Abstract
In order to evaluate the following potential mechanisms underlying atypical gaze following in autism, impaired reflexive gaze following, difficulty integrating gaze and affect, or reduced understanding of the referential significance of gaze, we administered three paradigms to young children with autism (N = 21) and chronological (N = 21) and nonverbal mental age (N = 21) matched controls. Children with autism exhibited impaired reflexive gaze following. The absence of evidence of integration of gaze and affect, regardless of diagnosis, indicates ineffective measurement of this construct. Reduced gaze following was apparent among children with autism during eye-tracking and in-person assessments. Word learning from gaze cues was better explained by developmental level than autism. Thus, gaze following may traverse an atypical, rather than just delayed, trajectory in autism.
Keywords: Response to joint attention, Autism, Reflexive gaze following, Word learning
Introduction
Under what conditions is responsiveness to joint attention (RJA), defined behaviorally as spontaneous gaze or point following, reduced among young children with autism? Conflicting accounts of when and why RJA is impaired in autism have arisen from studies examining different aspects of RJA among individuals of varying developmental levels (see Nation and Penny 2008 for a review). Given the heterogeneity of autism itself (e.g., Geschwind 2009), a reasonable way to resolve conflicting findings may be to administer a range of related tests to the same individuals. Such an approach yielded intriguing evidence that the ability to report where someone is looking may be intact among children with autism who do not spontaneously follow gaze (Leekam et al. 1997). However, the current study is the first, to our knowledge, to examine three potential underlying mechanisms of atypical RJA in autism among the same individuals: (a) atypical reflexive gaze following (Goldberg et al. 2008; Johnson et al. 2005; Ristic et al. 2005), (b) difficulty integrating gaze and affect (de Jong et al. 2008; Kasari et al. 1990; Uono et al. 2009), and (c) reduced recognition of the referential significance of gaze (Baron-Cohen et al. 1997; Preissler and Carey 2005).
These potential mechanisms are not necessarily independent and all could arise at least partially through reinforcement learning, which has been shown to play a role in RJA development (Corkum and Moore 1998). Similarly, implicit and explicit learning processes may contribute to the development of all three mechanisms. However, cues to elicit reflexive gaze following are typically presented for a shorter duration (and are thus more likely to be implicit) than are cues to emit spontaneous gaze following (e.g., Corkum and Moore 1998; Pruett et al. 2011; Ristic et al.2002). Verbal instructions were not provided to guide reflexive or spontaneous gaze behaviors because individuals with ASD who exhibit the ability to answer verbal questions about others’ minds may not exhibit spontaneous evidence of understanding others’ intentions (Senju et al. 2009).
How Does RJA Develop?
RJA typically begins to emerge very early in infancy (D’Entremont et al. 1997; Farroni et al. 2004; Hood et al.1998; Scaife and Bruner 1975) and develops gradually from birth to around 18 months of age (Butler et al. 2009; Butterworth and Jarrett 1991; Deák et al. 2000). Often emerging in the context of affective interactions (Adamson and Bakeman 1985; Reddy 2003; Teufel et al. 2010), RJA is an adaptive skill in that it provides information about what others find relevant in the environment. However, gaze following may not initially be synonymous with an understanding of others’ viewpoints (Corkum and Moore 1998).
RJA is not always observed in children with autism (e.g., Dawson et al. 2004; Loveland and Landry 1986; Sigman and Ruskin 1999). Indeed, reduced RJA is an early predictor of autism (Landa et al. 2007; Rozga et al. 2011; Sullivan et al. 2007; Yoder et al. 2009). Among individuals with ASCs (autism spectrum conditions), individual differences in RJA are related to concurrent linguistic and cognitive skills (e.g., Leekam et al. 1998; Sigman and Ruskin 1999) and subsequent cognitive (Sigman and Ruskin 1999), social-communicative (Mundy et al. 1990; Sigman and McGovern 2005) and adaptive development (Gillespie-Lynch et al. 2012).
However, reduced RJA is often not apparent among older or higher functioning individuals with autism. For example, RJA deficits were not observed in children with ASC who had a nonverbal mental age (NVMA) above 19 months (Mundy et al. 1994), a verbal mental age (VMA) above 47 months (Leekam et al. 1998; but see Leekam et al. 1997) or a nonverbal IQ in the normal range (Leekam et al. 2000). A delay in the development of RJA is apparent even when comparing children with autism to children with other types of developmental delay (e.g., Dawson et al. 2004; Leekam et al. 2000; Sigman and Ruskin 1999). Leekam et al. (2000) surmised that children with autism may require both more time and greater intellectual development in order to interpret the predictive meaning of gaze cues. Thus, it is important to consider developmental level when comparing across studies examining gaze following in autism. For example, most studies of reflexive gaze following, or orienting more quickly to targets cued by non-predictive gaze cues, were conducted with high-functioning participants in middle childhood or older.
Is Reflexive Gaze Following Impaired in Autism?
Evidence of reflexive gaze following, or looking more quickly toward objects cued by gaze, may be apparent within a few days of birth (Farroni et al. 2004). Investigations of whether reflexive gaze following is impaired in autism have typically used attention cueing paradigms wherein participants attend to a central face presenting gaze cues that are predictive, counter-predictive or non-predictive of the future location of a target (Posner 1980). A validity effect, or speeded detection of targets that are validly cued (in the location that the model’s eyes were looking toward) relative to those that are invalidly cued (in the location the eyes were looking away from), is taken as evidence of reflexive orienting. Participants are often instructed to attend to targets covertly (i.e., without moving their eyes from the center of the screen) and detection is generally indicated by pressing a button. The majority of the evidence suggests that high functioning children and adolescents (mean age of 9 to 11 years of age) and adults with autism demonstrate intact reflexive orienting to gaze when assessed with Posner-style paradigms (de Jong et al. 2008; Kylliainen and Hietanen 2004; Pruett et al. 2011; Rombough and Iarocci in press; Rutherford and Krysko 2008; Senju et al. 2004; Swettenham et al. 2003; Uono et al. 2009; Vlamings et al. 2003; but see Goldberg et al. 2008; Ristic et al. 2005). Even when reflexive gaze following is intact, however, people with autism may differentiate between gaze cues and non-social cues less than typically developing individuals do (Chawarska et al. 2003; Greene et al. 2011; Rombough and Iarocci in press; Senju et al. 2004; Vlamings et al. 2003; but see Kuhn et al. 2010; Ristic et al. 2002). Additionally, gaze cues may modulate action control, or reduce reaction time differences between responses that are congruent or incongruent with gaze cues, among typical adults but not those with ASD (Schilbach et al. 2012).
The aforementioned studies generally assessed covert reflexive orienting among high functioning individuals who would not be expected to exhibit reduced spontaneous RJA. Covert orienting may also be unimpaired relative to overt orienting, such as head movements, in autism (Gernsbacher et al. 2008). Less is known about overt reflexive gaze following, wherein participants are allowed to move their eyes toward the target following non-predictive gaze cues, among young children with autism. Kuhn et al. (2010) reported that overt reflexive orienting in response to schematic faces was intact among high functioning adults with autism. Two assessments of overt reflexive gaze cueing have been conducted with preschoolers with autism (Chawarska et al. 2003; Johnson et al. 2005). Chawarska et al. (2003) compared 15 cognitively delayed 2 year olds with autism to 12 controls matched for chronological age (CA). Although the children with autism exhibited reduced RJA during an in-person assessment, the interaction between reflexive gaze following and diagnosis was not statistically significant. However, children with autism attended to the target faster regardless of cue validity. Because no differences in reaction time were observed when the central cue was not social, the authors interpreted this as evidence for decreased engagement with a social stimulus. Johnson et al. (2005) found that 9 young children (mean age of 33 months) with autism also oriented more quickly to targets regardless of gaze cues relative to language delayed and typically developing controls, and post-hoc planned t tests after a marginally significant interaction between group and validity revealed that only the children with autism did not exhibit reflexive gaze following. A primary aim of the current study, therefore, was to determine if overt reflexive gaze following is intact among young children with autism.
Integrating Gaze and Affect
The preponderance of the evidence suggests that reflexive gaze following may be unimpaired in autism, yet even high functioning people with autism may have difficulty integrating emotional signals and gaze cues (de Jong et al.2008; Uono et al. 2009). Attention cueing paradigms typically present gaze cues in the context of neutral facial expressions. When gaze cues are paired with emotional expressions, typically developing adults may demonstrate more of a covert validity effect to fearful relative to happy or neutral gaze cues (Friesen et al. 2011; Graham et al. 2010; Pecchinenda et al. 2008; Tipples 2006). However, emotional faces may not enhance reflexive orienting among individuals with autism: 11 high-functioning adolescents with ASCs differed from age and IQ matched controls in that they did not show an enhanced covert validity effect for fearful relative to neutral gaze cues (Uono et al. 2009). Because children with autism may integrate positive affect with joint attention less than typically developing children and children with other disabilities (Kasari et al. 1990), a second aim of the current study was to examine overt reflexive gaze cueing in response to happy, fearful, and neutral expressions. Reduced gaze and emotion integration in autism could represent a core difficulty from which atypical RJA emerges. Indeed, mutual sharing of affect between infants and caregivers may give rise to later shared attention to objects (Reddy 2003).
Referential Significance of Gaze
Even if reflexive gaze following is unimpaired among young children with autism, reduced spontaneous gaze following may arise from difficulty interpreting the referential significance of gaze (Baron-Cohen et al. 1985). Intention reading is associated with gaze following among children with autism (Schietecatte et al. 2012). While an understanding of mental states is not necessary for gaze following, such an understanding could motivate gaze following (see Nation and Penny 2008). Difficulties using RJA to learn words in conjunction with typical reflexive gaze following would yield support for the theory that RJA impairments in autism arise from difficulties understanding referential intention but would be inconsistent with recent evidence suggesting that implicit social cognition may be impaired in ASD even when explicit social cognition is unimpaired (e.g., Schilbach et al. in press, 2012; Senju et al. 2009).
Gaze following is important for recognizing the referential nature of vocalizations in mapping words to objects (Baldwin 1991; Scaife and Bruner 1975; Seibert et al.1986). Difficulties using a speaker’s focus of attention to learn words have been documented among severely cognitively delayed children with autism (Baron-Cohen et al. 1997; Preissler and Carey 2005). Unlike typically developing and intellectually delayed children, school age children with autism were more likely to attach a label to the object they were attending to, or to use a listener’s direction of gaze (LDG) strategy, rather than attaching the label to the object a speaker was attending to, or a speaker’s direction of gaze (SDG) strategy.
Baron-Cohen et al. (1997) proposed that the LDG word learning strategy was part of a general difficulty sharing attention and understanding intentions in autism. Yet the LDG strategy may not be as common among children with autism as early studies suggested (Akechi et al. 2011; Luyster and Lord 2009; Parish-Morris et al. 2007). Luyster and Lord (2009) found that a LDG strategy was not apparent among younger (mean age 30 months) and higher functioning (with a mean NVIQ of 95) children with autism. Even among the 5 severely linguistically delayed children in the sample, one used the SDG strategy and 4 did not select any object. A similar pattern of avoiding mapping errors (but not necessary correctly mapping) is observed among typically developing infants (Baldwin 1991; Hollich et al. 2000), leading the authors to suggest that children with autism may follow a delayed but qualitatively typical path toward learning words from gaze cues.
Similar evidence of a delayed ability to use others’ attention to learn words was documented among slightly older (mean age of 5 years) moderately cognitively impaired children (with a mean IQ of 76) with autism in comparison to VMA and NVMA matched controls (Parish-Morris et al. 2007). Separate assessments used to measure joint attention and word learning in response to social cues revealed that children with autism exhibited less spontaneous gaze following than controls and unlike controls they did not use social cues to learn the labels of perceptually dull objects presented in conjunction with more salient objects.
These studies imply that the ability to learn words from gaze cues may be related to gaze following abilities, but gaze following during word learning was not assessed. A recent eye-tracking study suggests that subtle atypicalities in gaze following may underlie subtle difficulties with word learning among high functioning individuals (Akechi et al. 2011). Seventeen children with ASCs (mean age of 9 years) were compared to typically developing controls. Pairs of novel objects were presented on a computer screen while a schematic face looked toward and labeled either the object the child was attending to or the object the child was not attending to. Participants with and without ASCs more frequently looked from the face to the object the face was attending to relative to the other object. Typically developing participants, but not those with ASCs, differed from chance in the duration which they attended to the target relative to the other object (this analysis was a follow-up to a non-significant interaction between group and duration). While participants with and without ASC demonstrated above chance word comprehension, participants with ASC exhibited slightly less word learning than controls.
An eye-tracking study with 3-year-old siblings of children with autism suggests that understanding the referential intention behind gaze may be more important for word learning than gaze following (Gliga et al. 2012). Despite the absence of clear differences in gaze following (either duration or frequency) toward the object a model was labeling, siblings with poorer social-communication skills looked less to the target object during testing than typically developing children. While this study suggests that the frequency of gaze following may be less important for word learning than social-cognitive skills among the siblings of children with autism, the relative importance of developmental level and gaze following for word learning in response to gaze cues among individuals with autism remains an open question. The third aim of the current study was to clarify relations between gaze following, cognitive level and word learning in response to gaze cues using both an eye-tracker (video presentation) and a live model.
Hypotheses
Hypothesis 1: Intact Reflexive Gaze Following
We predicted that reflexive gaze following would be intact among children with ASC and typically developing children. We expected a main effect of validity and no moderating effect of diagnosis.
Hypothesis 2: Impaired Integration of Gaze and Affect
We predicted an interaction between diagnosis, emotion, and validity because typically developing children were expected to exhibit a larger validity effect to fearful relative to neutral or happy faces. In contrast, we predicted no emotional enhancement of the validity effect among children with ASC.
Hypothesis 3: Difficulty with the Referential Significance of Gaze is Related to Developmental Level Rather than Autism
We expected reduced spontaneous gaze following to be related to diagnosis after controlling for differences in age or NVMA but word learning following gaze cues to be attributable to NVMA rather than diagnosis.
Methods
Participants
Twenty-four children with autism and 42 children without an autism diagnosis participated. Children with autism (range 2.4–6.7 years) were individually matched to developing children in terms of CA (within 3 months) or NVMA (within 6 months). Gender was matched except in one case of CA and one case of NVMA. CA and NVMA age control groups were not independent of one another in that higher-functioning participants with autism were often matched with the same control for both CA and NVMA (n = 12). Comparisons of CA matches and children with ASCs are presented in Table 1 while comparisons of NVMA and children with ASCs are presented in Table 2.
Table 1.
Participant characteristics of CA matches and participants with ASCs: mean (SD)
| ASC (N = 21) | CA match (N = 21) | |
|---|---|---|
| Number female | 2 | 3 |
| CA in months | 56.48 (14.81) | 56.95 (15.27) |
| NVMA in months | 52.33 (23.49)* | 67.00 (17.62)* |
| VMA in months | 45.24 (21.75)* | 67.90 (18.00)* |
| Therapy in hours | 484.04 (401.25) | NA |
| Maternal ed. in years | 16.40 (1.77) | 17.52 (2.14) |
| % English in home | 87.59 (16.64) | 89.50 (22.12) |
Significant differences (p < .05)
Table 2.
Participant characteristics of NVMA matches and participants with ASCs: mean (SD)
| ASC (N = 21) | NVMA match (N = 21) | |
|---|---|---|
| Number female | 2 | 3 |
| CA in months | 56.48 (14.81)* | 44.29 (19.65)* |
| NVMA in months | 52.33 (23.49) | 52.33 (23.26) |
| VMA in months | 45.24 (21.75) | 51.72 (22.06) |
| Therapy in hours | 484.04 (401.25) | NA |
| Maternal ed. in years | 16.40 (1.77) | 17.35 (2.01) |
| % English in home | 87.59 (16.64) | 86.33 (22.45) |
Significant differences (p < .05)
Most of the children with autism (fifteen older siblings with autism and four at-risk siblings who developed autism) who participated in this study were recruited from families participating in an ongoing study of the longitudinal development of infant siblings of children with autism. In addition, four children with autism were recruited via fliers from the community. Two participants from the community had verified diagnoses of autism but no evidence that the children met criteria for ASC on the ADOS or the autism diagnostic interview (ADI-R); they were excluded from the analyses. For the remaining participants with ASCs (two female), clinical diagnoses were informed by the ADOS (n = 21) and the ADI-R (n = 15), 12 met the ADOS cut-off for autism and 9 met the ADOS cut-off for ASC.
Control participants were screened for autism symptoms with the Social Responsiveness Scale (SRS: if they were 4 years or older) and the Childhood Autism Rating Scale (CARS: if they were younger than 4). Two controls were excluded for elevated symptoms on the SRS. Three controls were excluded for a family history of autism. One control was excluded because the eye-tracker failed to follow his gaze due to thick glasses. Four controls were excluded because they did not match a child with autism in NVMA or CA.
Standardized Measures
CARS (Schopler et al. 2010)
The CARS includes a 36 item parent-report questionnaire that, in conjunction with examiner ratings, yields ratings of autism symptomatology. It has high internal consistency and inter-rater reliability.
Differential Ability Scales II (DAS: Elliot 1990)
The DAS can be used to assess nonverbal and verbal intelligence among children with a mental age between 2 years 6 months and 17 years 11 months (Elliot 1990). It has well established internal and external reliability. The DAS was used with most participants who were mature enough to receive age equivalence scores on the measure. Younger or more delayed children were assessed with the Mullen Scales of Early Learning (MSEL).
MSEL (Mullen 1995)
The MSEL is a standardized measure of VMA and NVMA from birth to 68 months of age. It has good test–retest reliability and high internal consistency. Participants who appeared to be below 2 years 6 months in mental age were administered the MSEL. The four younger siblings of children with autism who themselves developed autism were assessed with the MSEL as was one of their matched controls. The convergent validity of the DAS and the MSEL has been established in autistic and non-autistic populations (Bishop et al. 2011).
SRS (Constantino and Gruber 2002)
The SRS is a 65 item parent rating scale that assesses the severity of autism symptoms. It yields a dimensional measure of autism symptomatology as well as categorical cut-offs believed to indicate atypically high levels of symptoms. It can be used with individuals 4 to 18 years of age and has high internal consistency and test-retest reliability.
Gaze Following Assessments
Eye-Tracking Overview
Looking behaviors were recorded by a Tobii 1750 eye-tracker (Tobii Technology AB, Falls Church VA) integrated with a 17-in. monitor, while the child sat approximately 65 cm. from the monitor. Cameras beneath the monitor recorded reflections from an infrared light at a frequency of 50 Hz to assess the distance between the cornea and the pupil of both eyes. The spatial accuracy of these recordings approximates 1° of visual angle when participants’ point of gaze is calibrated according to the manufacturer’s recommendations (Morgante et al. 2012). A chin rest is not necessary to achieve this level of calibration accuracy and was not used in the current study. Stimuli were displayed with Tobii ClearView software. Fixations were defined as gaze within a 30 pixel radius for at least 100 ms. The “normal” ClearView validity filter averaging across both eyes was used. A five-point calibration was administered prior to the assessment. Rectangular areas of interest (AOIs), defined with ClearView software, subtended approximately 1° from the edge of all stimuli.
Participants were assigned to view the attention cueing and joint attention word learning eye-tracking paradigms in counterbalanced orders. Controls were assigned to the same version and order of each type of stimuli as their match with autism whenever possible (this occurred more frequently with chronological matches because such matches were more predictable prior to testing). Prior to each trial in either eye-tracking paradigm, the child’s attention was centered whenever possible with an animated attention getter. Trials prior to which the child’s attention was not centered were excluded from analysis.
Gaze Cueing
This paradigm was modeled after the study by Chawarska et al. (2003), which also measured overt gaze orienting in young children with autism. We also manipulated facial expression and stimulus onset asynchrony (SOA), the amount of time between the gaze cue and target onset, which was either 167 or 400 ms.
Each trial began with the model looking straight ahead with a neutral expression for 1,000 ms (see Fig. 1). This was followed by a static image of the model gazing to the left or right with one of the following facial expressions: happiness, fear, or a neutral expression. The model’s face measured 16.1° and 11.1° of vertical and horizontal angle. The model was selected from the Nim Stim catalogue (Tottenham et al. 2009) based on high validity ratings of the three facial expressions. A usable trial was defined by at least 100 ms. of attention to the head during the gaze cue. Then the face disappeared and was replaced by an object (a green balloon or a red bottle of bubbles) to the left or right of where the face had been which remained on the screen for 1,000 ms. The images of objects were selected from the Bank of Standardized Stimuli to equate them in terms of familiarity and object complexity (Brodeur et al. 2010). The objects measured 6.4° and 6.0° of vertical and horizontal angle.
Fig. 1.
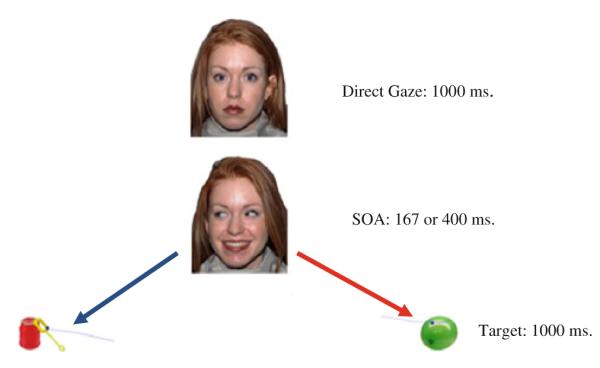
Gaze cueing stimuli. A different model than the one used for the stimuli is portrayed here due to copyright issues. The actual model used was model 14 in the NimStim set
The model’s gaze predicted the location of the target 50 % of the time. There were 48 trials. Two randomly-ordered versions of the stimuli were generated to counterbalance location cued, validity, target, and target location with the constraint that no stimulus type could occur more than twice in a row. This order was then transformed by replacing all fearful expressions with happy expressions, all happy with neutral and all neutral with fearful in order to create the second version of the stimuli.
Assessments of Joint Attention Word Learning
Participants were provided two opportunities for word learning, one in-person and one with the eye tracker. The eye-tracking word learning opportunity always occurred before the in-person opportunity because young children may learn words better from screens when screen-based word learning is preceded by an interaction (Roseberry et al. 2009). Thus a strong order effect could have occurred if the order of conditions were counterbalanced. Four conditions were created in which object-word pairings were counterbalanced across in-person and eye-tracking conditions.
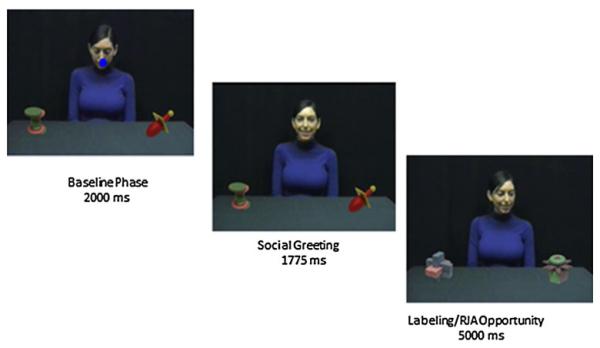
Two counterbalanced sets of training and test materials were assigned to either the in-person or eye-tracking opportunity for word learning. Within each set, two novel objects were always associated with the same two novel words during training. The novel words were monosyllabic yet phonologically distinct across languages to allow recruitment of children who were not monolingual. The two pairs of words were “bon” versus “dit” and “deet” versus “don.” During each training phase, children viewed a model seated between two novel objects. The objects always remained in the same location relative to the model. The model always turned in the following order LRRLRLLR and labeled each object once after she had turned to look at it. The word the model said as she looked to the left or right was counterbalanced (see Fig. 2).
Fig. 2.
Eye-tracking joint attention word learning stimuli
Eye-Tracking Assessment of Joint Attention Word Learning
Prior to word learning training, the two novel objects were shown on the screen in silence for 2 s to assess a baseline preference for either object, then repeated with the location of the objects switched. This was followed by a training session consisting of 8 word learning opportunities (4 for each object). Each trial began with a baseline phase wherein the model looked down (~2 s) followed by gazing and smiling in the direction of the child (~1.8 s). Then she turned toward one of the novel objects, and labeled it once while gazing at it and continuing to smile (~5 s). The model’s face measured 7.9° and 5.3° of vertical and horizontal angle. The objects measured between 7.6° and 9.0 ° of vertical and 6.5° and 9.3° of horizontal angle.
The training phase was followed by a testing phase during which only the objects were visible on the screen (2 s). During each of these trials, the child heard the model say a trained novel word once. Two same position trials (wherein the child heard each of the novel words while the location of the objects relative to one another was unchanged from training) were followed by two different position trials (wherein the location of the objects was reversed from training). This was followed by a “mutual exclusivity” trial wherein the child saw the two trained objects, a familiar object and a novel object that had not been seen before and heard a novel word that had not been heard before (i.e., “blick” or “bleek”). Lastly, two more same position and two more different position trials were presented. After the eye-tracking assessment, the child was presented with the two novel objects seen in the video and asked to select each novel word i.e., “Give me the deet.” This object selection was recorded by the examiner during testing but was not video-taped so it was not possible for a second coder to double-check them.
In-Person Assessment of Joint Attention Word Learning
This assessment was modeled after the eye-tracking measure. The child sat across the table from the model. The model secured the child’s attention before looking toward one of the objects while labeling it and smiling. Two independent coders (Cohen’s kappa .80) achieved reliability on 30 % of the sample for video coding of in-person joint attention. After eight training trials, the child was asked to select each object in its trained position i.e., “Show me the deet”, followed by two reversal trials, a mutual exclusivity trial, and two more opportunities to select the objects in the same position as during training. Mutual exclusivity trials were not analyzed. All word learning scores were assigned by the examiner during testing and double checked by a second coder.
Results
Despite careful attempts to individually match children with autism to controls, the number of children who provided usable data varied across measures. For each analysis, it will be noted if the intended dimensions of matching still hold given loss of data. Because the CA and NVMA matched groups were not independent of one another, comparisons between each type of control and children with autism will be reported separately. CA matches are older on average than NVMA matches. When comparison groups differed in CA or NVMA, the variable that differed was entered into models as a covariate. All significant main effects and interactions are reported.
Hypothesis 1: Intact Reflexive Gaze Following
The dependent variables used to investigation reflexive gaze following and integration of gaze and affect were gaze cueing usable trials and gaze cueing response time (RT). Gaze cueing usable trials were defined as the number of trials in which the child looked at the model’s head during the directional gaze cue and oriented to the target. If this was 0 for a given condition, no gaze cueing RT was available for that condition. Gaze cueing RT was calculated by averaging the RT in ms between the beginning of a gaze cue and the beginning of the first fixation to the target for at least 100 ms. Response time variables were log transformed due to excessive skew. Two RTs were calculated for each pairing of emotion and SOA: i.e., valid RT (following a valid gaze cue) and invalid RT. Each SOA was analyzed separately because different children provided usable data for each.
Analyses used to investigate the hypothesis of intact reflexive gaze following focus only on neutral gaze cues. Children with autism did not differ reliably from controls in the number of usable trials provided at either SOA. Among children who provided usable data at the short SOA, children with autism (N = 17) had marginally lower NVMA than CA matches (N = 18; p = .052) but did not differ reliably from NVMA matches (N = 17) in CA or NVMA. Focusing on CA matches at the short SOA, an ANCOVA with validity (valid RT and invalid RT) as a repeated measure, diagnosis as a between subjects factor, and NVMA as a covariate revealed no significant effects, although a trend toward an interaction between validity and diagnosis was observed (p = .096). An identical analysis conducted with NVMA matches (except without a covariate as the groups did not differ in CA or NVMA) revealed a main effect of diagnosis F (1, 32) = 5.500, p = .025 and an interaction between diagnosis and validity F (1, 32) = 4.459, p = .043 (see Fig. 3), which was in the same direction as the trend observed with CA matches. Post-hoc tests revealed a validity effect F (1, 16) = 6.444, p = .022 among typically developing participants but not children with autism. Children with ASCs (M = 2.494, SE = .020) were also faster to orient to targets regardless of cue validity than controls (M = 2.559, SE = .020; p = .025).
Fig. 3.
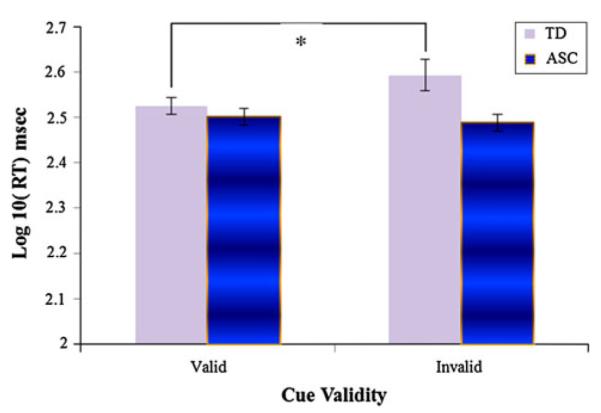
Impaired reflexive gaze cueing at the 167 ms SOA for NVMA matches. Error bars represent SEs
At the longer SOA, children with autism (N = 19) were significantly different than CA matches (N = 16) in NVMA (p = .017) but NVMA matches (N = 15) did not differ. Focusing on CA matches at the longer SOA, an ANCOVA with validity, diagnosis and NVMA as a covariate revealed an effect of diagnosis, F (1, 32) = 7.847, p = .003 and an interaction between validity and diagnosis F (1, 32) = 4.391, p = .044 (see Fig. 4). Children with autism (M = 2.543, SE = .013) were slower to orient to targets than typically developing children (M = 2.486, SD = .015; p = .009). Post-hoc tests revealed a validity effect F (1, 14) = 4.585, p = .050 and for typically developing children but not those with autism. No effects were observed when comparing NVMA matches. Contrary to our expectations, evidence of impairments in overt reflexive gaze following was evident in a well-matched comparison between children with and without autism, but not in a less well matched comparison.
Fig. 4.
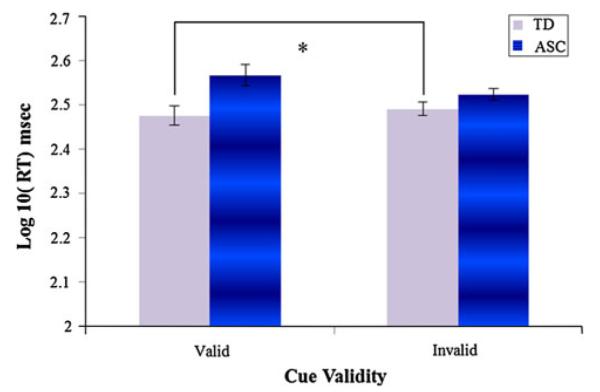
Impaired gaze cueing at the 400 ms SOA for CA matches after controlling NVMA. Error bars represent SEs
Hypothesis 2: Impaired Integration of Gaze and Affect
These analyses used the same dependent variables as those discussed for hypothesis 1 but focused on reflexive gaze following in response to neutral, happy and fearful cues. Children with autism for whom RT data were available for all emotion validity pairings did not differ reliably from controls in the number of usable trials provided at either SOA. CA matched controls who supplied data for emotion integration analyses were higher in NVMA at the long SOA (p = .048). No reliable differences in CA or NVMA were observed at either SOA among NVMA matches (short N = 16; long N = 15) and children with autism (short N = 11; long N = 13). Analyses focus on NVMA controls as they are better matched.
A repeated measures analysis at the short SOA with validity and emotion (fear, happy or neutral) as factors revealed an interaction between diagnosis and validity F (1, 25) = 4.376, p = .047, a main effect of emotion F (2, 50) = 5.279, p = .008, and an interaction between emotion and diagnosis F (2, 50) = 4.515, p = .016. However, the predicted interaction between validity, emotion and diagnosis was not observed. Post-hoc tests revealed that participants oriented to targets more slowly following fearful (M = 2.579, SE = .019) than neutral gaze cues (M = 2.529, SE = .017, p = .008). Post-hoc tests investigating the relation between emotion and diagnosis were not statistically significant.
A similar analysis of data from the longer SOA revealed a significant effect of emotion F (2, 42) = 6.963, p = .002 and an interaction between emotion and diagnosis F (2, 52) = 3.601, p = .034. Post-hoc tests revealed faster orienting to happy (M = 2.496, SE = .014) relative to neutral (M = 2.533, SE = .013; p = .001) or fearful (M = 2.536, SD = .016; p = .002) gaze cues, regardless of cue validity. A significant main effect of emotion on usable trials was observed wherein fearful cues (M = 2.51, SD = .17) yielded fewer usable trials than neutral cues (M = 2.98, SD = .153; F (2, 76) = 5.277, p = .007). Post-hoc tests investigating the relation between emotion and diagnosis were again not significant. Thus, we observed no evidence of integration of emotion and gaze regardless of diagnosis.
Hypothesis 3: Difficulty with the Referential Significance of Gaze is Related to Developmental Level Rather than Autism
Spontaneous gaze following was assessed with eye-tracking and in-person first look difference scores (Corkum and Moore 1998), or the number of training trials in which the child first looked from the model’s head to the object the model was attending to minus the number of trials wherein the child first looked to the other object.
Gaze Following Across Contexts
Children with autism (N = 16) did not differ reliably in CA or NVMA from CA matched controls (N = 18). NVMA matched controls (N = 18) were reliably lower in CA (p = .003). To compare performance of the ASC group to CA matches, we conducted a mixed ANOVA on first look difference scores (gaze following) with location of word learning as a repeated measure and diagnosis as a between subjects measure. Children with ASCs (M = 3.667, SE = .466) followed gaze less frequently overall than typically developing children across contexts (M = 5.417, SE = .425; F (1, 31) = 7.694, p = .009; see Fig. 5). Gaze following occurred less frequently in the eye-tracker (M = 4.050, SE = .331) than in person (M = 5.033, SE = .447; F (1, 31) = 4.362, p = .045). A similar ANCOVA conducted with NVMA matches (except that CA was entered as a covariate) revealed significant main effects of diagnosis F (1, 31) = 6.725, p = .014 and age F (1, 31) = 6.252, p = .018. Again, children with ASCs (M = 3.172, SE = .479) followed gaze less frequently overall than typically developing children (M = 4.986, SE = .448; p = .014). As hypothesized, children with ASCs exhibited less gaze following across contexts than typically developing children after controlling for differences in developmental level.
Fig. 5.
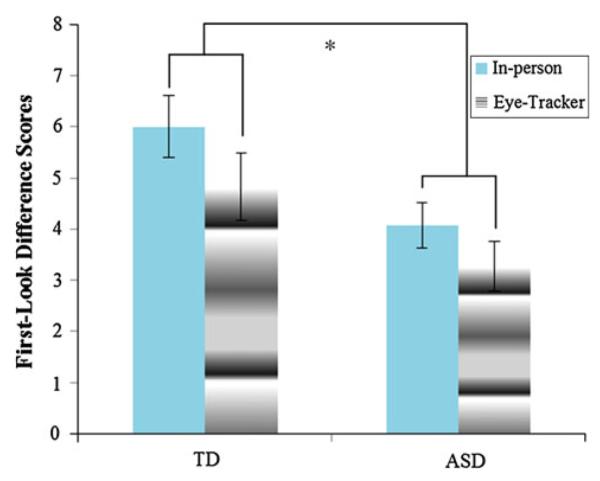
Gaze following across contexts for CA matches. Error bars represent SEs
Eye-Tracking Word Learning
Word learning from gaze cues during eye-tracking was assessed with eye-tracking referent preferences (based on Hollich et al. 2000), calculated by subtracting the summed duration of all looks of at least 100 ms. at the incorrect object from the summed duration of all looks at the correct object during test trials, and eye-tracking referent choices. Children received a score of 1 for eye-tracking referent choices if they correctly selected the referent of both novel words after testing and a score of 0 otherwise.
For CA and NVMA matches, eye-tracking referent preference was unrelated to referent choices after eye-tracking. Binary logistic regressions with CA or NVMA matches revealed no statistically significant associations between referent selection and diagnosis, first look difference scores (gaze following during training), age or NVMA. An ANCOVA conducted with CA matches predicting eye-tracking referent preference from the same set of predictors revealed an association between first look difference scores F (1, 28) = 8.770, p = .006, NVMA F (1, 28) = 6.371, p = .018 and referent preference. An identical ANCOVA with NVMA matches yielded no significant results. Thus, eye-tracking measures of word learning were not related to one another, referent selection after eye-tracking was not attributable to cognitive level or autism, and referent preference during the eye-tracking test phase was associated with gaze following during training and cognitive level (at least for CA matches).
In-Person Word Learning
Word learning following in person gaze cues was assessed with in-person referent choices. Children were assigned a score of 1 if they selected the referent on 6 out of 6 test trials—i.e., significantly better than chance with a binomial test- and 0 otherwise. For CA matches, NVMA was associated with in-person referent selection (whether or not the child selected the correct object on all test trials) (B = 1.138, SE = .061; p = .034). In-person first look difference scores, age, and diagnosis were not significantly associated with in-person referent selection. For NVMA matches, NVMA was associated with referent selection (B = 1.124, SE = .055, p = .036), but age, diagnosis, and difference scores were not. The third hypothesis, therefore was partially confirmed: gaze following difficulties were associated with autism while referent selection after in-person training and referent preference during eye-tracking were related to cognitive level. No relations between gaze following and referent selection were observed although gaze following was associated with referent preference.
Discussion
The current study examined three potential mechanisms underlying atypical gaze following in autism by using a range of measures to assess different aspects of gaze following in young children with and without autism. Results suggest that both reflexive and spontaneous gaze following may be atypical among young children with autism. In contrast, referential use of gaze may be more associated with developmental level than autism. No evidence for integration of gaze and affect was observed regardless of diagnosis.
Impaired Low-Level Gaze Following in Autism
Reflexive gaze following typically begins to emerge within days of birth (Farroni et al. 2004), but the children with autism we observed (at a mean age of 4.75 years) exhibited less evidence of overt reflexive orienting to gaze than typically developing controls. We observed atypical reflexive gaze following among young children with ASC when compared to younger controls (NVMA matches) at a short SOA and older controls (CA matches) at a longer SOA (after controlling for NVMA). These findings are consistent with post-hoc analyses following a marginally significant interaction at a long SOA by Johnson et al. (2005) but inconsistent with findings by Chawarska et al. (2003) of no interaction between validity and diagnosis at a short SOA. Like Chawarska et al., we did not find a significant interaction between validity and diagnosis with CA matches at a short SOA. Unlike Chawarska et al., we also matched our participants with and without ASCs in cognitive level. When matched in cognitive level, we found evidence of atypical reflexive gaze following in autism at a short SOA. Short SOAs may capture automatic aspects of gaze following while long SOAs may capture more volitional aspects of gaze following (see Pruett et al. 2011; Ristic et al. 2002). Indeed, the validity effect we observed at a longer SOA among older (but not younger) typically developing children was only apparent after controlling for NVMA.
Our sample size was larger and more stringently matched but also older than the children assessed by Chawarska et al. (2003). It is possible that overt reflexive gaze following becomes more atypical with development in autism due to atypical social interactions, but this is unlikely given strong evidence that covert reflexive gaze following is unimpaired among older individuals with autism. Future research should examine relations between covert and overt reflexive gaze following in autism. The current findings, in conjunction with previous research, imply that like spontaneous gaze following, reflexive gaze following may become more typical with development in autism. These findings suggest that short SOAs may be more effective than long SOAs for detecting atypicalities among infants at risk.
Children with autism were faster to disengage from neutral faces regardless of cue validity at a short SOA but slower at a longer SOA. Atypically fast disengagement by children with autism was observed by Chawarska et al. (2003) at a short SOA and by Johnson et al. (2005) at a longer SOA than that used here. Variation in the speed at which children with and without autism disengage from faces at different SOAs calls into question the idea that children with autism may generally have a “limited attentional bias for faces” (Chawarska et al. 2003).
No Evidence of Efficacy of the Emotion Integration Paradigm
The data presented here did not suggest that children with autism have particular difficulty integrating gaze and affect. However, typically developing children also did not show evidence of gaze emotion integration. Previous research demonstrating gaze emotion integration was conducted with adults using covert attention cueing paradigms (e.g., Bayliss et al. 2010; Friesen et al. 2011; Graham et al. 2010; Heitanen and Leppänen 2003). Enhanced reflexive orienting to emotional cues may emerge later in development. Because it is not always observed even in adulthood, some have asserted that emotion and gaze are processed independently early in visual processing (Heitanen and Leppänen 2003). Others have found evidence that the integration of gaze cues and emotion does occur, particularly when targets are emotionally salient (Bayliss et al. 2010; Friesen et al. 2011; Pecchinenda et al. 2008). However, emotionally enhanced validity effects have also been observed with neutral targets (Graham et al.2010; Tipples 2006). Relating performance on eye-tracking measures of emotion integration to expressive “emotion integration” (e.g., Kasari et al. 1990) would elucidate if similar or different mechanisms underlie the two. Examination of developmental relations between ecologically valid measures of emotion integration and referential understanding of gaze among infants who are at high risk for autism would shed light on the theory that affective understanding of others may precede cognitive representations of others’ minds (Reddy 2003).
Gaze Following but not Reference Associated with Autism
Contrary to what one would expect if reduced understanding of the referential significance of gaze underlies gaze following deficits in autism, reduced spontaneous gaze following was apparent among children with autism during in-person and eye-tracking assessments but word learning, as indexed by referent selection after in-person opportunities for joint attention, was related to cognitive level rather than autism or gaze following. Two possible reasons that referent selection after eye-tracking training trials was not associated with gaze following or cognitive level are the relative simplicity of the referent selection task after eye-tracking (an opportunity to select each object once) or the difference between training (presented via video) and test (presented in person). There was some evidence that learning may be more evident when assessed via the same modality as training; Gaze following during eye-tracking and cognitive level was only associated with looking toward the referent when it was labeled during the eye-tracking test phase and not in person referent selection. The current findings extend the Gliga et al. (2012) claim that gaze following is “necessary but not sufficient for successful word learning” by demonstrating that the frequency of gaze following may be less related to word learning in response to gaze cues than cognitive level among children with ASC.
Limitations
The majority of the participants in the current study were high functioning (only 4 had a NVIQ at or below 70). They were thus comparable to children assessed by Luyster and Lord (2009). The current study suggests that subtle difficulties learning words from gaze are attributable to developmental level. Comparisons of a range of children with autism (including more with severe intellectual delays) to typically developing and developmentally delayed children would yield further insights into links between word learning from social cues and developmental level in autism. Future research should also examine links between reflexive and spontaneous gaze following and symptom severity. Delayed assessment of memory for words learned in this manner could be administered to evaluate the long-term implications of findings.
Infrequent associations between gaze following and word learning in this study may be attributable to the model not adapting her social cues to the child’s focus of attention (e.g., Baldwin 1991) or to overly easy word learning tasks. While other studies with similar populations trained similar numbers of objects and used more clues to reference (i.e., Luyster and Lord 2009; Parish-Morris et al. 2007), a greater variety of training objects (such as used by Akechi et al. 2011) might also yield more sensitive measures of word learning. The lack of correspondence between the eye-tracking measure of word learning based on looking behaviors and the measure based on referent selection implies that care should be taken to validate eye-tracking measures of gaze following and word learning.
Given recent findings relating language level in ASC and deficits in volitional oculomotor control (Kelly et al. 2012), future studies examining gaze following in social contexts in ASC should also examine oculomotor control. While we chose 100 ms. as the minimum fixation duration because it is a common minimum in eye-tracking research (e.g., Gliga et al. 2012), different minimum durations and blinks (which were not accounted for in the current analyses) might influence results. The centralized attention getters used in the current study may also have obscured informative differences in spontaneous social attention between participants with and without ASC.
The ecological validity of eye-tracking measures of social interaction could be increased by using head mounted eye-trackers in conjunction with interactive partners. Head-mounted eye-trackers would also allow investigation of the relative contributions of social and non-social cues to word learning in ASC. Just as early gaze following may not indicate an understanding of other’s minds (Corkum and Moore 1998), early word learning may be attributable to sensory-motor aspects of naming situations, such as the relative size of objects in a learner’s view when they are named (Yu and Smith 2012). Gaze-contingent eye-tracking, in conjunction with neuroimaging, would allow investigation of neural activity underlying spontaneous social attention, orienting to and learning from gaze (Wilms et al. 2010).
The absence of predicted effects of the emotion integration paradigm among typically developing children is a significant limitation of this study that may be attributable to flaws with the gaze cueing paradigm, such as the impossibility of co-constructing affect with a pre-recorded video. Children often stated that they were bored with it or that it was weird. Morphing the emotion of the face and the shift in eye gaze would have led to more naturalistic stimuli. The shift between neutral affect and an emotional face during the emotional gaze cues was sudden and did not occur during neutral gaze cues. The disappearance of the gaze cue prior to the appearance of the target may have caused offset effects. However, previous studies examining reflexive gaze following in autism have used a similar design to avoid potential atypicalities of disengagement (e.g., Chawarska et al. 2003; Johnson et al. 2005). Other studies examining gaze cueing among young children used a blinking motion of the eyes to attract attention to the eyes prior to the gaze cue. This manipulation would likely increase data quantity. However, no diagnostic differences in whether or not children attended to the gaze cue were observed in the current sample.
Although not designed to assess the integration of affect and gaze following, our measures of spontaneous gaze following were administered by models who were smiling. Indeed, most standardized measures used to assess spontaneous gaze following, such as the Early Social Communication Scales, include a model who is smiling. In order to fully investigate the integration of emotion and affect, emotion should be varied during assessments of both reflexive and spontaneous gaze following. Indeed, reflexive and spontaneous gaze following may vary along a continuum rather than representing distinct concepts. For example, reflexive gaze following in response to non-predictive gaze cues at longer SOAs may share volitional characteristics with spontaneous gaze following in response to predictive gaze cues. Future research using more naturalistic measures of reflexive gaze following should vary SOA, emotion and how predictive cues are in order to gain a fuller understanding of the relations between spontaneous and reflexive gaze following in autism and typical development.
Perhaps the most important limitation of the current study is that these mechanisms were examined at one point in development. Longitudinal examination of relations between social attention, reflexive gaze following, emotion gaze integration and referential understanding of gaze could elucidate the role of reinforcement learning in RJA impairments in autism. Reduced responsiveness to social rewards due to atypical ventromedial activity and/or altered oxytocin levels may contribute to decreased interest in social stimuli in autism which could in turn reduce opportunities to respond to social cues (e.g., Dawson et al. 2012). Alternatively, atypical triadic interactions may precede dyadic impairments in autism. Autism may be associated with a preference for predictable over unpredictable contingencies (e.g., Gergely 2001). Opportunities to follow gaze may be less predictably rewarding than opportunities to act upon objects or engage in dyadic interactions. As social interactions become increasingly dominated by triadic rather than dyadic interactions, they may become increasingly unrewarding in autism. However, some evidence suggests that individuals with ASC may seek out unpredictable contingencies; they may be more interested in exploring alternatives and less interested in earning non-social rewards than controls (Yechiam et al. 2010). The developmental relationship between responsiveness to social and non-social reinforcement and subsequent symptoms of autism is an important question for future research.
Conclusion
The current study provides evidence that the development of gaze following may be atypical rather than simply delayed in autism: reflexive gaze following that typically begins to emerge in infancy is not evident among young children with autism. Despite subtle difficulties with both reflexive and spontaneous gaze following among children with ASCs, word learning following gaze was associated with developmental level rather than autism. Thus, children with autism may achieve reference despite or by means of atypical gaze following. These findings, in conjunction with previous research, suggest that both more implicit and more explicit gaze following may be impaired in autism in early childhood while neither may be impaired later in development despite continued difficulties with many aspects of implicit social understanding. The current study demonstrates the importance of controlling developmental level when comparing different paradigms believed to measure core symptoms in autism.
Acknowledgments
We are very grateful to all of the children and families who participated in our study. We would like to thank Devi Beck-Pancer and Brian Nguyen for their invaluable contributions to stimuli design. We would like to thank the following people for help with data collection and/or coding: Mithi del Rosario, Kia Dela Cruz, Lovella Gomez, Nancy Lê, Jane Lee, Jennifer Nicole Loa, Brigid McCarthy, Christine Park. Connie Kasari provided excellent guidance on study design. This work was supported by National Institutes of Health Grant R01-HD40432 to Scott P. Johnson, NICHD/NIH Autism Centers of Excellence (ACE) Grant Number P50-HD-055784 to Susan Bookheimer, and the FPR-UCLA Center for Culture, Brain and Development. We would also like to thank reviewers for their extremely helpful comments.
Contributor Information
K. Gillespie-Lynch, Department of Psychology, College of Staten Island, City University of New York, Staten Island, NY, USA; Department of Psychology, University of California, Los Angeles, CA, USA
R. Elias, Department of Psychology, University of California, Berkeley, Berkeley, CA, USA
P. Escudero, MARCS Institute, University of Western Sydney, Sydney, Australia
T. Hutman, Department of Psychiatry and Biobehavioral Sciences, Semel Insitute for Neuroscience and Human Behavior, University of California, Los Angeles, Los Angeles, CA, USA
S. P. Johnson, Department of Psychology, University of California, Los Angeles, CA, USA; Department of Psychiatry and Biobehavioral Sciences, Semel Insitute for Neuroscience and Human Behavior, University of California, Los Angeles, Los Angeles, CA, USA
References
- Adamson LB, Bakeman R. Affect and attention: Infants observed with mothers and peers. Child Development. 1985;56(3):582–593. [Google Scholar]
- Akechi H, Senju A, Kikuchi Y, Tojo Y, Osanai H, Hasegawa T. Do children with ASD use referential gaze to learn the name of an object? An eye-tracking study. Research in Autism Spectrum Disorders. 2011;5:1230–1242. doi: 10.1016/j.rasd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DA. Infants’ contribution to the achievement of joint reference. Child Development. 1991;62:875–890. [PubMed] [Google Scholar]
- Baron-Cohen S, Baldwin DA, Crowson M. Do children with autism use the speaker’s direction of gaze strategy to crack the code of language? Child Development. 1997;68(1):48–57. [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie A, Frith U. Does the autistic child have a theory of mind? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Bayliss AP, Schuch S, Tipper SP. Gaze cueing elicited by emotional faces is influenced by emotional context. Visual Cognition. 2010;18(8):1214–1232. doi: 10.1080/13506285.2010.484657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Guthrie W, Coffing M, Lord C. Convergent validity of the Mullen Scales of Early Learning and the differential ability scales in children with autism spectrum disorders. American Journal of Intellectual and Developmental Disabilities. 2011;16(5):331–343. doi: 10.1352/1944-7558-116.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur MS, Dionne-Dostie E, Montreuil T, Lepage M. The Bank of Standardized Stimuli (BOSS), a new set of 480 normative photos of objects to be used as visual stimuli in cognitive research. PLoS ONE. 2010;5(5):e10773. doi: 10.1371/journal.pone.0010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SC, Caron AJ, Brooks R. Infant understanding of the referential nature of looking. Journal of Cognition and Development. 2009;1(4):359–377. [Google Scholar]
- Butterworth G, Jarrett N. What minds have in common is space: Spatial mechanisms serving joint visual attention in infancy. British Journal of Developmental Psychology. 1991;9:55–73. [Google Scholar]
- Chawarska K, Klin A, Volkmar F. Automatic attention cueing through eye movement in 2-year old children with autism. Child Development. 2003;74(4):1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. The social responsiveness scale. Western Psychological Services; Los Angeles: 2002. [Google Scholar]
- Corkum V, Moore C. The origins of joint visual attention in infants. Developmental Psychology. 1998;34(1):28–38. doi: 10.1037/0012-1649.34.1.28. [DOI] [PubMed] [Google Scholar]
- D’Entremont B, Hains SMJ, Muir DW. A demonstration of gaze following in 3- to 6-month olds. Infant Behavior and Development. 1997;20(4):569–572. [Google Scholar]
- Dawson G, Bernier R, Ring RH. Social attention: A possible early indicator of efficacy in autism clinical trials. Journal of Neurodevelopmental Disorders. 2012;4(1) doi: 10.1186/1866-1955-4-11. doi: 10.1186/1866-1955-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271–282. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- de Jong MC, van Engeland H, Kemner C. Attentional effects of gaze shifts are influenced by emotion and spatial frequency, but not in autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(4):443–454. doi: 10.1097/CHI.0b013e31816429a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák GO, Flom RA, Pick AD. Effects of gesture and target on 12- and 18-month-olds joint visual attention to objects in front or behind them. Developmental Psychology. 2000;36(4):511–523. [PubMed] [Google Scholar]
- Elliot CD. Differential ability scales. The psychological corporation; San Antonio, TX: 1990. [Google Scholar]
- Farroni T, Massaccesi S, Pividori D, Johnson MH. Gaze following in newborns. Infancy. 2004;5(1):39–60. [Google Scholar]
- Friesen CK, Halvorson KM, Graham R. Emotionally meaningful targets enhance orienting triggered by a fearful face. Cognition and Emotion. 2011;25(1):73–88. doi: 10.1080/02699931003672381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely G. The obscure object of desire-‘Nearly, but clearly not, like me’: Contingency preference in normal children versus children with autism. Bulletin of the Menninger Clinic. 2001;65(3: special issue):411–426. doi: 10.1521/bumc.65.3.411.19853. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Stevenson JL, Khandakar S, Goldsmith HH. Why does joint attention look atypical in autism? Child Development Perspectives. 2008;2(1):38–45. doi: 10.1111/j.1750-8606.2008.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH. Advances in autism. Annual Review of Medicine. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie-Lynch K, Sepeta L, Wang Y, Marshall S, Gomez L, Sigman M, et al. Early childhood predictors of the social competence of adults with autism. Journal of Autism and Developmental Disorders. 2012;42(2):161–174. doi: 10.1007/s10803-011-1222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga T, Elsabbagh M, Hudry K, Charman T, Johnson MH. Gaze following, gaze reading, and word learning in children at risk for autism. Child Development. 2012;83(3):926–938. doi: 10.1111/j.1467-8624.2012.01750.x. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Mostow AJ, Vecera SP, Gidley Larson JC, Mostofsky SH, Mahone EH, et al. Evidence for impairments in using static line drawings of eye gaze cues to orient visual-spatial attention in children with high functioning autism. Journal of Autism and Developmental Disorders. 2008;38:1405–1413. doi: 10.1007/s10803-007-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R, Friesen CK, Fichtenholtz HM, LaBar KS. Modulation of reflexive orienting to gaze direction by facial expressions. Visual Cognition. 2010;18(3):331–368. [Google Scholar]
- Greene DJ, Colich N, Iacoboni M, Zaidel E, Bookheimer SY, Dapretto M. Atypical neural networks for social orienting in autism spectrum disorders. Neuroimage. 2011;56(1):354–362. doi: 10.1016/j.neuroimage.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitanen JK, Leppänen JM. Does facial expression affect attention orientation by gaze direction cues? Journal of Experimental Psychology. 2003;29(6):1228–1243. doi: 10.1037/0096-1523.29.6.1228. [DOI] [PubMed] [Google Scholar]
- Hollich GJ, Hirsh-Pasek K, Golinkoff RM, Brand RJ, Brown E, Chung HL, et al. Breaking the language barrier: An emergentist coalition model for the origins of word learning. Monographs of the Society for Research in Child Development. 2000;65(3):1–135. [PubMed] [Google Scholar]
- Hood BM, Willen JD, Driver J. Adult’s eyes trigger shifts of visual attention in young infants. Psychological Science. 1998;9(2):131–134. [Google Scholar]
- Johnson MH, Griffin R, Csibra G, Halit H, Farroni T, Haan M. The emergence of the social brain network: Evidence from typical and atypical development. Development and Psychopathology. 2005;17:599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Sigman M, Mundy P, Yirmiya N. Affective sharing in the context of joint attention interactions of normal, autistic, and mentally retarded children. Journal of Autism and Developmental Disorders. 1990;20(1):87–100. doi: 10.1007/BF02206859. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Walker R, Norbury CF. Deficits in volitional oculomotor control align with language status in autism spectrum disorders. Developmental Science. 2012;16(1):56–66. doi: 10.1111/j.1467-7687.2012.01188.x. [DOI] [PubMed] [Google Scholar]
- Kuhn G, Benson V, Fletcher-Watson S, Kovshoff H, McCormick CA, Kirkby J, et al. Eye movements affirm: Automatic overt gaze cueing effects for typical adults and adults with autism spectrum disorder. Experimental Brain Research. 2010;201:155–165. doi: 10.1007/s00221-009-2019-7. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Hietanen JK. Attention orienting by another’s gaze direction in children with autism. Journal of Child Psychology and Psychiatry. 2004;45(3):435–444. doi: 10.1111/j.1469-7610.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and late diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Leekam S, Baron-Cohen S, Perrett D, Milders M, Brown S. Eye-direction detection: A dissociation between geometric and joint attention skills in autism. British Journal of Developmental Psychology. 1997;15:77–95. [Google Scholar]
- Leekam SR, Hunnisett E, Moore C. Target and cues: Gaze-following in children with autism. Journal of Child Psychology and Psychiatry. 1998;39(7):951–962. [PubMed] [Google Scholar]
- Leekam SR, López B, Moore C. Attention and joint attention in preschool children with autism. Developmental Psychology. 2000;36(2):261–273. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- Loveland KA, Landry SH. Joint attention and language in autism and developmental language delay. Journal of Autism and Developmental Disorders. 1986;16(3):335–349. doi: 10.1007/BF01531663. [DOI] [PubMed] [Google Scholar]
- Luyster R, Lord C. Word learning in children with autism spectrum disorders. Developmental Psychology. 2009;45(6):1774–1786. doi: 10.1037/a0016223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante JD, Zolfaghari R, Johnson SP. A critical test of temporal and spatial accuracy of the Tobii T60XL eye tracker. Infancy. 2012;17:9–32. doi: 10.1111/j.1532-7078.2011.00089.x. [DOI] [PubMed] [Google Scholar]
- Mullen EM. In: Mullen Scales of Early Learning. AGS, editor. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders. 1990;20(1):115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. Joint attention, developmental level, and symptom presentation in autism. Development and Psychopathology. 1994;6:389–401. [Google Scholar]
- Nation K, Penny S. Sensitivity to eye gaze in autism: Is it normal? Is it automatic? Is it social? Development and Psychopathology. 2008;20:79–97. doi: 10.1017/S0954579408000047. [DOI] [PubMed] [Google Scholar]
- Parish-Morris J, Hennon EA, Hirsh-Pasek K, Golinkoff RM, Tager-Flusberg H. Children with autism illuminate the role of social intention in word learning. Child Development. 2007;78(4):1265–1287. doi: 10.1111/j.1467-8624.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- Pecchinenda A, Pes M, Ferlazzo F, Zoccolotti P. The combined effect of gaze direction and facial expression on cueing spatial attention. Emotion. 2008;8(5):628–634. doi: 10.1037/a0013437. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Preissler MA, Carey S. The role of inferences about referential intent in word learning: Evidence from autism. Cognition. 2005;97:B13–B23. doi: 10.1016/j.cognition.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Pruett JR, LaMacchia A, Hoertel S, Squire E, McVey K, Todd RD, et al. Social and non-social cueing of visuospatial attention in autism and typical development. Journal of Autism and Developmental Disorders. 2011;41(6):715–731. doi: 10.1007/s10803-010-1090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. On being the object of attention: Implications for self-other consciousness. TRENDS in Cognitive Sciences. 2003;7(9):397–402. doi: 10.1016/s1364-6613(03)00191-8. [DOI] [PubMed] [Google Scholar]
- Ristic J, Friesen CK, Kingstone A. Are eyes special? It depends on how you look at it. Psychonomic Bulletin Review. 2002;9(3):507–513. doi: 10.3758/bf03196306. [DOI] [PubMed] [Google Scholar]
- Ristic J, Mottron L, Friesen CK, Iarocci G, Burack JA, Kingstone A. Eyes are special: But not for everyone. Cognitive Brain Research. 2005;24:715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Rombough A, Iarocci G. Orienting in response to gaze and the social use of gaze among children with autism spectrum disorders. Journal of Autism and Developmental Disorders. :1–13. doi: 10.1007/s10803-012-1704-8. (in press) [DOI] [PubMed] [Google Scholar]
- Roseberry S, Hirsh-Pasek K, Parish-Morris J, Golinkoff RM. Live action: Can young children learn verbs from video? Child Development. 2009;80(5):1360–1375. doi: 10.1111/j.1467-8624.2009.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, et al. Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother–infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders. 2011;41:287–301. doi: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford MD, Krysko KM. Eye direction, not movement direction, predicts attention shifts in those with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:1958–1965. doi: 10.1007/s10803-008-0592-4. [DOI] [PubMed] [Google Scholar]
- Scaife M, Bruner JS. The capacity for joint visual attention in the infant. Nature. 1975;265:266. doi: 10.1038/253265a0. [DOI] [PubMed] [Google Scholar]
- Schietecatte I, Roeyers H, Warreyn P. Exploring the nature of joint attention impairments in young children with autism spectrum disorder: Associated social and cognitive skills. Journal of Autism and Developmental Disorders. 2012;42(1):1–12. doi: 10.1007/s10803-011-1209-x. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Cieslik EC, Kuzmanovic B, Vogelely K. Shall we do this together? Social gaze influences action control in a comparison group, but not in individuals with high-functioning autism. Autism. 2012;16(2):151–162. doi: 10.1177/1362361311409258. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K. Toward a second-person neuroscience. Behavioral and Brain Sciences. (in press) [Google Scholar]
- Schopler E, Van Bourgondien M, Wellman J, Love S. Childhood Autism Rating Scale—Second edition (CARS2): Manual. Western Psychological Services; Los Angeles: 2010. [Google Scholar]
- Seibert JM, Sliwin L, Hogan AE. Social and cognitive bases of early comprehension of object reference. Cognitive Development. 1986;1(4):391–404. [Google Scholar]
- Senju A, Southgate V, White S, Frith U. Mindblind eyes: An absence of spontaneous theory of mind in Asperger syndrome. Science. 2009;325(5942):883–885. doi: 10.1126/science.1176170. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and an arrow in children with and without autism. Journal of Child Psychology and Psychiatry. 2004;45(3):445–458. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Sigman M, McGovern CW. Improvement in cognitive and language skills from preschool to adolescence in autism. Journal of Autism and Developmental Disorders. 2005;35(1):15–23. doi: 10.1007/s10803-004-1027-5. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ruskin E. References. Monographs of the society for research in child development. 1999;64(1):109–113. doi: 10.1111/1540-5834.00002. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Finelli J, Marvin A, Garrett-Mayer E, Bauman M, Landa R. Response to joint attention in toddlers at risk for autism spectrum disorder: A prospective study. Journal of Autism and Developmental Disorders. 2007;37:37–48. doi: 10.1007/s10803-006-0335-3. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Condie S, Campbell R, Milne E, Coleman M. Does the perception of moving eyes trigger reflexive visual orienting in autism? Philosophical Transactions of the Royal Society of London, B. 2003;358:325–334. doi: 10.1098/rstb.2002.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel C, Gutmann A, Pirow R, Fischer J. Facial expression modulates the ontogenetic trajectory of gaze following among monkeys. Developmental Science. 2010;13(6):913–922. doi: 10.1111/j.1467-7687.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- Tipples J. Fear and fearfulness potentiate automatic orienting to eye gaze. Cognition and Emotion. 2006;26(2):309–320. [Google Scholar]
- Tottenham N, Tanaka JM, Leon AC, Mccarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uono S, Sato W, Toichi M. Dynamic fearful gaze does not enhance attention orienting in individuals with Asperger’s disorder. Brain and Cognition. 2009;71:229–233. doi: 10.1016/j.bandc.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Vlamings PHJM, Stauder JEA, van Son IAM, Mottron L. Atypical visual orienting to gaze- and arrow-cues in adults with high-functioning autism. Journal of Autism and Developmental Disorders. 2003;35(3):267–277. doi: 10.1007/s10803-005-3289-y. [DOI] [PubMed] [Google Scholar]
- Wilms M, Schilbach L, Pfeiffer U, Bente G, Fink GR, Vogeley K. It’s in your eyes- using gaze-contingent stimuli to create truly interactive paradigms for social cognitive and affective neuroscience. Scan. 2010;5:98–107. doi: 10.1093/scan/nsq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Arshavsky O, Shamay-Tsoory SG, Yaniv S, Aharon J. Adapted to explore: Reinforcement learning in autistic spectrum conditions. Brain and Cognition. 2010;72(2):317–324. doi: 10.1016/j.bandc.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Yoder P, Stone WL, Walden T, Malesa E. Predicting social impairment and ASD diagnosis in younger siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39:1381–1391. doi: 10.1007/s10803-009-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Smith LB. Embodied attention and word learning by toddlers. Cognition. 2012 doi: 10.1016/j.cognition.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]