Abstract
The dental follicle (DF) plays an essential role in tooth eruption via regulation of bone resorption and bone formation. Bone morphogenetic protein-6 (BMP6) expression in the DF is coincident with bone growth in the tooth crypt. Dental follicle stem cells (DFSCs) have been shown to possess strong osteogenic capability. This study aims to determine the expression of BMP6 in the DFSCs and to elucidate the role of BMP6 in osteogenesis of DFSCs. DFSCs and their non-stem cell counterpart dental follicle cells (DFC) were obtained from the DFs of rat pups. We showed that expression of BMP6 was significantly higher in the DFSCs than in the DFC. DFSCs lost osteogenic capability during in vitro expansion, and DFSCs at late passages had reduced BMP6 expression as compared to early passages of DFSCs when they were subjected to osteogenic induction. Addition of exogenous human recombinant BMP6 (hrBMP6) in the osteogenic medium dramatically enhanced the osteogenesis of the late passage DFSCs. Knockdown of BMP6 in the DFSCs of early passages by siRNA resulted in a decrease of osteogenesis, which could be restored by addition of hrBMP6. We concluded that DFSCs need to express high levels of BMP6 to maintain their osteogenesis capability. Increased BMP6 expression seen in vivo in the DF may reflect the activation of DFSCs for osteogenic differentiation for bone growth during tooth eruption.
Keywords: Bone morphogenetic protein-6, Dental follicle stem cells, Differentiation, Gene expression, Osteogenesis
Introduction
The dental follicle (DF), a loose connective tissue sac surrounding the unerupted tooth, plays a critical role in regulating tooth eruption. It has been determined that the DF produces molecules at critical times to upregulate osteoclastogenesis to promote bone resorption for the formation of an eruption pathway (Wise and King, 2008). Studies suggest that tooth eruption also requires bone formation. In a surgical study, when the basal-half DF of the dog fourth premolar was removed, no bone formation occurred in the base of the tooth crypt and the tooth did not erupt (Marks and Cahill, 1987), suggesting that the DF is essential for bone formation during tooth eruption. Studies in our lab have determined that the bone formation at the base of tooth crypt likely serves as an eruption force to push the tooth out of its bony crypt (Wise et al., 2007; Wise et al., 2011). To determine what molecules the DF uses to regulate the bone formation needed for tooth eruption, we examined the expression of bone morphogenetic proteins (BMPs) in the DF, and reported the expression of BMP2, BMP3 and BMP6 in the DF (Wise and Yao, 2006; Wise et al., 2011; Yao et al., 2010). In particular, an increase of BMP6 expression was coincident with the increase of bone formation seen in the base of the tooth crypt. BMP6 expression in the basal half of the rat DF was significantly higher than in the coronal half of the DF (Wise et al., 2011). Furthermore, knocking down BMP6 expression by transfection of BMP6-siRNA into the rat DF of the first mandibular molar resulted in reduction of bone formation at the base of the crypt of the molar and inhibited eruption of that molar (Wise et al., 2011). This indicated that BMP6 was a key molecule to promote the osteogenesis needed for tooth eruption.
Recently, multipotent stem cells have been isolated from the DF of various species (Honda et al., 2010). Given the fact that rat dental follicle stem cells (DFSCs) possess osteogenic differentiation capability (Yao et al., 2008) and that the DF is located adjacent to the alveolar bone, we have proposed that DFSCs might participate in the osteogenesis for bone formation during tooth eruption. Because studies have reported the capability of BMP6 in inducing osteogenic differentiation (Gruber et al., 2003; Kemmis et al., 2010), and because an increase of BMP6 expression in the DF is coincident with the bone growth at the base of tooth crypt (Wise et al., 2011), the objectives of this study were to evaluate the BMP6 expression in the DF-derived cell populations, and to elucidate its role in maintaining and regulating the osteogenic capability of DFSCs. The role of the DFSCs in tooth eruption also is discussed
Materials and methods
Culture and differentiation of dental follicle derived cells
DFs were isolated from first mandibular molars of rat pups and digested with trypsin to obtain the cell suspension. Animal use protocol was approved by the Institutional Animal Care and Use Committee. For cultures of non- stem cell dental follicle cells (DFC), cells were incubated in minimum essential medium (MEM) supplemented with 10% newborn calf serum (NCS) (Life Technologies, Grand Island, NY, USA) and 1mM sodium pyruvate. This culture condition resulted in homogenous fibroblast-like cells all expressing vimentin (Wise et al., 1992), which is a fibroblast marker (Sappino et al., 1990). Further studies showed the DFC cannot be induced to differentiate into other cell types (Yao et al., 2008), indicating that the DFC population did not contain stem/stromal cells. For DFSC cultures, cells were incubated in stem cell growth medium consisting of alpha-minimum essential medium (α-MEM) supplemented with 20% fetal bovine serum (FBS) (Atlanta Biologicals, Inc., Flowery Branch, GA, USA). When cells reached to about 90% confluence, they were detached by trypsin and passaged to fresh flasks at 1:3 ratio until the desired passages. The established cells were analyzed to determine the capability of osteogenic differentiation at passage 3. The DFSCs with strong differentiation capability and DFC with no differentiation ability were used for subsequent experiments. For all experiments, the cells were incubated at 37°C and 5% CO2 with medium change every 4 days.
To determine the osteogenic capability of DFSCs, DFSCs were seeded in 6-well plates at 80,000 cells/well, and incubated with osteogenic induction medium consisting of DMEM-LG, 10% FBS and osteogenic induction reagents (50 μg/ml ascorbate-2 phosphate, 10−5 mM dexamethasone and 10 mM β-glycerophosphate) when cells reached about 80 % confluence for osteogenesis. The control DFSCs were also cultured in DMEM-LG with10% FBS, but without addition of osteogenic induction reagents. After 2 weeks of induction, the cultures were fixed with neutral buffered formalin and stained with 1% Alizarin Red to detect calcium deposits. To ensure the staining was for calcium, some wells were treated with 10% EDTA prior to Alizarin Red staining. DFSCs were also collected for RNA isolation to determine expression of selected marker genes, bone sialoprotein (BSP), osteocalcin (OCN) and for assessment of differentiation after induction.
BMP6 expression in DFC and DFSCs
DFC and DFSCs established as described were seeded into T-25 flasks and cultured in α-MEM supplemented with 20% FBS for 1 week. Cells were collected for RNA and protein extraction to compare the expression of BMP6 using real-time RT-PCR and Western blotting. To study the BMP6 expression during osteogenesis, DFSCs of different passages were seeded into 6-well plates and incubated in osteogenic induction medium. The cells were collected after 7 days of induction and total RNA was extracted from the cells. Gene expression was assessed with real-time RT-PCR.
Effect of BMP6 on osteogenic differentiation of DFSCs
Two experiments were conducted to determine the effect of BMP6 on osteogenesis of DFSCs. For both experiments, DFSCs were seeded into 6-well plates and grown to about 80% confluence. The first experiment was designed to determine the effect of exogenous BMP6 on osteogenesis of DFSCs. To do that, DFSCs of passages 3, 7 and 11 were induced for osteogenic differentiation with or without the presence of 80 ng/ml human recombinant BMP6 (hrBMP6) purchased from PeproTech (Rocky Hill, NJ, USA) in osteogenic induction medium. Alizarin Red staining was conducted after 2–3 weeks of induction. The staining was analyzed using Image-Pro Analyzer 7.0 (Media Cybernetics, Inc. Rockville, MD) to quantitatively assess the degree of osteogenesis. Cells were also collected at 1 and 2 weeks, and total RNA was extracted for subsequent RT-PCR analysis to determine if BMP6 affects the expression of osteogenic genes, BSP and Runx 2.
The second experiment was to determine the effect of endogenous BMP6 on osteogenesis of DFSCs. For this experiment, a dicer substrate BMP6-siRNA (guide strand: 5′-rGrArArGrArArGrGrCrUrGrGrCrUrGrGrArArUrUrCrGrArCrA-3′; passenger strand: 5′-rUrGrUrCrGrArArUrUrCrCrArGrCrCrArGrCrCrUrUrCrUrUrCrGrG-3′) was designed based on the mRNA sequence of rat BMP6, and synthesized by Integrated DNA Technologies, INC (Coralville, Iowa, USA). DFSCs of passage 3 were transfected with the BMP6-siRNA using RNAiMAX™ (Life Technologies, Carlsbad, CA, USA). The control DFSCs were transfected with a scrambled siRNA or with mock transfection. Cells were collected at days 1, 2, 3, 4 and 5 post transfection to determine the BMP6 knockdown efficiency by Western blotting. Transfected cells in a separate plate were subjected to osteogenic induction for 2 weeks with a repeated BMP6-siRNA transfection at day 7. After 2 weeks of osteogenic induction, the cells were fixed and stained with Alizarin Red to detect osteogenesis. The staining was quantified by Image-Pro Analyzer 7.0.
RT-PCR and Western Blot to determine gene expression
For real-time RT-PCR, total RNA was isolated from the cells with an RNeasy mini kit (Qiagen, Valencia, CA, USA). RNA concentration was measured with a Nanodrop spectrophotometer after DNase I treatment. Equal amounts of RNA were reverse- transcribed into cDNA and the cDNA was used for real-time PCR to determine the CT values with gene specific primers. Relative gene expression (RGE) was calculated by the delta CT method using β-actin as the endogenous control. The primer pairs used for PCR analysis in this study were listed in the Table 1.
Table 1.
Primers used in this study
| Gene symbol | Gene bank accession# | Sequence (F-Forward, R-Reverse) |
|---|---|---|
| BMP6 | NM_013107 | F: 5′-CTTACA GGAGCATCAGCACAGA-3 R: 5-GTCACCACCCA CAGATTGCTA-3′ |
| BSP | AB001383 | F: 5′-ACGCTGGAAAGTTGGAGTTAGCTG-3′ R: 5′-TTCCTCTTCCTCGTCGCTTTCCTT-3′ |
| OCN | M23637 | F: 5′-ACTGCATTCTGCCTCTCTGACCT-3′ R: 5′-TATTCACCACCTTACTGCCCTCCT-3′ |
| F-Spondin | M88469 | F: 5′-GACCTACGAGTCACCAAACAA-3′ R: 5′-CACCTTCCGGGTCATAGAAAG-3′ |
| β-Actin | NM_031144 | F: 5′-CTAAGGCCAACCGTGAAAAGAT-3′ R: 5′-AGAGGCATACAGGGACAACACA-3′ |
For Western blotting, cells were lysed with ice-cold lysis buffer containing 15 mM sodium chloride, 1% TritonX-100, 0.1% SDS, 50 mM Tris and protease inhibitors. The cell lysate was centrifuged at 16,000g for 15 min, and the supernatant containing proteins was transferred into a fresh tube. Total protein was measured using the bicinchoninic acid method (Pierce, Rockford, IL, USA), and 10 μg of the protein was loaded to SDS-PAGE gel for electrophoresis. The protein then was transferred onto a PVDF membrane. Following incubation of the membrane in 5% skim milk for 30 min to reduce non-specific binding, the membrane was stained with mouse monoclonal primary antibodies reactive to rat BMP6 (Abcam-ab15640; Abcam, Cambridge, MA, USA) and rat β-actin (Sigma-A5441; Sigma-Aldrich, St. Louis, MO, USA), for 3 hours at room temperature or at 4°C overnight. The membrane was incubated with goat anti-rabbit IgG conjugated with HRP. Finally, protein was detected with an enhanced chemiluminescence detection method and imaging was captured with ChemiDoc (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Results were reported as means ± standard error. A student T-test was used for statistical analysis to obtain the p-values between two means for determining the significant differences in relative gene expression (RGE) between control and treatment, and in osteogenic quantitation between BMP6 treated and non-treated DFSCs. For comparison of RGE of BMP6 in the different passages of DFSCs, and the effect of BMP6 siRNA knockdown on osteogeneisis, ANOVA and least significant difference (LSD) were conducted with SAS programs, and means were considered to be significantly different at P≤0.05.
Results
Culture and osteogenic differentiation of dental follicle stem cells (DFSCs)
When the established DFSCs of passage 3 (P3) were incubated in osteogenic differentiation medium (DMEM + 10% FBS + osteogenic induction reagents) for about 2 weeks, they were capable of forming nodular deposits that could be stained with Alizarin Red (Fig. 1a). In contrast, when the cells were cultured in the DMEM medium without osteogenic induction reagents, no deposits were observed and Alizarin Red staining was negative (Fig 1a). To determine if the deposits were calcium, some wells were treated with 10% EDTA prior to Alizarin Red staining, and we found that the deposits could be completely removed by EDTA treatment such that no Alizarin Red staining could be seen in EDTA treated wells (Fig. 1b), indicating that these deposits were calcium.
Fig. 1.
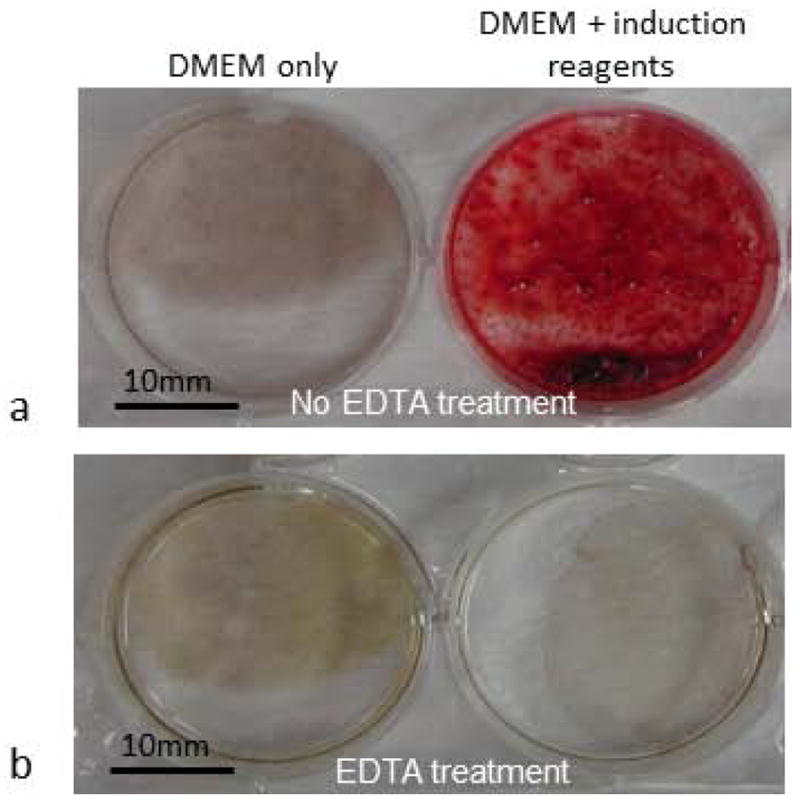
Osteogenic induction of DFSCs resulted in formation of calcium deposits as determined by Alizarin Red staining. (a) Alizarin Red positive staining was seen only when cells were cultured in the DMEM medium supplemented with induction reagents (right), and no Alizarin Red staining was seen in the DFSCs cultured in DMEM medium without induction reagents (left). (b) 10% EDTA treatment of the cultures prior to Alizarin Red staining completely removed the deposits and resulted in no staining in the induced cells (right) similar to the non-induced cells (left).
Because the DF is believed to harbor cells for development of the cementum and alveolar bone, and Alizarin Red staining cannot distinguish if the calcium-depositions were from the osteoblasts or from cementoblasts, we examined the expression of osteoblast and cementoblast markers in the DFSCs subjected to differentiation induction. Expression of osteoblast markers (BSP and OCN) and cementoblast marker (F-spondin) was determined with real-time RT-PCR. The DFSCs cultured in DMEM medium (basal medium) without osteogenic induction reagents expressed minimal OCN and BSP whereas an average of 3.8-fold increase of OCN expression and 174.8-fold increase of BSP were seen after 1 week of osteogenic induction. Expression of the markers continued to increase to an average of 278.7-fold for OCN and 403.8-fold for BSP after 2 weeks of induction (Table 2). In contrast, expression of F-spondin was decreased about 90% (i.e., RGE of 0.1) after 2 weeks of induction (Table 2). The changes of the expression of all these marker genes were statistically significant after 2 weeks of induction at P≤0.05. F-spondin has been reported to increase expression in human cementoblast-like cells and was identified as a promoting factor for cementoblastic differentiation (Kitagawa et al., 2006). Increased expression of the osteoblast markers and decreased expression of the cementoblast marker, together with calcium depositions seen in Alizarin Red staining, suggested that the DFSCs differentiated toward osteoblasts, but not cementoblasts under our osteogenic induction conditions.
Table 2.
Relative gene expression (RGE) of osteoblast and cementoblast markers during differentiation induction of DFSCs
| Culture medium | Osteocalcin (OCN) | Bone sialoprotein (BSP) | F-Spondin | |||
|---|---|---|---|---|---|---|
| 1 week | 2 weeks | 1 week | 2 weeks | 1 week | 2 weeks | |
| Basal medium (control) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Basal medium +induction reagents | 3.8±1.2 | 278.7±41.2** | 174.8±55.8* | 403.4±84.8** | 0.94±0.28 | 0.1±0.01** |
The RGEs were reported as mean ± SE (n=4).
indicates significant difference from the control at P≤0.05.
indicates highly significant difference from the control at P≤0.01.
Comparison of BMP6 Expression in the DFC and DFSCs
The expression of BMP6 in DFC and DFSCs was compared when both cells were grown in α-MEM +20%FBS medium. Real-time RT-PCR showed that BMP6 was highly expressed in the DFSCs. BMP6 expression in the DFSCs, on average, was about 17-fold higher than in the DFC (Fig. 2a). Statistical analysis indicated that this difference was highly significant (P=0.001). Western blotting showed that both cell types produced BMP6 protein, but DFSCs produced a higher amount of BMP6 protein than did the DFC (Fig. 2b) under the same culture conditions, which was consistent with the real-time RT-PCR results.
Fig. 2.
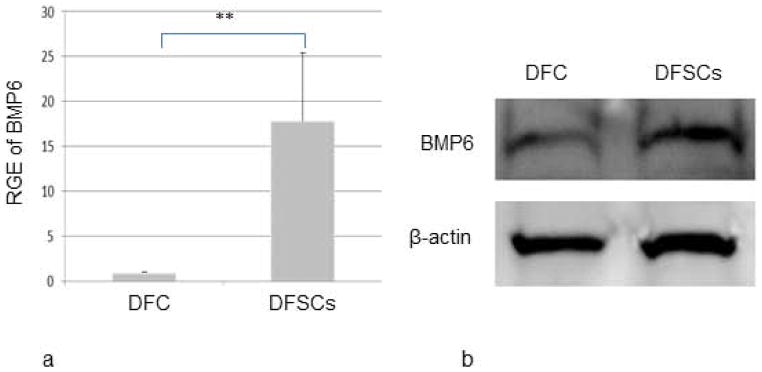
Comparison of BMP6 expression in DFC and DFSCs as determined by real-time RT-PCR (a) and by Western blotting (b). Both analyses indicated that DFSCs expressed a higher level of BMP6 than did the DFC. BMP6 expression in the DFSCs was greater than 17-fold when compared to the DFC as determined by the relative gene expression (RGE) in Real-time RT-PCR analysis, which was statistically significant at P≤0.01 (n=4).
Osteogenic capability of DFSCs during in vitro proliferation
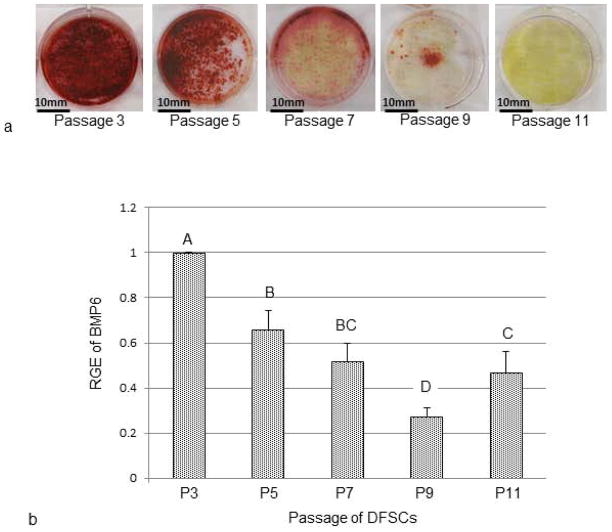
When different passages of DFSCs were subjected to osteogenic induction for 2 weeks, maximum calcium-deposition occurred in the DFSCs at passages 3 and 5 as revealed by Alizarin Red staining. A dramatic reduction of Alizarin Red staining was seen at passage 7. The staining was further reduced at passage 9 cells. For passage 11, Alizarin Red staining could only be seen occasionally (Fig. 3a). The results indicated that the DFSCs reduced their osteogenic capability during in vitro culture, and complete loss of the ability occurred around passage 11.
Fig. 3.
Evaluation of differentiation potential and BMP6 expression in different passages of DFSCs. (a) Osteogenic differentiation of different passages of DFSCs revealed the reduction of the osteogenic capability in later passages as shown by Alizarin Red staining of calcium deposits. (b) Expression of BMP6 in different passages of DFSCs during osteogenic differentiation indicated that DFSCs expressed significantly higher levels of BMP6 in the early passages than in late passages as determined by real-time RT-PCR. Statistical significances were denoted by letters (A, B, C and D) in the bar chart. Bars labeled with a same letter indicate no statistical difference of relative gene expression (RGE) at P≤0.05 (n=4).
Expression of BMP6 in different passages of DFSCs
The above experiment showed that the cultured DFSCs had reduced osteogenic capability with advancement of cell passaging. To determine if any changes of BMP6 expression occurred in later passages of DFSCs during osteogenic induction, different passages of DFSCs were placed in osteogenic induction medium for one week, and collected for real-time RT-PCR analysis. Maximal BMP6 expression was seen in the DFSCs of passage 3. BMP6 expression was decreased in other passages of DFSCs. Generally, the higher the cell passage, the lower the BMP6 expression was observed. On the average, BMP6 expression at passage 7 was decreased by 50% compared to passage 3. The expression further reduced to 25% of the passage 3 at passage 9 (Fig. 3b). This reduction of BMP6 expression seen in the late passages was statistically significant at P≤0.05.
Effect of BMP6 on osteogenesis of DFSCs
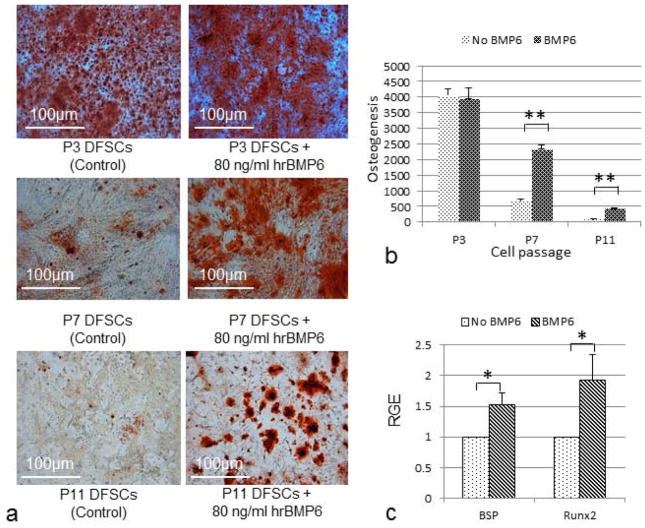
To further study the role of BMP6 on osteogenesis of DFSCs, hrBMP6 was added to the medium for induction of osteogenesis. The results showed that DFSCs at passage 3 (P3) possess strong osteogenesis regardless of the presence of hrBMP6 in the osteogenic induction medium; i.e., no obvious effect of hrBMP6 was observed for osteogenic induction of the DFSCs at passage 3 (Fig. 4a upper panel; Fig. 4b). In contrast, when hrBMP6 was added to the osteogenic medium for osteogenesis of DFSCs of passages 7 and 11 (P7 and P11), the passages in which the osteogenic capability and BMP6 expression were greatly reduced as compared to the passage 3 DFSCs, significant increase of osteogenesis was observed in hrBMP6 treatment as compared to the control without hrBMP6 after induction (Fig. 4a middle and lower panels; Fig. 4b). We noticed that such BMP6 effect on osteogenic differentiation of DFSCs was clearly shown after 2 weeks of induction for P3 and P7 DFSCs. But for P11 DFSCs, 3 weeks of induction was needed to show osteogenesis and obvious BMP6 effect as seen in Fig. 4. Furthermore, real-time RT-PCR analysis showed that BMP6 treatment significantly increased the expression of osteogenic genes BSP and Runx2 in passage 7 DFSCs (Fig. 4c).
Fig. 4.
Effect of exogenous BMP6 on osteogenesis of DFSCs. (a) Note that addition of hrBMP6 to the osteogenic induction medium resulted in no obvious effect on osteogenesis in passage 3 (P3) DFSCs (upper panel), but dramatically increased osteogenesis in passages 7 (P7) and 11 (P11) DFSCs (middle and lower right panel) as shown by Alizarin Red staining of calcium deposits. (b) Quantitation of osteogenesis by analyzing the Alizarin Red staining using Image-Pro Analyzer. Statistical analysis of the results showed a significant increase of osteogenesis by adding hrBMP6 to the medium for osteogenic induction of P7 and P11 DFSCs. Double asterisks (**) indicate a significant difference at P≤0.01 (n=3). (c) Expression of BSP and Runx2 in the passage 7 DFSCs after 2 weeks of osteogenic induction as determined by real-time RT-PCR. Increased expression of BSP and Runx2 were detected in the DFSCs when BMP6 was added to the induction medium. Asterisk (*) indicates a significant difference at P≤0.05 (n=3).
To determine if reduction of BMP6 expression affects osteogenesis, expression of BMP6 in the early passage DFSCs was knocked down by BMP6-siRNA transfection. The knockdown effect appeared to last for at least 5 days as determined by Western blotting analysis (Fig. 5a). When the DFSCs with BMP6 knockdown were subjected to osteogenic induction, dramatic reduction of osteogenesis was observed as compared to the mock transfection control or scrambled siRNA transfection control in Alizarin Red staining (Fig. 5b). The osteogenesis was quantified by analyzing the staining with an Image-Pro Analyzer. Knockdown of BMP6 expression resulted in about 90% reduction of osteogenesis as compared to the controls. Statistical analysis indicated that the reduction was statistically significant (Fig. 5c). Moreover, this reduction of osteogenesis by BMP6 knockdown could be restored by addition of exogenous hrBMP6 protein to the osteogenic induction medium (Fig. 5b, 5c).
Fig. 5.
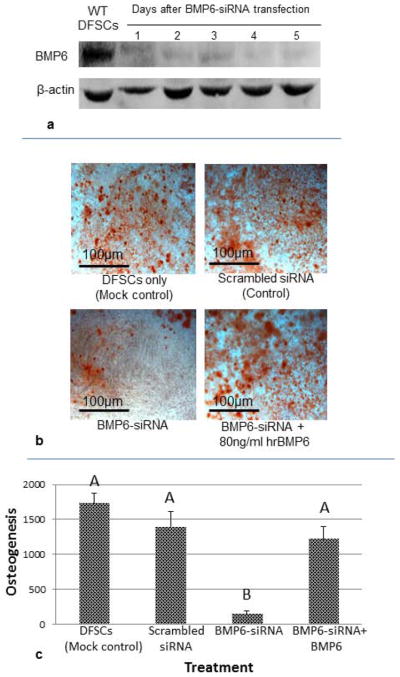
Effect of BMP6 knockdown on osteogenesis of DFSCs. (a) BMP6 expression was greatly knocked down by BMP6-siRNA as determined by Western blotting. (b) Knockdown of BMP6 reduced osteogenic differentiation capability of DFSCs as seen by comparing the BMP6-siRNA transfected DFSCs to the controls (untransfected and scrambled siRNA transfected DFSCs). Incubation of BMP6-siRNA transfected cells in osteogenic medium containing hrBMP6 protein restored the osteogenesis (lower right panel). (c) Osteogenesis was quantified by analyzing the Alizarin Red staining using Image-Pro Analyzer. Statistical analysis indicated significant reduction of osteogenesis after BMP6 knockdown, and addition of BMP6 could significantly restore osteogenesis (n=4). Bars labeled with a same letter indicate no significant difference at P≤0.05.
Discussion
The DFSCs have been shown to possess multilineage differentiation capability (Yao et al., 2008). When the DFSCs were cultured in the medium without induction reagents (i.e., without ascorbate-2 phosphate, dexamethasone and β-glycerophosphate), they did not form calcium deposits (Fig. 1a), indicating that the cell population contained no calcium depositing cells such as osteoblasts and cementoblasts. When the induction reagents were included in the medium, calcium-deposits were observed and expression of BSP and OCN (osteoblast markers) was dramatically increased, but no significant increased expression of F-spondin (cementoblast marker) was observed (Table 2). In fact, decreased expression of F-spondin was seen after 2 weeks of induction. These results suggested that the DFSCs were induced to differentiate into osteoblasts, but not cementoblasts.
The DFSCs used in this study were maintained in medium containing 20% FBS. FBS at the concentration of 10–20% remains as a common standard medium supplement in culturing mesenchymal stem cells for basic research, as well as for clinical studies (see review by Jung et al., 2012) although there are some concerns of using FBS in cell culture, including the concern that FBS can spontaneously induce osteogenic differentiation of stem cells. The results of this study revealed that the osteogenic induction reagents had to be included in the medium for induction of differentiation as shown by calcium deposits and increased expression of BSP and OCN (Fig. 1 and Table 2), suggesting that FBS alone cannot spontaneously induce osteogenic differentiation of DFSCs. Our observation was in agreement with other published results. A study by Shahdadfar et al. also showed that human mesenchymal stem cells maintained in FBS did not form calcium deposits without proper induction with the above mentioned induction reagents (Shahdadfar et al., 2005). Moreover, another study demonstrated that mesenchymal stem cells grown in FBS-containing medium remained undifferentiated, and that FBS concentration did not affect differentiation (Gong et al., 2009).
Although all experiments were conducted with FBS purchased from Atlanta Biologicals, Inc., different lots of FBS were used. In that vein, we observed variations of osteogenesis in given passages of DFSCs from experiment to experiment. It is possible that the variations were caused by different lots of FBS. Variation of differentiation at given passages also was seen when using different batches of DFSCs with the same lot of FBS, suggesting that DFSCs isolated from different litters may vary in their differentiation capability. However, the trend of loss of differentiation with advancement of passages was consistent regardless of different lots of FBS or of DFSCs derived from different litters.
BMP6 has been shown to stimulate new bone formation in vivo (Simic et al., 2006). Recently, we have reported that BMP6 is expressed in the DF of postnatal rat pups and its expression displays a continuous increase in the DF through day 11 postnatally (Wise et al., 2011). Knockdown of BMP6 by siRNA could result in reduction of bone formation in the base of tooth crypt and tooth impaction, suggesting the vital role of BMP6 in tooth eruption (Wise et al., 2011). We believe this is due to effect of BMP6 in regulating osteogenic differentiation.. In the current study, we compared the BMP6 expression in DF derived cells, namely DFC and DFSCs, and found that both types of cells produce BMP6, but DFSCs expressed a significantly higher amount of BMP6 than did the DFC at the same culture conditions (Fig. 2). However, this does not imply that DFSCs are the major BMP6 producer in the DF in vivo as the majority of cells in the DF are fibroblast-like cells (i.e., DFC) and stem cells only compose a small portion. BMP6 is a secreted molecule (Rickard et al., 1998), and BMPs exert their biological effect via binding to their receptors on cell membranes, including activin receptor-like kinase (ALK)-2 and BMP type II receptor (BMPR-II) (Ebisawa et al., 1999). In a study to determine the role of BMPs in the synthesis of follicle-stimulating hormone, BMP6 has been demonstrated to exert autocrine and paracrine regulation (Huang et al., 2001). High level expression of BMP6 in the DFSCs may favor them to undergo osteogenic differentiation by the autocrine pathway. This is supported by the in vitro osteogenic experiments showing that the osteogenic potential of DFSCs was correlated to BMP6 expression (Fig. 3), and knockdown of endogenous BMP6 reduced the osteogenesis of DFSCs (Fig 5). In addition, DFC also produced BMP6, although in lesser amounts (Fig 2). Thus, BMP6 secreted by DFC may serve as a supplemental source of BMP6 to upregulate osteogenesis of DFSCs via the paracrine pathway. Such osteogenesis of DFSCs might contribute to the overall bone growth seen in vivo in the tooth crypt.
Furthermore, DFSCs also may contribute to the bone formation by secreting and transporting BMP6 to the adjacent osteoblasts in alveolar bone, which could accelerate bone growth by stimulating the osteoblasts for mineralization. This is supported by the observation that BMP6 treatment promoted the formation of mineralized nodules in human primary osteoblasts (Grasser et al., 2007). In addition, BMP6 produced by both the DFSCs and normal DFC might stimulate osteogenesis of stem cells in alveolar bone. Other studies have revealed that when bone marrow-derived mesenchymal stem cells were induced for osteogenic differentiation in a medium containing exogenous BMP6, increased osteogenesis was observed (Lavery et al., 2008). This study showed that incubation of DFSCs in medium containing BMP6 could increase expression of BSP and Runx2 genes (Fig. 4b), which may promote osteogenic differentiation.
The in vitro osteogenic induction experiments demonstrated that addition of exogenous BMP6 could upregulate osteogenesis of DFSCs only when BMP6 expression was reduced either in the late passage cells (Fig. 4a, 4b) or when BMP6 expression was knocked down by siRNA (Fig. 5b, 5c). However, when BMP6 protein was added into the medium for osteogenic induction of the early passage DFSCs that express a high level of BMP6, no further increase of osteogenesis was observed (Fig. 4a, 4b). We reasoned that this is because the early passage DFSCs produced a maximal amount of endogenous BMP6 (Fig. 3), which was sufficient to mask the effect of exogenous BMP6. Therefore, addition of exogenous BMP6 resulted in no effect for induction of osteogenesis of DFSCs. Given that BMP6 is the most potent regulator of osteoblast differentiation of mesenchymal stem cells (Friedman et al., 2006), there likely is a threshold whereby additional BMP6 is not effective.
Loss of differentiation capability is a common phenomenon during in vitro expansion of adult stem cells, such as seen in dental pulp stem cells (Takeda et al., 2008). This study clearly showed that DFSCs gradually lose their differentiation capability during in vitro culture (Fig. 3a). Such loss of differentiation capability during in vitro expansion hampers the utilization of the stem cells for regenerative medicine. However, the reasons or mechanisms causing the stem cells to lose their differentiation capability have not been elucidated. Here, we found that when different passages of DFSCs were subjected to osteogenic induction, the early passage DFSCs (e.g., passage 3) expressed maximal levels of BMP6. In contrast, when the late passages of DFSCs were induced for osteogenesis, they expressed significantly lower BMP6 than did the early passage DFSCs (Fig. 3b), suggesting that high level expression of BMP6 is likely important for DFSCs to undergo osteogenesis. The failure to express sufficient BMP6 during osteogenic differentiation in the late passages of DFSCs may be responsible, at least partially, for their reduction or loss of osteogenic capability.
With this in mind, we conducted a BMP6 knockdown study. Knockdown of BMP6 expression in the DFSCs of early passage by BMP6-siRNA greatly reduced their osteogenic capability. Importantly, the reduction of osteogenic capability by siRNA could be restored by addition of exogenous hrBMP6 (Fig. 5). Together with the observation that the loss of osteogenic capability in late passage of DFSCs could be restored by exogenous BMP6 (Fig. 4a), we conclude that low level BMP6 expression in late passages of DFSCs during osteogenic induction might contribute to the loss or reduction of osteogenesis.
BMP6 knockdown dramatically reduced osteogenic differentiation of DFSCs, but did not completely inhibit osteogenesis. We speculate that this is because: (A) expression of BMP6 is greatly reduced, but not completely eliminated by siRNA transfection; (B) more likely, BMP6 is not the only factor regulating osteogenesis. Other osteogenic genes such as BMP2, BMP3 and BSP are also expressed in DF and DFSCs (data not shown). These factors may contribute to the osteogenic differentiation of DFSCs. Future studies may determine the role of these factors/genes in regulating DFSC differentiation and elucidate if BMP6 and those factors have an additive or synergistic effect. We showed that addition of human BMP6 protein to the culture of passage 11 DFSCs, whose endogenous BMP6 expression was greatly reduced, partially recovered osteogenesis (Fig. 4a, 4b), supporting the idea that BMP6 may not be the sole requirement for osteogenesis of DFSCs.
In conclusion, both DFC and DFSCs express BMP6, which would contribute to the overall BMP6 production in vivo in the DF. DFSCs produce a higher amount of BMP6 than did the DFC. High level expression of BMP6 appeared to be important for osteogenic differentiation of DFSCs and the low level of BMP6 expression in late passages may explain the reduction of osteogenesis in these passages. Increased expression of BMP6 seen in the DF would likely favor the osteogenesis of DFSCs, as well as the osteogenesis of cells in the alveolar bone adjacent to the DF.
Acknowledgments
This research was supported by a NIDCR grant 5R01DE008911-21 to GEW and SY. The authors would like to thank Dr. Xiaochu Wu and Mr. Gregory McCormick for their assistance in using Image-Pro Analyzer for quantification of osteogenesis.
List of Abbreviations
- α-MEM
alpha-minimum essential medium
- BMP6
bone morphogenetic protein-6
- BSP
bone sialoprotein
- DF
dental follicles
- DFSC
dental follicle stem cells
- DFC
dental follicle cells
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
fetal bovine serum
- OCN
osteocalcin
- NCS
newborn calf serum
- MEM
minimum essential medium
- RGE
relative gene expression
- Runx2
runt-related transcription factor 2
- RT-PCR
reverse transcription-polymerase chain reaction
- siRNA
short interfering RNA
References
- Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem. 2006;98:538–554. doi: 10.1002/jcb.20719. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;112:3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- Gong Z, Calkins G, Cheng EC, Krause D, Niklason LE. Influence of culture medium on smooth muscle cell differentiation from human bone marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15:319–330. doi: 10.1089/ten.tea.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasser WA, Orlic I, Borovecki F, Riccardi KA, Simic P, Vukicevic S, Paralkar VM. BMP-6 exerts its osteoinductive effect through activation of IGF-I and EGFpathways. Int Orthop. 2007;31:759–765. doi: 10.1007/s00264-007-0407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Graninger W, Bobacz K, Watzek G, Erlacher L. BMP-6-induced osteogenic differentiation of mesenchymal cell lines is not modulated by sex steroids and resveratrol. Cytokine. 2003;23:133–137. doi: 10.1016/s1043-4666(03)00223-0. [DOI] [PubMed] [Google Scholar]
- Honda MJ, Imaizumi M, Tsuchiya S, Morsczeck C. Dental follicle stem cells and tissue engineering. J Oral Sci. 2010;52:541–552. doi: 10.2334/josnusd.52.541. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Wu JC, Su P, Zhirnov O, Miller WL. A novel role for bone morphogenetic proteins in the synthesis of follicle-stimulating hormone. Endocrinology. 2001;142:2275–2283. doi: 10.1210/endo.142.6.8159. [DOI] [PubMed] [Google Scholar]
- Jung S, Panchalingam KM, Rosenberg L, Behie LA. Ex vivo expansion of human mesenchymal stem cells in defined serum-free media. Stem Cells Int. 2012 doi: 10.1155/2012/123030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmis CM, Vahdati A, Weiss HE, Wagner DR. Bone morphogenetic protein 6 drives both osteogenesis and chondrogenesis in murine adipose-derived mesenchymal cells depending on culture conditions. Biochem Biophys Res Commun. 2010;401:20–25. doi: 10.1016/j.bbrc.2010.08.135. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Kudo Y, Iizuka S, Ogawa I, Abiko Y, Miyauchi M, Takata T. Effect of F-spondin on cementoblastic differentiation of human periodontal ligament cells. Biochem Biophys Res Commun. 2006;349:1050–1056. doi: 10.1016/j.bbrc.2006.08.142. [DOI] [PubMed] [Google Scholar]
- Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283:20948–20958. doi: 10.1074/jbc.M800850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SC, Jr, Cahill DR. Regional control by the dental follicle of alterations in alveolar bone metabolism during tooth eruption. J Oral Pathol. 1987;16:164–169. doi: 10.1111/j.1600-0714.1987.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Rickard DJ, Hofbauer LC, Bonde SK, Gori F, Spelsberg TC, Riggs BL. Bone morphogenetic protein-6 production in human osteoblastic cell lines. Selective regulation by estrogen. J Clin Invest. 1998;101:413–422. doi: 10.1172/JCI119880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahdadfar A, Frønsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–1366. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- Sappino AP, Schürch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. [PubMed] [Google Scholar]
- Simic P, Culej JB, Orlic I, Grgurevic L, Draca N, Spaventi R, Vukicevic S. Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem. 2006;281:25509–25521. doi: 10.1074/jbc.M513276200. [DOI] [PubMed] [Google Scholar]
- Takeda T, Tezuka Y, Horiuchi M, Hosono K, Lida K, Hatakeyama D, Miyaki S, Kunisada T, Shibata T, Tezuka K. Characterization of dental pulp stem cells of human tooth germs. J Dent Res. 2008;87:676–681. doi: 10.1177/154405910808700716. [DOI] [PubMed] [Google Scholar]
- Wise GE, He H, Gutierrez DL, Ring S, Yao S. Requirement of alveolar bone formation for eruption of rat molars. Eur J Oral Sci. 2011;119:333–338. doi: 10.1111/j.1600-0722.2011.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise GE, Lin F, Fan W. Culture and characterization of dental follicle cells from rat molars. Cell Tissue Res. 1992;267:483–92. doi: 10.1007/BF00319370. [DOI] [PubMed] [Google Scholar]
- Wise GE, Yao S. Regional differences of expression of bone morphogenetic protein-2 and RANKL in the rat dental follicle. Eur J Oral Sci. 2006;114:512–516. doi: 10.1111/j.1600-0722.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Wise GE, Yao S, Henk WG. Bone formation as a potential motive force of tooth eruption in the rat molar. Clin Anat. 2007;20:632–639. doi: 10.1002/ca.20495. [DOI] [PubMed] [Google Scholar]
- Yao S, Pan F, Prpic V, Wise GE. Differentiation of stem cells in the dental follicle. J Dent Res. 2008;87:767–771. doi: 10.1177/154405910808700801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Prpic V, Pan F, Wise GE. TNF-alpha upregulates expression of BMP-2 and BMP-3 genes in the rat dental follicle--implications for tooth eruption. Connect Tissue Res. 2010;51:59–66. doi: 10.3109/03008200903019703. [DOI] [PMC free article] [PubMed] [Google Scholar]