Abstract
Background
Flexible cognition is a set of processes mediated by the prefrontal cortex (PFC), an area of the brain that continues to develop during adolescence and into adulthood. Adult rodents exhibit impairments specific to reversal learning across various dosing regimens of methamphetamine (mAMPH). For adolescent rodents, ongoing PFC development can be assessed by discrimination reversal learning, a task dependent on frontostriatal integrity. The task may also index an increased vulnerability for mAMPH sampling in adulthood.
Methods
The purpose of the present study was to investigate the long-term effects of escalating, adolescent mAMPH exposure on reversal learning, a PFC-dependent task (Experiment 1) and the likelihood of later sampling of mAMPH in adulthood (Experiment 2).
Results
Unlike previous research in adult-treated rats, our results show more generalized learning impairments after adolescent mAMPH exposure to include both attenuated visual discrimination as well as reversal learning. Additionally, we found that rats pre-exposed to mAMPH during adolescence consumed significantly more drug in adulthood. Intake of mAMPH was positively correlated with this learning.
Conculsion
Taken together, these findings show that even modest exposure to mAMPH during adolescence may induce general learning impairments in adulthood, and an enduring sensitivity to the effects of mAMPH.
Keywords: adolescence, reversal learning, plasticity, frontal cortex, cognitive flexibility
1. INTRODUCTION
A multitude of studies in human methamphetamine (mAMPH) users have documented cognitive impairments associated with protracted mAMPH abuse (Gonzalez et al., 2007; Woods et al., 2005; cf. Hart et al., 2012). Preclinical models of mAMPH exposure have been crucial in our understanding of the mechanisms by which mAMPH can lead to such impairments. Administration of either binge or escalating dose mAMPH in adult rats results in a broad array of learning and memory impairments (Belcher et al., 2005; Clark et al., 2007; Herring et al., 2010; Reichel et al., 2012; Kosheleff et al., 2012).
Cognitive flexibility and inhibitory control are constructs central to adaptive decision making, and the detrimental effects of mAMPH on these processes have received attention recently. These processes can be indexed, in part, by reversal learning across species (Izquierdo and Jentsch, 2012). Moderate to high binge doses of mAMPH result in impairments on response reversal (Cheng et al., 2007; cf. Daberkow et al., 2008), and visual discrimination reversal. Previously, we reported impairments specific to reversal learning after either binge dose, single dose, or escalating dose mAMPH, with discrimination learning and retention unaffected (Izquierdo et al., 2010; Kosheleff et al., 2012). Thus, as outlined here, there is abundant evidence for the long-term consequences of adult mAMPH exposure on cognitive flexibility as measured by reversal learning, yet this process in the adolescent period remains relatively unexplored. The study of this developmental period is of great value to understanding the progression to addiction since the initiation of drug use frequently occurs in adolescence in humans, particularly in the late teens (Patton et al., 2004; Schramm-Sapyta et al., 2009). Adolescents also represent a high prevalence of mAMPH users, with 1.4 million 12 years of age and older as documented users (SAMSHA, 2004).
The primary focus of the present study was to investigate the long-term effects of extended mAMPH exposure in adolescence on visual discrimination and reversal learning (Experiment 1). To our knowledge, there has not yet been a study that directly investigates the long-lasting effects of mAMPH in adolescence on this type of learning in adulthood. The secondary focus of the present study was to explore whether rats would exhibit differential voluntary mAMPH sampling in adulthood after being exposed to mAMPH during adolescence (Experiment 2). In conjunction with Experiment 1, our findings may add a novel, longitudinal dimension to the literature on the cognitive effects of mAMPH.
2. METHODS
2.1 Subjects
Eighteen male Long-Evans rats (Charles River Laboratories, Inc.) arrived at postnatal day (PND) 28 weighing between 76 and 100 g, and were socially housed 2 per cage, except during behavioral testing in Experiment 1 and during the 10-d duration of Experiment 2 (see section 2.7). Rats were habituated to the vivarium from PND 28–33, and experimenter handling began at PND 34. Each rat was handled for a minimum of 10 min, once per day, and weights were recorded 3 times per week. The vivarium maintained a 12-h light/12-h dark cycle, with the temperature constant at 22 °C. Food and water were available ad libitum until behavioral testing. Treatment and behavioral testing took place between 0800 and 1600 hours, as previously reported (Izquierdo et al., 2010; Kosheleff et al., 2011). All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council of the National Academies, 2013) and approved by the CSULA Institutional Animal Care and Use Committee. See Figure 1 for experimental timeline.
Figure 1.
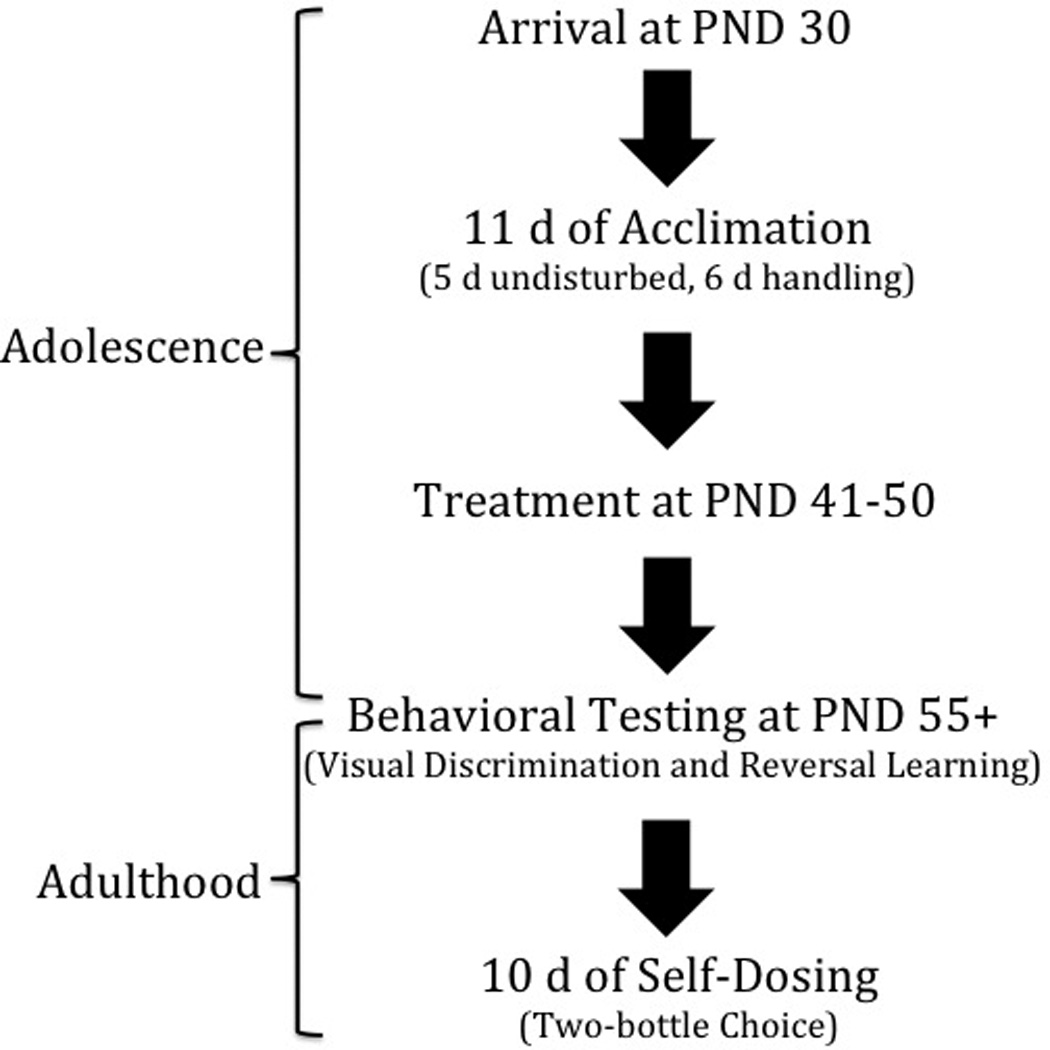
Experiment timeline. Rats arrive at PND 28 followed by 12 d of habituation to the viviarium. Handling and weighing of rats began on the 7th d. Treatment began at PND 41–50, followed by a 5-d washout period. Behavioral pretraining began at PND 55. The self-dosing phase started after behavioral testing was completed (PND 119–173).
2.2 Experiment 1
Rats in this experiment were treated with mAMPH or SAL from PND 41–50 (late adolescence), and were assessed on post-treatment learning assays in adulthood. The testing paradigm has been used in previous work from our lab (Izquierdo et al., 2010).
2.3 Apparatus
Operant conditioning chambers measuring 35 cm in length, 28 cm wide, and 34 cm high (#80004, Lafayette Instrument Co., Lafayette, IN) were housed within sound- and light-attenuating cubicles (#83018DDP, Lafayette Instrument Co.). Each chamber was equipped with a houselight, tone generator, and a 12” LCD touchscreen (EloTouch, Menlo Park, CA) in lieu of the wall opposing the pellet dispenser. The pellet dispenser delivered single 45 mg dustless sucrose pellets (BioServ, Frenchtown, NJ). Custom software (Ryklin Software Inc., NY) controlled touchscreen stimuli presentation, tone generation, houselight illumination, and pellet dispensation.
2.4 Drug treatment
Spear (2000) considered the age range of Post Natal Day (PND) 28–42 as the early adolescent period in the rat, paralleling human adolescence ages 12–18 years old. More recent studies affirm that PND 28–60 encapsulates the entire adolescent period in the rat (Laviola et al., 2003; Marco et al., 2011). Additionally, two other groups have treated rats with brief, high-dose mAMPH during late adolescence (PND 50–51) and have observed learning deficits (Vorhees et al., 2005; White et al., 2009). We chose our treatment period of PND 41–50, with these factors in mind. Rats were transferred from their housing colony room to a treatment room and given subcutaneous injections of d-mAMPH (Sigma, St. Louis, MO) or physiological saline (SAL) (10 ml/kg) once per d, for 10 consecutive d from PND 41–50. Before daily injections, rats were acclimated to the treatment room and left undisturbed for 30 min. Rats were randomly assigned to two treatment groups: (1) mAMPH group (n=10) received mAMPH beginning at 0.3 mg/kg and escalating in 0.3 mg/kg increments per d, culminating to 3.0 mg/kg, (2) SAL group (n=8) received a SAL treatment regimen identical to the mAMPH group. The order of injections was administered according to a latin-square design.
2.5 Behavioral testing
2.5.1 General
Rats were tested in three cohorts of 6 rats each. All behavioral training and testing took place five d per week, one session per d, with each session lasting a maximum of 45 min. After the last d of drug treatment (PND 50), rats received a 5-d washout period. During this period, rats were individually housed one per cage and left undisturbed with food and water ad libitum. During the last two d of this period, rats were fed 10 sucrose pellets in their homecage to familiarize them with the food rewards.
2.5.2 Food restriction
Beginning on the final d of the washout period, all rats were then single-housed and food-restricted to no less than 85% of their free-feeding body weight, while water was always available ad libitum. The weight of each rat was recorded three times per week to ensure a healthy body weight. New 85% minimum weights were calculated and observed throughout the study. Age-matched growth curves provided by the vendor were used for comparison to ensure mAMPH-treated rats fell within normal growth range.
2.5.3 Visual discrimination learning
After a series of pretraining phases outlined in detail previously (Izquierdo et al. 2012, 2013), rats were shown two concurrently-presented stimuli on each trial. One stimulus coincided with a reward and the other, a punishment. The designation of the reward stimulus was counterbalanced across each treatment group and the presentation of both stimuli alternated on the left and right side of the screen in a pseudorandom order predetermined by the custom software. The appearance of the stimuli remained on screen for 20 s, the absence of a nosepoke on either stimuli within the allotted time resulted in an ‘omitted’ trial. Each trial was separated with a 10-s inter-trial interval (ITI) before the initiation of the next trial. In order to advance to the next stage of testing, rats were required to reach a criterion of at least 85% correct nosepokes (minimum of 60 correct responses with all pellets consumed) within 45 min, for two consecutive d.
2.5.4 Reversal learning
Rats were required to respond to a reversal of the reward contingency: a nosepoke on the previously correct stimulus results in a punishment, and nosepoking the previously incorrect stimulus now results in a sucrose pellet reward. Methods and criterion were identical to those described above.
2.6 Experiment 2
Upon completion of behavioral testing, consumption of mAMPH vs H20 (5 d) and then quinine vs H20 (5 d) was measured, using methods similar to previous work performed in mice (Wheeler et al., 2009; Shabani et al., 2011).
2.6.1 Self-dosing
Rats that had received mAMPH or SAL during adolescence had voluntary access to consume pure H20 or the drug or bitter tastant dissolved in H20 for 18 h each d. H2O was offered during the remaining 6 h of each d. The 18-h period was based on studies in mice showing that mAMPH intake is greater under an intermittent access schedule, compared to 24-h access (Phillips, unpublished).
2.6.2 Drug and tastant
Ten mg of mAMPH or 5.6025 mg of quinine hemisulfate salt monohydrate (Sigma, St. Louis, MO) was dissolved in 1 liter of H20. Due to the bitter taste of mAMPH, the quinine tastant was used to ensure that taste could not account for a difference in consumption of the mAMPH between the adolescent exposure groups. These concentrations were reduced from those used in mice to account for somewhat greater drug sensitivity of rats, and to avoid rejection that can occur at higher concentrations (Shabani et al., 2011).
2.7 Two-bottle choice
2.7.1 General
Rats were first familiarized for 5 d drinking H20 from two “bottles:” 50 ml plastic centrifuge tubes, sealed with a 3.8 cm diameter rubber stopper embedded with a 6.3 cm open tip stainless steel sipper tube. Rats were then offered the opportunity to voluntarily consume mAMPH and quinine in a two-bottle choice design in which each rat had concurrent access in the homecage to: (1) one bottle of pure H20 and one bottle of H20 mixed with mAMPH, and then (2) one bottle of pure H20 and one bottle of H20 mixed with quinine. Tubes filled with H20 were placed in an empty cage to account for leakage.
2.7.2 Procedure
Beginning at 0700-h, the weights of each rat were recorded, and two full bottles of pure H20 were placed on top of each cage, on the left and right side. At 1300-h, fluid levels were recorded and then both bottles were removed from the cage. One full of pure H20 was then placed on one side of the cagetop, and one full bottle of H20 mixed with mAMPH was placed on the other side of the cagetop. This was repeated for 5 d of self-dosing of each type of solution (mAMPH and then quinine). The placement of the bottles was counterbalanced across all rats, and placements alternated on the 3rd and 5th d to account for potential drinking position preferences.
2.8 Data analyses
Data were analyzed using StatView statistical software (Version 5.0.1, Cary, NC). For Experiment 1, the data collected for analyses consisted of: (1) visual discrimination learning, and (2) reversal learning. Performance was analyzed according to: (1) accuracy as percent correct, and (2) time to learn as sessions to criterion. Specifically, accuracy was calculated as the percent correct trials (i.e., rewards) of total trials in each session and sessions to criterion was calculated as the total number of sessions required for an individual rat to reach the accuracy criterion (i.e., 85% correct over two consecutive sessions). Accuracy was analyzed using repeated measures analysis of variance (rmANOVA) and in the case of a significant interaction, ANOVA post-hocs for simple effects. Sessions to criterion were analyzed using an independent samples t-test.
For Experiment 2, self-dosing data consisted of the amounts of H20, mAMPH, and quinine consumed in ml. A Pearson product-moment correlation coefficient was computed to assess the relationship between sessions to criterion for both visual discrimination and reversal learning, and the amount of each solution consumed in ml. Independent samples t-test were used to analyze mean differences in ml consumed between rats treated with either mAMPH or SAL in adolescence, and average mg/kg consumed was also calculated across 5 d to determine the amount of mAMPH intake for both mAMPH and SAL pretreated groups. An alpha level of .05 was observed.
3. RESULTS
3.1 Experiment 1 General
Treatment groups did not differ in their body weights before mAMPH (or SAL) treatment [(t(16)=−1.322, p=0.20], or after treatment, immediately prior to behavioral testing [t(16)=−1.282, p=0.22].
3.1.1 Visual discrimination learning
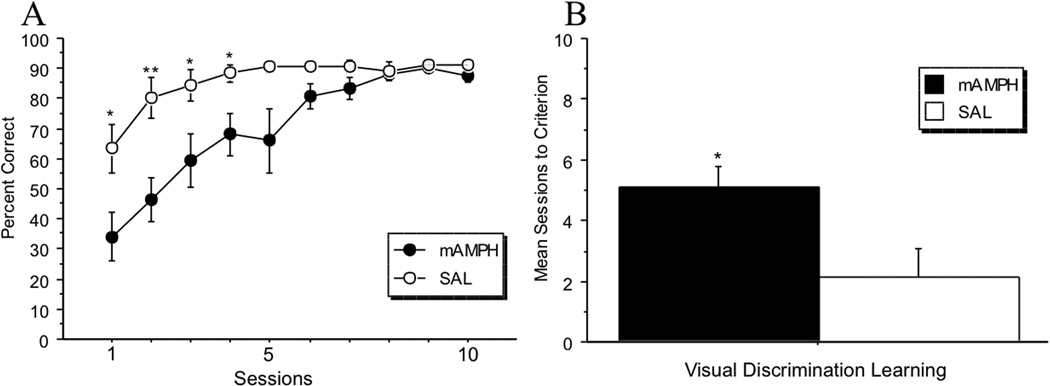
Visual discrimination learning was measured as percentage of correctly-performed trials over total trials, and number of sessions to reach criterion. A rmANOVA was conducted to test for differences in performance accuracy by treatment group (mAMPH vs. SAL) across the first 10 sessions of visual discrimination learning (Figure 2A). There was a significant main effect of treatment group [F(1,16)=8.44, p=.01], and a significant interaction of treatment group × session [F(1,9)=4.02, p=.0001]. Simple effects analyses revealed significant differences between treatment groups on session 1 (p=.02), session 2 (p=.004), session 3 (p=.04), and session 4 (p=.03). Group differences for sessions 5 (p=.06), and 6 (p=.07) approached, but did not reach, the alpha level required to conclude statistically significant differences. A significant within-subject effect of session was also found [F(1,9)=19.16, p<.0001], indicating improved accuracy of the group over this time period. For the mAMPH group, simple effects analyses confirmed a significant difference between: sessions 2 and 3 (p=.03), and sessions 3 and 4 (p=.01). For the SAL group, there was a significant difference between sessions 1 and 2 (p=.002) only. An independent samples t-test revealed a significant difference in mean sessions to criterion [t(16)=2.62, p=.02], with mAMPH-treated rats requiring more sessions to reach the 85% criterion compared to SAL-treated rats (Figure 2B).
Figure 2.
Visual discrimination learning. (A) Percentage correct across the first 10 sessions for visual discrimination learning. The mAMPH group was impaired on accuracy in comparison to the SAL group (significant main effect of treatment group, p=.01) (B) Mean number of sessions to reach criterion. The mAMPH group required significantly more sessions to reach performance criterion of 85% or higher (mAMPH = 5.10 vs. SAL = 2.13; **p=.01).
3.1.2 Reversal learning
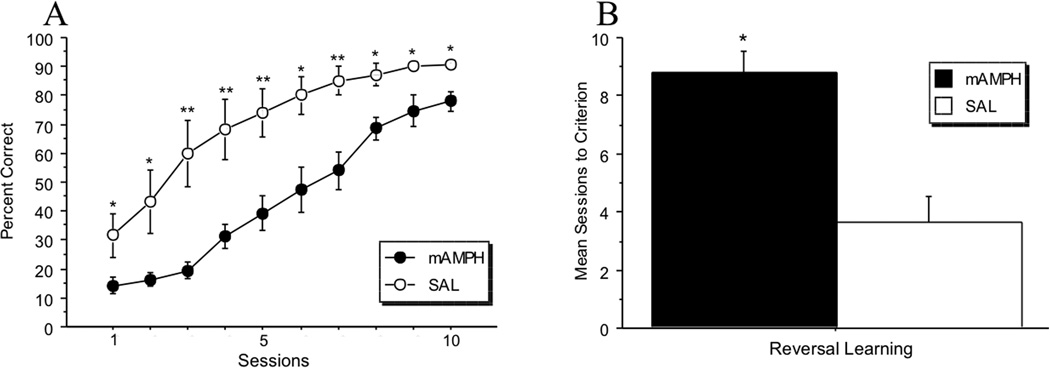
Reversal learning was also measured as percentage of correctly-performed trials over total trials, and number of sessions to criterion. A rmANOVA was conducted to test for differences in performance accuracy by treatment group (mAMPH vs. SAL) across the first 10 sessions of reversal learning (Figure 3A). A significant main effect of treatment group was found [F(1,16)=18.25, p=.0006], as well as a significant treatment group × session interaction [F(1,9)=2.59, p=.01]. Simple effects analyses showed significant differences between treatment groups on all 10 sessions: session 1 (p=.03), session 2 (p=.02), session 3 (p=.001), session 4 (p=.002), session 5 (p=.003), session 6 (p=.007), session 7 (p=.003), session 8 (p=.005), session 9 (p=.02), session 10 (p=.005). A significant within-subject effect of session was also found [F(1,9)=48.52, p<.0001]. For the mAMPH group, simple effects tests revealed significant differences between: sessions 3 and 4 (p=.0002), sessions 5 and 6 (p=.04), and sessions 8 and 9 (p=.005). For the SAL group, there was a significant difference of session between sessions 2 and 3 (p=.01) only. An independent samples t-test revealed a significant difference in mean sessions to criterion [t(16)=4.56, p=.0003], with mAMPH-treated rats requiring more sessions than SAL-treated rats to reach criterion (Figure 3B).
Figure 3.
Reversal learning. (A) Percentage correct across the first 10 sessions for reversal learning. The mAMPH group was impaired on accuracy compared to the SAL group (significant main effect of treatment group, p=.0006). (B) Mean number of sessions to reach performance criterion. The mAMPH group required significantly more sessions to reach the performance criterion of 85% (mAMPH = 8.80 vs. SAL = 3.63; **p=.003).
3.2 Experiment 2
3.2.1 Adolescent mAMPH pretreatment effects on adult mAMPH self-dosing
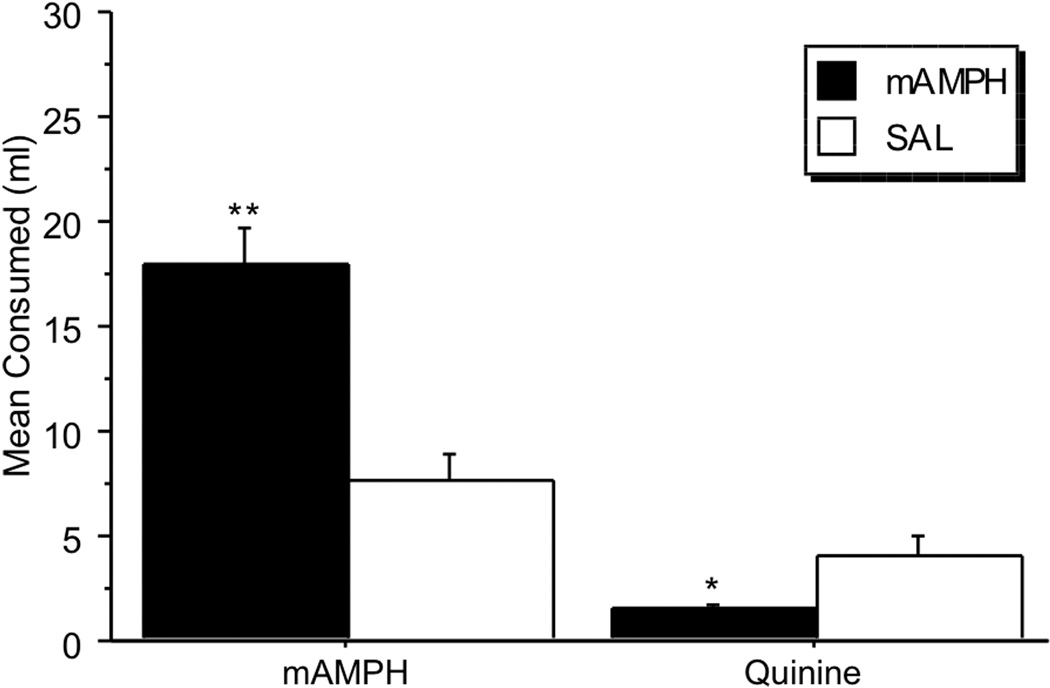
Average voluntary consumption (ml) was computed for the 5 days that each solution (mAMPH or quinine) was offered. An independent samples t-test revealed that rats that were pretreated with mAMPH in adolescence consumed significantly more mAMPH, compared to rats that were treated with SAL during adolescence [t(16)=4.42, p=.0004]. Less quinine, on the other hand, was consumed by the mAMPH pretreated than SAL pretreated rats [t(16)=−2.75, p=.01; Figure 4]. With regard to dose of mAMPH consumed, rats pretreated with mAMPH in adolescence consumed an average of 0.37 mg/kg of mAMPH, whereas SAL pretreated animals consumed an average of 0.13 mg/kg. Comparable amounts (0.1–0.5 mg/kg) of amphetamines are known to induce rewarding and behaviorally activating effects in rats, mice, and humans (Cunningham and Noble, 1992; Grilly and Loveland, 2001; Shabani et al., 2011; Wheeler et al., 2009). Preference ratio calculations (i.e., dividing the mean ml consumed of the mAMPH bottle by the mean total ml consumed from both mAMPH and H20 bottles) revealed that rats pretreated with mAMPH in adolescence had higher preference for the drug (0.5) compared to SAL pretreated rats (0.2). Both groups consumed similar amounts of total volume across a 5-d average (mAMPH=36.6 ml vs SAL=35.38 ml).
Figure 4.
Mean volume of mAMPH and quinine consumed in adulthood differed between mAMPH and SAL adolescent treatment groups. The mAMPH-pretreated animals consumed significantly more mAMPH (**p=.0004) and less Quinine (*p=.01) compared to SAL-pretreated animals. mAMPH: 10 mg d-mAMPH dissolved in 1 liter of H20. Quinine: 5.6025 mg of quinine hemisulfate salt monohydrate dissolved in 1 liter of H20.
3.3 Correlations
3.3.1 MAMPH self-dosing and visual discrimination learning
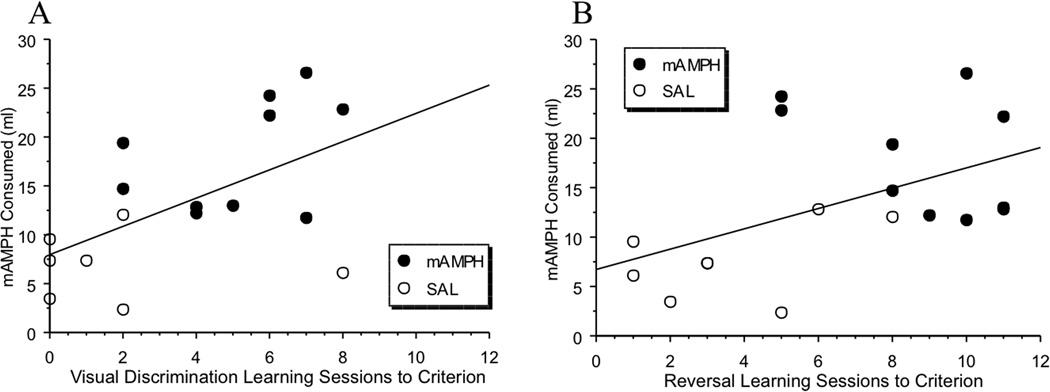
Using data from both the mAMPH and SAL pretreated groups, the amount of mAMPH consumed was positively and significantly correlated with sessions to criterion for visual discrimination learning (r=.56, p=.01; Figure 5A). There was also a significant negative correlation between consumption of H20 (when offered concurrently with mAMPH) and visual discrimination learning (r=−.51, p=.03); however, the correlation (also negative) between quinine consumption visual discrimination learning was nonsignificant (r=−.19, p=.62). Individual group consumption and learning correlations were not significant.
Figure 5. Learning is correlated with consumption.
(A)Visual discrimination learning was positively correlated with mAMPH self-dosing in adulthood. Rats that required more sessions to reach performance criterion consumed greater amounts of mAMPH, (r=0.56, p=.01). (B) Reversal learning was positively correlated with mAMPH self-dosing in adulthood. Rats with more sessions to reach performance criterion consumed more mAMPH, (r=.51, p=.03).
3.3.2 MAMPH self-dosing and reversal learning
Again, using data from both the mAMPH and SAL pretreated groups, there was a significant positive correlation between the amount of mAMPH consumed and sessions to criterion in reversal learning (r=.51, p=.03; Figure 5B). Additionally, the consumption of H20 (when offered concurrently with mAMPH) was negatively and significantly correlated with reversal learning (r=−.56, p=.01), whereas the negative correlation with learning and quinine consumption remained nonsignificant (r=−.36, p=.13). Individual group consumption and learning correlations were not significant.
4. DISCUSSION
An abundance of research in the rodent has documented a host of diverse cognitive and behavioral effects of mAMPH in the adult. To the best of our knowledge, no previous study has directly investigated mAMPH exposure in adolescence and its effects on visual discrimination reversal learning in adulthood, and the likelihood of intake in adulthood after pre-exposure to the drug. Thus, the current study offers novel evidence of long-term mAMPH-induced learning impairments. Specifically, animals pretreated with mAMPH in adolescence displayed robust impairments in both visual discrimination and reversal learning. Our findings also show an enhanced long-term sensitivity to mAMPH, as seen by the effects on mAMPH pre-exposed animals to consume more drug later in adulthood.
4.1 Visual discrimination learning
Converging evidence from previous (adult) rodent studies of mAMPH exposure indicate that the drug causes impairments specific to reversal learning, leaving discrimination learning unaffected. Our results revealed a significant impairment on the number of sessions required to reach criterion on visual discrimination learning, a more robust measure of learning not previously found impaired in the adult rodent (Izquierdo et al., 2010; Kosheleff et al., 2012; White et al., 2009) and non-human primate (Groman et al., 2012).
Importantly, discrimination learning impairments have not been observed after either binge or escalating mAMPH exposure in the adult. As such, our findings support the notion that the late adolescent period may represent a unique, increased vulnerability window to adverse cognitive effects of mAMPH. It should be noted, however, that although mAMPH-treated rats in the current study required more sessions to reach the 85% accuracy criterion, they continued to improve accuracy across sessions, showing that learning in this phase was attenuated, but not entirely blocked or permanently impaired.
4.2 Reversal learning
The existing literature establishes that adult animals can recover from mAMPH-induced impairments in reversal learning (Izquierdo et al., 2010; Kosheleff et al., 2012). Particularly in adult rats, significant impairments on accuracy were exclusively found within the first three test sessions (Izquierdo et al., 2010), with nonsignificant differences in overall number of sessions required to reach criterion (Kosheleff et al., 2012). Human mAMPH-dependent users show similar impairment patterns (Ghahremani et al., 2011). For practical reasons, we were unable to pretrain our young rats before treatment with mAMPH. One limitation is the small size of the rat, hindering adequate response to the stimuli. Additionally, due to the temporal sensitivity of the adolescent period, implementing the visual discrimination phase before treatment would have encroached upon our targeted treatment period (PND 41–50). Despite these methodological limitations, our findings still provide novel insight on mAMPH-induced impairments on acquisition, and are in accord with reports of impaired new skill learning in human substance users (Aharonovic et al., 2003; Fals-Stewart, 1993).
MAMPH-induced cognitive impairment may be an indication of other neuroplastic changes (metabolic or inflammatory) rather than frank dopamine (DA) neurotoxicity. Reversal learning is modulated in large part by DA D2 receptors, as observed in nonhuman primates (Groman et al., 2012). However, modifications to DA content or signaling after mAMPH exposure may recuperate significantly after extended abstinence in the striatum of rodents (Cass and Manning, 1999) and the substantia nigra of nonhuman primates (Harvey et al., 2000). Additionally, impaired reversal learning is not necessarily accompanied by reductions of the DA transporter (DAT) in adult rats (Kosheleff et al., 2012). Future experiments are aimed at uncovering alternate mechanisms for dysregulated circuitry after adolescent pretreatment.
4.3 Self-dosing
We observed a difference in the amount of mAMPH consumed by the mAMPH- and SAL-pretreated groups. The mAMPH-pretreated rats consumed an average of 19.74 ml, whereas SAL-pretreated rats consumed an average of 6.58 ml across 5 d (mAMPH doses of 0.37 and 0.13 mg/kg, respectively). This difference suggests that mAMPH pre-treated rats accrued an enhanced sensitivity to the rewarding effects of the drug upon adolescent exposure, similar to what has been found with other substances (Anker et al., 2011; Doremus et al., 2003). These findings are also consistent with other reports of increased sensitivity to the rewarding and reinforcing effects of mAMPH in a genetic mouse model of heightened oral self-administration (Shabani et al., 2011; 2012a). Such increased sensitivity is believed to result in an amplified resistance to drug extinction and increased drug-seeking behavior (Kitamura et al., 2006; Rogers et al., 2008), though this remains untested in the present study. Alternative interpretations are possible. For example, the SAL pre-treated rats were exposed to mAMPH for the first time in the self-administration phase, and pre-exposure during the adolescent period could have reduced an aversive component leading to higher mAMPH consumption. Low levels of mAMPH oral self-administration are associated with high sensitivity to some aversive effects of mAMPH in a genetic mouse model (Wheeler et al., 2009; Shabani et al., 2011; 2012b). Similarly, adult rats without pre-exposure to mAMPH in other studies have exhibited similar aversive self-administration patterns (Anker et al., 2012). It will be important to compare and assess the enduring effects of adolescent mAMPH pretreatment on long-term intravenous self-administration in the future.
We found that the amount of mAMPH consumed was significantly correlated with impaired learning in the visual discrimination reversal task. During the time that mAMPH was offered, H20 consumption was negatively correlated with learning. This correlation was likely driven by the correlation with mAMPH consumption because the animals that consumed more mAMPH, consumed less H2O during the same time period. No significant correlation was found for H2O consumption and learning during the time when quinine was offered. Thus, these findings establish an evident relationship between learning and mAMPH intake in adulthood, following adolescent mAMPH exposure. Interestingly, this association withstood an extended period of abstinence from the drug. Specifically, the self-dosing phase began once all rats within a cohort completed behavioral testing: 1) PND 173, 2) PND 119, and 3) PND 131. Despite the various ages of onset of the self-dosing phase, mAMPH consumption was not dampened in the oldest cohort (see range of data points in Figure 5). One testable hypothesis is that mAMPH pre-exposure in adolescence increases monoaminergic neurotransmission that underlies the reinforcing properties of the drug (for a review, see Adinoff, 2004; Labonte et al., 2011; Spear, 2000), generating long-lasting compulsive drug-seeking behavior that predisposes the animal to a greater potential for relapse (for a review, see Copeland and Sorensen, 2001).
Subsequent to mAMPH drinking, we found that the adolescent-exposed mAMPH group consumed less quinine than the SAL group. It is plausible that the consumption of quinine may have been altered by prior mAMPH drinking, such that the mAMPH pre-treated rats anticipated the rewarding/behavioral effects upon consuming quinine, and subsequently reduced quinine intake given the absence of the anticipated effects. This outcome also supports the notion that mAMPH pre-exposed animals did not consume more mAMPH because they had a greater liking for or reduced aversion to bitter tastes.
4.4 Conclusion
Low escalating exposure to mAMPH during the late adolescent period leads to a generalized learning impairment. Furthermore, the degree of mAMPH intake in adulthood is correlated with this impairment. Our findings provide insight into the cognitive-behavioral sequelae of mAMPH exposure during adolescence. Taken together, they suggest that the late adolescent period may serve as a critical temporal window in which the adolescent brain is particularly vulnerable to the neuroplastic alterations caused by mAMPH exposure.
Acknowledgements
Role of Funding Source
This work was supported by the NIH Minority Biomedical Research Support program at California State University, Los Angeles. Partial support also came from NIMH (Grant 5 SC2MH087974-03, to A.I.). T.J.P. received support from the Department of Veterans Affairs and P50 DA018165 during the conceptualization of this work and manuscript preparation. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
T.Y. and A.I. designed the study and T.J.P. shared the self-dosing protocol. T.Y. and H.P. conducted the behavioral testing and drug treatments. T.Y. and H.P. collected data and T.Y. conducted the statistical analysis. T.Y. and A.I. wrote the initial drafts of the manuscript. All authors contributed to and have approved of the final manuscript.
Conflict of Interest
All authors declare there are no actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations that could inappropriately influence, or be perceived to influence, this work.
REFERENCES
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harv. Rev. Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovic E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Baron TR, Zlebnik NE, Carroll ME. Escalation of methamphetamine self-administration in adolescent and adult rats. Drug Alcohol Depend. 2012;124:149–153. doi: 10.1016/j.drugalcdep.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Navin SF, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology. 2011;215:785–799. doi: 10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, O’Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- Cass WA, Manning MW. Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J. Neurosci. 1999;19:7653–7660. doi: 10.1523/JNEUROSCI.19-17-07653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, Etchegaray M, Meck WH. Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Res. 2007;1186:255–266. doi: 10.1016/j.brainres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Clark RE, Kuczenski R, Segal DS. Escalating dose, multiple binge methamphetamine regimen does not impair recognition memory in rats. Synapse. 2007;61:515–522. doi: 10.1002/syn.20397. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Sorensen JL. Differences between methamphetamine users and cocaine users in treatment. Drug Alcohol Depend. 2001;62:92–95. doi: 10.1016/s0376-8716(00)00164-2. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Methamphetamine-induced conditioned place preference or aversion depending on dose and presence of drug. Ann. N. Y. Acad. Sci. 1992;654:431–433. doi: 10.1111/j.1749-6632.1992.tb25989.x. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Effect of methamphetamine neurotoxicity on learning-induced Arc mRNA expression in identified striatal efferent neurons. Neurotox. Res. 2008;14:307–315. doi: 10.1007/BF03033855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol. Biochem. Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Norman G, Gaffan EA. The role of perirhinal cortex in visual discrimination learning for visual secondary reinforcement in rats. Behav. Neurosci. 2003;117:1318–1325. doi: 10.1037/0735-7044.117.6.1318. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W. Neurocognitive defects and their impact on substance abuse treatment. J. Addict. Offend. Counsel. 1993;13:46–57. [Google Scholar]
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED. Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology. 2011;36:950–959. doi: 10.1038/npp.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J. Clin. Exp. Neuropsychol. 2007;29:155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Grilly DM, Loveland A. What is a "low dose" of D-amphetamine for inducing behavioral effects in laboratory rats? Psychopharmacology. 2001;153:155–169. doi: 10.1007/s002130000580. [DOI] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD. Dysregulation of d2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J. Neurosci. 2012;32:5843–5852. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res. 2000;871:259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- Herring NR, Gudelski GA, Vorhees CV, Williams MT. (+)-methamphetamine-induced monoamine reductions and impaired egocentric learning in adrenalectomized rats is independent of hyperthermia. Synapse. 2010;64:773–785. doi: 10.1002/syn.20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Darling C, Manos N, Pozos H, Kim C, Ostrander S, Cazares V, Stepp H, Ruebeck PH. Basolateral amygdala lesions facilitate reward choices after negative feedback in rats. J. Neurosci. 2013;33:4105–4109. doi: 10.1523/JNEUROSCI.4942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kosheleff AR, Grimes M, O’Dell SJ, Marshall JF, Izquierdo A. Work aversion and associated changes in dopamine and serotonin transporter after methamphetamine exposure in rats. Psychopharmacology. 2011;219:411–420. doi: 10.1007/s00213-011-2367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosheleff AR, Rodriguez D, O’Dell SJ, Marshall JF, Izquierdo A. Comparison of single-dose and extended methamphetamine administration on reversal learning in rats. Psychopharmacology. 2012;224:459–467. doi: 10.1007/s00213-012-2774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte B, McLaughlin RJ, Dominguez-Lopez S, Bambico FR, Lucchino I, Ochoa-Sanchez R, Leyton M, Gobbi G. Adolescent amphetamine exposure elicits dose-specific effects on monoaminergic neurotransmission and behavior in adulthood. Int. J. Neuropsychopharmacol. 2011;15:1319–1330. doi: 10.1017/S1461145711001544. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Marco EM, Adriana W, Ruocco LA, Canese R, Sadile AG, Laviola G. Neurobehavioral adaptations to methylphenidate: the issue of early adolescent exposure. Neurosci. Biobehav. Rev. 2011;35:1722–1739. doi: 10.1016/j.neubiorev.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. Puberty and the onset of substance use and abuse. Pediatrics. 2004;114:300–306. doi: 10.1542/peds.2003-0626-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology. 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2004. Overview of Findings from the 2003 National Survey on Drug Use and Health. [Google Scholar]
- Schramm-Sapayta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology. 2009;84:344–352. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, Dobbs LK, Ford MM, Mark GP, Finn DA, Phillips TJ. A genetic animal model of differential sensitivity to methamphetamine reinforcement. Neuropharmacology. 2012a;62:2169–2177. doi: 10.1016/j.neuropharm.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Cunningham CL, Phillips TJ. Profound reduction in sensitivity to the aversive effects of methamphetamine in mice bred for high methamphetamine intake. Neuropharmacology. 2012b;62:1134–1141. doi: 10.1016/j.neuropharm.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Reed C, Cunningham CL, Phillips TJ. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011;10:625–636. doi: 10.1111/j.1601-183X.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, Skelton MR, McCrea AE, Rock SL, Williams MT. Periadolescent rats (P41–50) exhibit increased susceptibility to d-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21–30 or P31–40) or adult rats (P51–60) Neurotoxicol. Teratol. 2005;27:117–134. doi: 10.1016/j.ntt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8:758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IM, Minamoto T, O’dell JR, Mayhorn J, White W. Brief exposure to methamphetamine (METH) and phencyclidine (PCP) during late development leads to long-term learning deficits in rats. Brain Res. 2009;1266:72–86. doi: 10.1016/j.brainres.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, Cherner M, Heaton RK, Grant I HIV Neurobehavioral Research Center Group. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19:35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]