Abstract
Background
A phase I study was conducted to determine the maximum-tolerated dose, dose-limiting toxicities (DLTs), and pharmacokinetics of fenretinide (4-HPR) delivered in an oral powderized lipid complex (LXS) in patients with relapsed/refractory neuroblastoma.
Procedure
4-HPR/LXS powder (352 - 2210 mg/m2/day) was administered on Days 0 – 6, in 21-day courses, by standard 3+3 design.
Results
Thirty-two patients (median age = 8 years, range 3 – 27 years) enrolled with thirty evaluable for dose escalation. Prior therapies included stem cell transplantation/support (n = 26), 13-cis-retinoic acid (n = 22), 125/131I-MIBG (n = 13), and anti-GD2 antibody (n = 6). 170+ courses were delivered. Course 1 DLTs were a Grade 3 (n = 1) alkaline phosphatase at 352 mg/m2/day. Other major toxicities were Grade 4 (n = 1) alkaline phosphatases on Courses 5 and 6 at 774 mg/m2/day, and Grade 3 (n = 1) ALT/AST elevation on Course 2 at 1700 mg/m2/day. Of twenty-nine response-evaluable patients, six had stable disease (SD)(4 – 26 courses); four with marrow- or bone disease-only had complete responses (CR)(10 - 46 courses). 4-HPR plasma levels were several fold higher (P<0.05) than previously reported using capsular fenretinide. The Day 6 mean peak 4-HPR plasma level at 1700 mg/m2/day was 21 μM. An MTD was not reached.
Conclusions
4-HPR/LXS oral powder obtained higher plasma levels, with minimal toxicity and evidence of anti-tumor activity, than a previous capsule formulation. A recommended phase II schedule of 4-HPR/LXS powder is 1500 mg/m2/day, TID, on Days 0 – 6, of a 21-day course.
Keywords: fenretinide, neuroblastoma, pediatric, powder, Lym-X-Sorb™
INTRODUCTION
A synthetic retinoid, N-(4-hydroxyphenyl) retinamide (fenretinide, 4-HPR), has cytotoxicity in vitro against a wide variety of adult and pediatric cancer cell lines, including neuroblastoma [1-4]. The mechanism of 4-HPR cytotoxicity is complex and may include an increase of reactive oxygen species (ROS) [4-7], a time- and dose-dependent increase of dihydroceramide species [4, 8-13], angiogenesis inhibition [14], or increased natural killer cell activity [15]. Clinically, previous fenretinide studies have employed a corn oil-containing, oral capsule of limited bioavailability [16]. Low dose oral 4-HPR (200 - 900 mg/day; 1 - 3 μM plasma levels) was studied for cancer chemoprevention [17-19] with minimal toxicities that included reversible nyctalopia due to plasma retinol depletion, dry skin and rash [20-22]. In phase I and II studies, high dose oral 4-HPR capsules demonstrated a wide inter-patient variability of attained plasma levels (6 – 13 μM plasma) [23-26] with antitumor activity suggested in relapsed/refractory neuroblastoma based on multiple patients with prolonged stable disease. Side effects included reversible hepatic dysfunction, alkaline phosphatase elevations, hypertriglyceridemia, idiosyncratic pseudotumor cerebri, nausea, and mild thrombocytopenia [24, 25]. The modest toxicity of 4-HPR and in vitro data showing dose-related cytotoxicity suggested that increased drug levels could potentially improve anti-tumor activity. An oral powder delivering 4-HPR in an organized lipid complex, called LYM-X-SORB™ (LXS), was developed that increased 4-HPR levels in plasma (4-fold) and tissues (7-fold) in mice compared to the corn oil capsule [27]. A phase I trial was conducted in relapsed/refractory neuroblastoma to determine the maximal tolerated dosage (MTD) of this novel formulation beginning at a dose expected to achieve plasma levels similar to the capsular phase II dose. The dosing schedule of seven days, every three weeks, was modeled after two prior capsular studies that included patients with relapsed/refractory neuroblastoma [24, 25].
METHODS
Drug Sources and Formulation
N-(4-hydroxyphenyl)retinamide (Fenretinide, 4-HPR; NSC 374551) formulated as fenretinide/Lym-X-Sorb™ (LXS) oral powder was provided via a Rapid Access to Intervention Development (RAID) grant from the Developmental Therapeutics Program (DTP) of the National Cancer Institute (NCI) to B.J.M. (Study Chair and Sponsor, IND #68,254) as a powder 3% by weight 4-HPR, 55% wheat flour, 22% LXS lipid matrix (lysophosphatidylcholine, monoglycerides, and free fatty acids), and 20% sucrose. 4-HPR/LXS oral powder was formulated by Avanti Polar Lipids, Inc., Alabaster, AL, under agreement with BioMolecular Products, Inc., Newburyport, MA. As the fat content of foods was previously reported to affect the bioavailability of fenretinide formulated as corn oil capsules, oral powder doses were mandated to be mixed into a liquid nutritional supplement (Slim-Fast®, Unilever) to ensure a degree of uniformity of patient delivery. High milk-fat foods taken concurrently with daily doses were avoided to reduce possible destabilization of the LXS lipid matrix.
Patient Eligibility
Patients ≤ 30 years of age diagnosed with high-risk neuroblastoma with relapse at any time, refractory disease (less than partial response to frontline therapy based on International Neuroblastoma Response Criteria (INRC) [28]), or biopsy-documented neuroblastoma or ganglioneuroblastoma after a partial response to frontline therapy, were eligible. Patients were required to have at least one of the following: minimum of one centimeter soft tissue disease on CT/MRI scan, metaiodobenzylguanidine (MIBG) avid site(s), any amount of tumor in the bone marrow by standard histology. Patients with intraparenchymal brain or meningeal metastases were excluded. Specific organ function requirements included absolute neutrophil count (ANC) ≥ 750/μl, platelets ≥ 50,000/μl, creatinine < 1.5 × institutional upper limit of normal for age, and ALT/AST < 3 × institutional upper limit of normal for age. Postmenarchal females were required to have a negative beta-human chorionic gonadotropin and to utilize contraception. Morphological evidence of neuroblastoma in bone marrow was assessed by routine hematoxylin and eosin staining (i.e. routine morphology). The study protocol was approved by local human investigations committees in accord with an assurance filed with and approved by the U.S. Department of Health and Human Services and registered at www.clinicaltrials.gov as NCT00295919. ICH-GCP guidelines were followed. Written informed consent/assent from each participant or guardian was obtained prior to enrollment.
Treatment Plan and Dose Escalation
4-HPR/LXS oral powder was given at 352 mg to 2210 mg 4-HPR/m2/day, divided into two doses daily (BID), for 7 consecutive days, every three weeks. Dose escalation was by standard 3 + 3 design using fixed 30% dose escalation increments and no intrapatient dose escalation [29]. Pharmacokinetic sampling was required. Disease evaluations were done at entry, after Courses 2, 4, and 6, then every fourth course. An ophthalmologic examination with visual acuity assessment was performed at entry, every third course for two examinations, and then every fourth course. Patients were eligible to continue protocol therapy until tumor progression. Patients were advised to avoid prolonged exposure to sunlight due to possible 4-HPR-induced skin sensitivity and to use a sun-blocking agent (SPF 15 or greater) when out of doors.
Definitions of Dose Limiting Toxicity (DLT) and Maximum Tolerated Dose (MTD)
Adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Dose-limiting hematologic toxicity in patients with less than 20% tumor involvement of the bone marrow at the time of evaluation for persistent hematological toxicity was defined as platelets < 10,000 on one occasion, or more than one platelet transfusion required per week, or a Grade 4 ANC for more than two weeks. Dose-limiting non-hematologic toxicity was defined as Grade 3 or 4 nonhematologic toxicity possibly/probably-related to 4-HPR LXS oral powder excluding Grade 3 non-hematologic toxicity resolving to ≤ Grade 1, or baseline if > Grade1, by course Day 28; Grade 3 nausea/vomiting/diarrhea controllable by medical management; Grade 3 hepatic toxicity resolving by course Day 28; Grade 3 fever or infection; treatable Grade 3 headache not due to pseudotumor cerebri; central nervous system toxicity attributable to disease progression; and decreased adaptive dark vision. The MTD was defined as the dose at which at least five of six patients did not demonstrate dose-limiting toxicity (DLT) in Course 1.
Clinical Response Assessment
Neuroblastoma responses were graded using the NANT Response Criteria, (a modification of the INRC) [30]. Overall response was graded based on integration of CT/MRI response by RECIST Criteria v. 1.0 [31], metaiodobenzylguanidine (MIBG) response by Curie scale [32], and bone marrow response by morphology. Bone marrow responses of stable disease, complete response (requiring minimum of two negative consecutive bone marrows) or progressive disease were reported [33]. Radiological scans and bone marrow slides were centrally reviewed for patients with an overall partial or complete response, or stable disease for more than three courses. Marrows read as negative at entry per the treating site were not centrally reviewed unless overall response was CR/PR. Radiologic and bone marrow reports from the treating sites were centrally reviewed for all patients.
Pharmacokinetics
On Course One (Day 0 – 6), blood specimens were obtained by central catheter or venipuncture pre-dose (Hour 0) on Day 0, then at hours (hrs) +2, +4 , +6, and +8 after the first dose, on Day 6, pre-morning dose, then at hours +2, +4, +6, and +8, and once on Day 8; on Course Two, pre-morning dose on Day 0, then at hours +4 and +6, Day 2 at +6 hrs after the morning dose, and on Day 6, pre-morning dose, then at hours +4 and +6; on Course 6, pre-morning dose on Day 0, then at hours +4 and +6, on Day 6, pre-morning dose, then at hours +4 and +6. Plasma was stored at −80°C prior to analysis by the study reference pharmacokinetic laboratory. 4-HPR metabolites, N-(4- methoxyphenyl) retinamide (4-MPR), and in some cases, 4-oxo-4-HPR, were measured by a validated high-performance liquid chromatography (HPLC) assay with modification [34]. Briefly, N-(4-ethoxyphenyl)retinamide was added to the plasma samples as an internal standard prior to precipitation with ice-cold acetonitrile. Supernatant was analyzed on an Agilent Technology 1200 system with Photodiode Array Detector set at 354 nm wavelength using an Agilent Technologies ZORBAX Eclipse XDB C18 Column 4.6 × 150mm (3.5 μm). Gradient elution was with methanol and 0.5% acetic acid water at a flow rate of 1.0 ml/min with autosampler temperature at 20 °C. Peak level was defined as the highest value measured around a time point. Trough level was defined as the pre-dose level. Steady-state levels were defined as levels measured around the first dose on the seventh day (i.e., Day 6).
Statistical Methods
Statistical analysis of pharmacokinetic data between dosing groups was by one way ANOVA using the Holm-Sidak method with SigmaPlot® 11 software, Systat Software, Inc., San Jose, CA; pharmacokinetic analyses between CR responders and non-CR patients was by Wilcoxon test.
RESULTS
Patient Characteristics
Thirty-two eligible patients were enrolled (Table I/Supplemental Table 1). Two patients were inevaluable for dose escalation and response. One patient at 1,308 mg/m2/day (25 years old) refused further protocol therapy after seven days of therapy due to inability to comply with sunlight exposure restrictions and did not complete the required 14 day follow-up. One patient at 2,210 mg/m2/day (13 years old) was unable to take the drug on the first day of treatment and was replaced. One patient at 458 mg/m2/day (9 years old) was inevaluable for response due to withdrawing consent after completing the first seven days of drug without clinical progression to receive palliative care. Two additional patients withdrew consent during therapy but remained evaluable for dose escalation and response: one at 774 mg/m2/day (6 years old) in complete response after ten courses due to intolerance of the mandated liquid nutritional supplement carrier; and one at 1,006 mg/m2/day (27 years old) during Course 4 to receive another therapy.
Table I.
Enrollment and characteristics (n=32)
| Age at enrollment, years | |
| Median | 8 |
| Range | 3 - 27 |
| Gender (number of patients) | |
| Male | 21 |
| Female | 11 |
| Prior autologous stem-cell transplantation/support1 | |
| Yes | 26 |
| No | 6 |
| No. prior chemotherapy regimens | |
| 1 - 2 | 11 |
| 3 - 5 | 17 |
| 6+ | 4 |
| Prior radiation therapy | |
| Yes | 27 |
| No | 5 |
| MIBG-I125/131 | 15 |
| Prior therapy 13-cis-retinoic acid | |
| Yes | 23 |
| No | 9 |
| Prior anti-GD2 antibody therapy | |
| Yes | 6 |
| No | 26 |
| History of tumor | |
| Recurrent/progressive | 29 |
| Refractory | 2 |
| Persistent | 1 |
| Sites of tumor at entry | |
| Bone Marrow | |
| Yes* | 13 |
| No | 19 |
| Measurable soft tissue disease by CT/MRI Scan | |
| Yes | 13 |
| No | 19 |
| MIBG disease | |
| Yes | 23 |
| No | 7 |
| Not done | 1 |
| Non-avid | 1 |
| MIBG disease only | 8 |
| Bone marrow only | 5^ |
| Soft tissue only | 4@ |
| MIBG + bone marrow | 6 |
| Soft tissue + MIBG | 7 |
| Soft tissue + MIBG + bone marrow | 2 |
All stem cell transplantation/support was autologous.
Includes one patient with ganglioneuroma.
Includes one patient did not have MIBG done at study entry.
Includes one patient did not have MIBG done at study entry; another patient had MIBG done (results unknown) but was known to be MIBG non-avid
Toxicities
Tables II and III summarize the observed toxicities. In the single patient who experienced a DLT during Course 1, an isolated Grade 2 increase in alkaline phosphatase was noted on Day +19. Course 2 was delayed and, by Day +28, alkaline phosphatase had increased to Grade 3 with a Grade 1 AST elevation. The patient was asymptomatic; other hepatic function tests were normal. Alkaline phosphatase fractionation showed 40% liver/60% bone-derived isoenzymes. The patient was taken off protocol therapy for progressive disease (without bone sites) on Day 33 of Course 1. Alkaline phosphatase normalized by Day 47. Another patient had Grade 4 mixed alkaline phosphatase elevation at end of Course 5 without other hepatic abnormalities, and went off therapy at Course 6 for tumor progression without bone sites. Alkaline phosphatase normalized 3 months later. Among sixteen patients with Grade 1 or 2 transaminase elevations, ten resolved elevations in a median of 16 days (range 6 - 77) while transaminase elevations persisted in six at protocol completion. Of two patients with Grade 3 transaminases, one resolved in 15 days, while the other resolved AST elevation in 23 days but with an ongoing Grade 1 ALT elevation persisting at therapy completion. The 774 mg/m2/day dosing level was expanded after a tonic-clonic seizure in a febrile patient with concurrent, non-neutropenic, central catheter-associated Acinetobacter bacteremia. The patient was treated with levetiracetam and tolerated subsequent courses without a dose reduction. No patient had febrile neutropenia. One patient at 2,210 mg/m2/day had tumor progression after Course 2 and was also found to have a de novo Langerhans cell histiocytosis lesion of the skull with soft tissue extension [35] graded as unrelated to protocol therapy. No MTD was defined based on toxicity.
Table II.
All Grade 3 and 4 Toxicities (excluding unrelated toxicities)
| Daily Dose (mg/m2) | Patients entered (evaluable) | Patient Study Number | Course1 | Toxicity | Grade | Attribution | DLT* |
|---|---|---|---|---|---|---|---|
| 352 | 6 (6) | 01 | 2 | Platelet count decrease | 3 | Possible | No |
| 03 | 1 | Alkaline phosphatase increase | 3 | Possible | Yes | ||
| PTT | 3 | Unlikely | No | ||||
| 458 | 3 (3) | 08 | 1 | ALT | 3 | Probable | No |
| AST | 3 | Probable | No | ||||
| 595 | 3 (3) | 11 | 1 | Pain - Chest wall | 3 | Unlikely | No |
| 2 | Pain - Chest wall | 3 | Unlikely | No | |||
| 774 | 6 (6)2 | 17 | 5 | Alkaline phosphatase increase3 | 4 | Possible | Yes |
| 6 | Alkaline phosphatase increase | 4 | Possible | Yes | |||
| 15 | 1 | Catheter-related Infection with normal ANC; Grade 2 seizure | 3 | Unlikely | No@ | ||
| Hypokalemia | 3 | Possible | No | ||||
| Hyponatremia | 3 | Possible | No | ||||
| Hemoglobin decrease | 3 | Possible | No | ||||
| 1006 | 3(3) | N/A | N/A | N/A | N/A | N/A | N/A |
| 1308 | 4(3) | 22 | 1 | Hypokalemia | 3 | Possible | No |
| 1700 | 3 (3) | 27 | 2 | ALT | 3 | Probable | Yes |
| AST | 3 | Probable | Yes | ||||
| Diarrhea | 3 | Definite | No | ||||
| 2210 | 4(3) | N/A | N/A | N/A | N/A | N/A | N/A |
Only DLT occurring in Course 1 was utilized for determination of Maximal Tolerated Dose.
Dose Level expanded due to bacteremia which did not meet protocol DLT criteria.
Onset of Grade 1 Alkaline Phosphatase (AP) during Course 4; increased to Grade 4 at end of Course 5, patient went off-therapy after two doses during Course 6 due to tumor progression
Table III.
Grade 1 and 2 toxicities (excluding unrelated toxicities
| Daily Dose (mg/m2) | Toxicity | Number patients with maximal Toxicity Grade | |
|---|---|---|---|
| 1 | 2 | ||
| 352 (n=6) | Hepatic | 3 | 1 |
| Constitutional Symptoms | 1 | 0 | |
| Pain | 0 | 1 | |
| Coagulation | 1 | 0 | |
| Dermatology/Skin | 3 | 1 | |
| Metabolic/Laboratory | 5 | 0 | |
| Hematologic* | 1 | 0 | |
| 458 (n=3) | Hepatic | 1 | 1 |
| Constitutional Symptoms | 1 | 0 | |
| Renal/Genitourinary | 1 | 0 | |
| Dermatology/Skin | 2 | 1 | |
| Metabolic/Laboratory | 1 | 0 | |
| Hematologic* | 1 | 1 | |
| 595 (n=3) | Infection/Febrile Neutropenia | 0 | 1 |
| Pain | 0 | 0 | |
| Gastrointestinal | 1 | 0 | |
| Coagulation | 2 | 0 | |
| Dermatology/Skin | 0 | 1 | |
| Metabolic/Laboratory | 0 | 2 | |
| Ocular/Visual | 1 | 0 | |
| Hematologic* | 2 | 0 | |
| 774 (n=6) | Hepatic | 3 | 0 |
| Constitutional Symptoms | 0 | 1 | |
| Cardiovascular | 0 | 1 | |
| Infection/Febrile Neutropenia | 0 | 0 | |
| Pain | 1 | 0 | |
| Gastrointestinal | 3 | 0 | |
| Coagulation | 3 | 0 | |
| Dermatology/Skin | 0 | 2 | |
| Metabolic/Laboratory | 2 | 1 | |
| Neurology | 0 | 1 | |
| Hematologic* | 3 | 1 | |
| 1006 (n=3) | Hepatic | 0 | 1 |
| Pain | 2 | 0 | |
| Hemorrhage | 1 | 0 | |
| Gastrointestinal | 1 | 2 | |
| Dermatology/Skin | 1 | 1 | |
| Metabolic/Laboratory | 2 | 0 | |
| Hematologic* | 1 | 1 | |
| 1308 (n=4) | Hepatic | 1 | 1 |
| Constitutional Symptoms | 1 | 0 | |
| Pain | 2 | 1 | |
| Gastrointestinal | 1 | 1 | |
| Dermatology/Skin | 2 | 0 | |
| Metabolic/Laboratory | 1 | 1 | |
| Neurology | 0 | 1 | |
| Ocular/Visual | 2 | 0 | |
| Hematologic* | 2 | 1 | |
| 1700 (n=3) | Hepatic | 2 | 0 |
| Constitutional Symptoms | 1 | 0 | |
| Pain | 2 | 1 | |
| Hemorrhage | 1 | 0 | |
| Gastrointestinal | 2 | 0 | |
| Coagulation | 2 | 0 | |
| Dermatology/Skin | 0 | 1 | |
| Metabolic/Laboratory | 1 | 2 | |
| Musculoskeletal | 0 | 1 | |
| Neurology | 1 | 0 | |
| Ocular/Visual | 1 | 0 | |
| Hematologic* | 1 | 2 | |
| 2210# (n=4) | Hepatic | 3 | 0 |
| Constitutional Symptoms | 1 | 0 | |
| Pain | 1 | 0 | |
| Gastrointestinal | 2 | 2 | |
| Dermatology/Skin | 1 | 2 | |
| Metabolic/Laboratory | 3 | 0 | |
| Neurology | 1 | 0 | |
| Ocular/Visual | 0 | 1 | |
| Allergy | 1 | 0 | |
| Hematologic* | 0 | 2 | |
G1/G2 hematologic toxicities included leukopenia (n = 13), neutropenia (n = 7), lymphopenia (n = 10), thrombocytopenia (n = 10), and anemia (n = 11).
One patient able to take only half of initial dose due to nausea/vomiting was removed from study.
Tumor Response
Twenty-nine patients were evaluable for disease response (measurable soft tissue disease by CT/MRI scan, n = 11; MIBG avid sites and/or bone marrow involvement without measurable soft tissue disease, n = 18). There were four confirmed complete responses in patients with bone marrow or bone disease-only treated with 10 – 46 courses (Table IV). One of these (Patient 13) enrolled with relapsed marrow disease two months after completing ch14.18+IL-2 immunotherapy on ANBL0032 protocol (NCT00026312) and withdrew from protocol therapy in CR after 10 courses due to dissatisfaction with the liquid nutritional supplement carrier. This patient was still in CR at 36 months off protocol therapy without further treatment. Patient 28 enrolled with relapsed marrow disease after receiving frontline therapy with isotretinoin on the ANBL0032 protocol, received 46 courses of fenretinide, and remained in CR at end of therapy. Of the two other complete responders, Patient 18 enrolled with persistent MIBG avid sites after two courses of I-131 MIBG given 6 and 4 months prior followed by two courses of cyclophosphamide/zoledronic acid, and Patient 30 had bone marrow progression prior to transplant, was in a complete response following transplant, and then had another bone marrow relapse after three courses of isotretinoin on ANBL02P1 (NCT00070200) prior to enrollment. These two complete responders went off therapy for PD after 17 and 5 courses respectively. Six patients had stable disease (SD) for a median of 5.5 courses (range 4 – 26+ courses)(Table IV). One of these six patients (at 1006 mg/m2/day) withdrew from protocol therapy in SD after Course 4 to pursue other treatment options.
Table IV.
Patients with overall response of stable disease or better (n = 29 evaluable)
| Patient | Daily Dose (mg/m2) |
Number Courses |
Tumor Sites at Entry | Best Overall Response |
4-HPR (μM) C1 / C2 |
Comments and Follow-up |
||
|---|---|---|---|---|---|---|---|---|
| CT Longest Diameter |
MIBG Curie Score |
Marrow % Tumor* |
||||||
| 10# | 595¥ | 26 | 0 cm | 15 | <5% | SD | 19.3 / 18.2 | SD, Negative Marrow, at Exit |
| 17£ | 774¥ | 6 | 4.3 | 1 | Negative | SD | 2.2 / 3.1 | PD - Soft Tissue |
| 13@ | 774§ | 10 | 0 | 0 | <5% | CR | 17.6 / 13.8 | Withdrew, CR at Exit† |
| 18£ | 774¥§ | 17 | 0 | 3 | Negative | CR | 22.1 / 18.8 | PD |
| 15£ | 774 | 4 | 3.6 | 2% | Negative | SD | 9.1 / 5.7 | PD – Bone |
| 19£ | 1006¥ | 4 | 0 | 3 | Negative | SD | 5.3 / n.d. | Withdrew |
| 22£ | 1308 | 7 | 0 | 0 | Positive& | SD | 2.9$/ 15.2 | PD – Soft Tissue‡ |
| 28£ | 1700 | 46 | 0 | 0 | <1% | CR** | 17.6 / 14.9 | CR at Exit |
| 29£ | 2210 | 5 | 4.2 | Non-Avid | Negative | SD | 11.8 / 9.5 | PD - Soft Tissue |
| 30£ | 2210 | 10 | 0 | 0 | <5% | CR | 16.6 / 21.1 | PD |
C1 = Course 1, Day 6, peak plasma level; C2 = Course 2, Day 6, peak plasma level; SD, stable disease; CR, complete response; PD, progressive disease; CT, computed axial tomography scan, longest tumor diameter; MIBG, metaiodobenzylguanidine scan; n.d. = not done
Refractory disease at entry.
Persistent disease at entry.
Prior history of progressive disease.
Assessed by routine morphology
History of 131I-MIBG therapy
History of anti-GD2 antibody therapy
CR status at +36 months after withdrawal from protocol therapy without further treatment
Baseline MIBG image quality inadequate to grade, post-Course 2 Curie score was 2
Central review not possible.
Needed nasogastric tube for delivery for Courses 2+
Progressed with new intraparenchymal brain metastasis.
<1% tumor at entry, 8 subsequent negative marrows, last marrow done end of Course 43, stopped therapy after Course 46 due to prolonged CR.
Pharmacokinetics
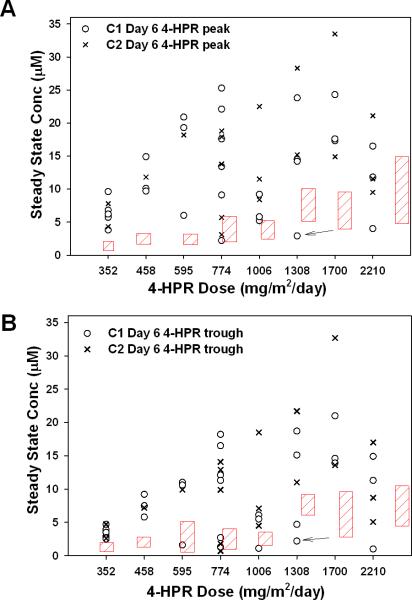
Plasma pharmacokinetics (PK) of 4-HPR, and metabolite 4-MPR, were determined for thirty-one patients around the first fenretinide dose on Day 0 and Day 6 of Course 1, and for eighteen patients on Day 0 and Day 6 of Course 2. Day 6 peak and trough plasma levels for 4-HPR for Courses 1 and 2 are shown in Fig. 1. Mean Day 6 4-HPR peak plasma levels (Fig. 1A) for Course 1 and/or Course 2 exceeded (P<0.05) those reported previously using similar doses and schedules of fenretinide delivered in corn oil capsules to pediatric patients at all dose levels except 2210 mg/m2/day [25]. Mean Day 6 4-HPR plasma trough levels (Fig. 1B) for Course 1 and/or Course 2 also exceeded (P<0.05) those reported previously using similar doses and schedules of fenretinide delivered in corn oil capsules. There was a decrease in plasma drug levels at 2210 mg/m2/day compared to the previous dose level. There was no clear difference in Course 1, Day 6, dose one, trough or peak fenretinide plasma levels between the four complete responders (Table V). There was a trend towards higher Course 1, Day 6, first dose, median trough or peak fenretinide plasma levels between the four complete responders (CR) and non-CR patients (CR trough = 14.4 μM vs. non-CR = 4.7 μM, P = 0.03; CR peak = 17.6 μM vs. non-CR = 9.2 μM, P = 0.06) but numbers were deemed inadequate to support definitive conclusions.
Fig. 1.
Steady-state fenretinide plasma concentrations. 4-HPR plasma concentration (A) peaks and (B) troughs of individual patients measured around the morning dose on the seventh day (Day 6), i.e. steady-state, of Course 1 (C1)(o) and Course 2 (C2)(X). Trough level was the plasma level measured immediately prior to the morning dose on Day 6; peak level was the highest plasma value measured after the morning dose on Day 6. Arrow indicates Course 1 value for a patient requiring nasogastric tube delivery for Courses 2+. Red hatching represent steady–state 4-HPR plasma levels (mean ± SD) previously reported in a pediatric phase I study using 4-HPR delivered in corn oil-containing capsules at similar dosing and schedule [25].
DISCUSSION
Previous trials in recurrent/resistant neuroblastoma using fenretinide (4-HPR) in corn oil capsules demonstrated modest anticancer activity [24, 25]. However, inter-patient variability in attained plasma drug levels may have confounded response rate assessment. The capsular formulation was also difficult to administer to younger children. Based on this rationale, we conducted a phase I study of a novel oral fenretinide formulation, 4-HPR/LXS oral powder. LYM-X-SORB™ (LXS) is an organized lipid complex that facilitates increased intestinal lymphatic drug absorption.
This trial demonstrated that 4-HPR/LXS powder attained two- to six-fold higher 4-HPR plasma levels (peak and trough) than equivalent 4-HPR doses delivered using corn oil capsules on similar schedules (Fig. 1) [24, 25]. In a phase I corn oil capsule study (COG 09709), Day 7 peak plasma levels around a single dose at the MTD of 2475 mg/m2/day divided into three doses were 9.9 ± 5.0 μM [25]. Similarly, a phase II corn oil capsule study using a dose of 2475 mg/m2/day (COG ANBL0321) reported Day 7 mean trough plasma levels of 7.3 μM (coefficient of variation 40 - 56%, range <1 – 13 μM) and Day 7 mean peak plasma levels of 8.3 μM (range 3 – 17 μM) [24]. In a phase I neuroblastoma study using corn oil capsules in a single dose, daily, for four weeks, followed by one week rest, Day +28 mean peak plasma levels were 12.9 ± 6.3 μM at 4,000 mg/m2/day [23]. Overall, these data suggest that 4-HPR/LXS oral powder can achieve higher fenretinide plasma levels than the corn oil capsule formulation.
Although a plateau in peak plasma levels was observed with 4-HPR/LXS above 774 mg/m2/day, trough plasma levels continued to trend upwards (Fig. 1). Plasma levels at 2210 mg/m2/day were lower than at the preceding dose levels. Based on considerations including total dosing volume and the apparent plateau of plasma drug levels, to optimize patient compliance, a dose of 1500 mg 4-HPR/m2/day, divided into three doses (TID), on Days 0 - 6 of repeating 21-day courses, was recommended for future trials
Inter-patient variability in plasma levels, previously reported with the corn oil capsules [25], was still observed with 4-HPR/LXS powder (Fig. 1), albeit at higher absolute plasma levels, suggesting that such variability might reflect intrinsic patient variation in 4-HPR metabolism (i.e., high and low metabolizers). This suggested that plasma levels might be further increased by concurrent administration of a P450 inhibitor, such as was attempted with trans-retinoic acid levels using ketoconazole [36]. Indeed, ketoconazole administered concurrently with 4-HPR/LXS powder increased 4-HPR plasma and tissue levels in mice [37]. To test this, an additional patient cohort of this study is concurrently receiving 4-HPR/LXS oral powder (1500 mg/m2/day) with ketoconazole (3 – 6 mg/kg/day).
The higher 4-HPR plasma levels obtained did not appreciably increase toxicity (Tables II and III) compared to previous studies. Moderate, reversible liver toxicity was the most significant. Hematopoietic toxicity was minimal. Diarrhea, which was dose-limiting in an adult study of 4-HPR/LXS powder, was not a DLT [38]. No maximal tolerated dosage was reached based on toxicity. Dose escalation was limited by the total drug volume required above the 2210 mg/m2/day dose level. Patient compliance was acceptable, although some expressed dissatisfaction with the liquid nutritional supplement carrier mandated to ensure uniformity of delivery for PK analysis. The protocol was subsequently amended to allow patient choice of dietary carrier, excluding whole-fat milk products which are known to destabilize the LXS lipid matrix (results pending). Of note, one patient unable to take 4-HPR/LXS powder orally successfully received drug via a naso-gastric feeding tube.
Four complete responses (CR) were observed among eighteen heavily pretreated patients treated at doses at or above 774 mg/m2/day (Table IV), two of which were sustained for 30+ and 36+ months. Six patients had stable disease for at least four courses, including patients with a Curie score of 15, and a soft tissue mass of 4.3 centimeters. Although all complete responses were in patients with limited marrow involvement or in I131mIBG-avid disease without RECIST defined measurable soft tissue disease, these results may have clinical significance since bone and bone marrow are the most common sites of relapse in high risk neuroblastoma patients [39], Curie scores are predictive of outcome in newly diagnosed patients [40, 41], and two previous studies using fenretinide corn oil capsules reported a shorter time to progression in patients who entered with marrow disease, with or without other sites of tumor, even in patients with minimal marrow-only disease [24, 25]. To address this question, several recent COG phase II neuroblastoma studies have grouped patients with or without measurable soft tissue disease as separate cohorts for response evaluation [42, 43], although currently there is still insufficient data to determine if response rates vary between these two cohorts. As accurate quantitation of bone marrow tumor is difficult in neuroblastoma due to inherent sampling variability, only complete responses (requiring at least two consecutive negative bone marrows) or stable disease were reported for bone marrow response. It is anticipated that real time quantitative polymerase chain reaction methodologies for the quantitation of bone marrow tumor currently being tested in clinical trials will improve future marrow assessment [40].
Responses on the phase II capsular formulation trial included 1 PR (I131mIBG-avid sites only) and 13 SD for median 15 (range 4 - 45+) courses among 59 evaluable patients [24]. Comparison of responses between the capsular and LXS powder formulations will require the analysis of additional patients being treated at the recommended phase II dosing of 4-HPR/LXS powder in ongoing expansion cohorts to the present study. However, all patients achieving CR with the LXS powder formulation had 4-HPR plasma levels of at least 15 μM (Table IV), suggesting that plasma drug levels may affect response and supporting strategies to further increase 4-HPR plasma levels (such as with concurrent ketoconazole administration as described above).
In summary, 4-HPR in an oral organized lipid complex obtained higher plasma levels than the previously-used capsule formulation with minimal toxicity. Complete tumor responses were observed in patients with tumor involvement limited to bone marrow and/or bone metastases, suggesting that future studies should further explore activity in this subgroup of patients. A recommended dose of 4-HPR/LXS oral powder for future studies is 1,500 mg/m2/day, divided TID, × 7 days, every three weeks.
Supplementary Material
ACKNOWLEDGMENTS
We recognize the late Dr. David W. Yesair, BioMolecular Products, the inventor of LYM-X-SORB™ and Drs. Walter A. Shaw and Stephen W. Burgess, of Avanti Polar Lipids, Inc., for their expertise in the development of 4-HPR/LXS oral powder. We thank Denice Tsao-Wei for statistical support.
Grant sponsors: National Cancer Institute, Developmental Therapeutics Program, Rapid Access to Intervention Development Program; National Cancer Institute, Grant numbers: R01 CA100895 and P01 CA81403. The Cancer Prevention and Research Institute of Texas, Grant number: RP10072. The Children's Neuroblastoma Cancer Foundation. Alex's Lemonade Stand Foundation. Children's Neuroblastoma Cancer Foundation. Pediatric Cancer Research Group. Dougherty Family Foundation. Evan T. J. Dunbar Neuroblastoma Foundation. Douglass Michael Fuller Foundation. Neuroblastoma Children's Cancer Society. The University of Southern California-Childrens Hospital Los Angeles Institute for Pediatric Research.
Footnotes
Presented, in part, at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29 – June 2, 2009, Orlando, FL (abstr. 10009).
Conflict of Interest
The Children's Hospital Los Angeles (CHLA) holds patents and/or patent applications on Fenretinide/LYM-X-SORB™ (LXS) oral powder (the study drug). CHLA and co-inventors of Fenretinide/LXS oral powder, Drs. Barry J. Maurer and C. Patrick Reynolds, Texas Tech University Health Sciences Center, Lubbock, TX, may potentially benefit financially from the development and future use of the study drug.
REFERENCES
- 1.Formelli F, Cleris L. Synthetic retinoid fenretinide is effective against a human ovarian carcinoma xenograft and potentiates cisplatin activity. Cancer Res. 1993;53:5374–5376. [PubMed] [Google Scholar]
- 2.Delia D, Aiello A, Lombardi L, et al. N-(4-hydroxyphenyl)retinamide induces apoptosis of malignant hemopoietic cell lines including those unresponsive to retinoic acid. Cancer Res. 1993;53:6036–6041. [PubMed] [Google Scholar]
- 3.Zou C-P, Kurie JM, Lotan D, et al. Higher potency of N-(4-hydroxyphenyl)retinamide than all-trans-retinoic acid in induction of apoptosis in non-small cell lung cancer cell lines. Clin Cancer Res. 1998;4:1345–1355. [PubMed] [Google Scholar]
- 4.Maurer BJ, Metelitsa LS, Seeger RC, et al. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-Hydroxyphenyl)-retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 5.Oridate N, Suzuki S, Higuchi M, et al. Involvement of reactive oxygen species in N-(4-hydroxyphenyl)retinamide-induced apoptosis in cervical carcinoma cells. J Natl Cancer Inst. 1997;89:1191–1198. doi: 10.1093/jnci/89.16.1191. [DOI] [PubMed] [Google Scholar]
- 6.Delia D, Aiello A, Meroni L, et al. Role of antioxidants and intracellular free radicals in retinamide-induced cell death. Carcinogenesis. 1997;18:943–948. doi: 10.1093/carcin/18.5.943. [DOI] [PubMed] [Google Scholar]
- 7.Sun SY, Li W, Yue P, et al. Mediation of N-(4-hydroxyphenyl)retinamide-induced apoptosis in human cancer cells by different mechanisms. Cancer Res. 1999;59:2493–2498. [PubMed] [Google Scholar]
- 8.Maurer BJ, Melton L, Billups C, et al. Synergistic Cytotoxicity in Solid Tumor Cell Lines Between N-(4-hydroxyphenyl)retinamide and Modulators of Ceramide Metabolism. J Natl Cancer Inst. 2000;92:1897–1909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell PH, Guo W, Reynolds CP, Maurer BJ. N-(4-hydroxyphenyl)retinamide increases ceramide and is cytotoxic to acute lymphoblastic leukemia cell lines, but not to non-malignant lymphocytes. Leukemia. 2002;16:902–910. doi: 10.1038/sj.leu.2402485. [DOI] [PubMed] [Google Scholar]
- 10.Batra S, Reynolds CP, Maurer BJ. Fenretinide Cytotoxicity for Ewing's Sarcoma (ES) and Primitive Neuroectodermal Tumor (PNET) Cell Lines is Decreased by Hypoxia and Synergistically Enhanced by Ceramide Modulators. Cancer Res. 2004:5415–5424. doi: 10.1158/0008-5472.CAN-04-0377. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Maurer BJ, Liu Y-Y, et al. N-(4-Hydroxyphenyl)retinamide increases dihydroceramides and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol Cancer Ther. 2008;7:2967–2976. doi: 10.1158/1535-7163.MCT-08-0549. [DOI] [PubMed] [Google Scholar]
- 12.Kraveka JM, Li L, Szulc ZM, et al. Involvement of the dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282:16718–16728. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahmaniyan M, Curley RW, Jr., Obeid LM, et al. Identification of Dihydroceramide Desaturase as a Direct in vitro target for Fenretinide. J Biol Chem. 2011;286:24754–24764. doi: 10.1074/jbc.M111.250779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribatti D, Alessandri G, Baronio M, et al. Inhibition of neuroblastoma-induced angiogenesis by fenretinide. Int J Cancer. 2001;94:314–321. doi: 10.1002/ijc.1441. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Z, Matsuura T, Popoff K, Ross AC. Effects of N-(4-hydroxyphenyl)-retinamide on the number and cytotoxicity of natural killer cells in vitamin-A-sufficient and - deficient rats. Nat Immun. 1994;13:280–288. [PubMed] [Google Scholar]
- 16.Gibbs IS, Kotwal PM. Pharmaceutical composition of N-(4-hydroxyphenyl) retinamide having increased bioavailability. 1987 U.S. Patent 4,665,098.
- 17.Costa A, Formelli F, Chiesa F, et al. Prospects of chemoprevention of human cancers with the synthetic retinoid fenretinide. Cancer Res. 1994;54:2032–2037. [PubMed] [Google Scholar]
- 18.Cobleigh MA, Dowlatshahi K, Deutsch TA, et al. Phase I/II trial of tamoxifen with or without fenretinide, an analog of vitamin A, in women with metastatic breast cancer. J Clin Oncol. 1993;11:474–477. doi: 10.1200/JCO.1993.11.3.474. [DOI] [PubMed] [Google Scholar]
- 19.Chiesa F, Tradati N, Marazza M, et al. Fenretinide (4-HPR) in chemoprevention of oral leukoplakia. J Cell Biochem Suppl. 1993;17F:255–261. doi: 10.1002/jcb.240531038. [DOI] [PubMed] [Google Scholar]
- 20.Formelli F, Clerici M, Campa T, et al. Five-year administration of fenretinide: pharmacokinetics and effects on plasma retinol concentrations. J Clin Oncol. 1993;11:2036–2042. doi: 10.1200/JCO.1993.11.10.2036. [DOI] [PubMed] [Google Scholar]
- 21.Costa A, Malone W, Perloff M, et al. Tolerability of the synthetic retinoid Fenretinide (HPR). Eur J Cancer Clin Oncol. 1989;25:805–808. doi: 10.1016/0277-5379(89)90124-7. [DOI] [PubMed] [Google Scholar]
- 22.Camerini T, Mariani L, de Palo G, et al. Safety of the synthetic retinoid fenretinide: long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J Clin Oncol. 2001;19:1664–1670. doi: 10.1200/JCO.2001.19.6.1664. [DOI] [PubMed] [Google Scholar]
- 23.Garaventa A, Luksch R, Piccolo MS, et al. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin Cancer Res. 2003;9:2032–2039. [PubMed] [Google Scholar]
- 24.Villablanca JG, London WB, Naranjo A, et al. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: A report from the Children's Oncology Group NSC #374551; IND# 40294. Clin Cancer Res. 2011;17:6858–6866. doi: 10.1158/1078-0432.CCR-11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villablanca JG, Krailo MD, Ames MM, et al. Phase I trial of oral Fenretinide in children with high risk solid tumors: A report from the Children's Oncology Group (CCG 09709). J Clin Oncol. 2006;24:3423–3430. doi: 10.1200/JCO.2005.03.9271. [DOI] [PubMed] [Google Scholar]
- 26.Schneider BJ, Worden FP, Gadgeel SM, et al. Phase II trial of fenretinide (NSC 374551) in patients with recurrent small cell lung cancer. Invest New Drugs. 2009;27:571–578. doi: 10.1007/s10637-009-9228-6. [DOI] [PubMed] [Google Scholar]
- 27.Maurer BJ, Kalous O, Yesair DW, et al. Improved oral delivery of N-(4-hydroxyphenyl)retinamide with a novel LYM-X-SORB organized lipid complex. Clin Cancer Res. 2007;13:3079–3086. doi: 10.1158/1078-0432.CCR-06-1889. [DOI] [PubMed] [Google Scholar]
- 28.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. Prog Clin Biol Res. 1994;385:363–9. 363–369. [PubMed] [Google Scholar]
- 29.Lin Y, Shih WJ. Statistical properties of the traditional algorithm-based designs for phase I cancer clinical trials. Biostatistics. 2001;2:203–215. doi: 10.1093/biostatistics/2.2.203. [DOI] [PubMed] [Google Scholar]
- 30.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. Prog Clin Biol Res. 1994;385:363–9. 363–369. [PubMed] [Google Scholar]
- 31.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 32.Ady N, Zucker JM, Asselain B, et al. A new 123I-MIBG whole body scan scoring method--application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer. 1995;31A:256–261. doi: 10.1016/0959-8049(94)00509-4. [DOI] [PubMed] [Google Scholar]
- 33.Matthay KK, Quach A, Huberty J, et al. Iodine131--metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: a new approaches to neuroblastoma therapy phase I study. J Clin Oncol. 2009;27:1020–1025. doi: 10.1200/JCO.2007.15.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vratilova J, Frgala T, Maurer BJ, Patrick RC. Liquid chromatography method for quantifying N-(4-hydroxyphenyl)retinamide and N-(4-methoxyphenyl)retinamide in tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;808:125–130. doi: 10.1016/j.jchromb.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 35.Rayburg M, Towbin A, Yin H, et al. Langerhans cell histiocytosis in a patient with stage 4 neuroblastoma receiving oral fenretinide. Pediatr Blood Cancer. 2009;53:1111–1113. doi: 10.1002/pbc.22200. [DOI] [PubMed] [Google Scholar]
- 36.Lee JS, Newman RA, Lippman SM, et al. Phase I evaluation of all-trans retinoic acid with and without ketoconazole in adults with solid tumors. J Clin Oncol. 1995;13:1501–1508. doi: 10.1200/JCO.1995.13.6.1501. [DOI] [PubMed] [Google Scholar]
- 37.Cooper JP, Hwang K, Singh H, et al. Fenretinide metabolism in humans and mice: utilizing pharmacological modulation of its metabolic pathway to increase systemic exposure. Br J Pharmacol. 2011;163:1263–1275. doi: 10.1111/j.1476-5381.2011.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kummar S, Gutierrez ME, Maurer BJ, et al. Phase I trial of fenretinide lym-x-sorb oral powder in adults with solid tumors and lymphomas. Anticancer Res. 2011;31:961–966. [PMC free article] [PubMed] [Google Scholar]
- 39.DuBois SG, Kalika Y, Lukens JN, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Naranjo A, Parisi MT, Shulkin BL, et al. Comparison of 123I-metaiodobenzylguanidine (MIBG) and 131I-MIBG semi-quantitative scores in predicting survival in patients with stage 4 neuroblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2011;56:1041–1045. doi: 10.1002/pbc.22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–2491. doi: 10.1200/JCO.2003.09.122. [DOI] [PubMed] [Google Scholar]
- 42.Bagatell R, London WB, Wagner LM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2011;29:208–213. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.