Abstract
Background
Efficient dietary interventions for hypertensive patients in clinical settings are needed.
Objective
To assess the separate and combined impact of a physician intervention (MD-I) and a patient intervention (PT-I) on dietary intakes of patients with hypertension.
Design
A nested 2 x 2 design, randomized controlled trial over 18-months.
Participants/setting
A total of 32 physicians and 574 outpatients with hypertension.
Intervention
MD-I included training modules addressing the JNC-7 hypertension management guidelines and lifestyle modification. PT-I included lifestyle coaching to adopt the DASH eating pattern, reduce sodium intake, manage weight, increase exercise, and moderate alcohol intake.
Main outcome measures
Dietary intakes measured by the Block Food Frequency Questionnaire. Concordance to the DASH dietary pattern was estimated by a DASH score.
Statistical analyses
The main effects of MD-I and PT-I, and their interaction, were evaluated using ANCOVA.
Results
After six months of intervention, MD-I significantly increased intakes of potassium, fruits, juices and carbohydrate; decreased intake of fat; and improved overall dietary quality by the Healthy Eating Index (HEI). PT-I resulted in increased intakes of carbohydrate, protein, fiber, calcium, potassium, fruits and fruit juices, vegetables, dairy and HEI, and decreased intakes in fat, saturated fat, cholesterol, sodium, sweets, added fats/oils/sweets, and glycemic index. In addition, PT-I improved overall DASH concordance score. The change in DASH score was significantly associated with the changes in Blood pressure and weight at six month. At 18 months, most changes reversed back toward the baseline levels including the DASH score.
Conclusions
Both MD-I and PT-I improved eating patterns at six months with some sustained effects at 18 months. Even though all dietary changes observed were consistent with the DASH nutrient targets or food group guidelines, only the PT-I was effective in improving the overall DASH concordance score. This finding affirms the role of medical nutrition therapy in long-term intensive interventions for hypertension risk reduction and weight management and underlines the need for development of maintenance strategies. Furthermore, this study emphasizes the importance of collaborations among physicians, dietitians and trained nutritionists, and lay health advisors while assisting patients to make healthy behavior changes.
Keywords: diet, lifestyle, intervention, hypertension
Introduction
Nutrition and lifestyle habits contribute substantially to the development and management of many chronic diseases and conditions. Primary care providers (PCP) are importantly positioned to address these topics. However, physicians often lack time or appropriate training to address these topics effectively and efficiently.1 In fact, one of the objectives of Healthy People 2020 was to increase the proportion of physician office visits made by patients with a diagnosis of cardiovascular disease (CVD), diabetes, or hyperlipidemia that include counseling or education related to diet or nutrition from the 20.8% in 2007 to 22.9% by 2020.2 Compounding this challenge is that the evidence base for PCPs addressing nutrition and physical activity is limited; studies have shown mixed or insignificant results of lifestyle intervention provided by PCPs.3 The U.S. Preventive Services Task Force (USPSTF) concluded from a meta-analysis that dietary and physical activity counseling by health care providers yields small improvements in adiposity, blood pressure (BP) and lipid levels. Regardless of this conclusion, a study showed that patients expect their PCPs to address these topics and infer that PCPs are not concerned about nutrition or physical activity when they do not address them.4 Overall, in 2007, about 52% of adults with obesity received advice from a health provider about healthy eating.5 In a study analyzing data from the 1996 Behavioral Risk Factor Surveillance System, 78% of overweight patients reported attempting to lose weight if they were advised by their physicians to lose weight compared to only 33% if their physicians did not discuss weight loss with them.6 Thus, PCPs should address these important topics. However, PCP counseling is necessary but likely not sufficient. Intensive lifestyle interventions provided by professionals like dietitians or behavioral interventionists have been shown to improve lifestyle behaviors.7 It is unknown whether these interventions are more effective when paired with an intervention aimed at optimizing the physician-patient interactions involving these issues. Thus, we compared the separate and combined impact of two interventions provided by physicians and interventionists on dietary intake in an 18-month lifestyle intervention study in patients with hypertension. Although the primary outcome of the study was BP which has been published elsewhere,8 it is important to investigate how the interventions, both physician-led (MD-I) and interventionist-led (PT-I), affect dietary behaviors because dietary change has the potential to also influence health risk factors other than BP.
Methods
Overview
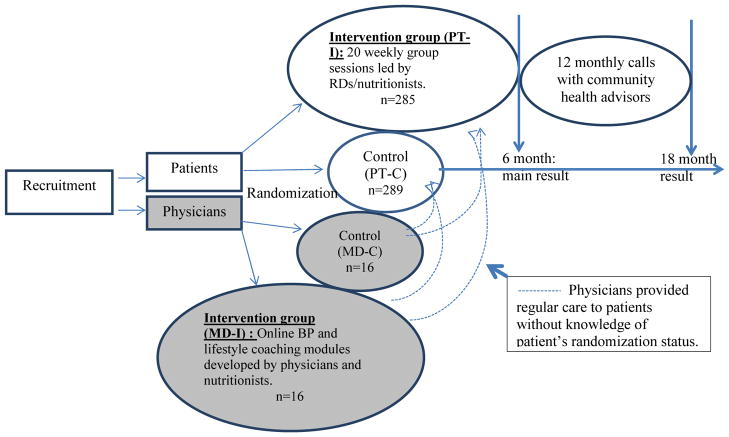
The Hypertension Improvement Project (HIP) study was a nested 2x2 randomized controlled trial of a physician intervention (MD-I), a patient intervention (PT-I), and both combined. The study protocol was approved by the Duke University Institutional Review Board; all physicians and patients in the study provided written informed consent. Primary care practices, matched for MD specialty and participant insurance status, were recruited and randomly assigned to either the MD-I or to the MD control (MD-C) group. All participating MDs within a given practice had the same randomization assignment. After enrolling practices and MDs, patients were then recruited from the patient panels of the enrolled MDs. Approximately ten to 15 patients cared for by each participating MD were enrolled and randomized to the PT-I or usual care (PT-C). A total of 8 practices (4 per treatment group), 32 physicians (3–5 per practice; 16 per treatment group), and 574 patients (289 control and 285 intervention) were enrolled. The main result and a detailed description of the intervention design have been reported elsewhere.8,9 Figure 1 illustrates the overall design of the study.
Figure 1.
Study Design
The active PT-I was based on key theoretical constructs developed to guide health behavior change efforts via lifestyle coaching, and on practical applications from previous trials of lifestyle change and CVD risk reduction.7,10 The PT-I lasted for five months, during which the patients met in small groups (n = 10–15 per group) weekly for 20 weeks. All intervention sessions were delivered by interventionists, either dietitians or nutritionists, trained in motivational interviewing and experienced in delivering lifestyle intervention. Specific behavior change strategies focused in PT-I included self-monitoring, goal setting, problem-solving, and social support. The specific lifestyle goals recommended to the patients included: 1) follow the DASH eating plan, 2) lose weight if overweight, 3) do moderate exercise up to 180 minutes a week or more, 4) reduce sodium intake to less than 2400 mg/d, 5) limit alcohol intake to one drink per day for women and two for men, 6) track food and beverage intake for 4 days per week or more, and 7) take BP medications as prescribed. The materials developed to deliver the intervention included a leader’s guide and a patient manual featuring self-monitoring tools. The leader’s guide provided a standardized framework and structure for each group session, as well as resource materials for session discussions. The interventionists were trained to follow the session outline as closely as possible, while appreciating the importance of interactions among group patients that may have taken the discussion in unanticipated directions. In addition, the intervention director regularly observed the group sessions and listened to the monthly calls and provided feedback to the interventionists to ensure delivery of consistent intervention to all the participants. The patient manual provided patients with the general format, outline, and worksheets for each session. It included information about diet and physical activity and emphasized lifestyle behavior changes. This manual was intended to complement and supplement the group session process and content, and to serve as a workbook during the sessions and as a reference between sessions.
The sessions occurred at or near the patients’ clinic site, providing a familiar location for these sessions. The study also employed volunteer community health advisors (CHAs) who were identified and recruited from the same communities as the target population. These “natural leaders” were trained to participate in all aspects of the patient intervention. They helped conduct the sessions with the interventionists and were asked to lead portions of the activities or discussion so that the intervention was delivered in conjunction with members of the community. The CHAs also made monthly calls during the 6 months while the intervention was delivered and during the 12 months after the intervention. Thus, the interventionists were the main “coach” during the first 6 months of the intervention with the assistance of the CHAs and then the CHAs served as the main contact during the last 12 months of follow up. The CHAs participated in a 20-hour training program consisting of 4 weekly sessions covering general information about hypertension, diet and physical activity interventions for BP control, community resources, facilitation of group education sessions, practical guidelines for helping peers, listening skills, lifestyle behavior change techniques, and skills in stress management, problem-solving, and goal setting. Certification and on-going supervision were similar to that provided to the interventionists. The CHAs served as familiar, non-authoritative resources for study patients, strengthening communication between the research group and the patients and providing additional social support. The intent was that the CHAs would develop sufficient knowledge and skills to serve as ongoing resources in their communities and to help sustain the effects of the project after completion.
The MD-I intervention consisted of 1) two continuing education training modules addressing the JNC-7 (The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) BP management guidelines with emphasis on lifestyle modification for BP control, 2) an evaluation and treatment algorithm that summarized the major BP management guidelines set forth in JNC-7 offered in a pocket size laminated card, and 3) a continuous quality improvement (CQI)-type procedure involving assessment of clinical process measures (CPMs) and quarterly feedback to MDs on their adherence to guidelines, including those related to lifestyle counseling. The ultimate goal of the MD-I is to equip the MDs to adhere to the JNC-7 guidelines for BP control which includes lifestyle intervention. The lifestyle modification portion of the MD intervention includes training for the MDs to deliver a brief discussion of the DASH dietary pattern, weight loss, sodium reduction and exercise. However, the MDs were not specifically asked to deliver the intervention to any particular patient. In fact, the MDs were not informed of their patients’ treatment assignment and the patients were instructed not to discuss their assignment with their MDs, nevertheless, strict blinding of the MDs was not possible to be enforced. The MD intervention was delivered mainly via the online modules and supplemented with the feedback provided. All physicians completed the continuing education modules before the first group session of the PT-I.
Patients randomized to the usual care control group had an individual brief visit with an interventionist after randomization, during which they received advice and brochures on lifestyle modification for BP control consistent with JNC-7. At the end of the study (18 months), after the final data collection visit, patients in the control group were offered an abbreviated version of the active intervention, which consisted of 6 weekly group sessions to help them make lifestyle changes to control BP (wait-list control).
Measurements
All study measurements obtained from patients were collected during face-to-face clinic visits by trained, certified study personnel who were blinded to intervention assignment. At each time point (baseline, 6- and 18-month follow-up), weight was measured with a digital scale, and BP was measured according to JNC-7 guidelines by an oscillometric BP machine. Dietary intake was assessed by administering the Block Food Frequency Questionnaire (FFQ, version 98.2)11 and the dietary intakes of food groups and nutrients of these patients were examined. The Healthy Eating Index (HEI)—an overall index for diet quality12--and dietary glycemic index and glycemic load were also estimated because an important feature of the DASH diet intervention was to reduce sugar-sweetened beverages and desserts. In addition, a DASH score was estimated incorporating 9 nutrient targets established in the original DASH study13. Intakes meeting the targets yield a score of 1. Intakes meeting levels intermediate between DASH targets and levels of the control diet in the original DASH study were assigned a score of 0.5 (Table 1). Intakes below the intermediate levels for the positive nutrients and greater than the intermediate levels for the negative nutrients were assigned a score of 0. The DASH score was the sum of the individual score for the 9 nutrients, thus with a maximum score of 9 and a minimum of 0. Urinary excretion of sodium, potassium and phosphorus were examined by standard methodology from a 24 hour urine collection. Urinary sodium was measured as a biomarker of dietary sodium intake, potassium as a biomarker for fruit and vegetable intake, and phosphorus as a biomarker for protein and dairy intake.
Table 1.
Nutrient targets for DASH score
| Nutrient | DASH diet targeta | Score 1 | Score 0.5 | Score 0 |
|---|---|---|---|---|
|
| ||||
| Total fat, %kcal | 27 | ≤27 | >27 & ≤32 | >32 |
| Saturated fat, %kcal | 6 | ≤6 | >6 & ≤11 | >11 |
| Cholesterol, mg | 150 | ≤150 | >150 & ≤225 | >225 |
| Protein, % kcal | 18 | ≥18 | <18 & ≥16.5 | <16.5 |
| Fiber, g | 31 | ≥31 | <31 & ≥20 | <20 |
| Magnesium, mg | 500 | ≥500 | <500 & ≥332 | <332 |
| Calcium, mg | 1240 | ≥1240 | <1240 & ≥844 | <844 |
| Potassium, mg | 4700 | ≥4700 | <4700 & ≥3221 | <3221 |
| Sodium, mg | 2400 | ≤2400 | >2400 & ≤2700 | >2700 |
Based on 2100 kcal/day.
Statistical analysis
The main effects of MD-I and PT-I as well as the interaction between the two interventions were evaluated for each of the nutrients using ANCOVA. The main effect for MD essentially compares dietary intakes in the MD-I/PT-I and MD-I/PT-C groups with the MD-C/PT-I and MD-C/PT-C groups, respectively. Similarly, the main effect for PT essentially compares dietary intakes in the MD-I/PT-I and MD-C/PT-I groups with the MD-I/PT-C and MD-C/PT-C groups, respectively.
For each outcome separate models were analyzed to evaluate: 1) baseline nutrient level, 2) change in nutrient level from baseline to 6 months and 3) change in nutrient level from baseline to 18 months. All models were adjusted for cohort, and baseline nutrient level was included for models evaluating change. Outcomes with significant results for the minimally adjusted models were re-analyzed with additional adjustment. To minimize the risk of over adjusting, additional covariates were chosen with careful consideration of their likely impact on dietary intake resulting in the inclusion of age, race, gender and baseline BMI. These covariates were available for all patients; thus, sample size was not reduced due to missing covariate data. In addition, spearman correlation analysis was conducted to explore how the changes in DAHS score may be associated with changes in weight and BP at 6 month when the greatest changes in dietary intakes were observed.
Due to our concern that reducing the Type I error rate may result in missing true associations between some outcomes and intervention, we chose not to adjust for multiple comparisons. Instead, significance was evaluated using combined criteria of: p≤0.05, consistent results between related nutrients, and effect sizes with direction and magnitude that meet clinical expectations. In general, the interactions were not significant, thus we focused on the main intervention effect for this report. All results are presented as mean ±SD. Least square means were adjusted for all factors in the models. Unadjusted means are presented in tables 2–5 for purposes of illustration and are intended to help explain the pattern of results, however, all of the statistical testing was conducted using ANCOVA models that adjusted for age, gender, race, cohort, and BMI.
Table 2.
Baseline characteristics of the study participants
| Characteristica | Total | MD-C/PT-C | MD-C/PT-I | MD-I/PT-C | MD-I/PT-I |
|---|---|---|---|---|---|
| n | 574 | 141 | 140 | 148 | 145 |
| Age, yr | 60.6 (11.1) | 60.9 (11.8) | 59.4 (12.0) | 61.6 (10.2) | 60.4 (10.5) |
| BMI, Kg/m2 | 32.6 (5.5) | 32.9 (5.7) | 31.8 (5.5) | 32.7 (5.5) | 33.1 (5.3) |
| SBP, mmHg | 132.8 (16.1) | 132.2 (15.0) | 131.1 (17.9) | 133.8 (14.2) | 134.0 (17.3) |
| DBP, mmHg | 73.8 (11.1) | 73.9 (10.7) | 71.6 (11.7) | 74.1 (11.0) | 75.6 (11.0) |
| Female, # (%) | 297 (62) | 74 (64) | 80 (71) | 76 (58) | 67 (56) |
| African American, # (%) | 182 (38) | 50 (43) | 48 (42) | 41 (31) | 43 (36) |
| Adequate Income, # (%) | 390 (85) | 92 (84) | 91 (86) | 111 (89) | 96 (82) |
| Education, completed high school, # (%) | 440 (93) | 106 (93) | 105 (95) | 119 (92) | 110 (92) |
| Hypertension, # (%) | 462 (96) | 114 (98) | 110 (97) | 126 (96) | 112 (93) |
| Medication adherence, # (%) | 369 (83) | 81 (79) | 85 (83) | 111 (88) | 92 (81) |
All values are expressed as either Mean (SD) or #(%). MD-C: Physician control, PT-C: patient control, MD-I: Physician intervention, PT-I: Patient intervention. There are no significant differences in the characteristics among the treatment groups.
Results
Sample characteristics
At baseline, the mean age of patients was 60.6 ±11.1 years, mean BMI was 32.6 ±5.5 kg/m2 and mean SBP/DBP was 132.8±16.1/73.8±11.1 mmHg (Table 2). Nearly two thirds of the patients were female (62%), more than one third (38%) were African American, most had “self-described” adequate income, had at least a high school education and almost all had hypertension (96%). There were no significant differences in the baseline characteristics among treatment groups. As reported in the main result paper8, 89% to 94% of the participants completed the six month visits and 87% to 91% completed the 18 month data collection. Compliance was not significantly different among the treatment groups. In addition, dietary intake, weight and urinary data also did not differ between those who completed the study and those who dropped out. Since many vitamins and minerals including vitamins A, B1, B2, niacin, B6, B12, folate, D, E, copper, iron, selenium and manganese were not influenced by the interventions significantly, this report will focus mainly on the food groups and nutrients that were targeted in the original DASH study (energy, carbohydrate, fat, protein, saturated fat, cholesterol, fiber, calcium, magnesium, potassium and sodium).11
Baseline dietary intakes were similar across treatment groups with the only exception that those receiving the patient intervention consumed significantly more energy from sweets at baseline (p<0.05, β=2.34) than those receiving usual care (Table 3). No other variable was significantly different between the intervention and control groups (Tables 3 and 4). The baseline DASH score also suggested that most participants consumed the DASH nutrients below the targets, thus the scores averaged slightly greater than 3 (possible maximum score of 9).
Table 3.
baseline and changes in nutrient intakes at 6 and 18 months
| Variables | Mos. | Unadjusted Mean(SD) | Parameter Estimates (β) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| MD-C/PT-C | MD-C/PT-I | MD-I/PT-C | MD-I/PT-I | MD-I vs. MD-C | PT-I vs. PT-C | ||
| Calories, Kcal | |||||||
| Ba | 1725(763) | 1664(843) | 1594(643) | 1780(826) | −55.4 | 71.4 | |
| 6 | −170.6(554) | −249.8(586) | −72.2(464) | −287.4(660) | 18.7 | −124.34* | |
| 18 | −119.1(698) | −158.8(535) | −73(543) | −260.8(741) | −43.75 | −90.94 | |
| Carbohydrate, % kcal | |||||||
| B | 48.6(9.5) | 48.2(10.3) | 46.8(9) | 48.8(8.4) | 0.12 | 0.7 | |
| 6 | −0.4(7.8) | 2.5(10.2) | 0.9(7.4) | 4.7(8.4) | 1.81* | 3.72** | |
| 18 | −0.9(8.3) | 2.2(9.9) | 1.1(7.9) | 3(8.6) | 1.58* | 2.83** | |
| Fat, % kcal (DASH target=27%)b | |||||||
| B | 38.1(8.2) | 38.4(7.9) | 39.8(8.4) | 37.5(6.8) | 0.08 | −0.98 | |
| 6 | 0.6(7.2) | −2.4(8.5) | −1(6.7) | −4.5(7) | −1.83* | −3.68** | |
| 18 | 0.9(7.7) | −1.7(7.9) | −1.2(6.5) | −2.6(7.8) | −1.67* | −2.44** | |
| Protein, % kcal (DASH target=18%) | |||||||
| B | 14.6(3) | 14.2(3) | 14.7(2.9) | 14.5(2.4) | 0.02 | −0.21 | |
| 6 | 0.02(3.2) | 0.8(3) | 0.2(2.5) | 0.8(2.5) | 0.08 | 0.56* | |
| 18 | 0.1(2.5) | 0.01(3.8) | 0.2(3.1) | 0.3(3) | 0.24 | −0.14 | |
| Energy from sweets, %kcal | |||||||
| B | 13.9(9.4) | 16(11.3) | 12.9(8.8) | 16(9.1) | −0.09 | 2.34* | |
| 6 | −0.9(8.2) | −4.5(11.7) | −0.5(5.7) | −5.4(7.6) | −0.31 | −3.16** | |
| 18 | −1.2(8.5) | −1.9(10.5) | −0.2(7) | −3(8.8) | 0.05 | −0.8 | |
| Saturated fat, g (DASH target≤13 g) | |||||||
| B | 20.9(11.6) | 20.5(12) | 19.9(10.6) | 21.5(12.6) | −0.9 | 0.69 | |
| 6 | −1.7(6.9) | −4.7(9.6) | −1(6.8) | −5.9(10.2) | −0.36 | −3.75** | |
| 18 | −1.1(9) | −3.4(8.3) | −1.2(8) | −4.7(11.6) | −0.97 | −2.71** | |
| Cholesterol, mg (DASH target=150 mg) | |||||||
| B | 209(129) | 198(138) | 196(104) | 217(135) | −9.7 | 8.6 | |
| 6 | −16.5(67.8) | −30.2(116) | −4.7(76.1) | −49.8(111) | −2.82 | −29.31** | |
| 18 | −8.5(96.6) | −26.9(99.5) | −5.9(115) | −43.5(115) | −8.38 | −25.68* | |
| Fiber, g (DASH target=28 g) | |||||||
| B | 17.2(9) | 16.3(9.4) | 15.9(8.2) | 16.4(8.4) | −1.03 | 0.04 | |
| 6 | −1.8(7.5) | 0.3(8.7) | −0.7(5.6) | 2(7.4) | 0.98 | 2.36** | |
| 18 | −0.9(9.9) | 0.2(8.6) | −0.5(6.7) | 0.6(7.3) | 0.15 | 0.99 | |
| Calcium, mg (DASH target=1131 mg) | |||||||
| B | 638(331) | 610(367) | 571(310) | 618(298) | −48.4 | 17.7 | |
| 6 | −51.8(259) | 27.9(274) | −2(196) | 53.3(317) | 23.32 | 71.29* | |
| 18 | −52.9(294) | −5.5(256) | 9.6(219) | −12(291) | 11.57 | 15.31 | |
| Magnesium, mg (DASH target=450 mg) | |||||||
| B | 279(127) | 264(136) | 262(110) | 269(124) | −13.9 | −0.76 | |
| 6 | −23.3(95.1) | −10.1(98.8) | −10.7(78.3) | 8.2(110) | 10.14 | 15.09 | |
| 18 | −17.6(126) | −10.2(98) | −3.3(89.6) | −6.5(107) | 4.03 | 1.02 | |
| Potassium, mg (DASH target=3932 mg) | |||||||
| B | 2662(1127) | 2475(1212) | 2470(925) | 2621(1079) | −99.5 | 13.1 | |
| 6 | −253(904) | −19.5(874) | −33.9(657) | 152(942) | 166.3* | 208* | |
| 18 | −205(1078) | −72(801) | −26.4(781) | −49.4(1007) | 67.86 | 53.59 | |
| Sodium, mg (HIP guideline: ≤2300 mg/d) | |||||||
| B | 2345(1114) | 2134(1215) | 2249(968) | 2346(1170) | −30.4 | −34.7 | |
| 6 | −217(792) | −234(902) | −114(703) | −338(1051) | 3.1 | −149* | |
| 18 | −163(968) | −145(828) | −114(858) | −352(1107) | −74.1 | −134 | |
| Urine Phosphorous, mg/dL | |||||||
| B | 825(387) | 730(342) | 846(570) | 824(400) | 10.6 | −49.4 | |
| 6 | −112(352) | −0.4(296) | −123(529) | −91.6(373) | −30.2 | 27.67 | |
| 18 | −64.2(335) | 79.1(337) | −62.7(556) | −37.1(354) | −51.01 | 41.66 | |
| Urine Potassium, mg/dL | |||||||
| B | 61.6(31.9) | 51.7(23) | 61.3(22.8) | 61.2(27.9) | 0.71 | −3.3 | |
| 6 | −8.1(27.5) | 4.7(19) | −3.1(23.1) | −0.2(26.3) | 1.24 | 5.4* | |
| 18 | −5.7(25.1) | 8.4(22.4) | 3(43.1) | −3.9(23) | −1.26 | 0.57 | |
| Urine Sodium, mg/24hr | |||||||
| B | 4018(1771) | 3471(1564) | 4030(1907) | 3917(1753) | 5.7 | −8.7 | |
| 6 | −524.4(1638) | −301.3(1431) | −542.8(1730) | −722.2(1833) | −7.59 | −8.15 | |
| 18 | −190.9(1934) | −552(1960) | −32.2(1608) | −644(1762) | −17.4* | −8.73 | |
B=baseline.
DASH daily targets tested in the original DASH feeding study at 2000 kcal level or HIP guidelines are included in parentheses.
p<0.05,
p<0.001, all models were adjusted for cohort, baseline nutrient intake, age, BMI, gender and race. All variables are dietary data from FFQ except as specified for the 24-hour urine variables.
Table 4.
Baseline and changes in food group consumption and dietary quality at 6 and 18 months
| Variables | Mos. | Unadjusted Mean(SD) | Parameter Estimates (β) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| MD-C/PT-C | MD-C/PT-I | MD-I/PT-C | MD-I/PT-I | MD-I vs. MD-C | PT-I vs. PT-C | ||
| Fruits and fruit juices, serv (DASH and HIP guideline: 4–6 servings/d)a | |||||||
| Bb | 1.3(0.9) | 1.3(1) | 1.3(0.9) | 1.4(1.1) | 0.005 | 0.09 | |
| 6 | 0.02(0.9) | 0.6(1.3) | 0.1(0.7) | 1(1.3) | 0.21* | 0.78** | |
| 18 | −0.02(0.9) | 0.4(1.1) | 0.01(0.8) | 0.5(1.2) | 0.06 | 0.53** | |
| Vegetables, serv (DASH and HIP guideline: 4–6 servings/d) | |||||||
| B | 2.9(1.9) | 3(2.2) | 2.8(1.6) | 3.1(2.4) | −0.12 | 0.23 | |
| 6 | 0.1(1.7) | 0.5(2.4) | 0.2(1.6) | 1(2.5) | 0.25 | 0.75** | |
| 18 | 0.3(2.5) | 0.03(1.8) | 0.04(1.8) | 0.3(2.5) | −0.09 | 0.07 | |
| Whole grains, serv | |||||||
| B | 1.7(1.6) | 1.4(1.2) | 1.5(1.1) | 1.3(1) | −0.15 | −0.21 | |
| 6 | −0.02(1.4) | 0(1.3) | 0.1(1.1) | 0.3(1.3) | 0.12 | −0.01 | |
| 18 | −0.03(1.6) | −0.1(1.3) | 0.1(1.3) | 0.1(1.2) | 0.12 | −0.17 | |
| Dairy, serv (DASH and HIP guideline: 2–3 servings/d) | |||||||
| B | 1(0.9) | 0.9(0.8) | 0.9(0.9) | 0.9(0.8) | −0.09 | −0.01 | |
| 6 | −0.04(0.7) | 0.1(0.6) | 0.02(0.5) | 0.2(0.7) | 0.07 | 0.16* | |
| 18 | −0.1(0.8) | −0.01(0.6) | 0.1(0.5) | 0.03(0.7) | 0.05 | −0.003 | |
| Added Fats/oils/sweets, serv (DASH and HIP guideline: ≤ 3 servings/d) | |||||||
| B | 2.9(1.5) | 2.8(1.6) | 2.8(1.4) | 3(1.7) | 0.06 | 0.09 | |
| 6 | −0.4(1.2) | −0.6(1.4) | −0.2(1.3) | −0.9(1.4) | 0.06 | −0.45** | |
| 18 | −0.1(1.5) | −0.2(1.4) | −0.1(1.4) | −0.6(1.6) | −0.16 | −0.28* | |
| Daily Glycemic Loadc | |||||||
| B | 102(49.1) | 96.9(50.9) | 89.9(35.8) | 106(51.3) | −2.45 | 5.28 | |
| 6 | −11.6(41.8) | −12.9(35.5) | −2.7(28.5) | −12.3(43.6) | 3.68 | −3.1 | |
| 18 | −11.5(45.2) | −6.4(34.5) | −3.4(32.1) | −13(45) | 0.03 | −0.2 | |
| Daily Glycemic Index | |||||||
| B | 53.8(4.3) | 53.5(4.2) | 54(4) | 53.5(4.2) | 0.25 | −0.56 | |
| 6 | 0.01(3.6) | −0.6(4.4) | −0.03(3.3) | −0.6(4.1) | 0.04 | −0.84* | |
| 18 | −0.3(3.7) | −0.5(4.5) | −0.5(3.3) | −0.5(3.6) | 0.08 | −0.43 | |
| HEI Score | |||||||
| B | 60.9(11.7) | 59.6(12.1) | 60.8(12.2) | 61.3(12.8) | 1.01 | −0.14 | |
| 6 | −0.2(9.4) | 5(12.2) | 0.9(10.5) | 8.9(11.5) | 2.64* | 6.75** | |
| 18 | −1.6(10.4) | 3.7(10.8) | −0.03(10.4) | 4.6(11.6) | 1.46 | 4.91** | |
| DASH score | |||||||
| B | 3.37(1.24) | 3.34(1.26) | 3.21(1.36) | 3.41(1.38) | −0.05 | 0.09 | |
| 6 | −0.04(1.09) | 0.41(1.45) | 0.06(1.20) | 0.68(1.29) | 0.19 | 0.54*** | |
| 18 | 0.03(1.37) | 0.16(1.12) | 0.07(1.21) | 0.27(1.40) | 0.07 | 0.16 | |
DASH guidelines published by National Heart Lung and Blood Institute or HIP guidelines are included in parentheses,
B=baseline.
Based on glucose scale.
p<0.05,
p<0.001,
p<0.0001, all models were adjusted for cohort, baseline nutrient intake, age, BMI, gender and race.
Effect of patient and physician interventions
At 6 months, as reported previously8, the main effect of MD-I on systolic BP, adjusted for baseline pressure, was 0.3 mm Hg (95% CI: −1.5 to 2.2; P=0.72). The main effect of the PT-I was −2.6 mm Hg (95% CI: −4.4 to −0.7; P=0.01). The interaction of the two interventions on BP was significant (P=0.03); the largest impact was observed with the combination of MD-I and PT-I (−9.7±12.7 mm Hg). Differences between treatment groups did not persist at 18 months. PT-I, but not MD-I, led to a significant reduction in weight (−6.1 and +0.6 lb, respectively; P<0.0001 for PT-I main effect).
Both MD-I and PT-I had a significant impact on the dietary intake of the patients (Tables 3 and 4) but the intensity of impact seemed to differ. Patients who were seen by physicians in the MD-I significantly increased intakes of energy from carbohydrate, potassium, fruits and juices, decreased energy from fat, and improved overall HEI (all p<0.05) but the overall concordance to the DASH diet did not improve significantly. Whereas, patients who received the PT-I significantly increased energy intakes from carbohydrate (p<0.05) and protein (p<0.05), increased intakes of fiber (p<0.001), calcium (p<0.05), potassium (p<0.05), fruits and fruit juices (p<0.001), vegetables (p<0.001), and dairy (p<0.05), improved HEI (p<0.001) and increased urinary potassium excretion (p<0.05). In addition, patients in the PT-I significantly decreased intakes of total energy (p<0.05), energy from fat (p<0.001), sweets (p<0.001), saturated fat (p<0.001), added fats/oils/sweets (p<0.001), cholesterol (p<0.001), and sodium (p<0.05), and lowered dietary glycemic index (p<0.05) as compared to the controls. Despite the significant changes from baseline, none of the mean dietary intakes reached the DASH nutrient targets or food group guidelines. However, the DASH score was increased significantly by PT-I on an average of 0.54 unit (p<0.0001). Furthermore, the changes in DASH score at 6 month was significantly correlated with the changes in systolic BP(r=−0.10, p=0.02) and weight (r=−0.15, p=0.001).
At 18 months, some of the dietary changes observed at 6 months remained but more than half of the changes reversed back toward baseline. Relative to the control group, patients who saw physicians in the MD-I arm still significantly increased energy intakes from carbohydrate (p<0.05) and decreased energy intakes from fat (p<0.05), however, no other changes were significant at 18 months. These patients also reduced urinary sodium excretion significantly at 18 months (p<0.05). The impact of the PT-I on carbohydrate energy, fat energy, saturated fat, cholesterol, fruits, added fats/oils/sweets and HEI were weakened but remained significant. All other changes in patients in the PT-I arm were not significant at 18 months. These changes were observed while controlling for covariates including cohort, age, BMI, gender and race. Similarly, the mean dietary intakes at 18 months did not reach the DASH nutrient targets nor the food group guidelines. The DASH score reversed back toward the baseline level and the increase observed at 18 months reduced by more than half from that observed at 6 months (NS).
Discussion
This study has several key findings. First, a detailed examination of the dietary intakes of the HIP patients revealed that both active interventions (MD-I and PT-I) improved patients’ eating pattern significantly at six months. The intensive PT-I impacted more aspects of dietary intake than MD-I did and improved the overall concordance to the DASH dietary pattern significantly at six month as reflected in the increased DASH score. In particular, the PT-I patients reduced intakes in energy, fat, and sweets, and increased intakes of fruits, vegetables and dairy, a pattern recommended by the DASH dietary pattern14. However, the concordance with the DASH dietary pattern was low throughout the study and was similar to that of the NHANES adults with hypertension15. The mean intakes did not reach the DASH nutrient targets or food group guidelines which may partially contribute to the modest but clinically meaningful impact observed in weight and BP. The significant correlation between changes in the DASH score and the changes in weight and systolic BP at six month supports the contribution of the dietary changes to the risk factors reduction. This finding also highlights the need for a greater emphasis on refining intervention programs at the individual, clinic and community levels to improve dietary quality more effectively.
While we did not separately test the combined MD-I/PT-I arm versus each of the two interventions alone, the magnitude of improvement (and maintenance of that improvement) was generally greater for most parameters in the combined arm. This underscores the importance of both interventions and steers us toward what may be the best overall strategy for maximal sustained improvement. Other research supports such a combined team approach intervention for behavior change16 where PCPs can endorse and deliver brief interventions to support the kind of intensive intervention required for more than minimal to moderate impact and that can be delivered by other allied health professionals. This is similar to the model tested in the current study and may be more feasible considering the capacity (time, skill and attitude) limitation of the PCPs. Thus, it is important to continue to develop efficient mechanism to allow dietitian or trained nutritionist led lifestyle intervention be made available to the patients in conjunction with endorsement from physicians. In addition, it is similarly important to develop effective brief lifestyle counseling for PCPs to use in clinical settings that address the common barrier17.
As is commonly seen with extended durations of follow-up, improvements were attenuated at the 18 month follow up. Other intervention programs have shown similar recidivism once intervention contact is reduced7,18. Previous studies8–10 have shown that frequent contact during intensive intervention is important for maximizing the intervention effect and when intervention contact is less frequent, as in the last 12-months of the PREMIER study and in the current study, adherence to the intervention guidelines is also reduced. Results from the current study also indicate that the impact of the MD intervention waned with time, implying that physicians may need frequent and persistent intervention just as patients do. Not-surprisingly, maintenance of changed behavior is difficult. Thus, it is important to develop strategies that can help patients to not only make behavioral changes but also to maintain the changes long term.
A limitation of the study include inherent issues with all dietary data collections and the fact that FFQ may not capture true intakes of the population. In addition, FFQ has been suggested to underestimate macronutrients like fat and overestimate micronutrient intakes.19 Although multiple 24 hour diet recalls is recognized as the preferred method for dietary assessment, FFQ has also been adopted by other studies widely especially in population-based studies.
In conclusion, this study supports previous findings that consuming DASH-concordant dietary pattern is associated with favorable changes in BP and weight. The dietitian/nutritionist led lifestyle intervention program (PT-I) was effective in improving patients’ eating patterns at six months and MD-I seemed to have enhanced the improvement further. However, the PT-I alone improved the overall concordance to the DASH dietary pattern as shown in the DASH score. Most changes reversed back toward the baseline and were not sustained at 18 months which underlines the need for strategies to sustain behavioral changes. This finding affirms the role of medical nutrition therapy in long-term intensive interventions for hypertension risk reduction and weight management. Furthermore, this study also emphasizes the importance of collaborations among physicians, dietitians and trained nutritionists, and lay health advisors while assisting patients to make healthy behavior changes.
Acknowledgments
This work was supported by National Institutes of Health grant R01-HL75373.
L.P.S., W.Y., K.P., R.D., B.B., G.S. and P.L. designed and conducted the research, J.M. and G.S. analyzed data and assisted with interpretation and provided critical review of manuscript, P.L. wrote the manuscript and had the primary responsibility for the final content of the manuscript. All authors approved the manuscript.
Footnotes
Conflict of interest disclosure
The authors have no conflict of interest to disclose.
Reprints not available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dennie S, Williams A, Taggart J, et al. Which providers can bridge the health literacy gap in lifestyle risk factor modification education: a systematic review and a narrative synthesis. BMC Fam Pract. 2012;13:44–73. doi: 10.1186/1471-2296-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. [Accessed on February 26, 2013];Healthy People 2020 Objectives. http://healthypeople.gov/2020/about/default.aspx.
- 3.Lin JS, O’Connor E, Whitlock EP, Beil TL. Behavioral counseling to promote physical activity and a healthful diet to prevent cardiovascular disease in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010 Dec 7;153(11):736–750. doi: 10.7326/0003-4819-153-11-201012070-00007. [DOI] [PubMed] [Google Scholar]
- 4.van Dillen SM, Hiddink GJ, Koelen MA, de Graaf C, van Woerkum CM. Understanding nutrition communication between health professionals and consumers: development of a model for nutrition awareness based on qualitative consumer research. Am J Clin Nutr. 2003 Apr;77(4 Suppl):1065S–1072S. doi: 10.1093/ajcn/77.4.1065S. [DOI] [PubMed] [Google Scholar]
- 5.2010 National Healthcare Quality & Disparities Reports. Agency for Healthcare Research and Quality; 2010. [Google Scholar]
- 6.Stafford RS, Farhat JH, Misra B, Schoenfeld DA. National patterns of physician activities related to obesity management. Arch Fam Med. 2000 Jul;9(7):631–638. doi: 10.1001/archfami.9.7.631. [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003 Apr 23–30;289(16):2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 8.Svetkey LP, Pollak KI, Yancy WS, Jr, et al. Hypertension improvement project: randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension. 2009 Nov 19;(6):1226–1233. doi: 10.1161/HYPERTENSIONAHA.109.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolor RJ, Yancy WS, Jr, Owen WF, et al. Hypertension Improvement Project (HIP): study protocol and implementation challenges. Trials. 2009;10:13. doi: 10.1186/1745-6215-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008 Mar 12;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 11.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epi. 1990;43(12):1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 12.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB. Evaluation of the Healthy Eating Index-2005. J Am Diet Assoc. 2008 Nov;108(11):1854–1864. doi: 10.1016/j.jada.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. New Eng J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 14.Karanja NM, Obarzanek E, Lin PH, et al. Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension trial. J Am Diet Assoc. 1999;99( suppl):S19–S27. doi: 10.1016/s0002-8223(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 15.Mellen PB, Gao SK, Vitolins MZ, Goff DC., Jr Deteriorating Dietary Habits Among Adults With Hypertension: DASH Dietary Accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168(3):308–314. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 16.McManus DD, Ockene IS. Brief supported lifestyle counseling: modest interventions yield modest effects. Arch Intern Med. 2008 Jan 28;168(2):129–130. doi: 10.1001/archinternmed.2007.7. [DOI] [PubMed] [Google Scholar]
- 17.Wynn K, Trudeau JD, Taunton K, Gowans M, Scott I. Nutrition in primary care: current practices, attitudes, and barriers. Can Fam Physician. 2010 Mar;56(3):e109–116. [PMC free article] [PubMed] [Google Scholar]
- 18.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 19.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block 98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9(1):84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]