZV replication in vitro, specialized functions, not only of complete VZV proteins but also small motifs, as well as single amino acids, are often crucial for pathogenesis in vivo. To protect these crucial functions, VZV has a high degree of genome stability69. At the same time, innate responses of uninfected cells modulate infection so that the host is not overwhelmed, which benefits both the host and the virus by ensuring that there are opportunities for transmission and persistence in the host population.
Despite these advances in understanding VZV–host interactions, many questions about VZV pathogenesis remain unresolved; for example, as is the case for other herpesviruses, the triggers of VZV reactivation from latency are unknown. DRG xenografts can be used to investigate VZV–neuron interactions, but axonal transport to skin cannot be examined in this model, and stimuli of viral reactivation from latency have not been explored. By contrast, simian varicella virus (SVV) can be studied in the natural non-human primate host99,100 and has been used to investigate reactivation that is associated with immunosuppression84. The SVV model avoids the need to acquire human tissue to make xenografts, which is a limitation of the SCID model, but genetic dissimilarities between SVV and VZV are likely to lead to differences in pathogenesis in their native hosts. Mechanisms of neuropathic pain cannot be assessed in DRG xenografts, but they can be investigated in the rat footpad model101, and infection of enteric neurons has recently been reported in guinea pigs102. Although cultured neurons lack the surrounding satellite cells that mediate neuronal homeostasis in vivo, viral protein functions of interest for further study in vivo can be identified in neuron cultures, as was shown for the ORF7 protein103. Although the xenograft models are valuable for probing intrinsic antiviral defences, advances in establishing a human immune system in SCID mice might make it possible to assess adaptive immune clearance of VZV in conjunction with innate control.
From a clinical perspective, VZV remains a medically important human herpesvirus despite major advances in vaccines and antiviral drugs to prevent or mitigate VZV infection. Live attenuated VZV vaccines are effective in healthy individuals but are not safe for immunocompromised patients, in whom they cause viraemia, and they can establish latency and reactivate in healthy and in immunodeficient individuals4–6,104,105, which is consistent with the lack of attenuation that is observed in T cell and DRG xenografts. The genetic basis of their attenuation is not defined58,59. The SCID mouse model can be exploited for rational VZV vaccine design by incorporating mutations that dampen replication in skin into the viral genome, similarly to the current vaccine, and, in contrast to the current vaccine, that also interfere with the capacity to infect T cells or to persist in neurons. Antiviral drugs, such as acyclovir and related agents, substantially reduce the risk of severe or fatal VZV infection in immunocompromised patients but have little or no effect on postherpetic neuralgia following zoster in the elderly106. Knowledge about functional motifs of VZV proteins and how the virus reprogrammes differentiated human cells in vivo might help in designing small-molecule inhibitors with antiviral activity that would also decrease postherpetic neuralgia. Understanding the principles of VZV pathogenesis at the molecular level has the potential to yield new approaches to prevent and treat VZV infections.
Acknowledgments
The authors thank M. Sommer, X. Che, L. Wang and M. Reichelt, past postdoctoral fellows and students and collaborators and many dedicated colleagues in the field of VZV research for their invaluable contributions. J. Moffat initiated the development of the SCID mouse model as a postdoctoral fellow in the Arvin laboratory. This work is supported by US National Institutes of Health (NIH) grants, AI20459, AI05346 and AI102546.
Glossary
- Dermatome
An area of skin that is primarily innervated by a single sensory ganglion of the spinal cord
- Trans-Golgi network (TGN)
An intracellular collection of tubules and vesicles that are located at the trans face of the Golgi stack; it is involved in processing glycoproteins
- Syncytia
Multinucleated cells that are created by fusion of membranes between cells with single nuclei; multinucleated cells in tissue are referred to as polykaryocytes
- Polykaryocytes
Cells that contain many nuclei
- Xenografts
Tissues from one species that have been transplanted into a different species
- Severe combined immunodeficiency (SCID)
A genetic defect that blocks the function of T cells and B cells, interfering with the capacity of the host to mount an effective immune response to foreign proteins
- Dorsal root ganglia (DRG)
Nodules on the dorsal root of the spinal cord that contain sensory nerve cell bodies
- Anastomosis
The end-to-end connection of tubular structures, such as capillaries
- Waldeyer’s ring
An annular arrangement of lymphoid tissue in the oropharynx; it consists of the pharyngeal, tubal, palatine and lingual tonsils
- Diapedesis
The passage of lymphocytes and cells of the immune system through intact vessel walls
- Major histocompatibility complex I
A protein that is present on the surfaces of most mammalian cells that functions to present peptide epitopes to T cells
- Satellite cells
Glial cells that surround the surfaces of neurons in the peripheral nervous system
- Neuropathic pain
Pain that is caused by damage to the somatosensory system; it is associated with increased sensitivity to touch, temperature and other stimuli
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Arvin AM, Gilden D. In: Fields Virology. 6. Knipe D, Howley P, editors. Lippincott Williams & Wilkins; 2013. pp. 2015–2057. [Google Scholar]
- 2.Ku CC, et al. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-α. J Exp Med. 2004;200:917–925. doi: 10.1084/jem.20040634. This paper shows the role of T cells in the transport of VZV and the control of replication by the potent innate response of skin cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilden DH, et al. Varicella-zoster virus DNA in human sensory ganglia. Nature. 1983;306:478–480. doi: 10.1038/306478a0. This study provides the first evidence of VZV latency in neurons, using in situ hybridization to detect VZV genomes. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi M. Clinical overview of varicella vaccine: development and early studies. Pediatrics. 1986;78:736–741. [PubMed] [Google Scholar]
- 5.Gershon AA, Gershon MD. Perspectives on vaccines against varicella-zoster virus infections. Curr Top Microbiol Immunol. 2010;342:359–372. doi: 10.1007/82_2010_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weibel RE, et al. Live attenuated varicella virus vaccine: efficacy trial in healthy children. N Engl J Med. 1984;310:1409–1415. doi: 10.1056/NEJM198405313102201. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JI. The varicella-zoster virus genome. Curr Top Microbiol Immunol. 2010;342:1–14. doi: 10.1007/82_2010_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JJ, et al. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell. 2004;119:915–926. doi: 10.1016/j.cell.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Suenaga T, et al. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci USA. 2010;107:866–871. doi: 10.1073/pnas.0913351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M, Hay J, Ruyechan WT. Varicella-zoster virus IE62 protein utilizes the human mediator complex in promoter activation. J Virol. 2008;82:12154–12163. doi: 10.1128/JVI.01693-08. This paper documents the VZV takeover of the host cell gene transcription complex for viral gene transcription in combination with the IE62 major viral transactivator. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruyechan WT. Roles of cellular transcription factors in VZV replication. Curr Top Microbiol Immunol. 2010;42:43–65. doi: 10.1007/82_2010_42. [DOI] [PubMed] [Google Scholar]
- 12.Heineman TC, Cohen JI. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J Virol. 1995;69:7367–7370. doi: 10.1128/jvi.69.11.7367-7370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffat JF, et al. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc Natl Acad Sci USA. 1998;95:11969–11974. doi: 10.1073/pnas.95.20.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besser J, et al. Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse. J Virol. 2003;77:5964–5974. doi: 10.1128/JVI.77.10.5964-5974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erazo A, Kinchington PR. Varicella-zoster virus open reading frame 66 protein kinase and its relationship to alphaherpesvirus US3 kinases. Curr Top Microbiol Immunol. 2010;342:79–98. doi: 10.1007/82_2009_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaap A, et al. T-cell tropism and the role of ORF66 protein in pathogenesis of varicella-zoster virus infection. J Virol. 2005;79:12921–12933. doi: 10.1128/JVI.79.20.12921-12933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaap-Nutt A, et al. ORF66 protein kinase function is required for T-cell tropism of varicella-zoster virus in vivo. J Virol. 2006;80:11806–11816. doi: 10.1128/JVI.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisfeld AJ, et al. Phosphorylation of the varicella-zoster virus (VZV) major transcriptional regulatory protein IE62 by the VZV open reading frame 66 protein kinase. J Virol. 2006;80:1710–1723. doi: 10.1128/JVI.80.4.1710-1723.2006. This paper shows the role of the ORF66 viral kinase in activating the transcriptional function of IE62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichelt M, Brady J, Arvin AM. The replication cycle of varicella-zoster virus: analysis of the kinetics of viral protein expression, genome synthesis, and virion assembly at the single-cell level. J Virol. 1999;83:3904–3918. doi: 10.1128/JVI.02137-08. This study documents the dynamics of VZV protein expression, genome replication and progeny virus assembly in newly infected cells from entry to virion release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grose C, Carpenter JE, Jackson W, Duus KM. Overview of varicella-zoster virus glycoproteins gC, gH and gL. Curr Top Microbiol Immunol. 2010;342:113–128. doi: 10.1007/82_2009_4. [DOI] [PubMed] [Google Scholar]
- 21.Maresova L, et al. Characterization of interaction of gH and gL glycoproteins of varicella-zoster virus: their processing and trafficking. J Gen Virol. 2000;81:1545–1552. doi: 10.1099/0022-1317-81-6-1545. [DOI] [PubMed] [Google Scholar]
- 22.Vleck SE, et al. Anti-glycoprotein H antibody impairs the pathogenicity of varicella-zoster virus in skin xenografts in the SCID mouse model. J Virol. 2010;84:141–152. doi: 10.1128/JVI.01338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vleck SE, et al. Structure–function analysis of varicella-zoster virus glycoprotein H identifies domain-specific roles for fusion and skin tropism. Proc Natl Acad Sci USA. 2011;108:18412–18417. doi: 10.1073/pnas.1111333108. This study maps the functional domains in the gH ectodomain that are required for replication and skin infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver SL, et al. Mutagenesis of varicella-zoster virus glycoprotein B: putative fusion loop residues are essential for viral replication, and the furin cleavage motif contributes to pathogenesis in skin tissue in vivo. J Virol. 2009;83:7495–7506. doi: 10.1128/JVI.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver SL, et al. An immunoreceptor tyrosine-based inhibition motif in varicella-zoster virus glycoprotein B regulates cell fusion and skin pathogenesis. Proc Natl Acad Sci USA. 2013;110:1911–1916. doi: 10.1073/pnas.1216985110. This paper shows that the gB cytoplasmic domain has a previously unrecognized motif that mimicks cellular ITIMs and that must be phosphorylated to control the fusogenic properties of gB and the detrimental effects of exaggerated cell fusion on VZV infection of skin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moffat JF, et al. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J Virol. 1998;72:965–974. doi: 10.1128/jvi.72.2.965-974.1998. This study is the first report to model VZV pathogenesis using T cell and skin xenografts in SCID mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zerboni L, et al. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci USA. 2005;102:6490–6495. doi: 10.1073/pnas.0501045102. This paper is the first report to model the neurotropism of VZV using DRG xenografts in SCID mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ku CC, et al. Tropism of varicella-zoster virus for human tonsillar CD4+ T lymphocytes that express activation, memory and skin homing markers. J Virol. 2002;76:11425–11433. doi: 10.1128/JVI.76.22.11425-11433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longnecker R, Kieff E, Cohen J. In: Fields Virology. 6. Knipe D, Howley P, editors. Lippincott Williams & Wilkins; 2013. pp. 1898–1957. [Google Scholar]
- 30.Abendroth A, et al. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J Virol. 2001;75:6183–6192. doi: 10.1128/JVI.75.13.6183-6192.2001. This paper shows the capacity of VZV to infect dendritic cells in skin and their capacity to transfer the virus into T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huch JH, et al. Impact of varicella-zoster virus on dendritic cell subsets in human skin during natural infection. J Virol. 2010;84:4060–4072. doi: 10.1128/JVI.01450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen N, et al. STAT3 activation and survivin induction by varicella zoster virus promotes viral replication and skin pathogenesis. Proc Natl Acad Sci USA. 2012;109:600–605. doi: 10.1073/pnas.1114232109. This paper shows that VZV, which is a lytic herpesvirus, induces STAT3 activation — leading to survivin expression, which was previously associated with oncogenic herpesviruses — and that takeover of this cellular pathway is necessary for VZV skin infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffat JF, et al. Functions of C-terminal domain of varicella-zoster virus glycoprotein E in viral replication in vitro and skin and T-cell tropism in vivo. J Virol. 2004;78:12406–12415. doi: 10.1128/JVI.78.22.12406-12415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Besser J, Ikoma M, Fabel K. Differential requirement for cell fusion and virion formation in the pathogenesis of varicella-zoster virus infection of skin and T cells. J Virol. 2004;78:13293–13305. doi: 10.1128/JVI.78.23.13293-13305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koropchak CM, et al. Investigation of varicella-zoster virus infection of lymphocytes by in situ hybridization. J Virol. 1989;63:2392–2395. doi: 10.1128/jvi.63.5.2392-2395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozaki T, et al. Viremic phase in nonimmunocompromised children with varicella. J Pediatr. 1984;104:85–87. doi: 10.1016/s0022-3476(84)80596-x. [DOI] [PubMed] [Google Scholar]
- 37.Arvin AM, et al. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr Top Microbiol Immunol. 2010;342:189–209. doi: 10.1007/82_2010_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berarducci B, et al. Deletion of the first cysteine-rich region of the varicella-zoster virus glycoprotein E ectodomain abolishes the gE and gI interaction and differentially affects cell–cell spread and viral entry. J Virol. 2009;83:228–240. doi: 10.1128/JVI.00913-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berarducci B, et al. Functions of the unique N-terminal region of glycoprotein E in the pathogenesis of varicella-zoster virus infection. Proc Natl Acad Sci USA. 2010;107:282–287. doi: 10.1073/pnas.0912373107. This study identifies a large non-conserved region of gE and maps its functions in skin infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallory S, Sommer M, Arvin AM. Mutational analysis of varicella-zoster virus (VZV) glycoproteins, gI and gE, in recombinant VZV strains. J Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, et al. Insulin degrading enzyme induces a conformational change in varicella-zoster virus gE, and enhances virus infectivity and stability. PLoS ONE. 2010;5:e11327. doi: 10.1371/journal.pone.0011327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moffat JF, et al. Glycoprotein I of varicella-zoster virus is required for viral replication in skin and T cells. J Virol. 2002;76:8468–8471. doi: 10.1128/JVI.76.16.8468-8471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito H, et al. Promoter sequences of varicella-zoster virus glycoprotein I targeted by cellular transactivating factors Sp1 and USF determine virulence in skin and T cells in SCIDhu mice in vivo. J Virol. 2003;77:489–498. doi: 10.1128/JVI.77.1.489-498.2003. This paper reports that cell transcription factors have an essential role as determinants of VZV pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Che X, Zerboni L, Sommer MH, Arvin AM. Varicella-zoster virus open reading frame 10 is a virulence determinant in skin cells but not in T cells in vivo. J Virol. 2006;80:3238–3248. doi: 10.1128/JVI.80.7.3238-3248.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baiker A, et al. The immediate-early 63 protein of varicella-zoster virus: analysis of functional domains required for replication in vitro and T cell and skin tropism in the SCIDhu model in vivo. J Virol. 2004;78:1181–1194. doi: 10.1128/JVI.78.3.1181-1194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinchington PR, et al. Virion association of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, requires expression of the VZV open reading frame 66 protein kinase. J Virol. 2001;75:9106–9113. doi: 10.1128/JVI.75.19.9106-9113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol. 2007;81:9034–9049. doi: 10.1128/JVI.00711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abendroth A, Kinchington PR, Slobedman B. Varicella zoster virus immune evasion strategies. Curr Top Microbiol Immunol. 2010;342:155–171. doi: 10.1007/82_2010_41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bontems S, et al. Phosphorylation of varicella-zoster virus IE63 protein by casein kinase influences its cellular localization and gene regulation activity. J Biol Chem. 2002;277:21050–21060. doi: 10.1074/jbc.M111872200. [DOI] [PubMed] [Google Scholar]
- 50.Sommer MH, et al. Mutational analysis of varicella-zoster virus ORF63/70 and ORF64/69. J Virol. 2001;75:8224–8239. doi: 10.1128/JVI.75.17.8224-8239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoover SE, et al. Downregulation of varicella-zoster virus (VZV) immediate-early ORF62 transcription by VZV ORF63 correlates with virus replication in vitro and with latency. J Virol. 2006;80:3459–3468. doi: 10.1128/JVI.80.7.3459-3468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuranski T, et al. Cell-type-dependent activation of the cellular EF-1α promoter by the varicella-zoster virus IE63 protein. Virology. 2005;338:35–42. doi: 10.1016/j.virol.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Niizuma T, et al. Construction of varicella-zoster virus recombinants from parent Oka cosmids and demonstration that ORF65 protein is dispensable for infection of human skin and T cells in the SCID-hu mouse model. J Virol. 2003;77:6062–6065. doi: 10.1128/JVI.77.10.6062-6065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng H, et al. Interaction between the varicella-zoster virus IE62 major transactivator and cellular transcription factor SP1. J Biol Chem. 2003;278:38068–38075. doi: 10.1074/jbc.M302259200. [DOI] [PubMed] [Google Scholar]
- 55.Berarducci B, Sommer M, Zerboni L, Rajamani J, Arvin AM. Cellular and viral factors regulate the varicella-zoster virus gE promoter during viral replication. J Virol. 2007;81:10258–10267. doi: 10.1128/JVI.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones JO, Arvin AM. Inhibition of the NF-κB pathway by varicella-zoster virus in vitro and in human epidermal cells in vivo. J Virol. 2006;80:5113–5124. doi: 10.1128/JVI.01956-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arvin AM, et al. The early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–429. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- 58.Zerboni L, et al. Analysis of varicella zoster virus attenuation by evaluation of chimeric parent Oka/vaccine Oka recombinant viruses in skin xenografts in the SCIDhu mouse model. Virology. 2005;332:337–346. doi: 10.1016/j.virol.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 59.Schmid DS. Varicella-zoster virus vaccine: molecular genetics. Curr Top Microbiol Immunol. 2010;342:323–340. doi: 10.1007/82_2010_14. [DOI] [PubMed] [Google Scholar]
- 60.Gershon MD, Gershon AA. VZV infection of keratinocytes: production of cell-free infectious virions in vivo. Curr Top Microbiol Immunol. 2010;342:173–188. doi: 10.1007/82_2010_13. This paper reviews the process of VZV replication and release of cell-free virus into cutaneous skin lesions that are needed for transmission to other susceptible individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sen N, et al. Varicella-zoster virus immediate-early protein 62 blocks interferon regulatory factor 3 (IRF3) phosphorylation at key serine residues: a novel mechanism of IRF3 inhibition among herpesviruses. J Virol. 2010;84:9240–9253. doi: 10.1128/JVI.01147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandevenne P, et al. The varicella-zoster virus ORF47 kinase interferes with host innate immune response by inhibiting the activation of IRF3. PLoS ONE. 2011;6:e16870. doi: 10.1371/journal.pone.0016870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambagala AP, Cohen JI. Varicella-Zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response. J Virol. 2007;81:7844–7851. doi: 10.1128/JVI.00325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carpenter JE, Jackson W, Benetti L, Grose C. Autophagosome formation during varicella-zoster virus infection following endoplasmic reticulum stress and the unfolded protein response. J Virol. 2011;85:9414–9424. doi: 10.1128/JVI.00281-11. This paper shows the role of autophagy in the intrinsic response of skin cells to VZV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reichelt M, et al. Entrapment of viral capsids in nuclear PML cages is an intrinsic antiviral host defense against varicella-zoster virus. PLoS Pathog. 2011;7:e1001266. doi: 10.1371/journal.ppat.1001266. This paper identifies a previously unrecognized function of PML in sequestering virions in the nuclei of neurons, satellite cells and epidermal cells and the antiviral effects of PML isoform IV that are mediated by this process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reichelt M, et al. 3D reconstruction of herpesvirus infected cell nuclei and PML nuclear cages by serial section array scanning electron microscopy and electron tomography. PLoS Pathog. 2012;8:e1002740. doi: 10.1371/journal.ppat.1002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L, et al. Disruption of PML nuclear bodies is mediated by ORF61 sumo-interacting motifs and required for varicella-zoster virus pathogenesis in skin. PloS Pathog. 2011;7:e1002157. doi: 10.1371/journal.ppat.1002157. This study shows that VZV must disrupt the architecture of PML-NBs in order to replicate in skin and documents the importance of the SUMO-interacting motifs of the ORF61 protein in disarming this antiviral mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Sommer M, Rajamani J, Arvin AM. Regulation of the ORF61 promoter and ORF61 functions in varicella-zoster virus replication and pathogenesis. J Virol. 2009;83:7560–7572. doi: 10.1128/JVI.00118-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Breuer J. VZV molecular epidemiology. Curr Top Microbiol Immunol. 2010;342:15–42. doi: 10.1007/82_2010_9. [DOI] [PubMed] [Google Scholar]
- 70.Santos RA, et al. Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures and SCID-hu mice. Virology. 2000;275:306–317. doi: 10.1006/viro.2000.0507. This paper provides the first evidence of a naturally occurring variant of VZV that has an increased capacity to replicate in skin. [DOI] [PubMed] [Google Scholar]
- 71.Oliver SL, et al. Mutagenesis of varicella-zoster virus glycoprotein I (gI) identifies a cysteine residue critical for gE/gI heterodimer formation, gI structure, and virulence in skin cells. J Virol. 2011;85:4095–4110. doi: 10.1128/JVI.02596-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sato B, et al. Mutational analysis of open reading frames 62 and 71, encoding the varicella-zoster virus immediate-early transactivating protein, IE62, and effects on replication in vitro and in skin xenografts in the SCID-hu mouse in vivo. J Virol. 2003;77:5607–5620. doi: 10.1128/JVI.77.10.5607-5620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Che X, et al. Functions of the ORF9-to-ORF12 gene cluster in varicella-zoster virus replication and in the pathogenesis of skin infection. J Virol. 2008;82:5825–5834. doi: 10.1128/JVI.00303-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Che X, et al. ORF11 protein interacts with the ORF9 essential tegument protein in varicella-zoster virus infection. J Virol. 2013;87:5106–5117. doi: 10.1128/JVI.00102-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X, Li Q, Dowdell K, Fischer ER, Cohen JI. Varicella-zoster virus ORF12 protein triggers phosphorylation of ERK1/2 and inhibits apoptosis. J Virol. 2012;86:3143–3151. doi: 10.1128/JVI.06923-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu X, Cohen JI. Varicella-zoster virus ORF12 protein activates the phosphatidylinositol 3-kinase/Akt pathway to regulate cell cycle progression. J Virol. 2013;87:1842–1848. doi: 10.1128/JVI.02395-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z, et al. Genome-wide mutagenesis reveals that ORF7 is a novel VZV skin-tropic factor. PLoS Pathog. 2010;6:e1000971. doi: 10.1371/journal.ppat.1000971. This study uses comprehensive screening to establish which VZV genes are required and which genes are dispensable for replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaudhuri V, Sommer M, Rajamani J, Zerboni L, Arvin AM. Functions of varicella-zoster virus ORF23 capsid protein in viral replication and the pathogenesis of skin infection. J Virol. 2008;82:10231–10246. doi: 10.1128/JVI.01890-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pevenstein SR, et al. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73:10514–10518. doi: 10.1128/jvi.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang K, et al. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single cell level. J Virol. 2005;79:14079–14087. doi: 10.1128/JVI.79.22.14079-14087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azarkh Y, Gilden D, Cohrs RJ. Molecular characterization of varicella zoster virus in latently infected human ganglia: physical state and abundance of VZV DNA, quantitation of viral transcripts and detection of VZV-specific proteins. Curr Top Microbiol Immunol. 2010;342:229–241. doi: 10.1007/82_2009_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nagel MA, et al. Varicella-zoster virus transcriptome in latently infected human ganglia. J Virol. 2011;85:2276–2287. doi: 10.1128/JVI.01862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahalingam R, et al. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci USA. 1996;93:2122–2124. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lungu O, et al. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA. 1998;95:7080–7085. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zerboni L, et al. Apparent expression of varicella-zoster virus proteins in latency resulting from reactivity of murine and rabbit antibodies with human blood group A determinants in sensory neurons. J Virol. 2012;86:578–583. doi: 10.1128/JVI.05950-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gowrishankar K, et al. Characterization of the host immune response in human ganglia after herpes zoster. J Virol. 2010;84:8861–8870. doi: 10.1128/JVI.01020-10. This paper shows the prominent characteristics of the host immune cell response to VZV reactivation in ganglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steain M, Gowrishankar K, Rodriguez M, Slobedman B, Abendroth A. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural varicella-zoster virus infection. J Virol. 2011;85:626–631. doi: 10.1128/JVI.01816-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steain M, Slobedman B, Abendroth A. Experimental models to study varicella-zoster virus infection of neurons. Curr Top Microbiol Immunol. 2010;342:211–228. doi: 10.1007/82_2010_15. [DOI] [PubMed] [Google Scholar]
- 89.Hood C, et al. Varicella-zoster virus ORF63 inhibits apoptosis of primary human neurons. J Virol. 2006;80:1025–1031. doi: 10.1128/JVI.80.2.1025-1031.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Janer A, et al. PML clastosomes prevent nuclear accumulation of mutant ataxin-7 and other polyglutamine proteins. J Cell Biol. 2006;174:65–76. doi: 10.1083/jcb.200511045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weinberg A, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reichelt M, Zerboni L, Arvin AM. Mechanisms of varicella-zoster virus neuropathogenesis in human dorsal root ganglia. J Virol. 2008;82:3971–3983. doi: 10.1128/JVI.02592-07. This paper reports that the capacity of VZV to induce cell fusion includes the fusion of satellite cells and neurons in infected DRG in addition to polykaryocte formation in skin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zerboni L, et al. Herpes simplex virus 1 tropism for human sensory ganglion neurons in the severe combined immunodeficiency mouse model of neuropathogenesis. J Virol. 2013;87:2791–2802. doi: 10.1128/JVI.01375-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esiri MM, Tomlinson AH. Herpes zoster: demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- 95.Zerboni L, et al. Varicella-zoster virus glycoprotein E is a critical determinant of virulence in the SCID mouse-human model of neuropathogenesis. J Virol. 2011;85:98–111. doi: 10.1128/JVI.01902-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zerboni L, Reichelt M, Arvin A. Varicella-zoster virus neurotropism in SCID mouse-human dorsal root ganglia xenografts. Curr Top Microbiol Immunol. 2010;342:255–276. doi: 10.1007/82_2009_8. [DOI] [PubMed] [Google Scholar]
- 97.Zerboni L, et al. Aberrant infection and persistence of varicella-zoster virus in human dorsal root ganglia in vivo in the absence of glycoprotein I. Proc Natl Acad Sci USA. 2007;104:14086–14091. doi: 10.1073/pnas.0706023104. This study documents the unexpected finding that the mutation of VZV proteins can result in chronic infectious processes in DRG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang ZH, et al. Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument. J Virol. 2001;75:323–340. doi: 10.1128/JVI.75.1.323-340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahalingam R, Messaoudi I, Gilden D. Simian varicella virus pathogenesis. Curr Top Microbiol Immunol. 2010;342:309–321. doi: 10.1007/82_2009_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ouwendijk WJ, et al. T-cell infiltration correlates with CXCL10 expression in ganglia of cynomolgus macaques with reactivated simian varicella virus. J Virol. 2013;87:2979–2982. doi: 10.1128/JVI.03181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kinchington PR, Goins WF. Varicella zoster virus-induced pain and post-herpetic neuralgia in the human host and in rodent animal models. J Neurovirol. 2011;17:590–599. doi: 10.1007/s13365-011-0069-7. This paper shows that VZV is transferred from local sites of inoculation into sensory ganglia and causes hypersensitivity to peripheral nerve stimulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen JJ, Gershon AA, Li Z, Cowles RA, Gershon MD. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J Neurovirol. 2011;17:578–589. doi: 10.1007/s13365-011-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Selariu A, et al. ORF7 of varicella-zoster virus is a neurotropic factor. J Virol. 2012;86:8614–8624. doi: 10.1128/JVI.00128-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oxman MN, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 105.Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316–323. doi: 10.1093/infdis/jiq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tyring S, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89–96. doi: 10.7326/0003-4819-123-2-199507150-00002. [DOI] [PubMed] [Google Scholar]
- 107.Cohen JI, Seidel KE. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc Natl Acad Sci USA. 1993;90:7376–7380. doi: 10.1073/pnas.90.15.7376. This paper reports the first use of VZV cosmids for generating mutant viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tischer BK, et al. A self-excisable infectious bacterial artificial chromosome clone of varicella-zoster virus allows analysis of the essential tegument protein encoded by ORF9. J Virol. 2007;81:13200–13208. doi: 10.1128/JVI.01148-07. This paper reports the first use of a VZV bacterial artificial chromosome (BAC) for generating mutant viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rowe J, Greenblatt RJ, Liu D, Moffat JF. Compounds that target host cell proteins prevent varicella-zoster virus replication in culture, ex vivo, and in SCID-Hu mice. Antiviral Res. 2010;86:276–285. doi: 10.1016/j.antiviral.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]