Abstract
Endothelial cells (ECs) are an essential component of the hematopoietic microenvironment, which maintains and regulates hematopoietic stem cells (HSCs). Although ECs can support the regeneration of otherwise lethally-irradiated HSCs, the mechanisms are not well understood. To further understand this phenomenon, we studied HSC regeneration from irradiated bone marrow using co-culture with human aortic endothelial cells (HAECs). Co-culture with HAECs induced a 24-fold expansion of long-term HSCs (CD150+, lineagelo, Sca-1+, c-Kit+; CD150+LSK cells) in vitro. These cells gave rise to functional hematopoietic stem and progenitor cells (HSPCs) with colony-forming activity, multilineage reconstitution and serial transplantation potential. Furthermore, HAECs significantly reduced DNA damage in irradiated LSK cells within 24 hours. Remarkably, we were able to delay the exposure of irradiated bone marrow to the regenerative, HAEC-derived signals for up to 48 hours and still rescue functional HSCs. G-CSF is the gold standard for promoting hematopoietic regeneration in vivo. However, when compared to HAECs, in vitro G-CSF treatment promoted lineage differentiation and regenerated 5-fold fewer CD150+LSK cells. Together, our results show that HAECs are powerful, direct mitigators of HSC injury and DNA damage. Identification of the HAEC-derived factors that rescue HSCs may lead to improved therapies for hematopoietic regeneration after radiation injury.
Introduction
The hematopoietic system is the most sensitive tissue in the body to the effects of ionizing radiation. Radiation-induced damage to hematopoietic stem cells (HSCs) results in bone marrow failure, which can cause anemia, infection and hemorrhage in irradiated individuals (Mauch et al., 1995; Chao, 2007). In addition to its acute effects, the induction of oxidative stress and DNA damage in HSCs are thought to underlie the increased risks that irradiated individuals have for developing long term complications, including myelofibrosis, myelodysplasia and acute leukemia (Wang et al., 2010; Yahata et al., 2011; Ivanov et al., 2012). Currently, hematopoietic failure following exposure to ionizing radiation is treated with the cytokine granulocyte colony-stimulating factor (G-CSF) (MacVittie et al., 2005; Dainiak, 2010); however, in the absence of endogenous hematopoietic recovery bone marrow transplantation is the only definitive cure. Thus, discovering the mechanisms responsible for regenerating HSCs and restoring functional hematopoiesis may improve future therapies for hematopoietic radiation injury.
HSCs reside in functional niches within the bone marrow microenvironment, where their asymmetric division and differentiation give rise to all blood cell lineages throughout life (for review, see (Wang and Wagers, 2011)). Coordinate signals from other cellular components of the hematopoietic microenvironment modulate HSC proliferation and differentiation through the elaboration of soluble factors and cell adhesion molecules (Chitteti et al., 2010; Chen et al., 2013; Nakamura-Ishizu and Suda, 2013). Endothelial cells (ECs) are microenvironmental components that modulate the proliferation, self-renewal, and differentiation of HSCs at the vascular niche (Kopp et al., 2005; Kobayashi et al., 2010). Our group and others have shown that ECs effectively restore hematopoiesis by regenerating irradiated HSCs both in vitro and in vivo (Chute et al., 2004; Muramoto et al., 2006; Hooper et al., 2009; Li et al., 2010). However, the mechanisms and practicality of EC-mediated hematopoietic regeneration are still largely unexplored.
In this study, we used a co-culture system to study the regeneration of functional murine HSCs by human aortic endothelial cells (HAECs) following whole body irradiation hours (WBI). We report that HAECs rescue hematopoiesis by reversing DNA damage in primitive hematopoietic cells and expanding long-term HSCs. Furthermore, we demonstrate that HAECs can rescue functional HSCs up to 48 hours following HSC radiation injury, whereas G-CSF cannot. Our results show that HAECs robustly support HSC regeneration following radiation injury, and that in vitro, their radiation mitigation is superior to G-CSF.
Materials and Methods
Mice
Congenic male and female 8–12 week old C57Bl/6 mice were used in this study. For transplantation experiments, CD45.2 (Ly5.1) or Ly5.1 EGFP+ (TgN(act-EGFP)OsbY01) mice provided donor bone marrow cells (BMC) and age-matched CD45.1 (Ly5.2) mice were used as transplant recipients. Recipient animals were maintained on acidified water (pH 2.2) for 1 week prior to irradiation and antibiotic-supplemented water for 4 weeks following BMC transplantation. Mice were maintained in accordance with the Institutional Animal Care and Use Committee at Oregon Health & Science University.
Cell Culture Experiments
HAECs (Lonza) were passaged to P3-P5 and then grown to confluence in EGM-2 (Lonza) in 25 cm2 tissue culture flasks. One day prior to the experiment, confluent HAEC monolayers were irradiated with 1200 cGy using a Shepherd 137cesium irradiator at a rate of 166 cGy/min. On the day of the experiment, BMC were harvested from mouse femurs immediately after 580 cGy whole body irradiation (WBI) and kept on ice. Femurs were flushed with ice-cold 3% serum-modified HBSS, and BMC were washed once before being stained with Turk’s solution and counted using a hemocytometer. BMC single cell suspensions (2 × 106 BMC/5 mL media) were prepared using EGM-2 supplemented with the following recombinant murine hematopoietic cytokines (purchased from Peprotech): 5 ng/mL IL-3, 5 ng/mL IL-6, 60 ng/mL stem cell factor, 50 ng/mL FMS-like tyrosine kinase 3 (Flt-3) ligand, 2 ng/mL GM-CSF, and 25 ng/mL thrombopoietin. BMC suspensions were cultured directly on HAEC monolayers (+EC) or plastic (−EC) for 7 days in a humidified 37 °C incubator at 5% CO2. In delayed rescue experiments, irradiated BMC were cultured in supplemented EGM-2 for 24 h or 48 h before being seeded on HAEC monolayers or plastic for a subsequent 7 days. For the G-CSF studies, 200 ng/mL human G-CSF (Neupogen) was added to EGM-2 supplemented with murine hematopoietic cytokines. At the end of the 7 day culture period, supernatants were collected, dishes washed once with Ca2+/Mg2+-free PBS, and treated with 0.25% trypsin-EDTA for 1 min at 37 °C to dissociate adherent cells. Cells were then collected by washing with ice-cold EGM-2, and BMC were counted as described above. HAECs were excluded from total counts by their large size. For lineage(Lin)lo, Sca-1+, c-Kit+ (LSK) cultures, equal numbers of FACS-sorted cells (2–3 × 104) were seeded into one well of a 24-well plate containing media only and one well containing media and an HAEC monolayer. After 24 h of culture, LSK cells were collected after vigorous pipetting and PBS washes.
Flow Cytometry and FACS
Flow cytometry analysis was performed on a BD LSR-II flow cytometer, and data files were analyzed with FlowJo software (Treestar). For CD150+LSK analysis, 1 × 106 BMC from each treatment group were stained with the following antibodies (purchased from eBiosciences unless otherwise indicated): phycoerythrin (PE)-conjugated CD3, CD4, CD5, CD8 (BD Biosciences), Mac-1/CD11b, Gr-1, B220, and Ter119 (Lin marker cocktail); CD150-Brilliant Violet (BV)-421, Sca-1-PE-Cyanine (Cy)7, and c-Kit-allophycocyanin (APC). All antibodies were used at 1:100 except Mac-1-PE, Gr-1-PE, B220-PE (1:200) and CD150-BV-421 (1:50). Propidium iodide (PI) staining was used to exclude dead cells. Absolute CD150+LSK cells were determined by multiplying the CD150+LSK cell proportion by the total BMC number in day 7 cultures for each experimental group. Peripheral blood (PB) leukocytes from transplant recipients were either assayed for EGFP fluorescence or stained with anti-Ly5.1-fluorescein isothiocyanate (FITC) and anti-Ly5.2-APC to determine host- and donor-derived PB cells; furthermore, the antibodies Mac-1/Gr-1-, CD3-, B220-PE were used to determine the proportion of myeloid, T, and B cells, respectively. For secondary transplant experiments, GFP+ c-Kit-APC+ cells were FACS purified from primary recipient BMC isolates using a BD Vantage cell sorter. For DNA damage studies, BMC were harvested immediately following radiation exposure and stained with Lin-PE cocktail, Sca-1-PECy7, c-Kit-APC, and PI, and live LSK cells were sorted to purity with a BD Influx cell sorter. Following culture, recovered cells were stained with Sca-1-PECy7, CD45-FITC and PI, and FACS sorted to purity prior to further analysis.
Methylcellulose Assays
Progenitor cell CFU activity was determined as described (Li et al., 2010). Briefly, BMC harvested from day 7 cultures were plated in mouse methylcellulose medium (Stem Cell Technologies, Inc.) at a density of 2 × 104 BMC/mL in duplicate. Cells were incubated at 37 °C for 7 days and then colonies were scored.
Transplantation Studies
BMC from day 7 cultures were collected as described above and transplanted into recipient mice via retro-orbital injection. 4 × 106 Ly5.1 or Ly5.1 GFP+ BMC/recipient mouse were transplanted in 3% serum-modified HBSS into Ly5.2 mice preconditioned with 750 cGy cesium irradiation, and recipients were followed for up to 30 weeks. For PB engraftment analysis, mice were anaesthetized with inhaled isoflurane and PB was collected from the retro-orbital venous plexus. Erythrocytes were sedimented in 3% dextran for 30 minutes, supernatants collected, and erythrocytes further excluded by hypotonic cell lysis using 0.2% NaCl. BMC engraftment and multilineage reconstitution analysis in transplant recipients were performed as described above.
Comet Assay
To quantify the extent of DNA damage in irradiated LSK cells after 24 h of co-culture, a Comet assay was performed under alkaline conditions as described previously (Olive and Banath, 2006), with the modifications detailed below. The alkaline lysis step was performed overnight at 4 °C. A total volume of 1 mL low melting point agarose containing LSK cells was used per slide at a density of 1500–2000 cells/slide. Slides were cooled on ice to facilitate the polymerization of agarose. All solutions were maintained at 4 °C with the exception of PI staining solution and washes, and all electrophoresis was also performed at 4 °C. Low melting point agarose and alkaline lysis solutions contained 2% DMSO to avoid damage by ion-catalyzed reactive oxygen species. At least 100 LSK cells per condition were scored using Comet IV software and an AVT Marlin Firewire camera. Comet IV software was used to calculate the Olive Tail Moment.
DNA Double Strand Break Analysis
Phosphorylation of the histone variant 2AX at Ser139 (γH2AX) was used to assess the presence of double-strand DNA breaks in LSK cells before and after 24 h of culture with or without HAECs. LSK cells were fixed for 10 minutes in 2% paraformaldehyde, centrifuged, and re-suspended in 150 μl PBS. Fixed LSK cells were placed on Fisher Superfrost Plus glass slides (at a minimum density of 2000 cells/slide), and allowed to settle overnight at 4 °C in a humidified chamber. Cells were permeabilized in PBS + 0.15% Triton-X-100 for 5 minutes, washed once, and blocked for 1 h at room temperature in PBS + 1% BSA. After blocking, a rabbit anti-γH2AX (1:50, Cell Signaling) was applied in PBS + 1% BSA for 1 h at room temperature. Slides were washed twice prior to the addition of secondary antibody (goat anti-Rabbit Cy3) at a dilution of 1:400, and incubated for 1 h at room temperature. After 2 washes, slides were mounted with 100 μL Prolong Gold + DAPI (Invitrogen), and imaged on a Zeiss Axiovert 200 fluorescent microscope. For a negative control, irradiated, sorted LSK cells were fixed at the start of the culture period and stained with secondary antibody only. Images were taken using an Axiocam MRM digital camera, at 100× magnification under oil immersion. Images were processed using Axiovision v4.8 software. For scoring γH2AX, at least 100 cells were counted for each condition using the criteria that <5 punctate spots was γH2AX negative, and ≥ 5 punctate spots was γH2AX positive.
Statistical Analysis
Data were analyzed using two-tailed Student’s t-tests. Statistical significance was considered at α ≤ 0.05.
Results
HAECs mediate the regeneration of phenotypic HSCs following radiation injury
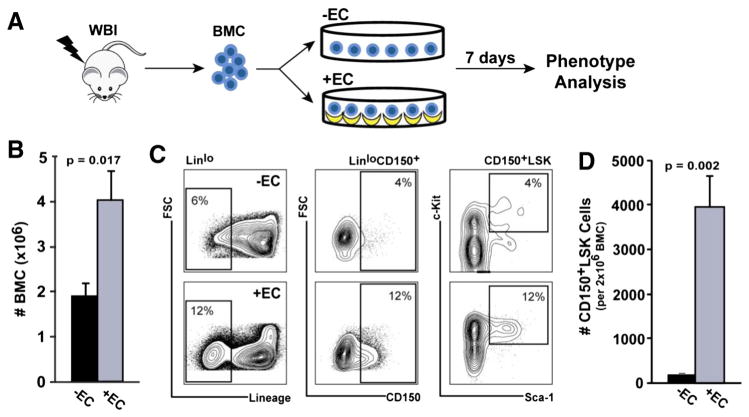
To determine whether HAEC monolayers could regenerate phenotypically-identifiable HSCs in vitro, bone marrow cells (BMC) were harvested from 580 cGy-irradiated mice and 2 × 106 input cells cultured in direct contact with HAECs or in the absence of HAECs for 7 days (Fig. 1A). Co-culture with HAECs resulted in a 2-fold increase in total BMC number (4.0 ± 0.65 ×106 BMC), whereas BMC cultured in the absence of HAECs did not expand relative to input (1.9 ± 0.28 ×106 BMC; p = 0.017 vs. +EC; Fig. 1B). To determine whether the increase in total BMC included an expansion of the HSC compartment, we analyzed the frequency of CD150+, Linlo, Sca-1+, c-Kit+ (CD150+LSK) cells, which are highly enriched for long-term HSCs (Kiel et al., 2005; Chen et al., 2008). Co-culture with HAECs induced an 18-fold increase in the proportion of CD150+LSK cells after 7 days. The CD150+LSK cell expansion was a consequence of a 2-fold increase in the frequency of Linlo cells, a 3-fold increase in the frequency of Linlo cells expressing CD150, and a 3-fold increase in the frequency of Sca-1+/c-Kit+ cells within the LinloCD150+ gate (Fig. 1C). In total, HAEC co-culture promoted a 24-fold overall increase in the absolute number of CD150+LSK cells relative to control (3922 ± 705 vs. 160 ± 16 CD150+LSK cells; p = 0.002; Fig. 1D). These results demonstrate that HAECs can robustly regenerate phenotypically-defined HSCs in vitro following radiation injury.
Figure 1.
HAECs promote the regeneration of cells with hematopoietic stem and progenitor phenotypes. (A) Bone marrow cells (BMC) were harvested from the femurs of mice treated with 580 cGy 137Cs whole body irradiation (WBI) and cultured in the absence (−EC, black bars) or presence (+EC, grey bars) of HAEC monolayers (input BMC: 2 × 106 cells). (B) After 7 days in culture, total BMC were counted and (C) HSCs (Linlo, CD150+, Sca-1+, c-Kit+ (CD150+LSK) cells) were identified by FACS. (D) The absolute number of CD150+LSK cells recovered on day 7 from 2 × 106 input BMC is shown. Error bars show SEM of 5 independent experiments.
Co-culture with HAECs rescues BMC containing functional hematopoietic stem and progenitor cells
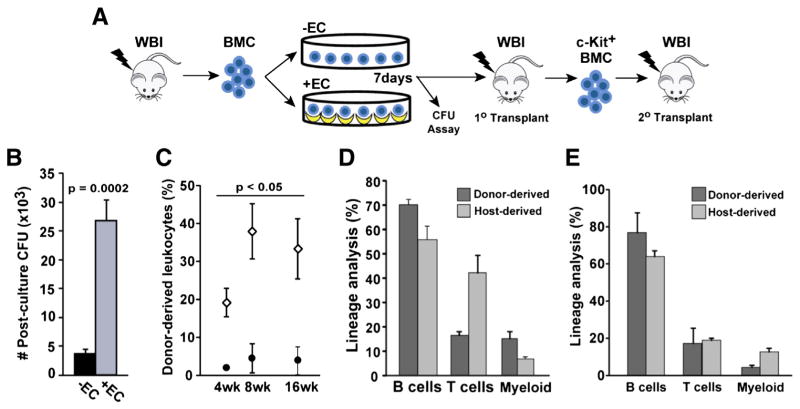
To query if the BMC regenerated during HAEC co-culture contained functional hematopoietic stem and progenitor cells (HSPCs), we assayed their colony forming activity in methylcellulose and performed serial bone marrow transplantation experiments (Fig. 2A). Irradiated BMC cultured in the presence of HAECs had significantly higher colony-forming activity when compared with control-cultured BMC (27 ± 4 ×103 vs. 3.8 ± 0.7 ×103 CFUs; p = 0.0002; Fig. 2B). Next, we examined HSC functional activity by transplanting BMC into sublethally irradiated congenic recipients. Transplantation of HAEC co-cultured BMC repopulated 20–40% of the peripheral blood (PB) in primary recipients over a 16 week period. In contrast, control cultured BMC contributed only 2.1–4.6% of recipient PB over the same time period (p < 0.05, Fig. 2C). Furthermore, analysis of primary recipient PB after 16 weeks of engraftment revealed that HAEC-treated BMC were capable of multilineage reconstitution (Fig. 2D). T-lymphocytes derived from HAEC-rescued HSCs constituted a lower frequency than host-derived T cells (p = 0.012), a finding consistent with our previous studies showing moderate lymphopenia in EC-rescued mice (Li et al., 2010). To determine whether the functional HSCs regenerated through HAEC co-culture were also self-renewing; donor-derived, c-Kit+ cells were FACS-sorted from primary recipient bone marrow and transplanted into sublethally irradiated secondary recipients. Multilineage hematopoietic reconstitution was detected for up to 16 weeks in these secondary recipients (Fig. 2E). These results indicate that ex vivo co-culture of BMC with HAECs provides an effective means to regenerate in vivo functional HSCs following injury by ionizing radiation.
Figure 2.
HAECs rescue long-term repopulating HSCs. (A) BMC harvested from 580 cGy-irradiated mice were cultured for 7 days in the presence or absence of HAECs and then assayed for CFU activity and in vivo hematopoietic potential. (B) BMC were plated in methylcellulose and absolute CFUs per 2 × 106 input BMC was determined (n = 3 independent experiments). (C) Peripheral blood (PB) engraftment of recipients transplanted with BMC cultured in the presence (open diamonds) or absence (closed circles) of HAECs (n = 5 recipients/group). (D) Primary transplant recipient donor- and host-derived, multilineage hematopoietic reconstitution of the PB by BMC cultured in the presence of HAECs. Donor-derived, c-Kit+ cells isolated from the bone marrow of primary recipients were transplanted into irradiated secondary recipients. (E) Multilineage hematopoietic reconstitution in secondary transplant recipients at 16 weeks. Error bars show SEM.
HAECs reverse radiation-induced DNA damage in LSK cells
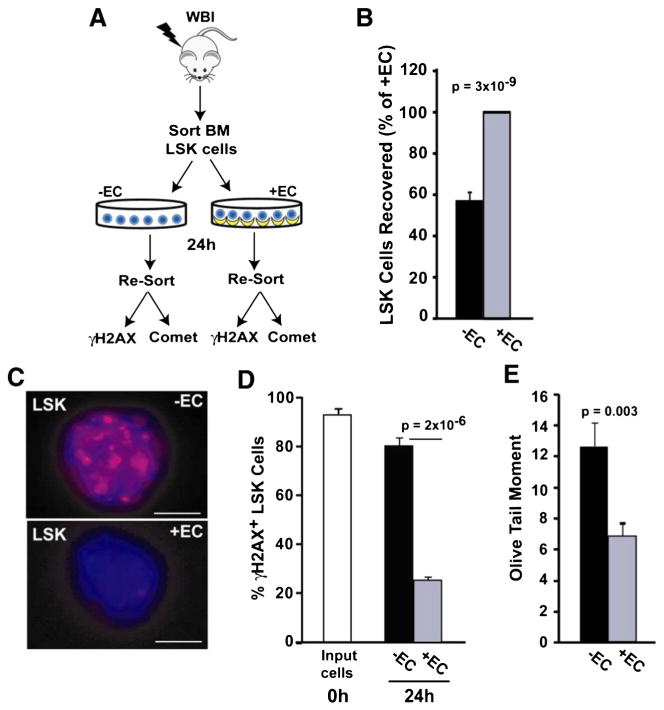
Ionizing radiation increases cellular oxidative stress and promotes DNA damage, including double strand breaks (DSBs). Because excessive DNA damage attenuates HSC self-renewal (Yahata et al., 2011), we hypothesized that HAECs may mitigate radiation-induced hematopoietic dysfunction by reducing DNA damage in HSPCs. To test this hypothesis, LSK cells were FACS-sorted from irradiated bone marrow and assayed for DNA damage following culture with or without HAECs (Fig. 3A). Consistent with the 7 day culture results (Fig. 1B), co-culture with HAEC for 24 h resulted in the recovery of 43% more LSK cells relative to controls (p = 3 × 10−9, Fig. 3B). Phosphorylation of the histone variant H2AX (γH2AX) is a sensitive, early response marker to DNA-DSBs (Mah et al., 2010); therefore, we assayed DNA-DSBs in irradiated LSK cells by detecting γH2AX immunofluorescence (Fig. 3C). Nearly all irradiated LSK cells had DNA-DSBs immediately after irradiation (Fig. 3D). Although we did not observe a reduction in DNA-DSBs after 3 h of HAEC co-culture (data not shown), extending the culture period to 24 h revealed that HAECs effectively reduce DSBs in LSK cells. Specifically, imaging 24 h after irradiation revealed the persistence of γH2AX foci in 79 ± 2.6% of LSK cells cultured in the absence of HAECs; in contrast, only 26 ± 1.1% of LSK cells cultured with HAECs showed any significant γH2AX signal (p = 2 × 10−6; Fig. 3D). To further validate these results, we also measured DNA strand breaks in single cells using an alkaline Comet assay. HAEC-rescued LSK cells exhibited a 54% reduction in olive tail moment relative to controls (p = 0.003; Fig. 3E), confirming that HAECs reduce DNA damage in HSPCs. Together, these two independent techniques demonstrate that co-culture with HAECs for only 24 h reverses radiation-induced DNA damage in a large proportion of LSK cells. These results suggest that the induction of DNA damage repair is an early event associated with HAEC-mediated mitigation of hematopoietic radiation injury.
Figure 3.
HAECs attenuate DNA damage in LSK cells. (A) LSK cells were FACS-sorted from 580 cGy-irradiated mouse bone marrow and cultured in the presence or absence of HAECs. After 24 h, LSK cells and their progeny were re-sorted based on Sca-1 and CD45 expression, and DNA damage was assessed using γH2AX immunofluorescence and a Comet assay. (B) LSK cells recovered after 24 h, with the + EC group normalized to 100% (n = 9 independent experiments). (C) Re-sorted LSK cells were assessed for DNA double strand breaks using γH2AX immunofluorescence. Representative images of irradiated LSK cells cultured in the absence (top) or presence (bottom) of HAECs (scale bar = 5 μm). (D) Quantification of γH2AX-positive cells (≥5 foci/cell) in LSK cells immediately after irradiation (white bar) and after 24 h culture in the presence (grey bar) or absence (black bar) of HAECs. At least 100 cells were scored per group (combined results from 4 independent experiments). (E) DNA damage in re-sorted LSK cells was also determined using a Comet assay. A representative experiment is shown where >100 LSK cells/group were scored for olive tail moment (n = 3 independent experiments).
HAECs can mitigate HSC loss and rescue functional hematopoiesis up to 48 hours following radiation injury
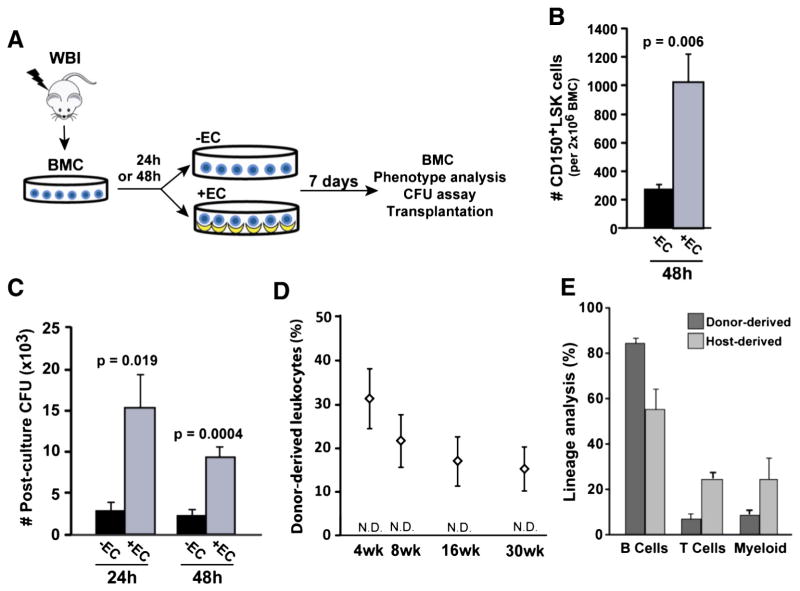
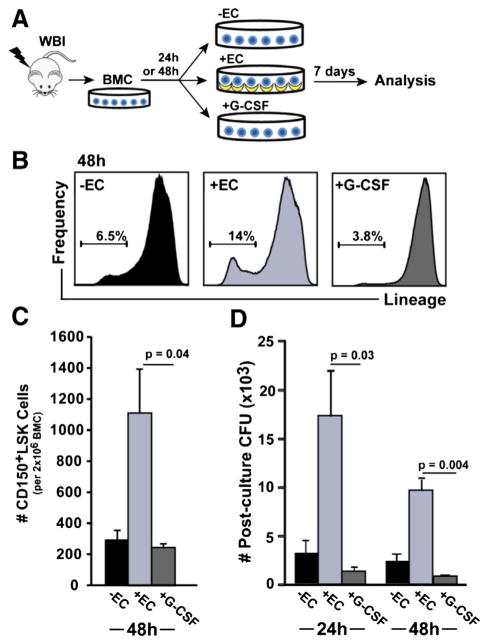
Very little is known about how long radiation-damaged HSCs can survive in the absence of environmental factors that promote their regeneration; however, this question has important biologic and therapeutic implications. Therefore, we sought to determine how long after a post-irradiation delay the surviving HSCs remained capable of regeneration by HAECs. To accomplish this, BMC harvested from irradiated mice were cultured under control conditions for 24 h or 48 h before transfer to co-culture with or without HAECs (Fig. 4A). After a 48 h post-irradiation delay, HAECs rescued nearly 4-fold more CD150+LSK cells than control culture conditions (1026 ± 200 vs. 276 ± 33 CD150+LSK cells; p = 0.006; Fig. 4B). We then tested the functional activity of rescued HSPCs with colony-forming assays and bone marrow transplantation. CFU activity was significantly higher in HAEC-treated BMC compared with control BMC after delays of both 24 h (15 ± 4.1 ×103 vs. 2.9 ± 1.1 ×103 CFUs; p = 0.019) and 48 h (9.4 ± 1.3 ×103 vs. 2.4 ± 0.7 ×103 CFUs; p = 0.0004; Fig. 4C). Moreover, HAEC co-culture rescued long-term repopulating HSCs that gave rise to 15–32% of total circulating leukocytes and multilineage hematopoietic reconstitution for up to 30 weeks following transplantation into host mice (Figs. 4D–E). In contrast, after a 48 h post-irradiation delay BMC cultured under control conditions were incapable of engrafting transplant recipients (Fig. 4D). These results highlight the existence of a 48 h window of opportunity during which endothelial-derived factors can regenerate functional hematopoiesis.
Figure 4.
HAECs rescue functional hematopoiesis up to 48 h following radiation injury. (A) BMC harvested from 580 cGy-irradiated mice were initially cultured for 24 h or 48 h in control conditions and then cultured in either the presence or absence of HAECs for 7 additional days. (B) Absolute CD150+LSK cells (per 2 × 106 input BMC) recovered after a 48 h post-irradiation delay and 7 days of culture (n = 5 independent experiments). (C) CFU activity (per 2 × 106 input BMC) after a 24 h or 48 h post-irradiation delay (n = 5–7 independent experiments). (D) PB engraftment by 48 h HAEC-rescued BMC for up to 30 weeks following transplantation (n = 5 recipients/group). BMC cultured in the absence of ECs provided no measurable level of engraftment (not detectable, N.D., sensitivity = 0.5%). (E) 30 week multilineage hematopoietic reconstitution of the PB by HAEC-rescued BMC after a 48 h delay. Error bars show SEM.
Regeneration of functional HSPCs by HAECs is superior to G-CSF
The current standard of care therapy for treating unintentional radiation exposure is G-CSF, which improves early hematopoietic recovery in part through HSC mobilization and differentiation (MacVittie et al., 2005; Dainiak, 2010). To directly compare their potential to regenerate HSPCs, HAECs or G-CSF were co-cultured with irradiated BMC after a 24 h or 48 h delay (Fig. 5A). Consistent with our previous results (Fig. 1C), HAECs expanded the proportion of Linlo cells 2-fold to 14 ± 1.0% of BMC, and this was significantly higher than both control (p = 0.009) and G-CSF (p = 0.001) treated groups (Fig. 5B). Further phenotypic analysis showed that HAECs rescue almost 5-fold more CD150+LSK cells than G-CSF after a 48 h post-irradiation delay (1107 ± 287 vs. 241 ± 31 CD150+LSK cells; p = 0.04; Fig. 5C). The superior ability of HAECs to rescue phenotypic HSPCs in culture correlated with increased functional activity, as HAEC co-cultured BMC formed 13-fold more colonies than G-CSF co-cultured BMC (17 ± 4.6 ×103 vs. 1.3 ± 0.5 ×103 CFUs; p = 0.03) after a 24 h delay prior to HAEC co-culture, and 11-fold more colonies (9.7 ± 1.1 ×103 vs. 0.86 ± 0.17 ×103 CFUs; p = 0.004) after a 48 h delay prior to co-culture (Fig. 5D). Together, these data show that following a post-radiation injury delay of up to two days, co-culture with HAEC is superior to G-CSF for enhancing the recovery of phenotypic and functional HSPCs.
Figure 5.
HAECs are superior to G-CSF for promoting HSPC regeneration for up to 48 h after radiation injury. (A) After a 24 h or 48 h delay, irradiated BMC were cultured in the presence or absence of HAECs, or with G-CSF. (B) Analysis of BMC by FACS after 7 days of culture showed a relative depletion of Linlo BMC in 48 h delayed, G-CSF-treated cultures (representative flow histograms and a mean of 3 experiments are shown). (C) Quantification of CD150+LSK cells following culture in the presence or absence of HAECs, or with G-CSF (n = 3 independent experiments). (D) CFU activity of BMC cultured in the presence or absence of EC, or the presence of G-CSF, after the indicated delay period (n = 4 independent experiments). Error bars show SEM.
Discussion
We have shown that HAECs mediate the recovery of hematopoietic function following radiation injury by promoting the proliferation of functional HSCs and reducing DNA damage. Relative to control culture conditions, HAEC co-culture regenerated significantly more CD150+LSK cells from irradiated bone marrow; furthermore, HAEC-rescued BMC had increased long-term hematopoietic reconstitution potential and contained self-renewing, multilineage-reconstituting HSCs. For phenotypic identification of HSCs we included the SLAM family member CD150, which has been shown to enrich for long-term HSCs within LSK populations (Kiel et al., 2005; Chen et al., 2008). HAECs expanded the proportion of CD150+LSK cells in culture by 24-fold (Fig. 1D), and this increase correlated with a log-fold engraftment advantage for HAEC-treated BMC relative to control (Fig. 2C).
A remarkable finding from our study is the long window of opportunity during which irradiated HSCs can be rescued. Despite the persistence of substantial amounts of DNA damage in LSK cells (Fig. 3D), a subpopulation of these cells survive for up to 48 h and are responsive to HAEC-derived factors that promote HSC regeneration. In the case of unanticipated exposure to ionizing radiation, the possibility that healthcare intervention may not be immediate is clinically important. Our results show that in the absence of HAEC-derived signals, irradiated HSCs completely lose their ability to repopulate the blood of radiation-conditioned recipients after a 48 h culture delay (Fig. 4D). Notably, the degree to which irradiated BMC remained capable of producing CD150+LSK cells and active progenitors was inversely proportional to the length of the post-irradiation delay. Although we recovered fewer absolute CD150+LSK cells from BMC cultures that were delayed 48 h prior to co-culture with HAECs, the percentage of CD150+LSK cells in day 7 cultures did not change significantly when compared to BMC cultured immediately on HAECs (data not shown). Thus, our functional studies show that HSCs regenerated by HAECs immediately after irradiation (Fig. 2C) have comparable engraftment potential on a per-cell basis as HSCs regenerated by HAECs after a post-irradiation delay of 48 h (Fig. 4D). These results suggest that HSC death, rather than an intrinsic alteration to the quality and engraftment potential of HSCs, is limiting for the delayed rescue of HSCs through HAEC co-culture.
Currently, G-CSF is the standard of care for the treatment of bone marrow failure from unanticipated radiation exposure or following chemotherapy. The main therapeutic benefit of G-CSF is to enhance neutrophil recovery (Dainiak, 2010). It is less clear whether G-CSF is a direct mitigator of HSPC radiation damage (Drouet and Herodin, 2010). We found that relative to HAECs, treatment of isolated, irradiated BMC with G-CSF after a 48 h delay was less efficacious at promoting the rescue of CD150+LSK cells. In fact, when G-CSF was added to control cultures we noted a potentially detrimental effect on the regeneration of HSPCs. Whereas HAECs significantly expanded Linlo BMC populations relative to both control and G-CSF culture, G-CSF actually induced a shift in BMC populations to the Linhi phenotype, and this caused a non-significant but pronounced (34%) loss of Linlo cells in day 7 cultures (Fig. 5B). Consistent with this loss of Linlo cells, treatment with G-CSF did not expand active progenitors (Fig. 5D). This disparate effect of G-CSF treatment in vitro may be accounted for by other regulatory signals within the hematopoietic microenvironment that modulate the granulocytic differentiation or asymmetric division of HSCs upon G-CSF stimulation in vivo. Indeed, co-culture of irradiated BMC with HAECs + G-CSF yielded intermediate levels of CD150+LSK cells and colony-forming activity compared to either treatment alone (data not shown), suggesting that HAECs and G-CSF may induce opposing signaling networks in regenerating HSCs. These data highlight that the continued discovery of endogenous signals that regenerate HSCs may improve our ability to rescue high-fidelity, long-term HSCs with combinatorial pharmacologic treatment.
We have demonstrated that ECs reverse radiation-induced DNA damage in HSPCs, and that this reversal is associated with enhanced bone marrow engraftment and serial transplantation potential (Figs. 2C–E). We believe these are the first data to suggest that ECs can induce DNA damage repair pathways through paracrine or cell-contact signaling. The identity of the endothelial-derived factor(s) with this activity remains to be determined. Both basic fibroblast growth factor (FGF-2) and epidermal growth factor (EGF) have been shown to promote DNA damage repair in other cell types (Harfouche et al., 2010; Bai et al., 2012). Interestingly, systemic delivery of EGF to irradiated mice promotes hematopoietic recovery following radiation injury (Doan et al., 2013). We did not detect EGF mRNA in HAECs (data not shown), thus ruling out EGF as a candidate HAEC-derived radiation mitigator; however, we cannot rule out the possibility of other ligands signaling through the EGF receptor. Notably, our basal endothelial culture medium contains both FGF-2 and EGF. Thus, the presence of these factors may account for the reduction in the percentage of HSPCs with DSBs (relative to input cells) following culture in the absence of HAEC (Fig. 3D). Because HAECs promote a much greater degree of DNA repair as well as HSPC expansion in prolonged cultures, we postulate that additional factor(s) from HAECs may be working together with the EGF and/or FGF-2 present in the tissue culture media. To identify these potential candidates, we have undertaken both transcriptome and secretome analyses of HAECs.
In conclusion, our findings add to a growing body of evidence supporting the role of endothelium in HSC maintenance and regeneration following radiation injury (Montfort et al., 2002; Chute et al., 2004; Muramoto et al., 2006; Li et al., 2010). Continued efforts toward identifying endothelial-derived factors that promote the regeneration of HSPCs may lead to improvements in long-term hematopoietic outcomes for individuals exposed to ionizing radiation.
Highlights.
Endothelial cells (ECs) regenerate irradiated hematopoietic stem cells (HSCs).
Hematopoietic regeneration involves the reversal of radiation-induced DNA damage.
HSC regeneration by ECs can be delayed up to 48 h after radiation exposure.
In vitro, ECs are superior to G-CSF at rescuing irradiated HSCs.
Acknowledgments
Special thanks to Pamela Canaday, Dorian LaTocha, and Miranda Boyd of the Flow Cytometry core at OHSU for assistance with cell sorting. Special thanks to Hyunjung Lee for technical support with housing and maintenance of mice. This work was supported by a NIH grants R01-HL069113 (W.H.F), U19-AI091175 (W.H.F., C.G.) and D.K.Z. was supported as trainee on T32-HL007781.
References
- Bai J, Guo XG, Bai XP. Epidermal growth factor receptor-related DNA repair and radiation-resistance regulatory mechanisms: a mini-review. Asian Pac J Cancer Prev. 2012;13:4879–4881. doi: 10.7314/apjcp.2012.13.10.4879. [DOI] [PubMed] [Google Scholar]
- Chao NJ. Accidental or intentional exposure to ionizing radiation: biodosimetry and treatment options. Exp Hematol. 2007;35:24–27. doi: 10.1016/j.exphem.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Chen J, Ellison FM, Keyvanfar K, Omokaro SO, Desierto MJ, Eckhaus MA, Young NS. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice. Exp Hematol. 2008;36:1236–1243. doi: 10.1016/j.exphem.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–265. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitteti BR, Cheng YH, Poteat B, Rodriguez-Rodriguez S, Goebel WS, Carlesso N, Kacena MA, Srour EF. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. 2010;115:3239–3248. doi: 10.1182/blood-2009-09-246173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute JP, Fung J, Muramoto G, Erwin R. Ex vivo culture rescues hematopoietic stem cells with long-term repopulating capacity following harvest from lethally irradiated mice. Exp Hematol. 2004;32:308–317. doi: 10.1016/j.exphem.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Dainiak N. Rationale and recommendations for treatment of radiation injury with cytokines. Health Phys. 2010;98:838–842. doi: 10.1097/HP.0b013e3181b3fce5. [DOI] [PubMed] [Google Scholar]
- Doan PL, Himburg HA, Helms K, Russell JL, Fixsen E, Quarmyne M, Harris JR, Deoliviera D, Sullivan JM, Chao NJ, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013 doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet M, Herodin F. Radiation victim management and the haematologist in the future: time to revisit therapeutic guidelines? Int J Radiat Biol. 2010;86:636–648. doi: 10.3109/09553001003789604. [DOI] [PubMed] [Google Scholar]
- Harfouche G, Vaigot P, Rachidi W, Rigaud O, Moratille S, Marie M, Lemaitre G, Fortunel NO, Martin MT. Fibroblast growth factor type 2 signaling is critical for DNA repair in human keratinocyte stem cells. Stem Cells. 2010;28:1639–1648. doi: 10.1002/stem.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VK, Tsyb AF, Khait SE, Kashcheev VV, Chekin SY, Maksioutov MA, Tumanov KA. Leukemia incidence in the Russian cohort of Chernobyl emergency workers. Radiat Environ Biophys. 2012;51:143–149. doi: 10.1007/s00411-011-0400-y. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- Li B, Bailey AS, Jiang S, Liu B, Goldman DC, Fleming WH. Endothelial cells mediate the regeneration of hematopoietic stem cells. Stem Cell Res. 2010;4:17–24. doi: 10.1016/j.scr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Jackson W., III Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys. 2005;89:546–555. doi: 10.1097/01.hp.0000173143.69659.5b. [DOI] [PubMed] [Google Scholar]
- Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, Deeg HJ. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- Montfort MJ, Olivares CR, Mulcahy JM, Fleming WH. Adult blood vessels restore host hematopoiesis following lethal irradiation. Exp Hematol. 2002;30:950–956. doi: 10.1016/s0301-472x(02)00813-5. [DOI] [PubMed] [Google Scholar]
- Muramoto GG, Chen B, Cui X, Chao NJ, Chute JP. Vascular endothelial cells produce soluble factors that mediate the recovery of human hematopoietic stem cells after radiation injury. Biol Blood Marrow Transplant. 2006;12:530–540. doi: 10.1016/j.bbmt.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A, Suda T. Hematopoietic stem cell niche: an interplay among a repertoire of multiple functional niches. Biochim Biophys Acta. 2013;1830:2404–2409. doi: 10.1016/j.bbagen.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- Wang LD, Wagers AJ. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol. 2011;12:643–655. doi: 10.1038/nrm3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–356. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata T, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, Sheng Y, Onizuka M, Ito M, Kato S, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]