Abstract
Cigarette smoking has been linked to almost all major types of cancer. Emerging evidence suggests that smoking initiates transformed cell growth and migration by disrupting cell–cell interactions in the polarized mucosal epithelium. Together with other adherens junction proteins, p120-catenin (p120ctn) maintains cell–cell adhesion through its direct interaction with E-cadherin (E-cad). Mislocalization and/or loss of p120ctn have been reported in all lung cancer subtypes and are related to poor prognosis. Here, we showed that p120ctn modulates smoke-induced cell migration via the EGFR/Src-P pathway. Chemical blockade of EGFR/Src signaling inhibited smoke-induced activation of cofilin (an actin severing protein) and promoted cell migration in the presence of p120ctn but had little effect on blocking migration in the absence of p120ctn. These data suggested that smoke-induced cell migration was mediated via an EGFR/Src-dependent signaling pathway in cells that expressed p120ctn, but upon loss of p120ctn, migration continued to occur via an alternative, EGFR/Src-independent pathway. Thus, gradual loss of membrane p120ctn with lung cancer progression may contribute to reduced effectiveness of conventional chemotherapies, such as those directed against EGFR.
Keywords: Cigarette smoke, Adherens junction, p120-Catenin, Cell migration
1. Introduction
Smoking represents the single most important carcinogenic exposure and is the leading cause of cancer-related mortalities [1]. Smoke promotes lung carcinogenesis through triggering genetic mutations [2], epigenomic changes [3], inflammation [4] and epithelial-mesenchymal transition (EMT) [5,6]. EMT is involved in normal embryonic development, during which cells switch from a polarized, immobile epithelial phenotype to a highly motile, fibroblastic or mesenchymal phenotype. In the airway, inappropriate activation of embryonic pathways by smoke can promote EMT and tumorigenesis [5,6]. Signaling via the epithelial growth factor receptor (EGFR) and Src has been established as a major pathway modulating smoke-induced cell migration, invasion and EMT. EGFR exposed to cigarette smoke is aberrantly activated and promotes cell migration and invasion [7–9]. Nicotine, a major component of cigarette smoke, has been reported to promote EMT and accelerate cell migration and invasion through Src signaling [10,11]. In these events, Src has been reported as a downstream effector of EGFR [8,11].
In order to undergo EMT, epithelial cells must first be released from their interactions with neighboring cells in the polarized epithelial sheet. One important component that maintains cell–cell interactions in mucosal epithelia is the adherens junction. p120-Catenin (p120ctn) is an armadillo repeat-containing protein. Together with other adherens junction proteins such as cadherins, β-catenin (β-ctn) and α-catenin, p120ctn plays a critical role in regulating cell–cell adhesion [12]. Previous studies have demonstrated a crucial role for 120ctn in stabilizing the adherens junction through its direct interaction with E-cadherin (E-cad). Loss of p120ctn disrupts adherens junctions by promoting the internalization and turnover of E-cadherin [13–16], while loss of E-cadherin represents a major hallmark of EMT [17] and tumor malignancy [18]. Accordingly, mislocalization, downregulation and loss of p120ctn have been reported in all lung cancer subtypes, and are related to poor prognosis [12,14]. Out of 1081 human genes predicted to affect cell migration and adhesion, high-throughput siRNA screening recently identified p120ctn as the most confident gene inhibiting cell migration in MCF-10A breast cancer cells [19].
The similarities that exist between smoke and p120ctn in mediating EMT and cell migration, raised two intriguing questions: Is p120ctn a target of smoke? And, if so, does p120ctn play a functional role in modulating cell migration provoked by smoke? Here, we identified p120ctn as a membrane target of cigarette smoke. Using primary human bronchial epithelial (HBE) cells with and without functional p120ctn, we identified a smoke-induced EGFR/Src signaling pathway that stimulated cell migration in the presence of membrane p120ctn but failed to do so in p120ctn-knockdown cells. Accordingly, EGFR and Src specific inhibitors abolished smoke-induced cell migration in wild-type HBE cells but failed to block migration in p120ctn-deficient cells. This indicated the existence of an EGFR/Src-independent signaling pathway that continued to promote migration in cells lacking p120ctn despite treatment with conventional chemotherapies, such as those directed against EGFR. Thus, loss of p120ctn with tumor progression in smokers may provide a novel mechanism of chemoresistance.
2. Materials and methods
2.1. Cell culture
Human tissue was handled according to the Declaration of Helsinki and was approved by the University of California Committee for Human Research. Primary HBE cells were isolated from tracheal tissue strips of human donors and cultured using previously established methods [20]. HBE cells were cultured in BEGM BulletKit (Lonza, Basel, Switzerland) according to vendor’s instructions.
2.2. p120ctn knockdown
p120siRNA pool 1 was composed of p120ctn siRNA1 [13], p120ctn siRNA2 (s3725) and p120ctn siRNA3 (s3727, Ambion Inc., Austin, TX). p120siRNA pool 2 was composed of p120ctn siRNA1 [13] and p120ctn siRNA4 [21], synthesized by Sigma–Aldrich (St. Louis, MO). Scrambled siRNA control was purchased from Ambion Inc. (Austin, TX). HBE cells were transfected with the above siRNAs by Amaxa NHBE nucleofector Kit (Lonza, Basel, Switzerland) according to the manufacturer’s protocol. Experiments were generally performed at 48 h posttransfection.
2.3. Smoke and inhibitor treatment
Smoke-conditioned medium was prepared as previously described [22]. Concentrated smoke exposed medium (93 mg/m3 total suspended particles (TSP)) was prepared in DMEM and diluted with culture medium to final concentrations of 12, 24 and 48 mg/m3 TSP. AG1478 was purchased from Cell Signaling Technology Inc. (Danvers, MA). PP2 was obtained from Sigma–Aldrich (St. Louis, MO). Cells were starved in basal medium without growth factor for 2 h and pretreated with the above inhibitors for 1 h, followed by incubation in smoke-free or smoke medium for 3 h or 24 h as indicated in the text. Concentrations of all inhibitors were titrated to have no effect on cellular toxicity as determined by trypan blue exclusion assay.
2.4. Immunofluorescent analysis
Cells were fixed with 4% paraformaldehyde, permeabilized with 0.3% triton X-100 and incubated with primary antibodies at 4 °C overnight. Antibodies directed against p120ctn, β-ctn and E-cad were obtained from BD Transduction Laboratories (San Jose, CA) and used as instructed. Cells were stained with Alexa fluor-488 or -594 conjugated secondary antibodies (Invitrogen, Carlsbad, CA). Nuclei were stained with DAPI. Images were photographed with a Nikon-TiE fluorescence microscope.
2.5. Western blot
Protein concentrations of cell lysates were determined using the Bradford protein assay (Bio-Rad, Hercules, CA). Western blot analysis was performed using the Novex gel system (Invitrogen, Carlsbad, CA). The following primary antibodies were obtained from Cell Signaling Technology Inc. (Danvers, MA) and used as instructed: phospho-Tyr416 Src, total Src, phospho-Ser3 Cofilin, phospho-Limk1 (Thr508)/Limk2(Thr505) and GAPDH. Phospho-Tyr 4G10 was obtained from Millipore (Billerica, MA). Total EGFR was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Western blot signals were detected with secondary HRP-conjugated antibodies and ECL reagent (Invitrogen, Carlsbad, CA), followed by densitometric quantitation with ImageJ (National Institutes of Health, Bethesda, MD).
2.6. Cell migration assay
HBE migration was measured with 24-well Boyden chambers containing Transwell filters with 8 μm pores (BD Biosciences, Bedford, MA). p120ctn siRNA- or scrambled siRNA-transfected cells were starved and pretreated with inhibitors as above at 48 h posttransfection. Subsequently, 2 * 104 cells suspended in growth factor-free medium without and with inhibitor were loaded onto the filter. Smoke-free and smoke medium were applied to the lower chambers. After 4 h of incubation at 37 °C, the non-migratory cells on upper surface of the filters were removed with a cotton swab. Cells that migrated to the bottom of filters were fixed, stained with 0.01% crystal violet, washed with 5% acetic acid/5% methanol. The number of migratory cells was estimated with colorimetric readings taken at OD 595 nm.
2.7. Statistical analysis
Three to four independent repeats were conducted in all experiments. Data were presented as mean ± SEM. A Student’s t-test was used and a p value of <0.05 was considered significant.
3. Results
3.1. p120ctn-knockdown in HBE cells disrupts adherens junctions
Primary human bronchial epithelial (HBE) cells were incubated with smoke-free medium (Ctrl) or smoke medium at 24 mg/m3 TSP (Smk) overnight. In unexposed HBE cells, p120ctn was localized to adherens junctions on the basolateral membranes of adjacent HBE cells (Fig. 1A, Ctrl panel). In contrast, smoke exposure caused the cytoplasmic translocation and loss of p120ctn (Fig. 1A, Smk panel). Having observed smoke’s effects on membrane p120ctn, we explored adherens junction integrity in the presence and absence of p120ctn. HBE cells were transfected with p120ctn siRNA or scrambled siRNA and cultured as monolayers. Immunostaining 48 h posttransfection confirmed p120ctn knockdown (p120KD) in over 70% of p120ctn siRNA-treated cells compared to scrambled siRNA-transfected (p120WT) cells. Loss of membrane p120ctn following siRNA treatment (p120KD) mimicked the loss that occurred following exposure to smoke (Fig. 1B, leftmost column). Moreover, basolateral localization of adherens junction components, β-ctn and E-cad, was disrupted in p120KD cells (Fig. 1B, middle and rightmost columns).
Fig. 1.
siRNA knockdown of p120ctn in primary HBE cells disrupts adherens junctions. (A) Immunofluorescent staining of adherens junction markers p120-catenin (p120ctn) in untreated cells (Ctrl) and cells treated with smoke medium at 24 mg/m3 TSP (Smk) for 24 h. (B) Immunofluorescent staining of p120ctn, β-ctn, and E-cad in scrambled siRNA-transfected cells (p120WT) and p120ctn knockdown cells (p120KD) at 48 h posttransfection. Bar represents 50 μm. (C) Cell lysates from p120WT and p120KD cells (transfected with p120siRNA pool 1 or p120siRNA pool 2) were analyzed by Western blots probed with p120ctn, β-ctn and E-cad antibodies at 48 h posttransfection. GAPDH was used as a loading control. (D) Quantitation of p120ctn, β-ctn and E-cad levels in p120WT and p120KD cells at 48 h posttransfection by densitometry. The levels of proteins in p120KD cells (black bars) were normalized to those in p120WT cells (white bars, designated 1-fold). Results shown are the average of three independent experiments. **p < 0.01, p120KD versus p120WT cells.
Western blot results confirmed loss of adherens junction components in p120KD cells at 48 h posttransfection (Fig. 1C). Using densitometry, p120ctn, β-ctn and E-cad were estimated to decrease by 75%, 50% and 60%, respectively, compared to p120WT cells (Fig. 1D). p120siRNA pool 1 was composed of a mixture of siRNAs targeting three non-overlapping sequences of human p120ctn gene. p120siRNA pool 2 consisted of two siRNAs targeting different sequences of the human p120ctn gene. Similar p120ctn knockdown effects and experimental results were achieved using either pool, suggesting that the observed consequences were specific to p120ctn ablation. These results demonstrated the feasibility of depleting endogenous p120ctn from primary HBE cells and supported previous tumor cell studies where loss of p120ctn led to destabilization of adherens junctions and increased cell migration [13–15].
3.2. EGFR/Src activation in response to smoke occurred in the presence and absence of p120ctn
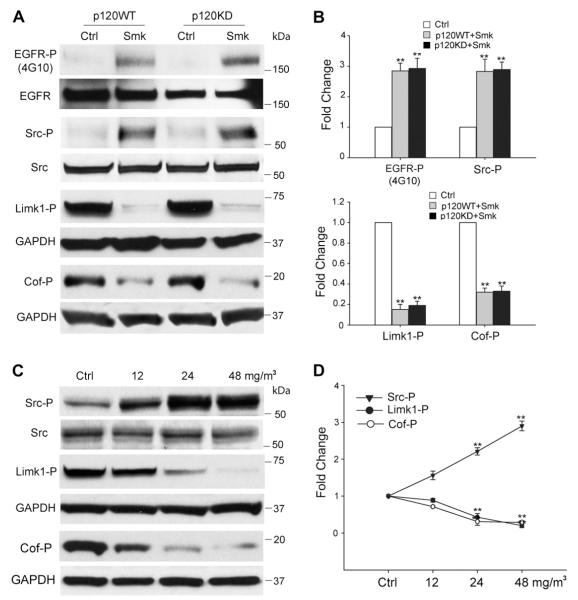
Previous studies showed smoke-mediated cell migration occurred through intracellular signaling pathways involving EGFR and Src [23,24]. To investigate the involvement of p120ctn in the activation of EGFR/Src signaling by smoke, we examined kinase phosphorylation in p120WT and p120KD HBE cells. Compared to control cells, phosphoryation of EGFR (EGFR-P) and Src (Src-P) peaked at 3 h of smoke exposure. Activation was similar in the presence and absence of p120ctn (Fig. 2A). Quantitative analysis based on a minimum of three independent experiments confirmed that EGFR-P and Src-P were significantly increased in the presence of smoke (Fig. 2B, top bar graph). Src activation appeared to occur downstream to EGFR as AG1478 can block Src phosphorylation in response to smoke ([8,25] and data not shown).
Fig. 2.
Smoke-mediated signaling events in HBE cells. (A–D) Smoke causes p120ctn-independent phosphorylation of EGFR/Src and dephosphorylation of Limk1/Cofilin (Cof) in HBE cells after 3 h of exposure. (A) Cell lysates obtained from p120WT and p120KD cells after a 3 h exposure to smoke-free (Ctrl) or smoke medium at 48 mg/m3 TSP (Smk) were analyzed by Western blot using antibodies directed against Tyr-phosphorylated EGFR (EGFR-P), Tyr416-phosphorylated Src (Src-P), Thr508-phosphorylated Limk1 (Limk1-P) and Ser3-phosphorylated Cofilin (Cof-P). Equal loading of lysates was confirmed using antibodies directed against total EGFR, Src and GAPDH. Blots are representative of three independent experiments. (B) Relative fold change in EGFR-P, Src-P, Limk1-P and Cof-P following smoke exposure in p120WT (grey bar) and 120KD cells (black bar) was normalized relative to Ctrl (white bars, designated 1-fold). Results shown are the mean ± SEM of three independent experiments. **p < 0.01, p120KD or p120WT versus Ctrl cells. (C) HBE cell lysates exposed to smoke-free (Ctrl) or smoke medium at 12, 24 and 48 mg/m3 TSP (Smk) for 3 h were analyzed by Western blot using antibodies directed against Src-P, Limk1/2-P and Cof-P. Levels of total Src and GAPDH were used as loading controls. (D) Relative fold change in Src, Limk1 and Cof phosphorylation induced by smoke was normalized to Ctrl (designated 1-fold) at 3 h and plotted as dose-response curves. Results shown are the mean ± SEM of three independent experiments. **p < 0.01, Smk-exposed versus Ctrl cells.
3.3. Lim kinase/cofilin suppression in response to smoke occurred in the presence and absence of p120ctn
Cofilin (Cof) is an actin binding protein that regulates the disassembly of actin during cell migration. In its phosphorylated form, the severing capacity of cofilin is inhibited. Dephosphorylation restores its severing capacity and promotes cell migration [26]. Smoke-induced dephosphorylation of cofilin at Ser3 (Cof-P) was detected as early as 3 h post-exposure (Fig. 2A). Cofilin is a downstream target of Lim kinases (Limk1 and Limk2). Phosphorylated Lim kinases inhibit the actin severing activity of cofilin by phosphorylating cofilin at Ser3 [26,27]. Accordingly, smoke promoted the dephosphorylation of Limk1 at Thr508 (Limk1-P) in HBE cells (Fig. 2A). Quantitative analysis based on a minimum of three independent experiments confirmed that Cof-P and Limk1-P were significantly decreased in the presence of smoke (Fig. 2B, bottom bar graph). Next, we treated HBE cells with increasing doses of smoke to demonstrate that a dose-dependent increase in Src-P occurred in conjunction with dose-dependent decreases in the phosphorylation of Limk1 and cofilin (Fig. 2C). Limk1-P and Cof-P were inhibited ~80% in both p120WT and p120KD cells after 3 h of smoke exposure, closely mirroring the activation of Src-P under identical conditions (Fig. 2D).
3.4. Smoke-induced cell migration is p120ctn-dependent
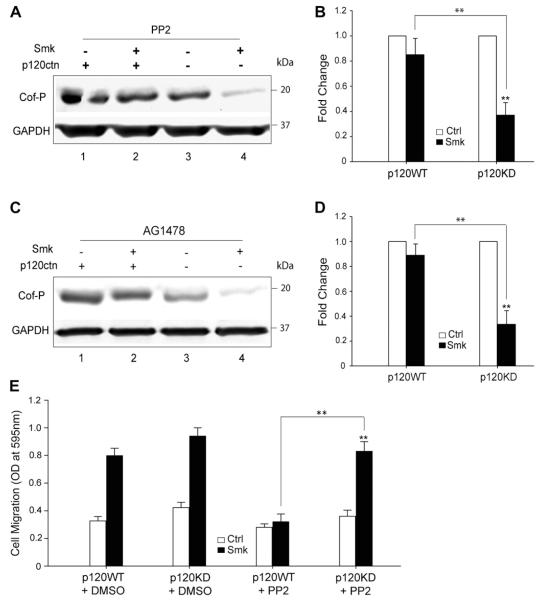
To link smoke-mediated activation of EGFR/Src to dephosphorylation of cofilin (pro-migration), and to explore the potential role of p120ctn in modulating this link, we treated p120WT and p120KD cells with Ctrl or smoke medium at 48 mg/m3 TSP for 3 h in the presence and absence of chemical inhibitors, AG1478 (EGFR inhibitor), or PP2 (Src inhibitor). Interestingly, AG1478 blocked smoke-mediated dephosphorylation of cofilin in p120WT cells, but failed to inhibit the effect in p120KD cells (Fig. 3A and B). Similarly, PP2 only prevented cofilin dephosphorylation by smoke in the presence of p120ctn (Fig. 3C and D). Similar results were noted in Boyden Chamber assays, where blocking Src only inhibited migration in the presence of p120ctn (Fig. 3E). These results were intriguing in that well-established smoke-induced signaling pathways functioning downstream of EGFR and Src required p120ctn to mediate essential elements of cytoskeletal reorganization in migrating cells. Conversely, in the absence of p120ctn, migration persisted despite treatment with conventional chemotherapies, such as those directed against EGFR. A proposed model of smoke-modulated cell migration in the presence and absence of p120ctn is described in Fig. 4.
Fig. 3.
EGFR and Src inhibitors fail to block decreased Cof-P and increased cell migration in response to smoke when cells lack p120ctn. (A–D) p120WT and p120KD cells were treated with smoke-free (Ctrl) or smoke medium at 48 mg/m3 TSP (Smk) for 3 h in the presence of 1 μM AG1478 (A and B) or 12.5 μM PP2 (C and D) to inhibit EGFR or Src, respectively. Western blot (A and C) and densitometric analysis (B and D) of Cof-P in Smk-treated cells were normalized to Ctrl (designated 1-fold). Data are expressed as the mean ± SEM of three independent experiments. (E) P120WT and p120KD cells were loaded on Boyden chambers and incubated with Ctrl (white bar) or smoke medium at 48 mg/m3 TSP (black bar) in the presence of DMSO (vehicle control) or 12.5 μM PP2 for 4 h. Migrating cells were quantified by measuring the OD at 595 nm and plotted as the mean ± SEM of three independent chambers. **p < 0.01, smoke-treated p120WT or p120KD cells versus respective control. **p < 0.01, smoke-treated p120KD cells versus smoke-treated p120WT cells.
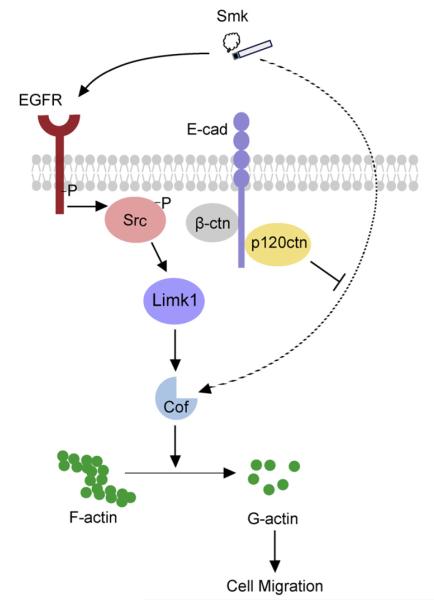
Fig. 4.
Smoke mediates EGFR/Src-dependent and independent cell migration. In the normal human airway, adherens junctions (p120ctn, β-ctn and E-cad) are intact on the epithelial cell membrane. Smoke induces rapid activation of membrane Src through EGFR. Src activation promotes cell migration through suppression of Cof-P. When airway epithelial cells express p120ctn, EGFR and Src specific inhibitors (as those used in chemotherapy) help to stabilize p120ctn on the membrane where it functions as a tumor suppressor. In the advanced stages of lung cancer, p120ctn is translocated into the cytoplasm by smoke where it is degraded or modified. Membrane loss of p120ctn results in disruption of adherens junctions and decreased cell–cell adhesion. Src continues to be activated through EGFR but EGFR and Src inhibitors fail to block cell migration because loss of tumor-suppressing membrane p120ctn, activates an alternative EGFR/Src-independent pathway (denoted with a dotted-line). Cell migration continues via this alternative pathway in the absence of p120ctn, bypassing traditional EGFR/Src signaling and thus providing a potential mechanism of chemotherapeutic resistance.
4. Discussion
We used primary human bronchial epithelial cells to decipher events associated with lung cancer initiation, including disruption of adherens junctions and increased cell motility [28,29]. Given the strong association between loss of p120ctn and poor prognosis in lung cancer [30], we explored the potential involvement of p120ctn in smoke-induced migration. Using p120WT and p120KD HBE cells treated with tobacco smoke, we provide compelling evidence that chemical inhibitors directed against the well-established EGFR/Src signaling pathway do not effectively block smoke-induced actin depolarization or cell migration in the absence of membrane p120ctn. Thus, as membrane p120ctn is lost with tumor progression, an alternative, EGFR/Src-independent signaling pathway appears to be engaged that renders migrating cells resistant to chemical inhibitors directed against EGFR and Src.
p120ctn has been suspected as a tumor suppressor mainly based on its physical and functional connection with cadherins, especially E-cad. p120ctn mediated stability and turnover of E-cad [13,15], which has been proposed as the most potent mediator of cell–cell adhesion [17,18]. Loss of p120ctn may endow cancer cells an advantage in cell migration through abrogation of cell adhesion. Consistently, membrane loss, downregulation or mislocalization of p120ctn has been reported in almost all major types of cancer, including lung, prostate, breast, pancreas, colon, skin, bladder, and endometrium [30,31]. Neoplastic lesions grow in the oral cavity, esophagus, and forestomach of p120ctn conditional knockout mice [32], and intestinal adenoma have been identified in a mouse model with limited p120ctn ablation [33]. These studies established a causal role for p120ctn as a tumor suppressor and are in line with our reported findings.
Although the mechanism whereby smoke promotes membrane loss of p120ctn is currently unknown, it is possible that Src actively elicits it promigratory function by virtue of disrupting p120ctn. Interestingly, both AG1478 [29] and PP2 [34] have been reported to stabilize adherens junctions. Destabilization of p120ctn following Src activation may be achieved through its phosphorylation since membrane p120ctn was originally identified as a substrate of Src [35]. Our data suggest that in the early stages of lung cancer, intact membrane p120ctn maintains cell–cell adhesion until smoke-induced Src-P negates its effect to promote cell migration. With advanced disease, loss of membrane p120ctn releases its role as a tumor suppressor and unveils an alternative pathway (Fig. 4). Activation of this pathway promotes cell migration independent of EGFR/Src; thus, providing a mechanism whereby EGFR or Src inhibitors lose their therapeutic efficiency. Our preliminary studies suggest this pathway may involve the activation/inhibition of cofilin’s upstream signaling partners Limk1, ROCK and the Rho GTPases, RhoA and Rac1. Inhibition of ROCK and Limk1 has been shown to promote cofilin dephosphorylation and migration independent of p120ctn [27]. Identification of alternative pathways linking smoke to cell migration in a primary cell system is essential given most lung cancer patients exhibit intrinsic resistance or develop acquired resistance to conventional tyrosine kinase inhibitors targeting EGFR or Src after a median of 10–14 months [36]. A deeper understanding of smoke-induced cell migration may provide a platform for screening new drug candidates that suppress tumor progression in the setting of increasing chemoresistance and decreasing expression of membrane p120ctn.
Acknowledgment
This work was supported by American Cancer Society grant 115502-RSG-08-136-01-CNE.
References
- [1].Shafey O EM, Ross H, Mackay J. The Tobacco Atlas. American Cancer Society; Atlanta (GA): 2009. [Google Scholar]
- [2].Pleasance ED, Stephens PJ, O’Meara S, McBride DJ, Meynert A. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463:184–190. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu F, Killian JK, Yang M, Walker RL, Hong JA, et al. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene. 2010;29:3650–3664. doi: 10.1038/onc.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barr S, Thomson S, Buck E, Russo S, Petti F. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clinical and Experimental Metastasis. 2008;25:685–693. doi: 10.1007/s10585-007-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dasari V, Gallup M, Lemjabbar H, Maltseva I, McNamara N. Epithelialmesenchymal transition in lung cancer: is tobacco the “smoking gun”? American Journal of Respiratory Cell and Molecular Biology. 2006;35:3–9. doi: 10.1165/rcmb.2006-0051SF. [DOI] [PubMed] [Google Scholar]
- [7].Lemjabbar H, Li D, Gallup M, Sidhu S, Drori E, Basbaum C. Tobacco smoke-induced lung cell proliferation mediated by tumor necrosis factor alpha-converting enzyme and amphiregulin. Journal of Biological Chemistry. 2003;278:26202–26207. doi: 10.1074/jbc.M207018200. [DOI] [PubMed] [Google Scholar]
- [8].Khan EM, Lanir R, Danielson AR, Goldkorn T. Epidermal growth factor receptor exposed to cigarette smoke is aberrantly activated and undergoes perinuclear trafficking. FASEB Journal. 2008;22:910–917. doi: 10.1096/fj.06-7729com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Du B, Leung H, Khan KM, Miller CG, Subbaramaiah K, Falcone DJ, Dannenberg AJ. Tobacco smoke induces urokinase-type plasminogen activator and cell invasiveness: evidence for an epidermal growth factor receptor dependent mechanism. Cancer Research. 2007;67:8966–8972. doi: 10.1158/0008-5472.CAN-07-1388. [DOI] [PubMed] [Google Scholar]
- [10].Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, Banerjee S, Carless M, Kim E, Coppola D, Haura E, Chellappan S. Nicotine induces cell proliferation. invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines, International Journal of Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pillai S, Rizwani W, Li X, Rawal B, Nair S, Schell MJ, Bepler G, Haura E, Coppola D, Chellappan S. ID1 facilitates the growth and metastasis of non-small cell lung cancer in response to nicotinic acetylcholine receptor and epidermal growth factor receptor signaling. Molecular and Cellular Biology. 2011;31:3052–3067. doi: 10.1128/MCB.01311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–7956. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- [13].Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. Journal of Cell Biology. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120-catenin in E-cadherin function. Journal of Cell Biology. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120-catenin function as a set point for cadherin expression levels in microvascular endothelial cells. Journal of Cell Biology. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, Ikura M. Dynamic and static interactions between p120-catenin and E-cadherin regulate the stability of cell–cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- [17].Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nature Reviews Molecular Cell Biology. 2003;4:657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- [18].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [19].Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, Brugge JS. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nature Cell Biology. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- [20].Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. American Journal of Physiology. 1992;262:L713–724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- [21].Liu Y, Li QC, Miao Y, Xu HT, Dai SD, Wei Q, Dong QZ, Dong XJ, Zhao Y, Zhao C, Wang EH. Ablation of p120-catenin enhances invasion and metastasis of human lung cancer cells. Cancer Science. 2009;100:441–448. doi: 10.1111/j.1349-7006.2008.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Witschi H, Espiritu I, Maronpot RR, Pinkerton KE, Jones AD. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997;18:2035–2042. doi: 10.1093/carcin/18.11.2035. [DOI] [PubMed] [Google Scholar]
- [23].Xu L, Deng X. Protein kinase Ciota promotes nicotine-induced migration and invasion of cancer cells via phosphorylation of micro- and m-calpains. Journal of Biological Chemistry. 2006;281:4457–4466. doi: 10.1074/jbc.M510721200. [DOI] [PubMed] [Google Scholar]
- [24].Zhang Q, Tang X, Zhang ZF, Velikina R, Shi S, Le AD. Nicotine induces hypoxia-inducible factor-1alpha expression in human lung cancer cells via nicotinic acetylcholine receptor-mediated signaling pathways. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:4686–4694. doi: 10.1158/1078-0432.CCR-06-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gensch E, Gallup M, Sucher A, Li D, Gebremichael A, Lemjabbar H, Mengistab A, Dasari V, Hotchkiss J, Harkema J, Basbaum C. Tobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNK. Journal of Biological Chemistry. 2004;279:39085–39093. doi: 10.1074/jbc.M406866200. [DOI] [PubMed] [Google Scholar]
- [26].Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- [27].Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- [28].Lemjabbar-Alaoui H, Dasari V, Sidhu SS, Mengistab A, Finkbeiner W, Gallup M, Basbaum C. Wnt and Hedgehog are critical mediators of cigarette smoke-induced lung cancer. PLoS ONE. 2006;1:e93. doi: 10.1371/journal.pone.0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen YT, Gallup M, Nikulina K, Lazarev S, Zlock L, Finkbeiner W, McNamara N. Cigarette smoke induces epidermal growth factor receptor-dependent redistribution of apical MUC1 and junctional beta-catenin in polarized human airway epithelial cells. American Journal of Pathology. 2010;177:1255–1264. doi: 10.2353/ajpath.2010.091129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu Y, Wang Y, Zhang Y, Miao Y, Zhao Y, Zhang PX, Jiang GY, Zhang JY, Han Y, Lin XY, Yang LH, Li QC, Zhao C, Wang EH. Abnormal expression of p120-catenin. E-cadherin, and small GTPases is significantly associated with malignant phenotype of human lung cancer, Lung Cancer. 2009;63:375–382. doi: 10.1016/j.lungcan.2008.12.012. [DOI] [PubMed] [Google Scholar]
- [31].van Hengel J, van Roy F. Diverse functions of p120ctn in tumors. Biochimica et Biophysica Acta. 2007;1773:78–88. doi: 10.1016/j.bbamcr.2006.08.033. [DOI] [PubMed] [Google Scholar]
- [32].Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, Klein-Szanto A, Lee JS, Katz JP, Diehl JA, Reynolds AB, Vonderheide RH, Rustgi AK. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell. 2011;19:470–483. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Smalley-Freed WG, Efimov A, Short SP, Jia P, Zhao Z, Washington MK, Robine S, Coffey RJ, Reynolds AB. Adenoma formation following limited ablation of p120-catenin in the mouse intestine. PLoS One. 2011;6:e19880. doi: 10.1371/journal.pone.0019880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nam JS, Ino Y, Sakamoto M, Hirohashi S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clinical Cancer Research. 2002;8:2430–2436. [PubMed] [Google Scholar]
- [35].Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Molecular and Cellular Biology. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].OxnardGR AM, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to egfr tyrosine kinase inhibitors in lung cancer. Clinical Cancer Research. 2011;17:5530–5537. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]