Abstract
Introduction
After definitive treatment of esophageal cancer, patients are at high risk for recurrence. Consistent follow-up is important for detection and treatment of recurrence. The optimal surveillance regimen remains undefined. We investigated posttreatment recurrence patterns and methods of detection in survivors of esophageal cancer.
Methods
We retrospectively studied a cohort of patients who had undergone surgical resection for esophageal cancer at our institution between 1996 and 2010. Routine computed tomography (CT) scan and upper endoscopy were performed for surveillance.
Results
In total, 1147 patients with resected esophageal adenocarcinoma or squamous cell carcinoma were included (median follow-up, 46 months). Of these, 723 (63%) had received neoadjuvant therapy before surgery. During follow-up, there were 595 deaths (52%) and 435 recurrences (38%) (distant [55%], locoregional [28%], or both [17%]). Half of recurrences were detected as a result of symptoms (n = 217), 45% by routine chest and abdominal CT scan (n = 194), and 1% by surveillance upper endoscopy (n = 6). The recurrence rate decreased from 27 per 100 person-years in posttreatment year 1 to 4 per 100 person-years in year 6. In the first 2 years, the rate of recurrence was higher among patients who had received neoadjuvant therapy (35 per 100 person-years) than among those who had not (14 per 100 person-years) (p < 0.001).
Conclusions
The incidence of recurrence is high after esophagectomy for cancer. Surveillance endoscopy has limited value for detection of asymptomatic local recurrence. The yield from follow-up scans diminishes significantly after the sixth year; surveillance scans after that point are likely unnecessary.
Keywords: Esophageal Cancer, Surveillance, Endoscopy
INTRODUCTION
Esophageal cancer is the seventh leading cause of cancer death in men in the United States. Nearly 90% of patients with this malignancy will eventually die of the disease.1 Even after treatment with curative intent, recurrence often develops in patients. Regular follow-up after definitive treatment is believed to be an important component of cancer care, potentially allowing for earlier detection and better management of recurrences. Scant evidence exists on the optimal follow-up regimen and its impact. As a result, guidelines differ on the method and interval of follow-up for posttreatment surveillance.2,3 The use of computed tomography (CT) scan, positron emission tomography (PET)/CT scan, upper endoscopy, and serum carcinoembryonic antigen levels for surveillance have all been reported.4,5 Although endoscopic ultrasound has been shown to be more sensitive than endoscopy alone for detection of locoregional recurrences, it has not been widely used for surveillance of asymptomatic patients.6-8 The efficacy of each surveillance modality has not been systematically assessed in large cohorts. Likewise, although there is no consensus on the optimal frequency of follow-up, several series have demonstrated that most recurrences occur in the first 2 years after completion of treatment.4,5 The purpose of this study was to evaluate (1) the incidence of esophageal cancer recurrence, over time, after treatment with curative intent; (2) the means of detection of recurrence; and (3) the outcomes after recurrence. The ultimate goal was to provide data to support a rational follow-up regimen for surveillance of patients after treatment of esophageal cancer with curative intent.
METHODS
We conducted a single-institution, retrospective cohort study in which we reviewed patients who had undergone esophagectomy for pathologic stage I to III esophageal adenocarcinoma or squamous cell carcinoma at Memorial Sloan-Kettering Cancer Center (MSKCC) between 1996 and 2010. Staging was performed using the 7th edition of the American Joint Committee on Cancer Cancer Staging Manual.9 We extracted baseline information from a prospectively maintained database, including demographic variables, pathologic details, preoperative staging and treatment details, and postoperative disease status and vital status. Of note, at MSKCC, it is our practice to use preoperative chemoradiation therapy in patients with clinical evidence of locoregional advanced disease (clinical stages II and III). Details on recurrences were obtained from medical records from MSKCC and outside institutions, when available, as well as from documented patient communications. In some instances, questionnaires regarding recurrences and long-term complications were mailed every 2 to 3 years to patients who were not receiving follow-up at MSKCC. Recurrence status was censored on the date of the last MSKCC clinic visit or outside communication. Vital status was confirmed using the Social Security Death Index. The study was approved by the MSKCC institutional review board.
Definition of Recurrence
After surgery, patients received regular follow-up from their surgeon and/or medical oncologist. Clinic visits took place every 4 to 6 months for the first 2 years after surgery then yearly thereafter. Each visit consisted of a medical history, physical examination, and chest and abdominal CT scan. In general, surveillance upper endoscopy was performed every 6 months for 2 years then yearly thereafter, by either the primary surgeon or a gastroenterologist. Once a recurrence was suspected, patients underwent further workup that included PET/CT scan, endoscopic ultrasound, upper endoscopy, biopsy, or other modalities specific to the suspected site of recurrence. The date of detection of recurrence was defined as the date at which the initial abnormal surveillance study or symptomatic presentation led to further workup and diagnosis of recurrence. Diagnosis of recurrence was adjudicated by pathologic confirmation or by findings by other study modalities that led to changes in treatment. Locoregional recurrence was defined as a recurrence isolated to the area of the anastomosis (perianastomotic) or in lymph nodes in the mediastinum and upper abdomen (supraceliac). Distant recurrence was defined as any spread of disease beyond a locoregional recurrence. Most patients with confirmed recurrences received chemotherapy; some received radiation therapy, when possible. Occasionally, patients received both. In patients who developed an isolated anastomotic recurrence and could tolerate further operation, surgical resection was performed.
Statistics
Summary statistics are presented as prevalence, mean ± SD, and median. The average hazard rate of recurrence for each year after surgery was calculated at 12-month intervals, as number of recurrences per person-years of follow-up during that interval. Time to recurrence and overall survival were estimated using the Kaplan-Meier method. Patients were followed from the date of surgery until documented recurrence (for time to recurrence analysis) or death (for overall survival analysis). Patients who did not experience the corresponding event during the study period were censored at the time of the last available follow-up. In addition, in the group of patients who experienced recurrence, we estimated postrecurrence survival. The log-rank test was used for univariate comparison between groups. Multivariate analysis for postrecurrence survival was performed using Cox proportional hazards regression, investigating the effect of recurrence type (locoregional vs. distant, early vs. late), controlling for age, disease stage, and neoadjuvant therapy use. The α value was set at 0.05. SAS version 9.3 was used to perform all statistical analyses (SAS Institute, Cary, NC).
RESULTS
Of the 1373 patients who underwent esophagectomy at our institution between 1996 and 2010, 1147 were included in the study. Exclusion criteria were histologic type other than squamous cell carcinoma or adenocarcinoma (n = 36), Barrett's esophagus or carcinoma in situ (n = 64), R2 resection (n = 95), stage IV disease (n = 25), primary resection not performed at MSKCC (n = 4), and nonesophageal primary cancer (n = 2). Follow-up was performed through February 2012. The median follow-up for those alive and without recurrence at study end was 46 months (range, 0–192 months). Table 1 summarizes patient and disease characteristics. Adenocarcinoma was the predominant histologic type (n = 942 [82%]). A total of 723 patients (63%) had received neoadjuvant therapy before surgery. Combined chemoradiation therapy was more common than chemotherapy alone. Only 7% (n = 77) of patients had R1 resection. At the time of review, 435 patients (38%) had developed a recurrence, and 595 patients (52%) had died (Figure 1). Of the 435 patients with evidence of recurrence, 241 (55%) had distant recurrence, 121 (28%) had locoregional recurrence, and 73 (17%) had both types.
Table 1.
Patient and Disease Characteristics (N = 1147)
| Characteristic | N (%)a |
|---|---|
| Sex | |
| Male | 888 (77.4) |
| Female | 259 (22.6) |
| Age, years | |
| Mean (SD) | 63 (10.7) |
| Range | 21–89 |
| Histologic type | |
| Squamous cell carcinoma | 205 (17.9) |
| Adenocarcinoma | 942 (82.1) |
| Induction therapy | |
| Chemotherapy | 67 (5.8) |
| Chemoradiation therapy | 656 (57.2) |
| None | 424 (37.0) |
| Margins | |
| R0 | 1070 (93.3) |
| R1 | 77 (6.7) |
| Pathologic stage | |
| IA | 194 (16.9) |
| IB | 172 (15.0) |
| IIA | 79 (6.9) |
| IIB | 264 (23.0) |
| IIIA | 140 (12.2) |
| IIIB | 71 (6.2) |
| IIIC | 73 (6.4) |
| Recurrence | 435 (37.9) |
| Distant | 241 (55.4) |
| Locoregional | 121 (27.8) |
| Distant + locoregional | 73 (16.8) |
Percentage of all recurrences.
Figure 1.
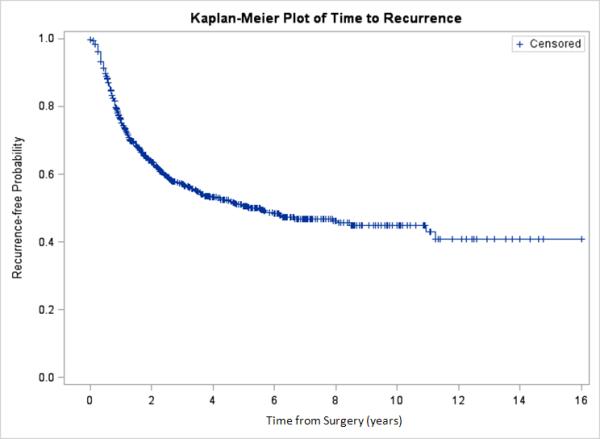
Time to recurrence in all patients.
Detection of Recurrence
For half of the patients (50%), suspicious symptoms (weight loss, dysphagia, shortness of breath, neurologic symptoms) or abnormal physical examinations (cervical or supraclavicular adenopathy) ultimately led to the diagnosis of recurrence (Table 2). Surveillance chest and abdominal CT scans detected 45% of recurrences. Among asymptomatic patients, only 6 cases of recurrence (1%) were detected by surveillance upper endoscopy. Interestingly, the type of recurrence (distant vs. locoregional) did not correlate with clinical or subclinical detection (50% vs. 56% detected clinically in distant and locoregional recurrences, respectively; p = 0.24). During 6 years of follow-up, the pattern of detection of recurrences remained unchanged (p = 0.85).
Table 2.
Method of Detection in All Patients with a Recurrence (N = 435)
| Method of Detection | N (%) |
|---|---|
| Clinical (symptoms)a | 217 (49.9) |
| Computed tomography | 194 (44.6) |
| Upper endoscopy | 6 (1.4) |
| Otherb | 2 (0.5) |
| Unknown | 16 (3.7) |
Clinical detection includes symptoms and/or abnormal physical examinations.
Other detection methods include tests not routinely performed at Memorial Sloan-Kettering Cancer Center: positron emission tomography/computed tomography, carcinoembryonic antigen level, chest X-ray, and magnetic resonance imaging.
Upper Endoscopy Surveillance
Because upper endoscopy surveillance may have been performed and recorded less reliably at outside centers, we limited our examination of its efficacy to the periods of continuous follow-up at MSKCC. During follow-up at MSKCC (median, 31 months; range, 0–192 months), 367 recurrences were diagnosed, and only 6 (2%) were initially detected by surveillance upper endoscopy. More than half of the patients (n = 215 [59%]) with a documented recurrence underwent at least 1 endoscopy within 3 months (before or after) of the diagnosis of recurrence, either for screening or for further evaluation of possible recurrence; only 46 of these endoscopies (21%) detected any evidence of malignancy. Of the 40 patients who developed a perianastomotic recurrence, 6 (15%) had their recurrence initially detected by surveillance upper endoscopy, whereas most (n = 26 [65%]) presented with symptoms first (Table 3). Of the 6 patients whose perianastomotic recurrence was detected by upper endoscopy, 1 underwent a second resection with colon interposition, 1 received chemoradiation therapy, 2 underwent chemotherapy alone, and 2 did not receive further treatment.
Table 3.
Method of Detection and Upper Endoscopy in Patients with Perianastomotic Recurrence (N = 40)
| Method of Detection/Upper Endoscopy | N (%) |
|---|---|
| Method of detection | |
| Clinical (symptoms) | 26 (65.0) |
| Computed tomography | 5 (12.5) |
| Upper endoscopy | 6 (15.0) |
| Unknown | 2 (5.0) |
| Chest X-ray | 1 (2.5) |
| Upper endoscopy <3 months after diagnosis of recurrence | |
| Yes | 37 (92.5) |
| No | 3 (7.5) |
| Upper endoscopy findings (diagnostic or surveillance) | |
| Normal | 5 (13.5) |
| Abnormal | 32 (86.5) |
Time to Recurrence
In total, 75% of all recurrences occurred in the first 2 years after surgery; this proportion was lower (63%) for patients who had not received neoadjuvant therapy than for those who had (83%) (p < 0.001). The median time to recurrence was 5.5 years (95% confidence interval [CI], 3.8–8.1 years). The overall recurrence rate was 27 per 100 person-years in postoperative year 1 (95% CI, 23–31 per 100 person-years) and then rapidly decreased to 4 per 100 person-years by postoperative year 6 (95% CI, 2–8 per 100 person-years) (Figure 2). A similar pattern was observed when the analysis was limited to patients who had received neoadjuvant therapy. In this latter group of patients, however, recurrence occurred at a higher initial rate (35 per 100 person-years in postoperative year 1; 95% CI, 30–40 per 100 person-years) (Figure 3). In contrast, patients who had not received neoadjuvant therapy developed recurrences at a lower initial rate (14 per 100 person-years; 95% CI, 10–19 per 100 person-years) but then experienced a slower rate of decline over time.
Figure 2.
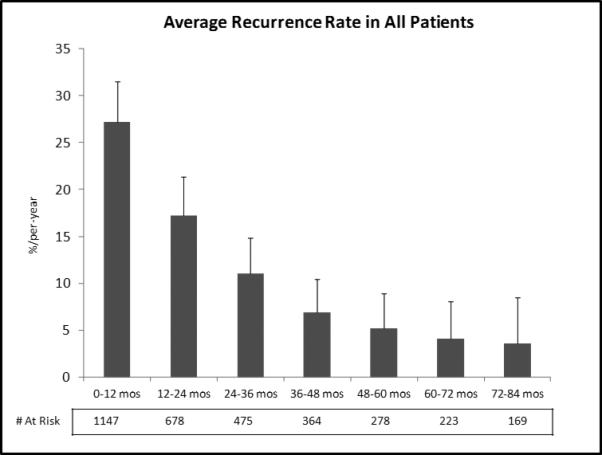
Average hazard rate of recurrence in all patients.
Figure 3.
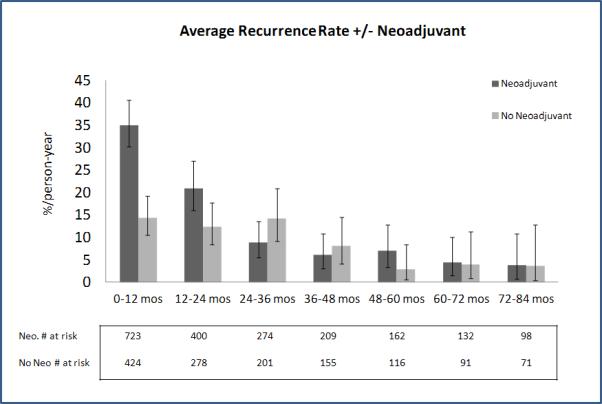
Recurrence rates in patient with and without neoadjuvant therapy.
Survival
Supplementary Figures 1 and 2 show overall survival following surgery among patients who had and had not received neoadjuvant therapy, stratified by nodal stage and pathologic stage, respectively. Median postrecurrence survival among the 237 patients who experienced recurrence was 11 months (Supplementary Figure 3). Patients whose recurrence was initially detected by radiographic or laboratory tests had longer survival after diagnosis of recurrence than those who presented with symptoms and received clinical diagnosis (p = 0.01) (Supplementary Figure 4). Likewise, the site of the first recurrence and the time to diagnosis of recurrence were associated with postrecurrence survival. Those with only locoregional disease (n = 121) (p < 0.001) and those whose recurrence was detected >2 years after surgery (n = 99) (p = 0.003) had longer survival, even after age, disease stage, and neoadjuvant therapy status were controlled for.
DISCUSSION
Esophageal cancer is an aggressive malignancy with high rates of recurrence, even after completion of therapy with curative intent. Current National Comprehensive Cancer Network guidelines2 recommend more-frequent follow-up, with medical history and physical examination, during the first 5 years. Surveillance modalities such as CT scans, laboratory tests, and upper endoscopies are recommended only as clinically indicated. Many institutions (including ours), however, perform routine imaging and endoscopic examinations for surveillance of asymptomatic patients.4,10 The assumed value of a surveillance program is that the detection of recurrences at an earlier time might result in improved survival and quality of life. The benefits of intensive surveillance, however, must be weighed against costs and potential side effects. At present, there is no consensus on the optimal follow-up regimen after esophagectomy for cancer. The aim of this study was to evaluate recurrence patterns after esophagectomy for cancer and, on the basis of these findings, to propose a rational follow-up surveillance program.
Methods of Detection
Half (50%) of the patients who developed a recurrence initially presented with symptoms, even while undergoing routine surveillance imaging; 45% of patients who developed a recurrence were diagnosed by surveillance CT scan. This pattern of detection of recurrences remained the same throughout the follow-up period. Furthermore, recurrences detected clinically were associated with significantly worse survival, compared with recurrences detected subclinically, through surveillance screening. Because all patients underwent a similar follow-up regimen and therefore had the same chance for early detection by surveillance, the association between method of diagnosis and survival is likely attributable to a more aggressive tumor biology in patients diagnosed clinically, rather than to any benefit of “earlier” detection by surveillance screening. Nonetheless, our experience with routine CT scan points to its important role in the detection of recurrences before clinical presentation of symptoms. Our results differ from those of Abate et al.5 In their retrospective series on follow-up after treatment of esophageal adenocarcinoma, only 17% of recurrences were diagnosed as a result of symptomatic presentation; 60% and 18% of recurrences were diagnosed by routine CT and PET scan, respectively. The difference in detection of recurrences between their study and ours may be attributable to several factors: the retrospective nature of both studies, the differences in follow-up protocols, and the definitions of “symptomatic recurrence” used. In contrast to the surveillance regimen in our study, their surveillance regimen included more-frequent follow-up and routine PET/CT scans in some patients. Despite the differences in results, both series demonstrate that intensive follow-up with routine imaging effectively identifies subclinical recurrences.
Unlike CT scans, surveillance upper endoscopies rarely detected asymptomatic recurrences. Although there has been no published evidence on the efficacy of surveillance upper endoscopies, our follow-up regimen has included this modality because of its presumed ability to detect locoregional recurrences. Furthermore, perianastomotic disease recurrences are conceivably amenable to a second curative resection in select patients.11 Although upper endoscopy confirmed perianastomotic recurrence (when present) in the majority of patients (80%), the initiating event (ie, symptom) that prompted the endoscopy was typically dysphagia (65%), rather than detection by any surveillance modality. In asymptomatic patients with perianastomotic recurrences, upper endoscopy rarely detected relapse (15%), which is likely attributable to the fact that this recurrence pattern often represents extraluminal nodal disease without endoluminal extension. However, most patients with perianastomotic recurrences first presented with symptoms. Furthermore, only 1 of 6 patients whose recurrence was detected by surveillance endoscopy ultimately underwent further surgery with curative intent. Given the cost and invasiveness of this modality, surveillance upper endoscopy appears to be of limited use for the follow-up of patients after curative treatment of esophageal cancer.
Timing of Recurrence
A rational follow-up regimen for surveillance of patients after esophagectomy for cancer should correspond to the likelihood of recurrence in the patients at risk (ie, the recurrence rate), rather than the absolute number of patients experiencing recurrence. Our current screening regimen includes more-intense follow-up in the first 2 years—reflecting the belief that most recurrences occur during that period—followed by yearly CT scans thereafter, for an indefinite period. Indeed, we found that, overall, 54% and 21% of recurrences were diagnosed in the first and second years after surgery, respectively; these findings are similar to those described by Mariette et al.4 The recurrence rate was highest in postoperative year 1 (27 per 100 person-years). This rate then dropped quickly in year 2 (17 per 100 person-years), after which it declined more slowly, to 4 per 100 person-years by year 6. When the same analysis was performed on patients on the basis of whether they had received neoadjuvant therapy, we found that patients who had received neoadjuvant therapy had a very high initial rate of recurrence, followed by a sharp drop in the second year and a further drop in the third year; the rates then remained stable until year 5, before dropping thereafter. Among patients who had not received neoadjuvant therapy, the risk of recurrence was highest during the first year, followed by a stable risk of recurrence until year 4, with a decline in risk thereafter. This discordance in risk of recurrence over time likely reflects the initial stage of the disease. Neoadjuvant therapy was offered to patients with clinically advanced disease (clinical stage II and III) but not to those with an earlier clinical stage.
As the follow-up regimen should reflect the risk of recurrence, a more rational follow-up regimen for postinduction patients should therefore consist of short-interval follow-up scans for 2 years, followed by yearly CT scans thereafter—whereas, for noninduction patients, yearly CT scans should be sufficient. The ultimate duration of follow-up is debatable. However, after year 6, only 4% of patients at risk developed a recurrence (<2% of all recurrences), perhaps indicating a time point after which follow-up should be clinical only.
Recurrence Pattern
Previous studies have evaluated recurrence patterns in esophageal cancer.4,5,10 Similar to other investigators, we found that distant, locoregional, and mixed recurrences represented 55%, 28%, and 17% of all new events, respectively. The site of the first recurrence was correlated with survival: patients with locoregional disease did the best, and patients with both distant and locoregional disease did the worst. This finding likely represents the overall tumor burden at the time of detection.
Limitations
Several limitations in our study should be noted. The details on recurrences were retrospectively obtained and are therefore subject to significant bias. As this is a single-institution experience, our results may have limited generalizability to other practices. Most importantly, this study was not designed to evaluate the effect of intensive follow-up on survival or patient-oriented outcomes. Although routine CT scans detected subclinical recurrences, prospective studies are needed to assess the effects of early diagnosis on survival, treatment outcomes, and quality of life.
Conclusion
Surveillance upper endoscopy rarely detected subclinical recurrences in survivors of esophageal cancer. Considering the costs and potential complications of the procedure, we do not recommend its routine use for follow-up of asymptomatic patients after surgical resection. CT scans of the chest and abdomen, on the other hand, were effective at identifying subclinical recurrences. For patients who have undergone preoperative treatment of clinically advanced disease (clinical stage II or III), follow-up should be more frequent during the first 2 years (ie, every 4 to 6 months), followed by yearly screening thereafter. For patients with earlier clinical stage disease, follow-up should be most frequent during the first year, followed by yearly CT scans afterward. As the yield from follow-up scans diminishes significantly after the sixth year, surveillance scans after that point are likely unnecessary.
Supplementary Material
Supplementary Figure 1. Overall survival in patients with neoadjuvant treatment, by nodal stage (p < 0.001).
Supplementary Figure 2. Overall survival in patients without neoadjuvant therapy, by pathologic stage (p < 0.001).
Supplementary Figure 3. Postrecurrence survival (median, 11 months [95% confidence interval, 11–14 months]).
Supplementary Figure 4. Postrecurrence survival by method of detection of recurrence (p < 0.001).
Acknowledgments
Funding: None
Footnotes
Conflicts of interest: All authors declare no conflicts of interest.
References
- 1.Seigel R, Ward E, Brawley O, et al. Cancer statistics, 2011. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa S, Kanda T, Kosugi S, et al. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198:205–211. doi: 10.1016/j.jamcollsurg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Moyes L, Anderson JE, Forshaw MJ. Proposed follow up programme after curative resection for lower third oesophageal cancer. World J Surg Oncol. 2010;8:75–87. doi: 10.1186/1477-7819-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mariette C, Balon JM, Piessen G, et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrence disease. Cancer. 2003;97:1616–1623. doi: 10.1002/cncr.11228. [DOI] [PubMed] [Google Scholar]
- 5.Abate E, DeMeester ST, Zehetner J, et al. Recurrence after esophagectomy for adenocarcinoma: defining optimal follow-up intervals and testing. J Am Coll Surg. 2010;210:428–435. doi: 10.1016/j.jamcollsurg.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Catalano M, Sivak MV, Rice TW, Dam JW. Postoperative screening for anastomotic recurrence of esophageal carcinoma by endoscopic ultrasonography. Gastrointest Endosc. 1995;42:540–544. doi: 10.1016/s0016-5107(95)70007-2. [DOI] [PubMed] [Google Scholar]
- 7.Fockens P, Manshanden CG, van Lanschot JJ, Obertop H, Tytgat GN. Prospective study on the value of endosonographic follow-up after surgery for esophageal carcinoma. Gastrointest Endosc. 1997;46:487–491. doi: 10.1016/s0016-5107(97)70001-4. [DOI] [PubMed] [Google Scholar]
- 8.Lightdale C, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol. 2005;23:4483–4489. doi: 10.1200/JCO.2005.20.644. [DOI] [PubMed] [Google Scholar]
- 9.Rice T, Blackstone EH, Rusch VW. 7th Edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa S, Kanda T, Kosugi S, et al. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198:205–211. doi: 10.1016/j.jamcollsurg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Schipper P, Cassivi SD, Deschamps C, et al. Locally recurrent esophageal carcinoma: when is re-resection indicated? Ann Thorac Surg. 2005;80:1001–1006. doi: 10.1016/j.athoracsur.2005.03.099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Overall survival in patients with neoadjuvant treatment, by nodal stage (p < 0.001).
Supplementary Figure 2. Overall survival in patients without neoadjuvant therapy, by pathologic stage (p < 0.001).
Supplementary Figure 3. Postrecurrence survival (median, 11 months [95% confidence interval, 11–14 months]).
Supplementary Figure 4. Postrecurrence survival by method of detection of recurrence (p < 0.001).


